When Incorporated into Fruit Sorbet Matrix, Are the Fructans in Natural Raw Materials More Beneficial for Bone Health than Commercial Formulation Added Alone?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
- Validation model, with two groups of rats fed a diet with recommended Ca dose (RC) [24] or Ca-deficient diet (LC) for 12 weeks;
- Treatment, with six groups of rats fed a low-calcium diet containing 8% fructans, for 12 weeks: LC-JA, LC-Y, and LC-F (raw materials added to the diets alone), as well as LC-JAS, LC-YS, and LC-FS (raw materials added to the diets as a components of strawberry sorbets).
2.2. Sample Collection and Storage
2.3. Biochemical Parameter Assays
2.4. Bone Densitometric Measurements
2.5. Statistical Analysis
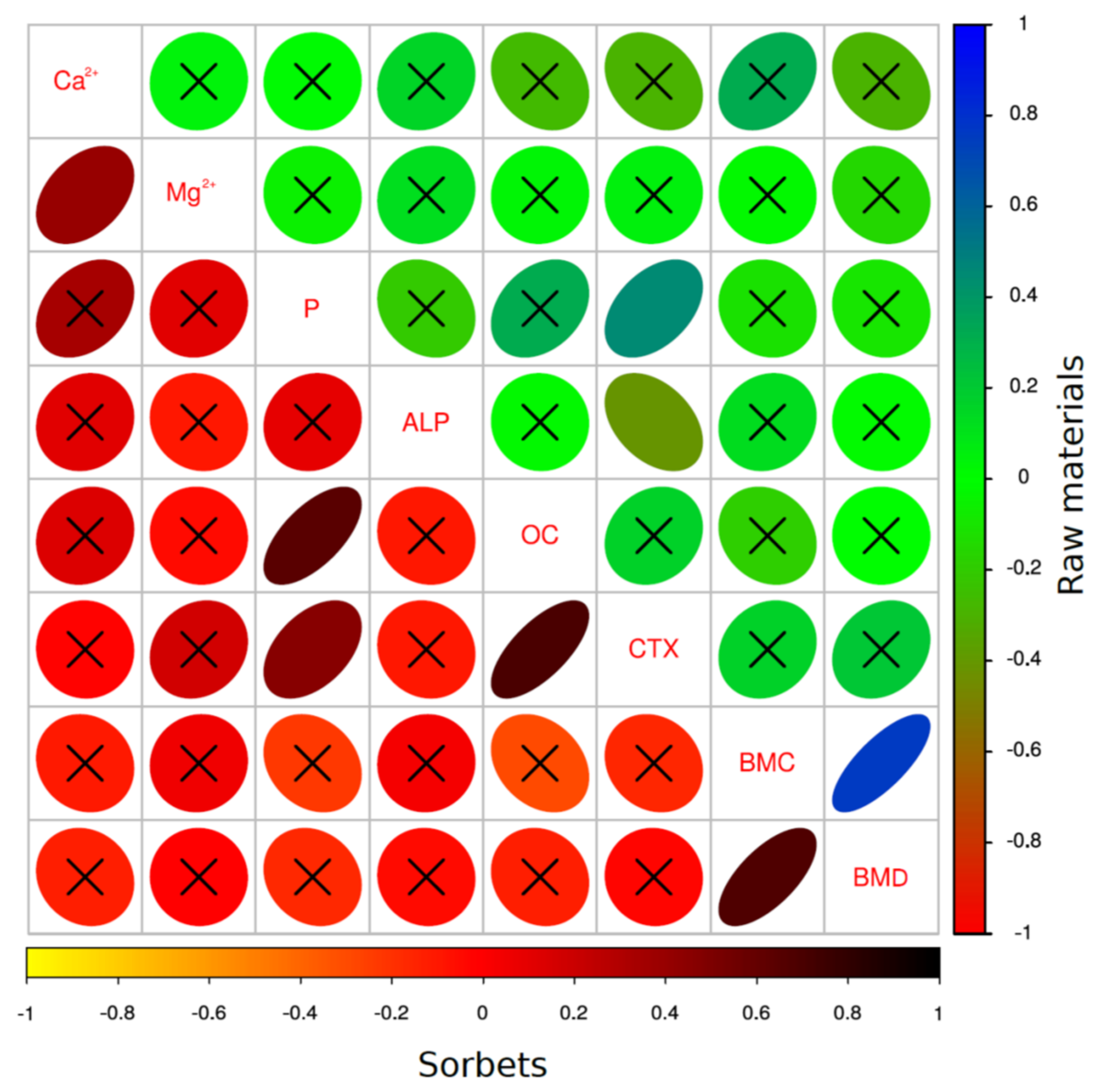
3. Results
3.1. The Changes in Dependence on Dietary Calcium Dose of Examined Parameters
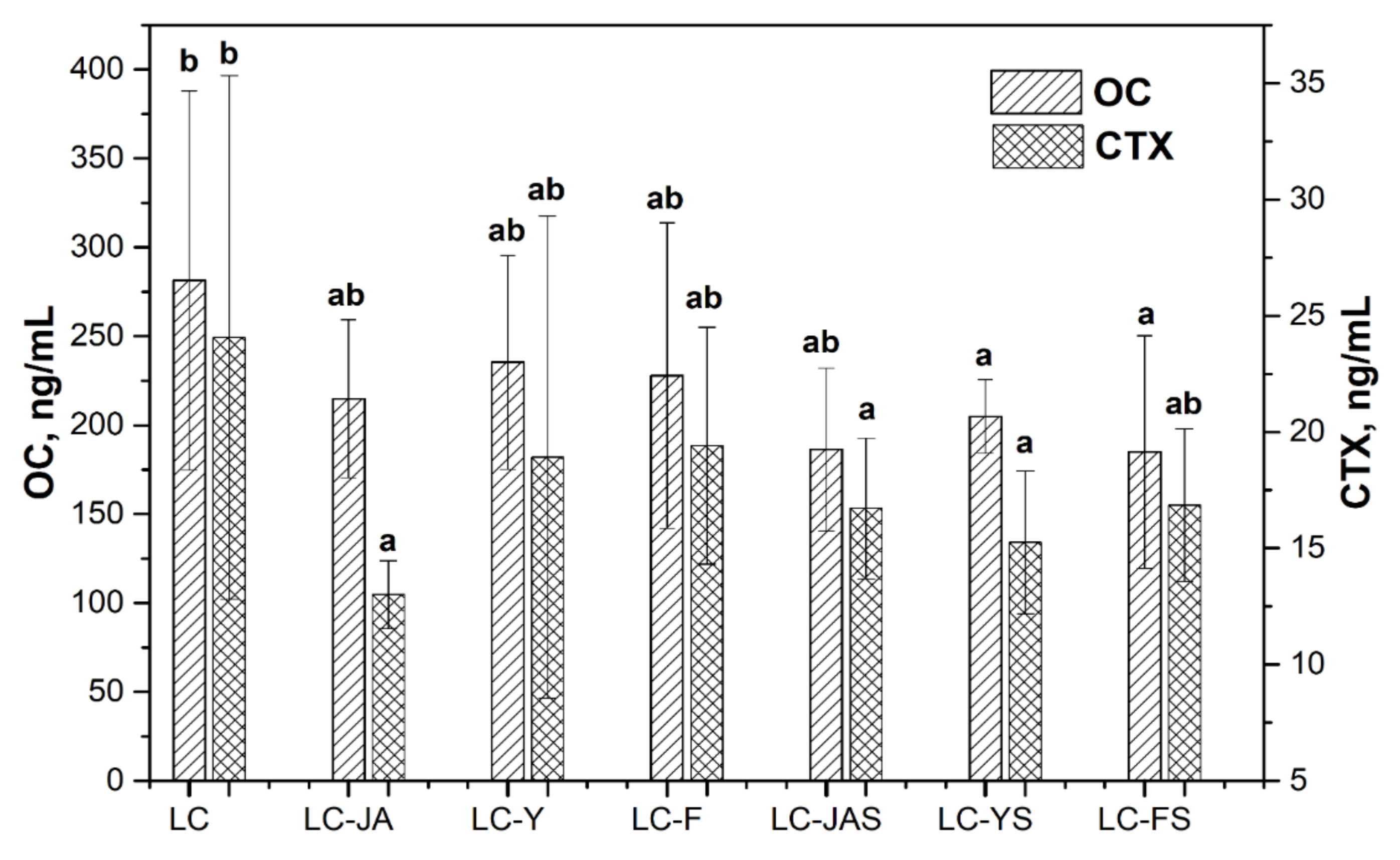
3.2. The Effect of Fructan-Enriched Diets
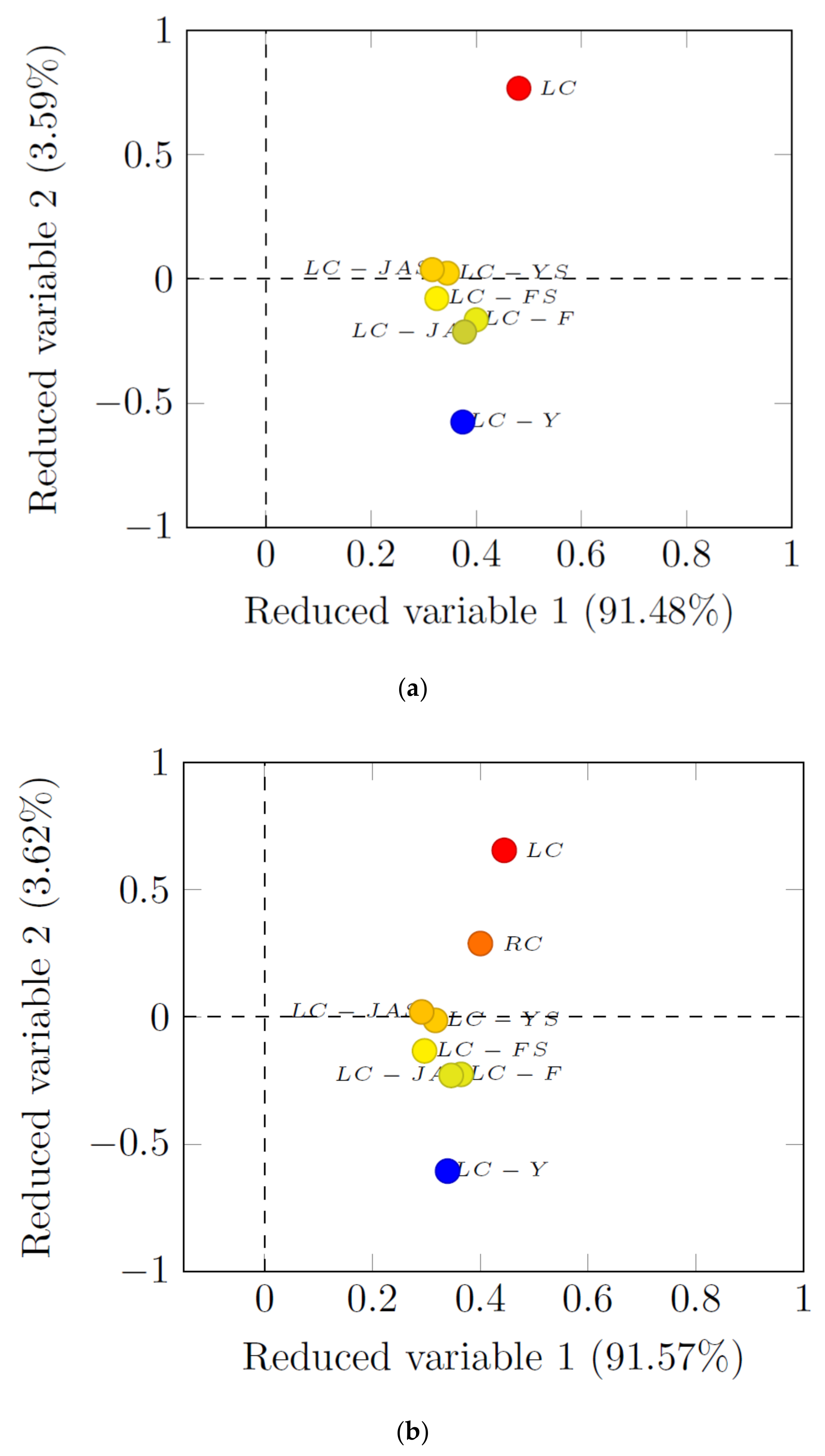
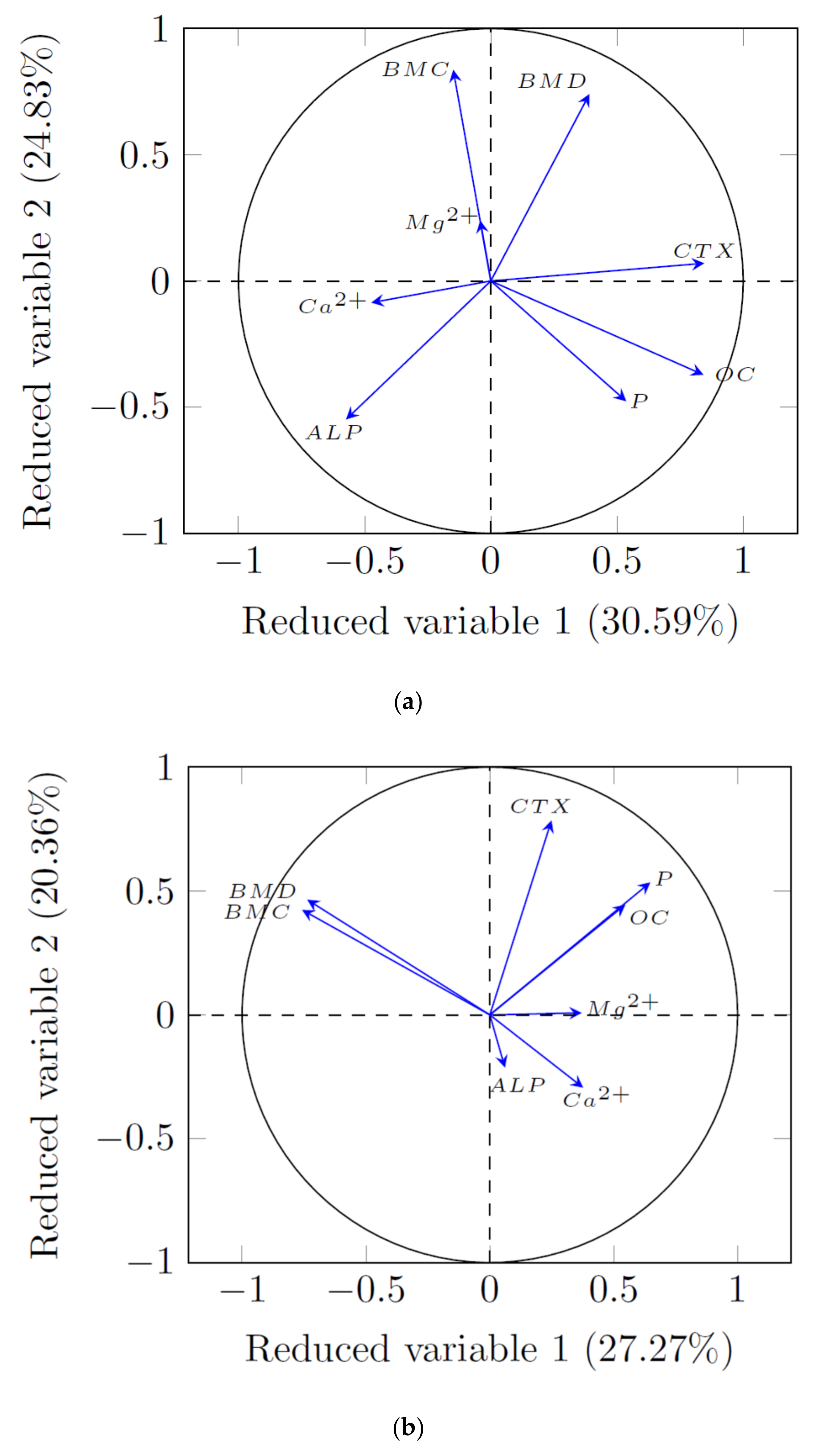
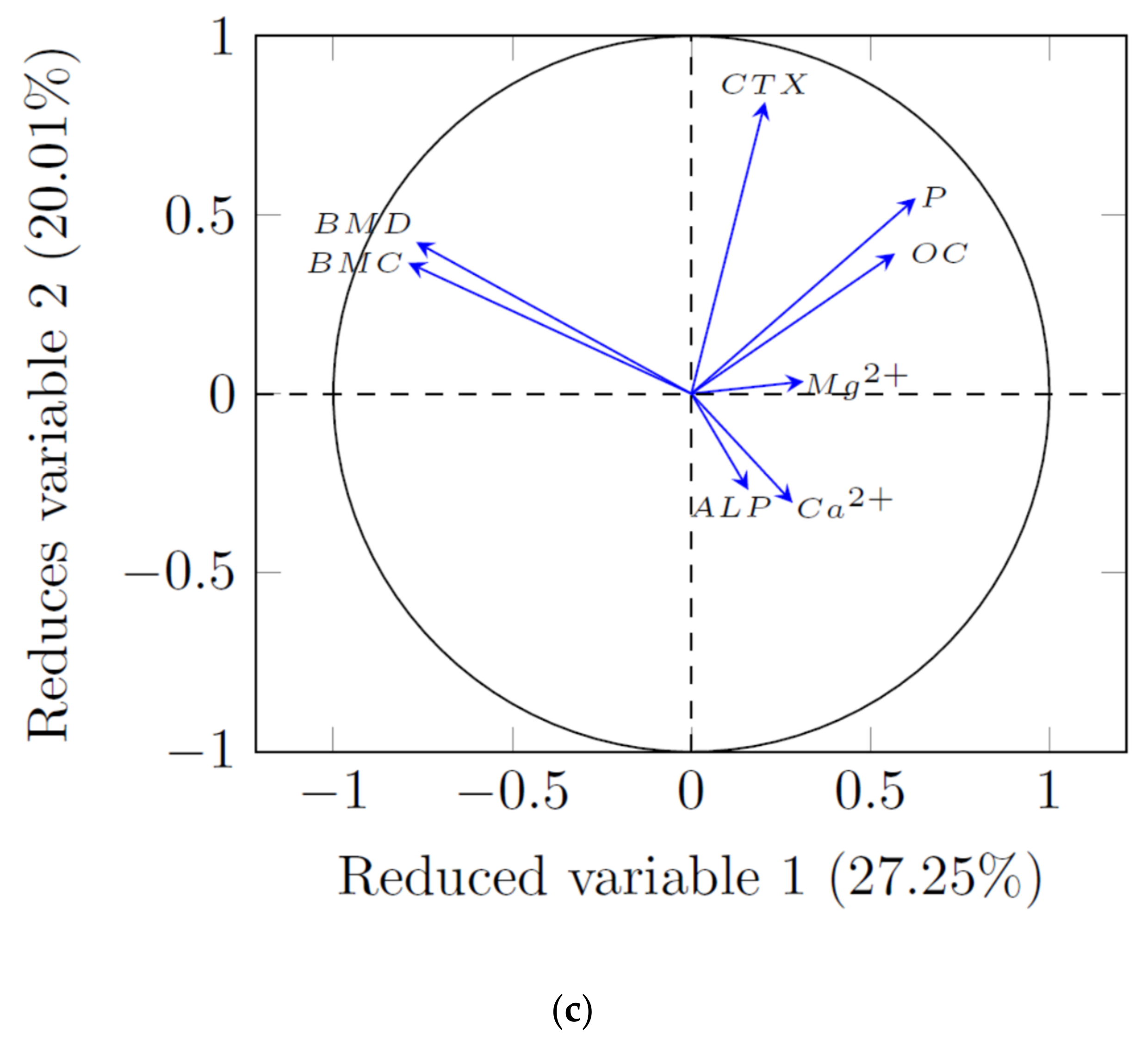
3.3. PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Al Anouti, F.; Taha, Z.; Shamim, S.; Khalaf, K.; Al Kaabi, L.; Alsafar, H. An insight into the paradigms of osteoporosis: From genetics to biomechanics. Bone Rep. 2019, 11, 100216. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, M.C.; Brown, K.E.; Collett, G.; Layton, L.; Schollum, L.M. The Effect of Fructooligosaccharides with Various Degrees of Polymerization on Calcium Bioavailability in the Growing Rat. Exp. Biol. Med. 2003, 228, 683–688. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Hoppler, M.; Membrez Scalfo, F.; Vuichoud, J.; Vigo, M.; Offord, E.A.; Ammann, P. Peak bone strength is influenced by calcium intake in growing rats. Bone 2014, 68, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J. Effects of taurine supplementation on bone mineral density in ovariectomized rats fed calcium deficient diet. Nutr. Res. Pract. 