Investigation of the Efficacy of Dithiothreitol and Glutathione on In Vitro Fertilization of Cryopreserved Large White Boar Semen
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
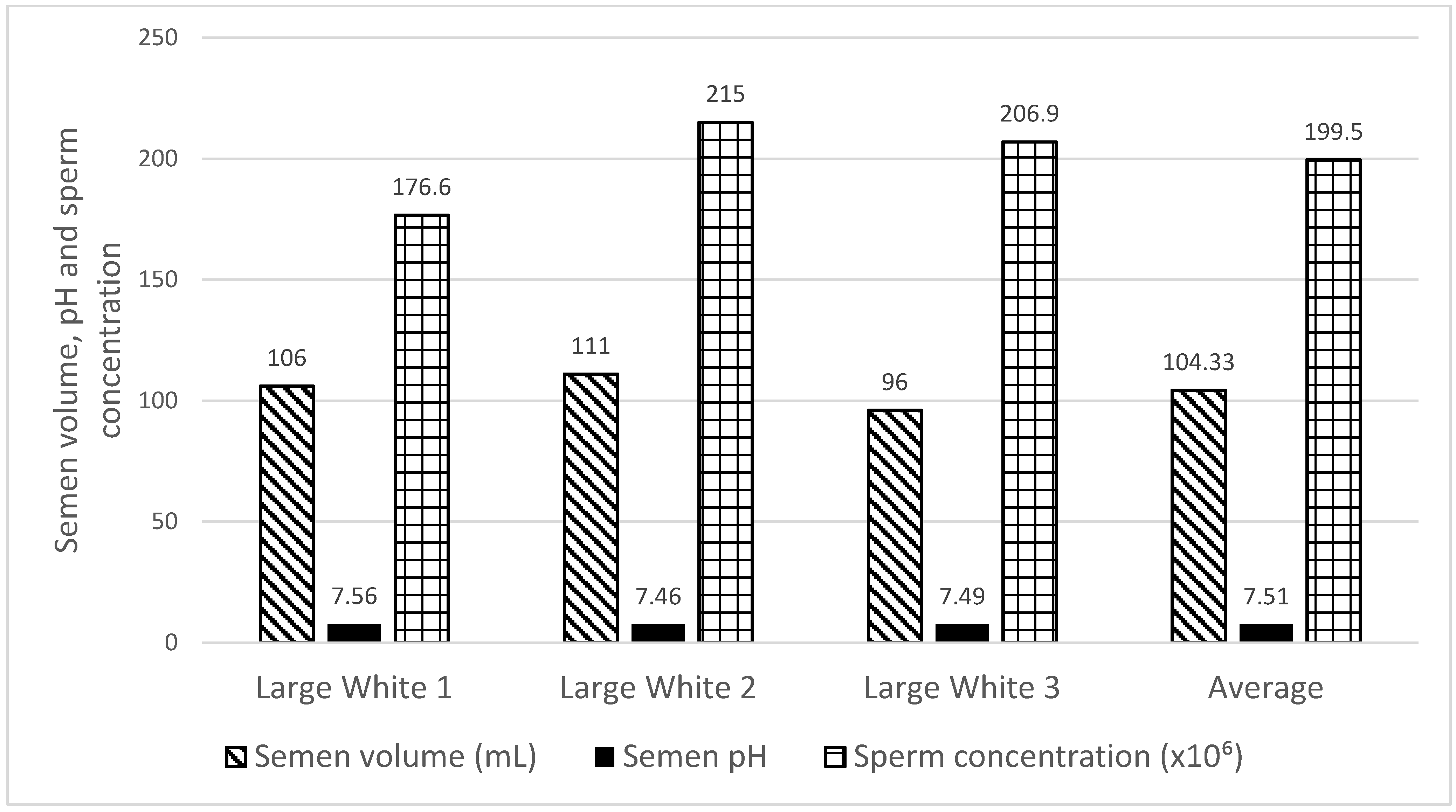
2.2. Boars Semen Collection
2.3. Experiment I
2.4. Experiment II
2.4.1. Cryopreservation of Large White Boar’s Semen
2.4.2. Boars Semen Thawing and Evaluation
2.4.3. Evaluation of Sperm Acrosome Integrity
2.5. Experiment III
2.5.1. Ovaries Collection
2.5.2. In Vitro Maturation of Oocytes
2.5.3. In Vitro Fertilization of Matured Oocytes and Thawing of Semen
2.5.4. Assessment of the Pronucleus following In Vitro Fertilization of Porcine Oocytes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Experimental Location and Ethical Clearance
References
- Holt, W.V. Basic aspects of frozen storage of semen. Anim. Reprod. 2000, 62, 3–22. [Google Scholar] [CrossRef]
- Namula, Z.; Isumi, Y.; Sato, Y.; Le, Q.A.; Lin, Q.; Takebayashi, K.; Hirata, M.; Tanihara, F.; Thongkittidilok, C.; Otoi, T. Improvement of the in vitro fertilization and embryo development using frozen–thawed spermatozoa of microminipigs. Arch. Anim. Breed. 2021, 64, 265–271. [Google Scholar] [CrossRef]
- Bathgate, B. Antioxidant mechanisms and their benefit on post-thaw boar sperm quality. Reprod. Domest. Anim. 2011, 46, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.F.; Silva, P.F.N.; Gadella, B.M. New assay for detection and localization of endogenous lipid peroxidation products in living boar sperm after BTS dilution or after freeze-thawing. Theriogenology 2005, 63, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Winn, E.; Whitaker, B.D. Quercetin supplementation to the thawing and incubation media of boar sperm improves post-thaw sperm characteristics and the in vitro production of pig embryos. Reprod. Biol. 2020, 20, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Sarıözkan, S.; Bucak, M.N.; Tuncer, P.B.; Ulutas, P.A.; Bilgen, A. The influence of cysteine and taurine on microscopic-oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology 2009, 58, 134–138. [Google Scholar] [CrossRef]
