Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rearing Conditions
2.3. Proximate Analysis
2.4. Amino Acid Analysis
2.5. Mineral Analysis
2.6. Fatty Acid Analysis
2.7. ANN Modelling
2.8. Global Sensitivity Analysis
2.9. Statistical Analyses
2.10. Multi-Objective Optimization (MOO)
3. Results
3.1. ANN Models
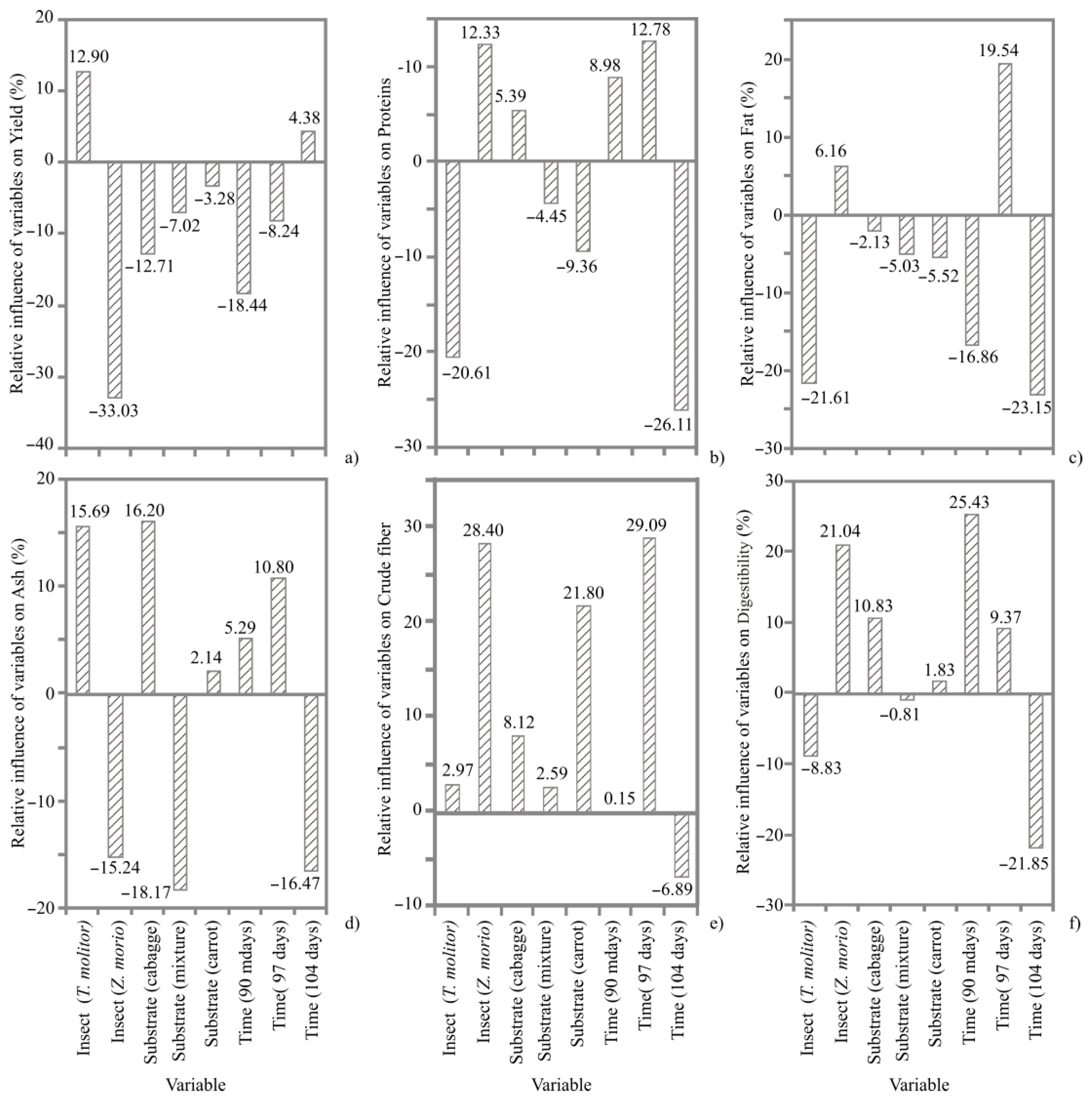
3.2. Proximate Composition
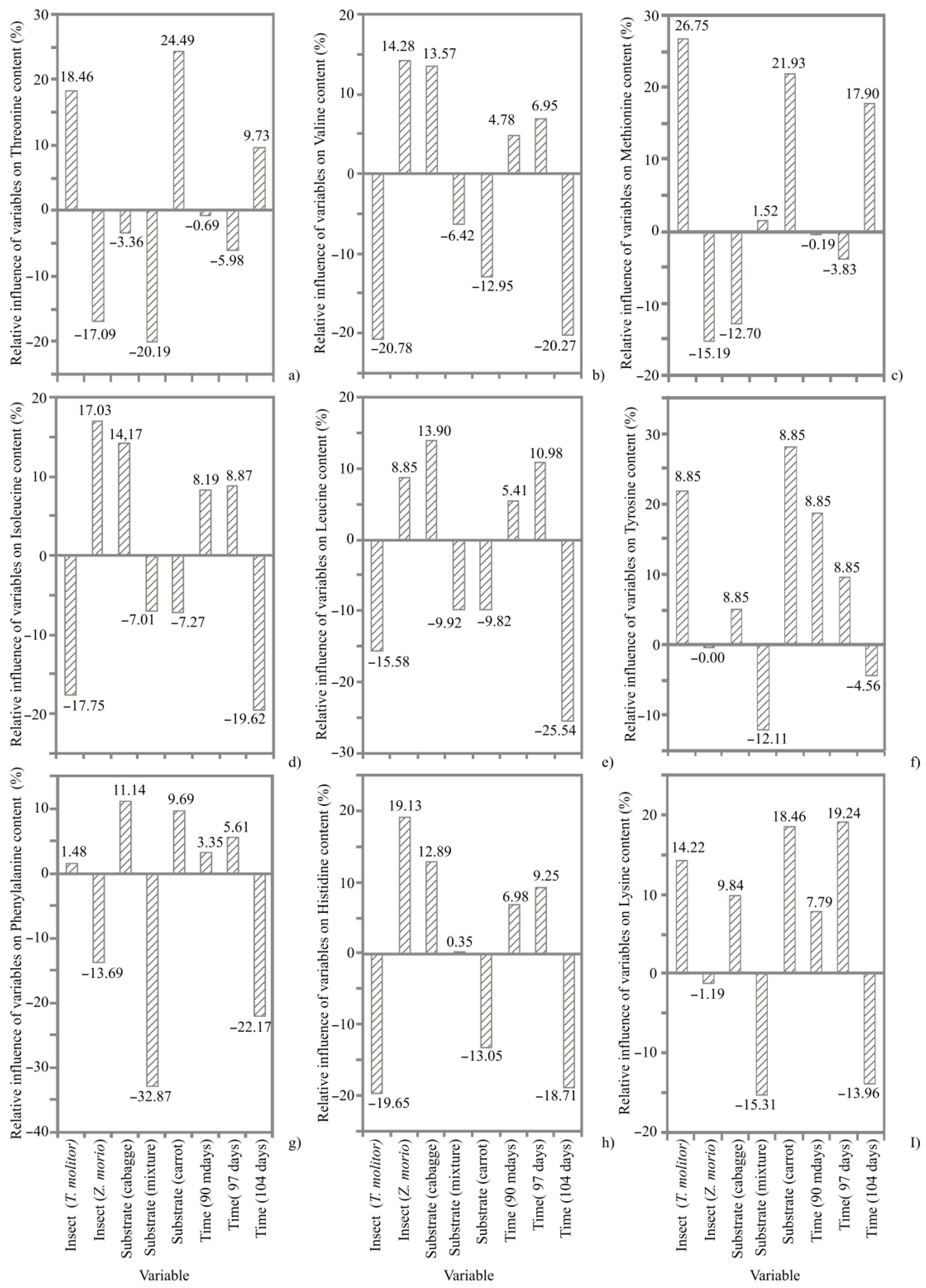
3.3. Amino Acid Composition
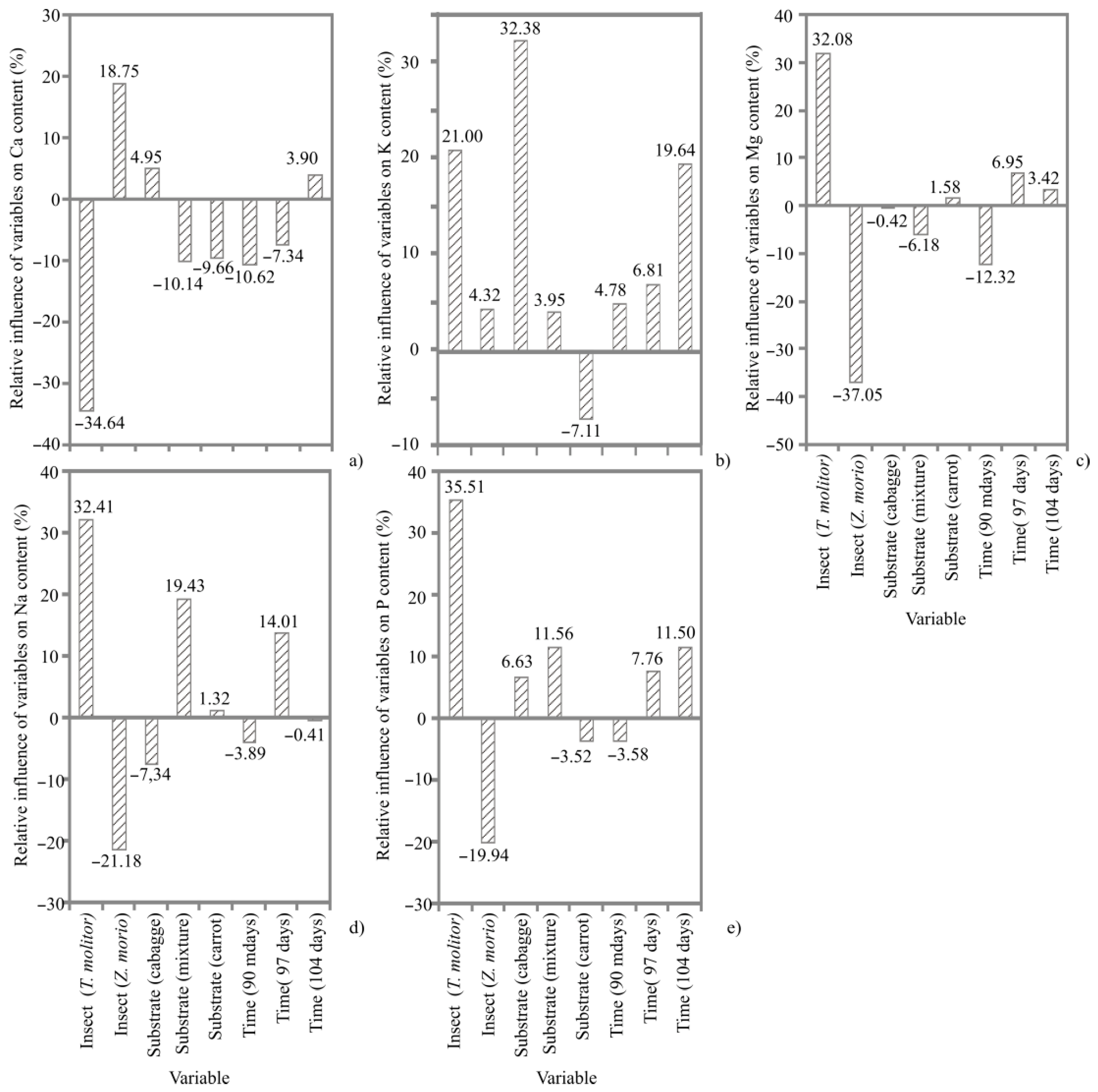
3.4. Mineral Content
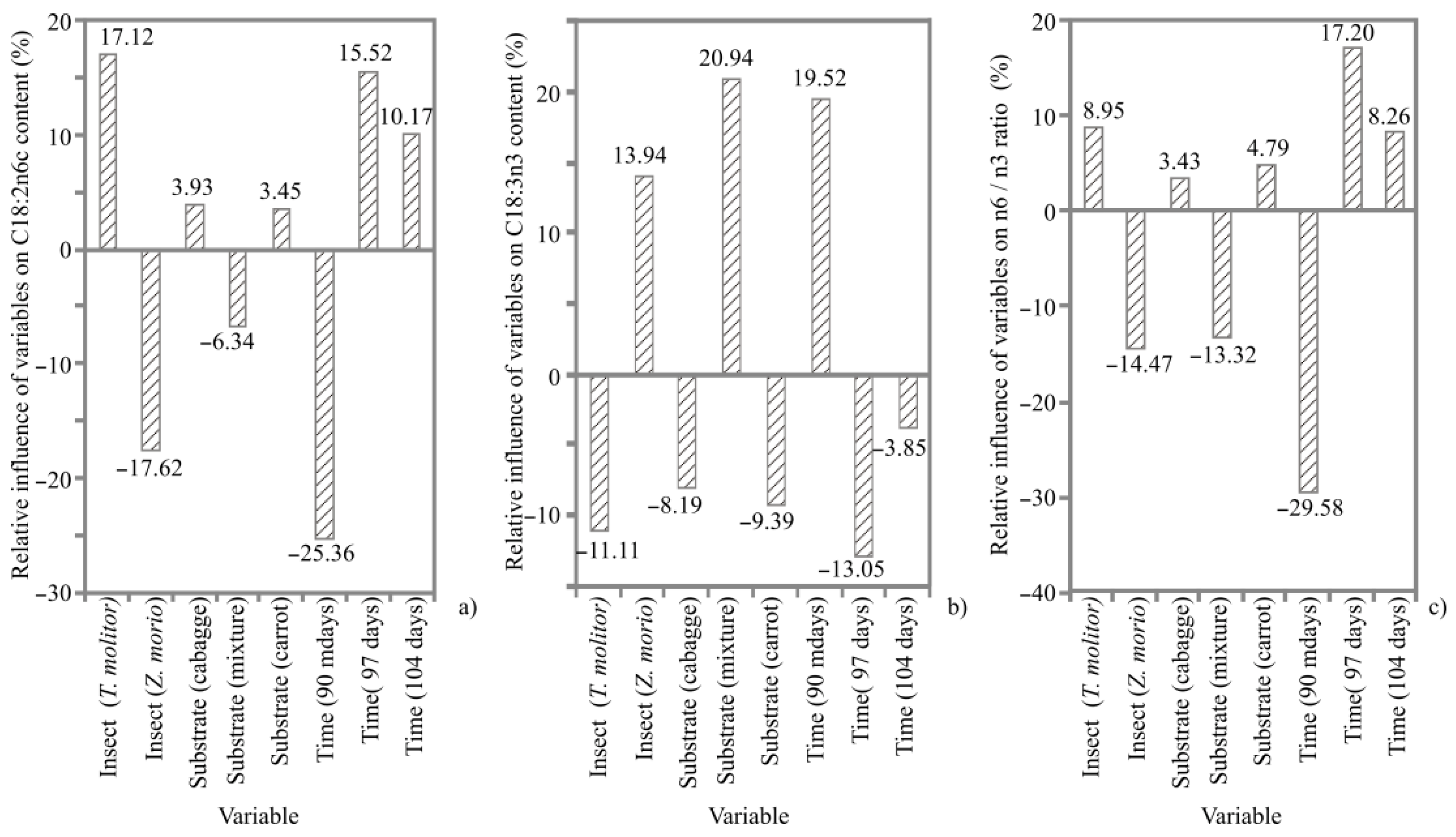
3.5. Fatty Acid Composition
3.6. Multi-Objective Optimization of the Rearing Conditions of the ANN
4. Discussion
4.1. Proximate Composition
4.2. Amino Acid Composition
4.3. Mineral Content
4.4. Fatty Acid Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food and Agriculture: Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; ISBN 978-92-5-131789-1. [Google Scholar]
- Verbeke, W.; Spranghers, T.; De Clercq, P.; De Smet, S.; Sas, B.; Eeckhout, M. Insects in animal feed: Acceptance and its determinants among farmers, agriculture sector stakeholders and citizens. Anim. Feed Sci. Technol. 2015, 204, 72–87. [Google Scholar] [CrossRef]
- Meyer-Rochow, V. Can insects help to ease the problem of world food shortage? Search 1975, 6, 261–262. [Google Scholar]
- Van Huis, A. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Garino, C.; Zagon, J.; Nesic, K. Novel real-time PCR protocol for the detection of house cricket (Acheta domesticus) in feed. Anim. Feed Sci. Technol. 2021, 280, 115057. [Google Scholar] [CrossRef]
- Pyo, S.J.; Kang, D.G.; Jung, C.; Sohn, H.Y. Anti-Thrombotic, Anti-Oxidant and Haemolysis Activities of Six Edible Insect Species. Foods 2020, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Gjerris, M.; Gamborg, C.; Röcklinsberg, H. Ethical aspects of insect production for food and feed. J. Insects Food Feed 2016, 2, 101–110. [Google Scholar] [CrossRef]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Yi, L.; Han, R.; Giroud, L.; Jullien, R.L. Insect Mass Production Technologies. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J., Guadalupe Roja, M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 154–201. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.; Barroso, F.G.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm ( Tenebrio molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jędras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Kulma, M.; Kou, L.; Homolková, D.; Plachý, V. Effect of developmental stage on the nutritional value of edible insects. A case study with Blaberus craniifer and Zophobas morio. J Food Compost Anal. 2020, 92, 103570. [Google Scholar] [CrossRef]
- Prachom, N.; Boonyoung, S.; Hassaan, M.S.; El-Haroun, E.; Davies, S.J. Preliminary evaluation of Superworm (Zophobas morio) larval meal as a partial protein source in experimental diets for juvenile Asian sea bass, Lates calcarifer. Aquac. Nutr. 2021, 27, 1304–1314. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C. The Superworm, Zophobas morio (Coleoptera: Tenebrionidae): A ‘Sleeping Giant’ in Nutrient Sources. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Veldkamp, T.; Bosch, G. Insects: A protein-rich feed ingredient in pig and poultry diets. Anim. Front. 2015, 5, 45–50. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Commercially Available Feeder Insects Fed Enhanced Diets During Growth. Zoo Biol. 2015, 564, 554–564. [Google Scholar] [CrossRef]
- Da Silva Lucas, A.J.; de Oliveira, L.M.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska–Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef]
- Çetin, N.; Saglam, C. Rapid detection of total phenolics, antioxidant activity and ascorbic acid of dried apples by chemometric algorithms. Food Biosci. 2022, 47, 101670. [Google Scholar] [CrossRef]