2009, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.; Castillo, L. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2017, 102, 443–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pikosky, M.; Cifelli, C.; Agarwal, S.; Fulgoni, V. Do Americans Get Enough Nutrients from Food? Assessing Nutrient Adequacy with NHANES 2013–2016 (P18-040-19). Curr. Dev. Nutr. 2019, 3 (Suppl. 1), nzz039-P18. [Google Scholar] [CrossRef]
- Kosendiak, A.; Bronkowska, M.; Felińczak, A.; Biernat, J. Ocena spożycia wybranych produktów mlecznych jako źródeł wapnia przez osoby przygotowujące się do maratonu. Bromat. Chem. Toksykol. 2016, 49, 10–15. (In Polish) [Google Scholar]
- Kwiatkowska, I.; Lubawy, M.; Formanowicz, D. Nutritional procedure in osteoporosis prevention in older people. Geriatria 2019, 13, 177–183. [Google Scholar]
- Koczara, O.; Rumińska, M.; Czerwonogrodzka-Senczyna, A. The effect of dietary calcium intake and dairy products on body weight in children aged 7–10 years. Pediatr. Endocrinol. 2018, 17, 183–190. [Google Scholar]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzoli, R. Nutritional aspects of bone health. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark Res. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martirosyan, D.; Singh, J. A New Definition of Functional Food by FFC: What Makes a New Definition Unique? Funct. Food Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- Saeed, F.; Pasha, I.; Arshad, M.; Rasheed, R.; Nasir, M.; Shafique, B. Physiological and Nutraceutical Perspectives of Fructan. Int. J. Food Prop. 2015, 18, 1895–1904. [Google Scholar] [CrossRef]