- Yavaş, I.; Bozkurt, Y.; Yıldız, C. Effect of different antioxidants on motility, viability and fertilizing capacity of cryopreserved scaly carp (Cyprinus carpio) Semen. Isr. J. Aquac. 2014, 66, 1066. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Storey, B.T. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol. Reprod. 1983, 29, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Durairajanayagam, D.; Du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yi, K.; Chen, C.; Hou, X.; Zhou, X. Application of antioxidants and centrifugation for cryopreservation of boar spermatozoa. Anim. Reprod. Sci. 2012, 132, 123–128. [Google Scholar] [CrossRef]
- Gadea, J.; Sellés, E.; Marco, M.A.; Coy, P.; Matás, C.; Romar, R.; Ruiz, S. Decrease in glutathione content in boar sperm after cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 2004, 62, 690–701. [Google Scholar] [CrossRef]
- Gadea, J.; Gumbao, D.; Matás, C.; Romar, R. Supplementation of the thawing media with reduced glutathione improves function and the in vitro fertilizing ability of boar spermatozoa after cryopreservation. J. Androl. 2005, 26, 6. [Google Scholar] [CrossRef] [Green Version]
- Foote, R.H.; Brockett, C.C.; Kaproch, M.T. Motility and fertility of bull sperm in whole milk extender containing antioxidants. Anim. Reprod. Sci. 2002, 71, 13–23. [Google Scholar] [CrossRef]
- Funahashi, H.; Sano, T. Select antioxidants improve the function of extended boar semen stored at 10 degrees C. Theriogenology 2005, 63, 1605–1616. [Google Scholar] [CrossRef]
- Watanabe, H.; Fukui, Y. Effects of dithiothreitol and boar on pronuclear formation and embryonic development following intracytoplasmic sperm injection in pigs. Theriogenology 2006, 65, 528–539. [Google Scholar] [CrossRef]
- Deshpande, V.S.; Kehrer, J.P. Oxidative stress-driven mechanisms of nordihydroguaiaretic acid-induced apoptosis in FL5.12 cells. Toxicol. Appl. Pharmacol. 2006, 214, 230–236. [Google Scholar] [CrossRef]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Blanchette, S.; Gagnon, C.; Sirard, M.A. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology 2001, 56, 275–286. [Google Scholar] [CrossRef]
- Kikuchi, K.; Kaneko, H.; Nakai, M.; Somfai, T.; Kashiwazaki, N.; Nagai, T. Contribution of in vitro systems to preservation and utilization of porcine genetic resources. Theriogenology 2016, 86, 170–175. [Google Scholar] [CrossRef]
- Kathiravan, P.; Kalatharan, J.; Edwin, M.J.; Veerapandian, C. Computer automated motion analysis of crossbred bull spermatozoa and its relationship with in vitro fertility in zona-free hamster oocytes. Anim. Reprod. Sci. 2008, 104, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Vyt, P.; Maes, D.; Quinten, C.; Rijsselaere, T.; Deley, W.; Aarts, M.; De Kruif, A.; Van Soom, A. Detailed motility evaluation of boar semen and its predictive value for reproductive performance in sows. Vlaams. Diergeneeskd. Tijdschr. 2008, 77, 291–298. [Google Scholar]