- Türkoglu, S.; Zengin, A.; Ozturk, M.; Çagrı-Mehmetoglu, A. Mathematical modelling of biocontrol effect of Cryptococcus albidus on the growth of Penicillium expansum on Fuji apple fruits. Biol. Control 2022, 170, 104908. [Google Scholar] [CrossRef]
- Wenning, M.J.; Piotrowsk, T.; Janzen, J.; Nießing, B.; Schmitt, R.H. Towards monitoring of a cricket production using instance segmentation. J. Insects Food Feed 2022, 1–10. [Google Scholar] [CrossRef]
- Baur, A.; Koch, D.; Gatternig, B.; Delgado, A. Noninvasive monitoring system for Tenebrio molitor larvae based on image processing with a watershed algorithm and a neural net approach. J. Insects Food Feed 2022, 1–8. [Google Scholar] [CrossRef]
- Kröncke, N.; Baur, A.; Böschen, V.; Demtröder, S.; Benning, R.; Delgado, A. Automation of Insect Mass Rearing and Processing Technologies of Mealworms (Tenebrio molitor). In African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Mariod, A.A., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 123–139. ISBN 978-3-030-32951-8. [Google Scholar]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Harsányi, E.; Juhász, C.; Kovács, E.; Huzsvai, L.; Pintér, R.; Fekete, G.; Varga, Z.I.; Aleksza, L.; Gyuricza, C. Evaluation of organic wastes as substrates for rearing Zophobas morio, Tenebrio molitor, and Acheta domesticus larvae as alternative feed supplements. Insects 2020, 11, 604. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Kojić, J.S.; Kojić, P.S.; Pezo, L.L.; Banjac, V.V.; Krulj, J.A.; Bodroža Solarov, M.I. Multiobjective process optimization for betaine enriched spelt flour based extrudates. J. Food Process Eng. 2018, 42, e12942. [Google Scholar] [CrossRef] [Green Version]
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Cullere, M.; Kovitvadhi, A.; Chundang, P.; Dalle Zotte, A. Effect of different killing methods on physicochemical traits, nutritional characteristics, in vitro human digestibility and oxidative stability during storage of the house cricket (Acheta domesticus L.). Innov. Food Sci. Emerg. Technol. 2020, 65, 102444. [Google Scholar] [CrossRef]