- Franco-Robles, E.; Ramírez-Emiliano, J.; López, M.G. Agave fructans and oligofructose decrease oxidative stress in brain regions involved in learning and memory of overweight mice. Nat. Prod. Res. 2018, 33, 1527–1530. [Google Scholar] [CrossRef]
- Topolska, K.; Bieńko, M.; Filipiak-Florkiewicz, A.; Radzki, R.P.; Cieślik, E. The effect of fructan-enriched diet on bone turnover parameters in ovariectomized rats under calcium restriction. Ann. Agric. Environ. Med. 2020, 27, 219–224. [Google Scholar] [CrossRef]
- Jamieson, J.A.; Ryz, N.R.; Taylor, C.G.; Weiler, H.A. Dietary long-chain inulin reduces abdominal fat but has no effect on bone density in growing female rats. Br. J. Nutr. 2008, 100, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Topolska, K.; Radzki, R.P.; Filipiak-Florkiewicz, A.; Florkiewicz, A.; Leszczyńska, T.; Cieślik, E. Fructan-Enriched Diet Increases Bone Quality in Female Growing Rats at Calcium Deficiency. Plant. Foods Hum. Nutr. 2018, 73, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Topolska, K.; Radzki, R.P.; Filipiak-Florkiewicz, A.; Bieńko, M.; Florkiewicz, A.; Cieślik, E. Could fructan sources in strawberry matrix be more effective as a tool for improvement of bone structure than these compounds added to diet alone?—Study on osteopenic rat model. Ann. Agric. Environ. Med. 2020, 27, 19–28. [Google Scholar] [CrossRef]
- Bakirhan, H.; Karabudak, E. Effects of inulin on calcium metabolism and bone health. Int. J. Vit. Nutr. Res. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kalala, G.; Kambashi, B.; Everaert, N.; Beckers, Y.; Richel, A.; Pachikian, B.; Neyrinck, A.M.; Delzenne, N.M.; Bindelle, J. Characterization of fructans and dietary fibre profiles in raw and steamed vegetables. Int. J. Food Sci. Nutr. 2018, 69, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C. Inulin, oligofructose and bone health: Experimental approaches and mechanisms. Brit. J. Nutr. 2005, 93, S99–S103. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Topolska, K.; Filipiak-Florkiewicz, A.; Florkiewicz, A.; Cieślik, E. Fructan stability in strawberry sorbets in dependence on their source and the period of storage. Eur. Food Res. Technol. 2017, 243, 701–709. [Google Scholar] [CrossRef]
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The Upper Tail Probabilities of Spearman’s\(\rho\) . Appl. Stat. 1975, 24, 377–379. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Vienna, Austria. 2012. Available online: https://www.R-project.org (accessed on 29 October 2021).
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Mechanisms of Disease. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [Green Version]
- Centeno, V.; de Barboza, G.D.; Marchionatti, A.; Rodríguez, V.; Tolosa de Talamoni, N. Molecular mechanisms triggered by low-calcium diets. Nutr. Res. Rev. 2009, 22, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Kasukawa, Y.; Baylink, D.J.; Wergedal, J.E.; Amaar, Y.; Srivastava, A.K.; Guo, R.; Mohan, S. Lack of insulin-like growth factor I exaggerates the effect of calcium deficiency on bone accretion in mice. Endocrinology 2003, 144, 4682–4689. [Google Scholar] [CrossRef]
- Coudray, C.; Rambeau, M.; Feillet-Coudray, C.; Tressol, J.C.; Demigne, C.; Gueux, E.; Mazur, A.; Rayssiguier, Y. Dietary inulin intake and age can significantly affect intestinal absorption of calcium and magnesium in rats: A stable isotope approach. Nutr. J. 2005, 4, 29. [Google Scholar] [CrossRef]
- Bryk, G.; Coronel, M.Z.; Pellegrini, G.; Mandalunis, P.; Rio, M.E.; de Portela, M.L.; Zeni, S.N. Effect of a combination GOS/FOS® prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur. J. Nutr. 2015, 54, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Kapoor, N.; Bondu, J.D.; Thomas, N.; Paul, T.V. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 2016, 20, 846–852. [Google Scholar] [CrossRef]