- Somi, S.S.; Kluger, E.; Knapp, D.; Klein, C.; Aurich, A. Effects of semen extender and semen processing on motility and viability of frozen-thawed dog spermatozoa. Theriogenology 2006, 66, 173–182. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Pilane, C.M.; Bopape, M.A.; Bovula, N.; Mapeka, M.H. Buck semen does not easily succumb to oxidative stress. Open J. Anim. Sci. 2019, 9, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Pilane, C.M.; Bopape, M.A.; Mapeka, M.H.; Netshirovha, T.R. Assessment of the susceptibility of boar semen to oxidative stress. Open J. Anim. Sci. 2016, 6, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Kaeoket, K.; Tantiparinyakul, K.; Kladkaew, W.; Chanapiwat, P.; Techakumphu, M. Effect of different antioxidants on quality of cryopreserved boar semen in different breeds. Thai. J. Agric. Sci. 2008, 41, 1–9. [Google Scholar]
- Rath, D.; Niemann, H. In vitro fertilization of porcine oocytes with fresh and frozen-thawed ejaculated or frozen-thawed epididymal semen obtained from identical boars. Theriogenology 1996, 47, 785–793. [Google Scholar] [CrossRef]
- Reichman, D.E.; Jackson, K.V.; Racowsky, C. Incidence and development of zygotes exhibiting abnormal pronuclear disposition after identification of two pronuclei at the fertilization check. Int. J. Fertil. Steril. 2009, 94, 965–970. [Google Scholar] [CrossRef]
- Casillas, F.; Betancourt, M.; Cuello, C.; Ducolomb, Y.; Lopez, A.; Juarez-Rojas, L.; Retana-Marquez, S. An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porc. Health. Manag. 2018, 4, 16. [Google Scholar] [CrossRef]
- Grupen, C.G. The evolution of porcine embryo in vitro production. Theriogenology 2014, 81, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Causio, F.; Fischetto, R.; Sarcina, E.; Geusa, S.; Tartagni, M. Chromosome analysis of spontaneous abortions after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Eur. J. Obstet. Gynecol. Reprod. 2002, 105, 44–48. [Google Scholar] [CrossRef]
- Yoshizawa, M. Chromosomal abnormalities and embryonic development into the blastocyst stage in mammalian embryos derived in vitro. A review. J. Mamm. Ova. Res. 2003, 20, 7–15. [Google Scholar] [CrossRef]