- Larouche, J.; Deschamps, M.H.; Saucier, L.; Lebeuf, Y.; Doyen, A.; Vandenberg, G.W. Effects of Killing Methods on Lipid Oxidation, Colour and Microbial Load of Black Soldier Fly (Hermetia illucens) Larvae. Animals 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC Int.: Gaithersburg, MD, USA, 2006. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC Press: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Janssen, R.; Vincken, J.; van den Broek, L.; Fogliano, V.; Lakemond, C. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Spackman, D.H.; Stein, W.H.; Moore, S. Automatic Recording Apparatus for Use in the Chromatography of Amino Acids. Anal. Chem. 1958, 30, 1190–1206. [Google Scholar] [CrossRef]
- Karlovic, S.; Bosiljkov, T.; Brncic, M.; Jezek, D.; Tripalo, B.; Dujmic, F.; Dzineva, I.; Skupnjak, A. Comparison of artificial neural network and mathematical models for drying of apple slices pre-treated with high intensity ultrasound. Bulg. J. Agric. Sci. 2013, 19, 1372–1377. [Google Scholar]
- Prevolnik, M.; Andronikov, D.; Žlender, B.; Font-i-Furnols, M.; Novič, M.; Škornjac, D.; Čandek-Potokar, M. Classification of dry-cured hams according to the maturation time using near infrared spectra and artificial neural networks. Meat Sci. 2014, 96, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pezo, L.L.; Ćurčić, B.L.; Filipović, V.S.; Nićetin, M.R.; Koprivica, G.B.; Mišljenović, N.M.; Lević, L.B. Artificial neural network model of pork meat cubes osmotic dehydration. Hem. Ind. 2013, 67, 465–475. [Google Scholar] [CrossRef]
- Iqbal, A.; Valous, N.A.; Sun, D.; Allen, P. Parsimonious classification of binary lacunarity data computed from food surface images using kernel principal component analysis and artificial neural networks. Meat Sci. 2011, 87, 107–114. [Google Scholar] [CrossRef]