- Grigoryan, A.V.; Dimitrova, A.A.; Kostov, K.G.; Russeva, A.L.; Atanasova, M.A.; Blagev, A.B.; Betova, T.M.; Trifonov, R.G. Changes of Serum Concentrations of Alkaline Phosphatase and Metalloproteinase-9 in an Ovariectomized Wistar Rat Model of Osteoporosis. J. Biomed. Clin. Res. 2017, 10, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Lee, M.H.; Rhee, M.H. Studies on the effects of biomedicinal agents on serum concentration of Ca2+, P and ALP activity in osteoporosis-induced rats. J. Vet. Sci. 2003, 4, 151–154. [Google Scholar] [CrossRef]
- Cho, J.A.; Baek, S.Y.; Cheong, S.H.; Kim, M.R. Spirulina Enhances Bone Modeling in Growing Male Rats by Regulating Growth-Related Hormones. Nutrients 2020, 12, 1187. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shirota, H.; Kuwada, M.; Terato, K.; Katayama, K. Plasma and Bone Osteocalcin Levels in Rats with Type I1 Collagen-Induced Arthritis. Arthritis Rheum. 1988, 31, 1413–1420. [Google Scholar] [CrossRef]
- Talbott, S.M.; Chowdhury, H.; Shapses, S.A. Urinary 3H-tetracycline and pyridinium crosslinks differ in their response to calcium restriction in mature and aged rats. Calcif. Tissue Int. 1999, 64, 352–356. [Google Scholar] [CrossRef]
- Creedon, A.; Cashman, K.D. The effect of calcium intake on bone composition and bone resorption in the young growing rat. Br. J. Nutr. 2001, 86, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Nzeusseu, A.; Dienst, D.; Haufroid, V.; Depresseux, G.; Devogelaer, J.P.; Manicourt, D.H. Inulin and fructo-oligosaccharides differ in their ability to enhance the density of cancellous and cortical bone in the axial and peripheral skeleton of growing rats. Bone 2006, 38, 394–399. [Google Scholar] [CrossRef]