- Somfai, T.; Ozawa, M.; Noguchi, J.; Kaneko, H.; Karja, N.W.K.; Fahrudin, M.; Nakai, M.; Maedomari, N.; Dinnyés, A.; Nagai, T.; et al. In vitro development of polyspermic porcine oocytes: Relationship between early fragmentation and excessive number of penetrating spermatozoa. Anim. Reprod. Sci. 2008, 107, 131–147. [Google Scholar] [CrossRef]
- Han, Y.M.; Wang, W.H.; Abeydeera, L.R.; Petersen, A.L.; Kim, J.H.; Murphy, C.; Day, B.N.; Prather, R.S. Pronuclear location before the first cell division determines ploidy of polyspermic pig embryos. Biol. Reprod. 1999, 61, 1340–1346. [Google Scholar] [CrossRef] [Green Version]
- Funahashi, H. Polyspermic penetration in porcine IVM-IVF systems. Reprod. Fertil. Dev. 2003, 15, 167–177. [Google Scholar] [CrossRef]
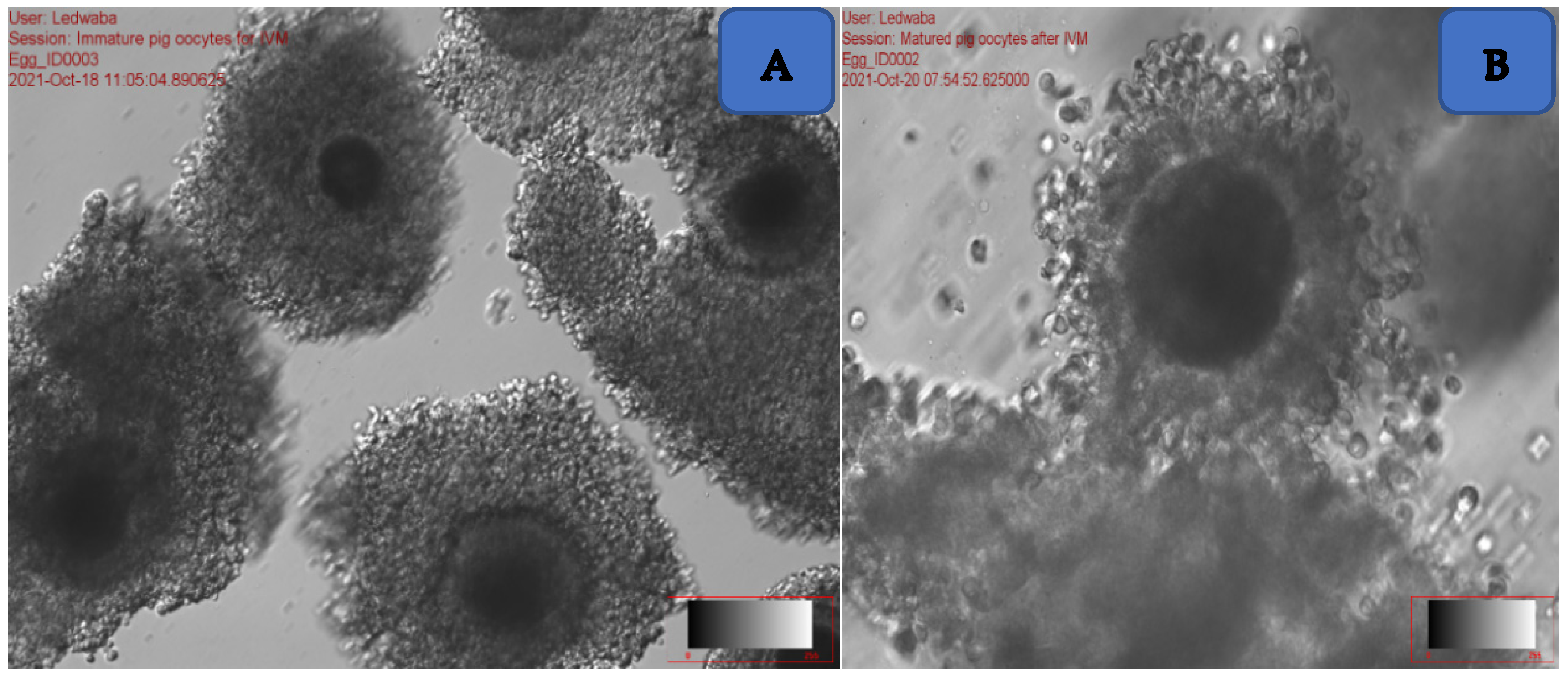

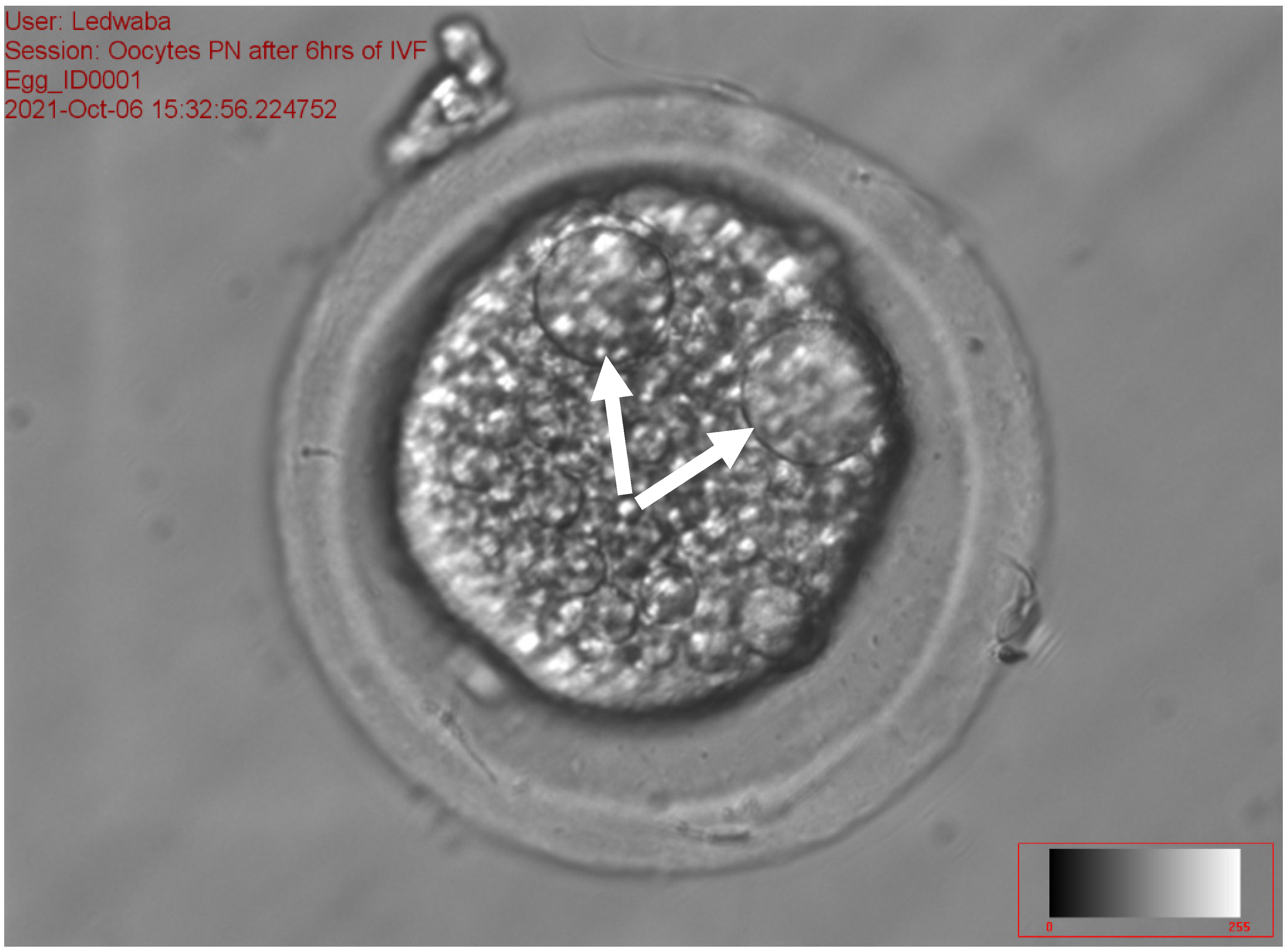
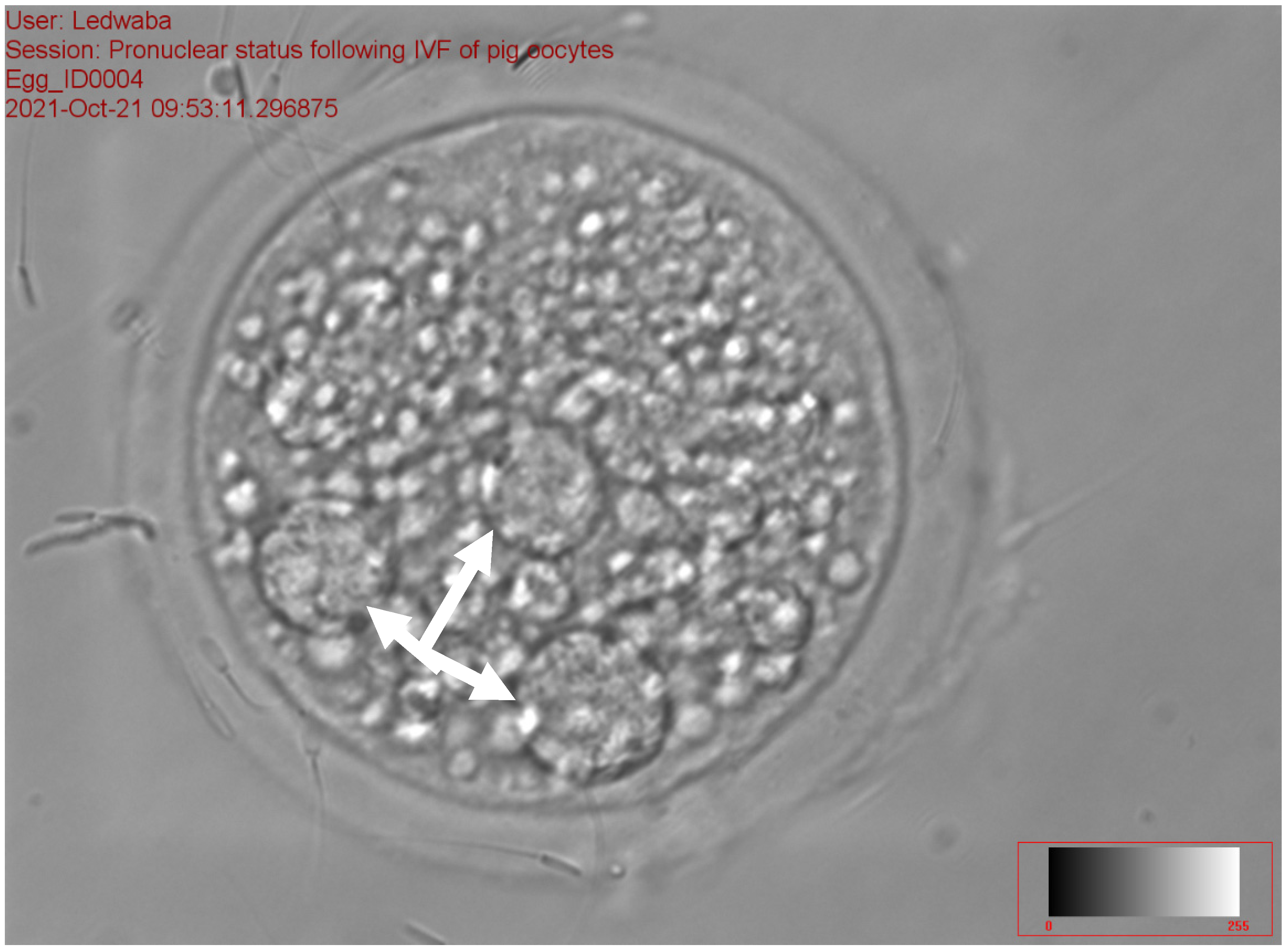
| Parameter | Abbreviation | Definition | Unit | Reference |
|---|---|---|---|---|
| Total motility | TM | The ratio of motile sperm to the total cell concentration is expressed as a percentage. | % | Kathiravan et al. [21] |
| Progressive motility | PM | Percent of sperm moving rapidly and in a straight path. | % | Kathiravan et al. [21] |
| Non-progressive motility | NPM | The percentage of sperm not moving forward in a straight path. | % | Vyt et al. [22] |
| Static | STC | Percentage static sperm (not moving during the analysis). | % | Vyt et al. [22] |
| Rapid motility | RAP | Percentage of rapidly moving sperm. | % | Vyt et al. [22] |
| Slow | SLW | Percentage of sperm moving at 1–10 μm/second. | % | Vyt et al. [22] |
| Medium | MED | Percentage of sperm moving at 11–25 μm/second. | % | Vyt et al. [22] |
| Curvilinear velocity | VCL | The instantaneously recorded sequential progression along the whole trajectory of the sperm per unit of time. | μm/s | Somi et al. [23] |
| Straight-line velocity | VSL | The straight trajectory of the sperm per unit of time (=straight line distance from beginning to end of track divided by the time taken). | μm/s | Somi et al. [23] |
| Average path velocity | VAP | The mean trajectory of the sperm per unit of time. | μm/s | Somi et al. [23] |
| Linearity | LIN | The ratio of the straight displacement in the sum of elementary displacements during the time of the measurement is defined as (VSL/VCL) × 100. | % | Somi et al. [23] |
| Straightness | STR | The ratio of projected length to the average velocity of sperm head along a spatial trajectory, STR = VSL/VAP. | % | Somi et al. [23] |
