Alleviation of Cr(VI) Toxicity and Improve Phytostabilization Potential of Vigna radiata Using a Novel Cr(VI) Reducing Multi-Stress-Tolerant Plant Growth Promoting Rhizobacterial Strain Bacillus flexus M2
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection and Bacterial Isolation
2.2. Cr(VI) Reduction Assay
2.3. Multi-Metal Tolerance and Antibiotic Resistance
2.4. Molecular Identification and Phylogenetic Analysis of the Rhizobacterial Isolate M2
2.5. Fourier Transform Infra-Red (FTIR) Spectroscopy, Raman Spectroscopy, and Transmission Electron Microscopy–Energy Dispersive X-ray Spectroscopy (TEM-EDX) Analysis
2.6. Plant-Growth-Promoting Ability of the Rhizobacterial Strain M2
2.6.1. Indole Acetic Acid, Ammonia, and Exocellular Polysaccharide Production
2.6.2. Phosphate Solubilization, Siderophore, Catalase, Protease, Amylase, and Lipase Production
2.7. Influence of the Strain M2 Inoculation on V. radiata Growth under Cr(VI) Stress
2.7.1. Estimation of Photosynthetic Pigments
2.7.2. Estimation of Proline and Hydrogen Peroxide Contents
2.7.3. Antioxidant Enzymes Extraction from Roots and Leaves of V. radiata