- Zafar, T.A.; Weaver, C.M.; Zhao, Y.; Martin, B.R.; Wastney, M.E. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J. Nutr. 2004, 134, 399–402. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.P.; Delmas, P.D.; Malaval, L.; Edouard, C.; Chapuy, M.C.; Meunier, P.J. Serum bone Gla-protein: A specific marker for bone formation in postmenopausal osteoporosis. Lancet 1984, 1, 1091–1093. [Google Scholar] [CrossRef]
- Lobo, A.R.; Colli, C.; Alvares, E.P.; Filisetti, T.M. Effects of fructans-containing yacon (Smallanthus sonchifolius Poepp and Endl.) flour on caecum mucosal morphometry, calcium and magnesium balance, and bone calcium retention in growing rats. Brit. J. Nutr. 2007, 97, 776–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.X.; Li, Q.; Wang, Q.; Li, Y.; Ling, J.; Liu, L.; Chen, X.; Bi, K. Simultaneous determination of seven bioactive components in Oolong Tea Camellia sinensis: Quality control by chemical composition and HPLC fingerprints. J. Agric. Food Chem. 2012, 60, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Weifeng, D.; Xiaoning, L.; Ying, Y.; Xianke, Y.; Dongjing, J.; Weihong, G.; Baochang, C. Quantitative determination, principal component analysis and discriminant analysis of eight marker compounds in crude and sweated Dipsaci Radix by HPLC-DAD. Pharm. Biol. 2017, 55, 2129–2135. [Google Scholar] [CrossRef] [Green Version]
| Group Shortcut | Group Description | Number of Animals |
|---|---|---|
| RC | Animals fed a diet with recommended calcium dose (100% Ca) | 8 |
| LC | Animals fed a Ca-deficient diet (60% Ca) | 8 |
| LC-JA | Animals fed a Ca-deficient diet (60% Ca), with Jerusalem artichoke pulp added alone | 8 |
| LC-Y | Animals fed a Ca-deficient diet (60% Ca), with yacon root powder added alone | 8 |