| Wobble | WOB | The oscillation of the curvilinear trajectory upon the mean trajectory is defined as (VAP/VCL) × 100. | % | Somi et al. [23] |
| Beat-cross frequency | BCF | The number of lateral oscillatory movements of the sperm head around the mean trajectory. | Hz | Somi et al. [23] |
| Amplitude of lateral head displacement | ALH | The mean width of sperm head oscillation. | μm | Somi et al. [23] |
| Boar Semen | TM (%) | PM (%) | NPM (%) | STC (%) | RAP (%) | MED (%) | SLW (%) | VCL (µm/s) | VSL (µm/s) | VAL (µm/s) | LIN (%) | STR (%) | WOB (%) | ALH (µm/s) | BCF (HZ) | HPA (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh semen | 97.93 ± 2.14 | 53.40 ± 10.79 | 44.53 ± 9.84 | 2.07 ± 2.14 | 40.63 ± 14.53 | 53.61 ± 13.05 | 2.15 ± 1.69 | 110.58 ± 18.25 | 27.46 ± 3.17 | 62.21 ± 9.03 | 27.07 ± 4.33 | 46.74 ± 5.50 | 56.45 ± 3.42 | 2.66 ± 0.37 | 26.23 ± 1.55 | 16.27 ± 8.38 |
| Boar Semen | Sperm Viability (%) | Live Sperm with Abnormalities (%) | ||||
|---|---|---|---|---|---|---|
| Live | Dead | Head | Tail | Proximal Droplets | Distal Droplets | |
| Fresh semen | 92.80 ± 8.26 | 3.70 ± 4.31 | 0.50 ± 0.71 | 1.80 ± 0.92 | 0.70 ± 0.67 | 0.50 ± 0.71 |
| Antioxidants Treatments | TM (%) | PM (%) | NPM (%) | STC (%) | RAP (%) | MED (%) | SLW (%) | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | WOB (%) | ALH (µm/s) | BCF (Hz) | HPA (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 93.32 ± 7.74 | 71.28 ± 17.86 a | 22.04 ± 15.42 c | 8.35 ± 7.81 | 64.92 ± 21.60 a | 22.40 ± 17.50 b | 6.35 ± 2.90 | 158.91 ± 52.38 a | 29.29 ± 5.39 ab | 87.69 ± 23.89 a | 20.58 ± 5.76 b | 35.86 ± 8.48 b | 55.60 ± 4.63 | 3.44 ± 1.07 a | 27.83 ± 3.11 | 27.71 ± 12.28 |
| 5 µM H2O2 | 86.70 ± 10.24 | 49.88 ± 16.81 c | 36.82 ± 16.06 a | 13.30 ± 10.24 | 40.76 ± 19.69 b | 38.69 ± 17.28 a | 6.03 ± 3.45 | 112.60 ± 26.79 b | 24.61 ± 5.16 b | 62.71 ± 12.50 b | 25.09 ± 5.68 ab | 43.27 ± 6.15 ab | 56.42 ± 6.21 | 2.64 ± 0.50 b | 26.87 ± 2.99 | 21.81 ± 16.37 |
| 5 µM DTT | 89.72 ± 10.19 | 54.14 ± 12.63 bc | 35.58 ± 11.46 ab | 10.28 ± 10.19 | 43.48 ± 16.27 b | 39.70 ± 16.02 a | 6.55 ± 3.29 | 121.74 ± 22.37 b | 23.81 ± 3.79 b | 66.03 ± 10.52 b | 24.40 ± 8.40 ab | 43.03 ± 9.85 ab | 54.24 ± 8.36 | 2.84 ± 0.60 ab | 26.39 ± 2.41 | 20.47 ± 9.94 |
| 5 µM GSH | 88.15 ± 9.47 | 63.81 ± 12.88 ab | 24.34 ± 13.08 bc | 11.85 ± 9.47 | 53.46 ± 17.06 ab | 30.26 ± 18.63 ab | 4.38 ± 3.52 | 136.57 ± 30.15 ab | 31.50 ± 9.46 a | 75.76 ± 14.31 ab | 24.77 ± 5.29 ab | 43.60 ± 8.18 ab | 55.68 ± 8.03 | 3.09 ± 0.78 ab | 26.91 ± 1.77 | 25.12 ± 12.86 |