2.7.4. Estimation of Superoxide Dismutase, Catalase and Peroxidase Activities in V. radiata
2.7.5. Analysis of Cr Accumulation in Plant Tissues
2.8. Statistical Analysis
3. Results and Discussion
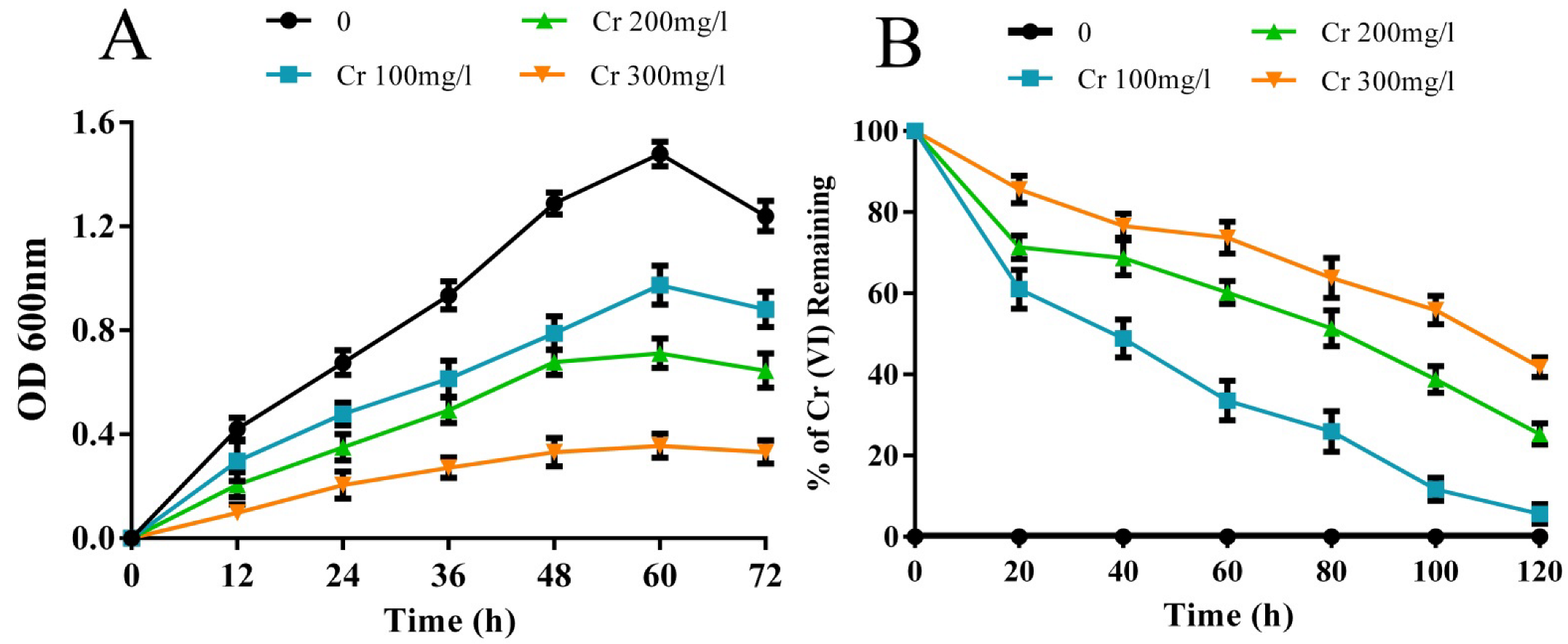
3.1. Cr(VI) Tolerance and Reduction Ability of the Rhizobacterial Strain M2
3.1.1. Effect of Temperature, pH, NaCl, and PEG on Cr(VI) Reduction
3.1.2. Multi-Metal Tolerance and Antibiotic Resistance of the Rhizobacterial Strain M2
3.2. Molecular Identification and Phylogenetic Analysis of the Rhizobacterial Strain M2
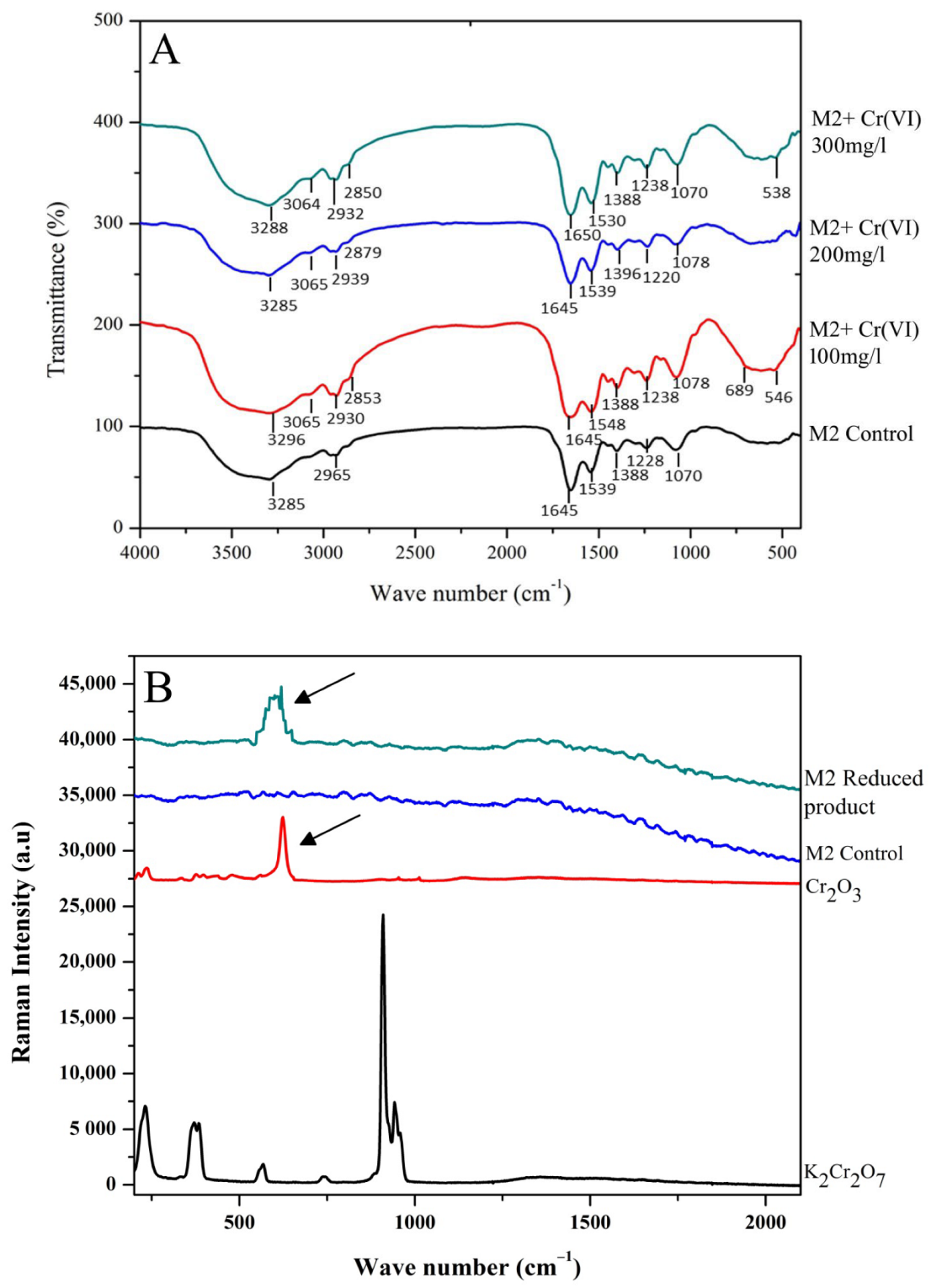
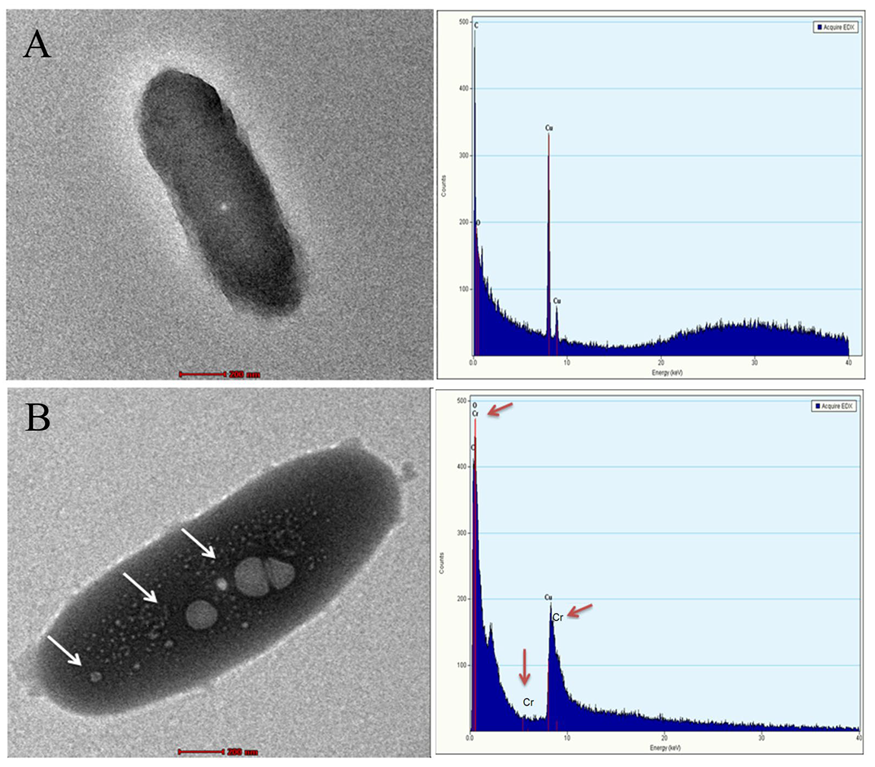
3.3. FTIR, Raman Spectroscopy and TEM-EDX Analysis on the Rhizobacterial Strain M2
3.4. Plant-Growth-Promoting Activities of the Rhizobacterial Strain M2 under Cr Stress
3.4.1. Indole Acetic Acid, Ammonia, and EPS Production
3.4.2. Phosphate Solubilization, Siderophore, Catalase, Protease, Amylase, and Lipase Production
3.5. Effect of the Rhizobacterial Strain M2 and Cr(VI) on V. radiata Growth and Antioxidant Response
3.5.1. Effect of Strain M2 and Cr(VI) on Seed Germination, Root and Shoot Length of V. radiata