- Yoon, Y.; Swales, G.; Margavio, T.M. A comparison of discriminant analysis versus artificial neural networks. J. Oper. Res. Soc. 1993, 44, 51–60. [Google Scholar] [CrossRef]
- Goldberg, D. Genetic Algorithms in Search, Optimization and Machine Learning, 1st ed.; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1989; pp. 121–179. [Google Scholar]
- Kim, S.Y.; Chung, T.; Kim, S.; Song, S.; Kim, N. Recycling Agricultural Wastes as Feed for Mealworm (Tenebrio molitor). Korean J. Appl. Entomol. 2014, 53, 367–373. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Mikołajczak, Z.; Urbański, A.; Nogowski, L.; Józefiak, D. Insect fat in animal nutrition—A review. Ann. Anim. Sci. 2020, 20, 1217–1240. [Google Scholar] [CrossRef]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Yoo, J.S.; Cho, K.H.; Hong, J.S.; Jang, H.S.; Chung, Y.H.; Kwon, G.T.; Shin, D.G.; Kim, Y.Y. Nutrient ileal digestibility evaluation of dried mealworm (Tenebrio molitor) larvae compared to three animal protein by-products in growing pigs. Asian Australas. J. Anim. Sci. 2019, 32, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Boulos, S.; Anina, T.; Nyström, L. Nitrogen-to-Protein Conversion Factors for Edible Insects on the Swiss Market: T. molitor, A. domesticus, and L. migratoria. Front. Nutr. 2020, 7, 89. [Google Scholar] [CrossRef]
- Kar, S.K.; Jansman, A.J.; Boeren, S.; Kruijt, L.; Smits, M.A. Protein, peptide, amino acid composition, and potential functional properties of existing and novel dietary protein sources for monogastrics. J. Anim. Sci. 2016, 94, 30–39. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.; Choi, W.H.; Hong, S.; Kim, N.J. Nutritional Value of Mealworm, Tenebrio molitor as Food Source. Int. J. Ind. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- Kulma, M.; Petříčková, D.; Kurečka, M.; Kotíková, Z.; Táborský, J.; Michlová, T.; Kouřimská, L. Effect of carrot supplementation on nutritional value of insects: A case study with Jamaican field cricket (Gryllus assimilis). J. Insects Food Feed 2021, 1–10. [Google Scholar] [CrossRef]