| 5 µM H2O2 + 5 µM DTT | 86.71 ± 11.22 | 64.70 ± 14.29 ab | 22.01 ± 10.85 c | 13.29 ± 11.22 | 54.44 ± 20.38 ab | 28.70 ± 17.03 ab | 4.46 ± 3.14 | 131.24 ± 25.61 ab | 29.58 ± 3.69 ab | 73.93 ± 8.99 b | 26.01 ± 8.07 ab | 43.14 ± 7.35 ab | 57.75 ± 8.56 | 2.95 ± 0.57 ab | 27.61 ± 1.55 | 29.74 ± 13.93 |
| 5 µM H2O2 + 5 µM GSH | 90.54 ± 6.74 | 59.31 ± 14.32 abc | 27.41 ± 10.25 abc | 9.46 ± 6.74 | 42.36 ± 22.80 b | 38.49 ± 14.77 a | 6.66 ± 4.77 | 116.68 ± 31.31 b | 31.76 ± 10.32 a | 68.19 ± 13.19 b | 28.81 ± 12.91 a | 48.93 ± 15.09 a | 59.68 ± 11.42 | 2.67 ± 0.66 b | 26.14 ± 4.80 | 19.30 ± 13.93 |
| Antioxidants Treatments | Sperm Viability (%) | Live Sperm with Abnormalities (%) | ||||
|---|---|---|---|---|---|---|
| Live | Dead | Head | Tail | Proximal Droplets | Distal Droplets | |
| Control | 86.00 ± 4.22 a | 9.80 ± 3.99 bc | 0.33 ± 0.71 b | 2.20 ± 1.23 | 0.70 ± 0.82 b | 0.60 ± 0.52 |
| 5 µM H2O2 | 76.00 ± 10.46 b | 20.30 ± 6.78 a | 0.30 ± 0.48 b | 2.80 ± 1.62 | 0.50 ± 0.53 b | 0.10± 0.32 |
| 5 µM DTT | 85.10 ± 6.51 a | 8.60 ± 2.59 c | 0.70 ± 0.95 ab | 3.22 ± 1.39 | 0.60 ± 0.70 b | 0.40± 0.70 |
| 5 µM GSH | 77.40 ± 10.46 b | 14.80 ± 6.55 ab | 0.89 ± 0.78 ab | 3.89 ± 2.09 | 0.50 ± 0.53 b | 0.50± 0.70 |
| 5 µM H2O2 + 5 µM DTT | 75.80 ± 10.22 b | 16.70 ± 6.68 a | 1.10 ± 1.10 a | 3.22 ± 2.44 | 1.40 ± 1.07 a | 0.44± 0.73 |
| 5 µM H2O2 + 5 µM GSH | 79.00 ± 9.46 ab | 15.70 ± 9.78 a | 0.70 ± 0.48 ab | 3.60 ± 2.07 | 0.50 ± 0.53 b | 0.50± 0.53 |
| Antioxidants Treatments | TM (%) | PM (%) | NPM (%) | STC (%) | RAP (%) | MED (%) | SLW (%) | VCL (µm/s) | VSL (µm/s) | VAP (µm/s) | LIN (%) | STR (%) | WOB (%) | ALH (µm/s) | BCF (Hz) | HPA (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 32.09 ± 6.66 a | 19.13 ± 6.96 a | 12.95 ± 4.78 | 67.92 ± 6.66 b | 17.04 ± 6.55 a | 8.79 ± 3.45 | 6.25 ± 2.89 ab | 111.63 ± 18.29 | 22.77 ± 4.66 | 50.74 ± 11.20 | 21.31 ± 3.08 | 44.72 ± 4.80 | 44.96 ± 4.33 | 3.19 ± 0.39 | 17.67 ± 5.22 | 18.66 ± 12.62 |
| 5 mM DTT | 28.33 ± 10.66 ab | 16.48 ± 5.74 a | 13.17 ± 6.57 | 71.67 ± 10.66 ab | 13.41 ± 4.94 ab | 8.25 ± 3.95 | 6.61 ± 2.52 a | 114.67 ± 21.49 | 24.75 ± 5.36 | 53.59 ± 17.05 | 23.39 ± 3.89 | 47.81 ± 5.91 | 46.01 ± 2.32 | 3.19 ± 0.69 | 18.43 ± 5.38 | 21.49 ± 16.84 |