| LC-F | Animals fed a Ca-deficient diet (60% Ca), with the formulation Beneo Orafti Synergy 1 added alone | 8 |
| LC-JAS | Animals fed a Ca-deficient diet (60% Ca), with Jerusalem artichoke pulp added in a strawberry sorbet matrix | 8 |
| LC-YS | Animals fed a Ca-deficient diet (60% Ca), with yacon root powder added in a strawberry sorbet matrix | 8 |
| LC-FS | Animals fed a Ca-deficient diet (60% Ca), with the formulation Beneo Orafti Synergy 1 added in a strawberry sorbet matrix | 8 |
| Parameter | Validation Group | |
|---|---|---|
| RC | LC | |
| Ca 2+ (mg/dL) | 5.98 A ± 0.27 | 5.93 A ± 0.32 |
| Mg2+ (mg/dL) | 3.52 A ± 0.18 | 3.41 A ± 0.48 |
| P (mg/dL) | 5.41 A ± 1.75 | 7.51 B ± 2.15 |
| ALP (mg/dL) | 94.00 A ± 19.86 | 80.65 A ± 16.35 |
| OC (ng/mL) | 252.61 A ± 54.67 | 281.31 A ± 106.69 |
| CTX (ng/mL) | 14.81 A ± 2.67 | 24.07 B ± 11.26 |
| BMC (g) | 0.36 A ± 0.02 | 0.36 A ± 0.02 |
| BMD (g/cm2) | 0.11 A ± 0.02 | 0.11 A ± 0.01 |
| Parameter (unit) | LC | Fructan Source | |||||
|---|---|---|---|---|---|---|---|
| Type of Raw Material | Type of Sorbet | ||||||
| LC-JA | LC-Y | LC-F | LC-JAS | LC-YS | LC-FS | ||
| Ca 2+ (mg/dL) | 5.93 ab ± 0.32 | 6.28 c ± 0.37 | 5.91 ab ± 0.35 | 6.06 bc ± 0.38 | 6.28 c ± 0.27 | 6.17 b ± 0.28 | 5.66 a ± 0.41 |
| Mg 2+ (mg/dL) | 3.41 a ± 0.48 | 3.84 bc ± 0.48 | 3.77 bc ± 0.17 | 4.12 c ± 0.54 | 3.59 ab ± 0.25 | 3.79 bc ± 0.27 | 3.59 ab ± 0.22 |
| P (mg/dL) | 7.51 c ± 2.15 | 5.60 a ± 0.79 | 6.01 ab ± 0.69 | 6.87 abc ± 0.75 | 5.35 a ± 1.02 | 6.40 ab ± 1.20 | 6.00 a ± 1.96 |
| ALP (mg/dL) | 80.65 b ± 16.35 | 99.15 c ± 11.02 | 96.11 c ± 16.84 | 73.23 b ± 15.34 | 70.67 ab ± 18.15 | 64.10 a ± 13.58 | 67.78 ab ± 8.89 |
| BMC (g) | 0.36 ab ± 0.02 | 0.37 ab ± 0.02 | 0.39 b ± 0.04 | 0.35 a ± 0.02 | 0.39 b ± 0.04 | 0.38 ab ± 0.02 | 0.36 ab ± 0.02 |