- EC. Regulation (EC) No 767/2009 of the European Parliament and of the Council of 13 July 2009 on the placing on the market and use of feed, amending European Parliament and Council Regulation (EC) No 1831/2003 and repealing Council Directive 79/373/EEC, Commission Directive 80/511/EEC, Council Directives 82/471/EEC, 83/228/EEC, 93/74/EEC, 93/113/EC and 96/25/EC and Commission Decision 2004/217/EC. Off. J. Eur. Union 2009, 229, 1–36. [Google Scholar]
- Selenius, O.V.O.; Korpela, J.; Salminen, S.; Gallego, C.G. Effect of Chitin and Chitooligosaccharide on In vitro Growth of Lactobacillus rhamnosus GG and Escherichia coli TG. Appl. Food Biotechnol. 2018, 5, 163–172. [Google Scholar] [CrossRef]
- Hussain, I.; Khan, S.; Sultan, A.; Chand, N.; Khan, R.; Alam, W.; Naseer, A. Meal worm (Tenebrio molitor) as potential alternative source of protein supplementation in broiler. Int. J. Biosci. 2017, 10, 255–262. [Google Scholar] [CrossRef]
- Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. Invitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. [Google Scholar] [CrossRef] [PubMed]
- Dossey, A.T.; Tatum, J.T.; McGill, W.L. Modern Insect-Based Food Industry: Current Status, Insect Processing Technology, and Recommendations Moving Forward. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 113–152. ISBN 978-0-12-802856-8. [Google Scholar]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Bosch, G.; Zhang, S.; Oonincx, D.G.A.B.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, e29. [Google Scholar] [CrossRef] [Green Version]
- Kovitvadhi, A.; Chundang, P.; Thongprajukaew, K.; Tirawattanawanich, C.; Srikachar, S.; Chotimanothum, B. Potential of Insect Meals as Protein Sources for Meat-Type Ducks Based on In Vitro Digestibility. Animals 2019, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Jabir, M.D.A.R.; Razak, S.A.; Vikineswary, S. Chemical Composition and Nutrient Digestibility of Super Worm Meal in Red Tilapia Juvenile. Pak. Vet. J. 2012, 32, 489–493. [Google Scholar]