| 5 mM GSH | 26.04 ± 9.41 ab | 15.24 ± 5.71 ab | 10.95 ± 5.59 | 73.23 ± 9.68 ab | 12.43 ± 6.04 ab | 8.20 ± 4.64 | 5.59 ± 2.74 ab | 114.72 ± 25.18 | 24.55 ± 4.24 | 53.63 ± 14.23 | 21.74 ± 3.67 | 47.11 ± 8.83 | 45.78 ± 4.48 | 3.29 ± 0.57 | 16.72 ± 4.09 | 18.25 ± 13.06 |
| 2.5 mM DTT + 2.5 mM GSH | 22.45 ± 11.14 b | 10.41 ± 4.59 b | 9.49 ± 3.83 | 77.55 ± 11.14 a | 9.20 ± 3.55 b | 6.53 ± 2.76 | 4.05 ± 2.31 b | 123.01 ± 24.79 | 23.12 ± 3.92 | 55.43 ± 16.26 | 21.97 ± 3.11 | 47.07 ± 8.08 | 44.88 ± 2.96 | 3.69 ± 0.62 | 16.43 ± 5.52 | 20.29 ± 15.52 |
| Antioxidants Treatments | Acrosome Integrity (%) | Viability (%) | Live Sperm Abnormalities (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Reacted Acrosome | Non-Reacted Acrosome | Live | Dead | Head Defects | Tail Defects | Proximal Droplets | Distal Droplet | |
| Control | 25.20 ± 5.39 | 74.80 ± 5.39 | 34.90 ± 6.51 a | 62.50 ± 6.42 b | 0.33 ± 0.50 | 1.22 ± 0.67 | 0.20 ± 0.42 | 0.30 ± 0.48 |
| 5 mM DTT | 22.40 ± 13.16 | 77.60 ± 13.16 | 29.80 ± 5.20 a | 67.40 ± 5.78 b | 0.20 ± 0.42 | 1.70 ± 0.82 | 0.50 ± 0.53 | 0.40 ± 0.52 |
| 5 mM GSH | 24.40 ± 11.65 | 75.60 ± 11.65 | 29.40 ± 6.38 a | 68.40 ± 6.79 b | 0.50 ± 0.53 | 1.10 ± 0.74 | 0.30 ± 0.48 | 0.30 ± 0.48 |
| 2.5 mM DTT + 2.5 mM GSH | 24.50 ± 14.14 | 75.50 ± 14.14 | 21.67 ± 6.91 b | 76.00 ± 7.48 a | 0.30 ± 0.48 | 1.70 ± 0.67 | 0.33 ± 0.50 | 0.10 ± 0.32 |
| Antioxidants Treatments | IVM Oocytes (n) | IVF Oocytes (n) | Pronucleus | Total Number of Fertilization Rate (%) | Total Number of Non-Fertilization Rate (%) | |||
|---|---|---|---|---|---|---|---|---|
| 0 PN n (%) | 1 PN n (%) | 2 PN n (%) | >2 PN n (%) | |||||
| Fresh semen | 125 | 97 | 53.96 ± 11.83 | 22.50 ± 7.13 | 10.02 ± 8.76 | 11.84 ± 9.47 a | 46.04 ± 11.83 | 53.96 ± 11.83 |
| Control | 129 | 80 | 51.52 ± 17.02 | 28.12 ± 9.34 | 13.32 ± 7.22 | 7.04 ± 5.10 ab | 48.48 ± 17.02 | 51.52 ± 17.02 |
| 5 mM GSH | 116 | 82 | 68.06 ± 8.65 | 18.32 ± 3.92 | 11.18 ± 5.06 | 0.86 ± 1.92 b | 31.94 ± 8.65 | 68.06 ± 8.65 |
| 5 mM DTT | 135 | 114 | 55.26 ± 15.75 | 22.76 ± 17.93 | 19.76 ± 9.90 | 2.22 ± 4.96 b | 44.74 ± 15.75 | 55.26 ± 15.75 |
| 2.5 mM DTT + 2.5 mM GSH | 120 | 79 | 51.28 ± 16.07 | 22.80 ± 9.55 | 11.82 ± 11.57 | 14.10 ± 10.49 a | 48.72 ± 16.07 | 51.28 ± 16.07 |
| Traits | 0 PN % | 1 PN % | 2 PN % | <2 PN % | FR % | TM % | PM % | NPM % | STC % | RAP % | MED % | SLW % | VCL µm/s | VSL µm/s | VAP µm/s | LIN % | STR % | WOB % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 PN% | 1.00 | |||||||||||||||||
| 1 PN% | −0.59 | 1.00 | ||||||||||||||||
| 2 PN% | −0.56 | −0.02 | 1.00 | |||||||||||||||