3.5.2. Effect of the Strain M2 and Cr(VI) on Chlorophyll and Carotenoid Contents of V. radiata
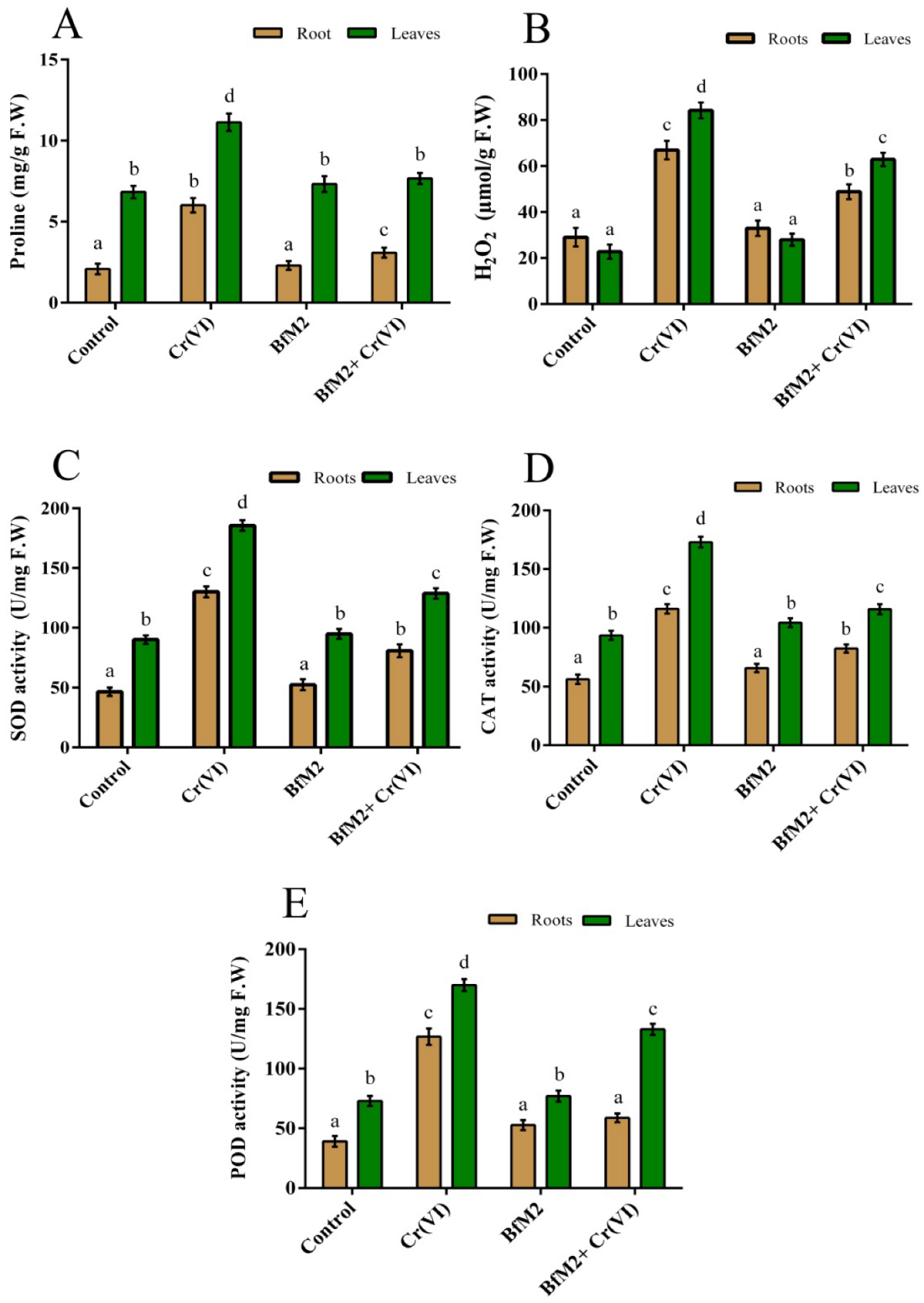
3.5.3. Effect of the Strain M2 and Cr(VI) on Proline and H2O2 Contents of V. radiata
3.5.4. Effect of the Strain M2 and Cr(VI) on the Activity of SOD, CAT, and POD in V. radiata
3.5.5. Effect of the Strain M2 Inoculation of Cr Accumulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bahadur, A.; Ahmad, R.; Afzal, A.; Feng, H.; Suthar, V.; Batool, A.; Khan, A.; Mahmood-ul-Hassan, M. The influences of Cr-tolerant rhizobacteria in phytoremediation and attenuation of Cr (VI) stress in agronomic sunflower (Helianthus annuus L.). Chemosphere 2017, 179, 112–119. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Health Advisory—Chromium; Office of Drinking Water, US Environmental Protection Agency: Washington, DC, USA, 1987. [Google Scholar]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Greger, M. Metal Availability and Bioconcentration in Plants. In Heavy Metal Stress in Plants: From Molecules to Ecosystems; Prasad, M.N.V., Hagemeyer, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–27. [Google Scholar]