| BMD (g/cm2) | 0.11 a ± 0.01 | 0.12 a ± 0.01 | 0.12 a ± 0.03 | 0.11 a ± 0.02 | 0.12 a ± 0.04 | 0.12 a ± 0.02 | 0.11 a ± 0.02 |
| Parameter | Factor 1 (Type of Fructan Source) | Factor 2 (Form of Fructan Source) | Factor 1 and Factor 2 |
|---|---|---|---|
| Ca2+ | 0.006 | ns | ns |
| Mg2+ | 0.004 | 0.037 | ns |
| P | 0.020 | ns | ns |
| ALP | ns | 0.000 | 0.005 |
| OC | 0.010 | ns | ns |
| CTX | 0.005 | ns | ns |
| BMC | 0.035 | ns | ns |
| BMD | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topolska, K.; Bienko, M.; Ptaszek, P.; Florkiewicz, A.; Radzki, R.P.; Filipiak-Florkiewicz, A. When Incorporated into Fruit Sorbet Matrix, Are the Fructans in Natural Raw Materials More Beneficial for Bone Health than Commercial Formulation Added Alone? Animals 2022, 12, 1134. https://doi.org/10.3390/ani12091134
Topolska K, Bienko M, Ptaszek P, Florkiewicz A, Radzki RP, Filipiak-Florkiewicz A. When Incorporated into Fruit Sorbet Matrix, Are the Fructans in Natural Raw Materials More Beneficial for Bone Health than Commercial Formulation Added Alone? Animals. 2022; 12(9):1134. https://doi.org/10.3390/ani12091134
Chicago/Turabian StyleTopolska, Kinga, Marek Bienko, Paweł Ptaszek, Adam Florkiewicz, Radoslaw P. Radzki, and Agnieszka Filipiak-Florkiewicz. 2022. "When Incorporated into Fruit Sorbet Matrix, Are the Fructans in Natural Raw Materials More Beneficial for Bone Health than Commercial Formulation Added Alone?" Animals 12, no. 9: 1134. https://doi.org/10.3390/ani12091134
APA StyleTopolska, K., Bienko, M., Ptaszek, P., Florkiewicz, A., Radzki, R. P., & Filipiak-Florkiewicz, A. (2022). When Incorporated into Fruit Sorbet Matrix, Are the Fructans in Natural Raw Materials More Beneficial for Bone Health than Commercial Formulation Added Alone? Animals, 12(9), 1134. https://doi.org/10.3390/ani12091134