| <2 PN% | −0.43 | −0.18 | −0.06 | 1.00 | ||||||||||||||
| FR% | −1.00 | 0.59 | 0.56 | 0.43 | 1.00 | |||||||||||||
| TM% | 0.06 | −0.09 | −0.22 | 0.23 | −0.06 | 1.00 | ||||||||||||
| PM% | 0.11 | −0.18 | −0.23 | 0.26 | −0.11 | 0.97 | 1.00 | |||||||||||
| NPM% | −0.01 | 0.05 | −0.02 | −0.02 | 0.01 | 0.58 | 0.47 | 1.00 | ||||||||||
| STC% | −0.06 | 0.08 | 0.15 | −0.14 | 0.06 | −0.90 | −0.87 | −0.86 | 1.00 | |||||||||
| RAP% | 0.12 | −0.20 | −0.25 | 0.28 | −0.12 | 0.92 | 0.98 | 0.38 | −0.79 | 1.00 | ||||||||
| MED% | −0.04 | 0.01 | −0.18 | 0.25 | 0.04 | 0.96 | 0.88 | 0.62 | −0.87 | 0.81 | 1.00 | |||||||
| SLW% | 0.04 | 0.04 | 0.14 | −0.26 | −0.04 | −0.12 | −0.16 | 0.72 | −0.32 | −0.25 | −0.09 | 1.00 | ||||||
| VCL µm/s | 0.15 | −0.21 | −0.17 | 0.17 | −0.15 | −0.05 | 0.14 | −0.23 | 0.05 | 0.25 | −0.17 | −0.17 | 1.00 | |||||
| VSL µm/s | 0.13 | −0.25 | 0.01 | 0.06 | −0.13 | −0.03 | 0.08 | −0.20 | 0.07 | 0.12 | −0.09 | −0.17 | 0.48 | 1.00 | ||||
| VAP µm/s | 0.24 | −0.31 | −0.30 | 0.27 | −0.24 | 0.15 | 0.32 | −0.24 | −0.05 | 0.41 | 0.06 | −0.38 | 0.90 | 0.42 | 1.00 | |||
| LIN% | 0.19 | −0.11 | −0.12 | −0.06 | −0.19 | 0.16 | 0.05 | 0.07 | −0.07 | −0.04 | 0.23 | −0.06 | −0.54 | 0.35 | −0.37 | 1.00 | ||
| STR% | −0.02 | 0.001 | 0.22 | −0.21 | 0.02 | −0.11 | −0.21 | 0.10 | 0.07 | −0.28 | −0.05 | 0.21 | −0.49 | 0.45 | −0.57 | 0.79 | 1.00 | |
| WOB% | 0.23 | −0.18 | −0.50 | 0.34 | −0.23 | 0.51 | 0.49 | 0.01 | −0.29 | 0.46 | 0.57 | −0.47 | −0.01 | 0.02 | 0.39 | 0.40 | −0.20 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledwaba, M.R.; Mphaphathi, M.L.; Thema, M.A.; Pilane, C.M.; Nedambale, T.L. Investigation of the Efficacy of Dithiothreitol and Glutathione on In Vitro Fertilization of Cryopreserved Large White Boar Semen. Animals 2022, 12, 1137. https://doi.org/10.3390/ani12091137
Ledwaba MR, Mphaphathi ML, Thema MA, Pilane CM, Nedambale TL. Investigation of the Efficacy of Dithiothreitol and Glutathione on In Vitro Fertilization of Cryopreserved Large White Boar Semen. Animals. 2022; 12(9):1137. https://doi.org/10.3390/ani12091137
Chicago/Turabian StyleLedwaba, Mahlatsana Ramaesela, Masindi Lottus Mphaphathi, Mamonene Angelinah Thema, Cyril Mpho Pilane, and Tshimangadzo Lucky Nedambale. 2022. "Investigation of the Efficacy of Dithiothreitol and Glutathione on In Vitro Fertilization of Cryopreserved Large White Boar Semen" Animals 12, no. 9: 1137. https://doi.org/10.3390/ani12091137