- Araujo, R.R.S.; dos Santos Benfica, T.A.R.; Ferraz, V.B.; Santos, E.M. Nutritional composition of insects Gryllus assimilis and Zophobas morio: Potential foods harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Anderson, S.J. Increasing calcium levels in cultured insects. Zoo Biol. 2000, 19, 1–9. [Google Scholar] [CrossRef]
- Spasevski, N.; Čolović, D.; Rakita, S.; Ikonić, P.; Đuragić, O.; Banjac, V.; Vukmirović, Đ. Fatty acid composition and β -carotene content in egg yolk of laying hens fed with linseed, paprika and marigold. Contemp. Agric. 2016, 65, 15–22. [Google Scholar] [CrossRef] [Green Version]
- St-Hilaire, S.; Cranfill, K. Fish Offal Recycling by the Black Soldier Fly Produces a Foodstuff High in Omega-3 Fatty Acids. J. World Aquac. Soc. 2007, 38, 309–313. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragojlović, D.; Đuragić, O.; Pezo, L.; Popović, L.; Rakita, S.; Tomičić, Z.; Spasevski, N. Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior? Animals 2022, 12, 1277. https://doi.org/10.3390/ani12101277
Dragojlović D, Đuragić O, Pezo L, Popović L, Rakita S, Tomičić Z, Spasevski N. Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior? Animals. 2022; 12(10):1277. https://doi.org/10.3390/ani12101277
Chicago/Turabian StyleDragojlović, Danka, Olivera Đuragić, Lato Pezo, Ljiljana Popović, Slađana Rakita, Zorica Tomičić, and Nedeljka Spasevski. 2022. "Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior?" Animals 12, no. 10: 1277. https://doi.org/10.3390/ani12101277
APA StyleDragojlović, D., Đuragić, O., Pezo, L., Popović, L., Rakita, S., Tomičić, Z., & Spasevski, N. (2022). Comparison of Nutritional Profiles of Super Worm (Zophobas morio) and Yellow Mealworm (Tenebrio molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior? Animals, 12(10), 1277. https://doi.org/10.3390/ani12101277