- DesMarias, T.L.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in Agricultural Soils and Crops: A Review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Kanwar, V.S.; Sharma, A.; Srivastav, A.L.; Rani, L. Phytoremediation of toxic metals present in soil and water environment: A critical review. Environ. Sci. Pollut. Res. 2020, 27, 44835–44860. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Gao, J.; Wu, S.; Liu, Y.; Wu, S.; Jiang, C.; Li, X.; Wang, R.; Bai, Z.; Zhuang, G.; Zhuang, X. Characterization and transcriptomic analysis of a highly Cr(VI)-resistant and -reductive plant-growth-promoting rhizobacterium Stenotrophomonas rhizophila DSM14405T. Environ. Pollut. 2020, 263, 114622. [Google Scholar] [CrossRef]
- Prakash, J. Chapter 9—Plant growth promoting rhizobacteria in phytoremediation of environmental contaminants: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Kumar, V., Saxena, G., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–218. [Google Scholar]
- Danish, S.; Kiran, S.; Fahad, S.; Ahmad, N.; Ali, M.A.; Tahir, F.A.; Rasheed, M.K.; Shahzad, K.; Li, X.; Wang, D.; et al. Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2019, 185, 109706. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Husna; Murad, W. Phytohormones producing rhizobacterium alleviates chromium toxicity in Helianthus annuus L. by reducing chromate uptake and strengthening antioxidant system. Chemosphere 2020, 258, 127386. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 149. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Chaudhary, H.J.; et al. PGPR-Mediated Plant Growth Attributes and Metal Extraction Ability of Sesbania sesban L. in Industrially Contaminated Soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Karthik, C.; Elangovan, N.; Kumar, T.S.; Govindharaju, S.; Barathi, S.; Oves, M.; Arulselvi, P.I. Characterization of multifarious plant growth promoting traits of rhizobacterial strain AR6 under Chromium (VI) stress. Microbiol. Res. 2017, 204, 65–71. [Google Scholar] [CrossRef]
- Baldiris, R.; Acosta-Tapia, N.; Montes, A.; Hernández, J.; Vivas-Reyes, R. Reduction of Hexavalent Chromium and Detection of Chromate Reductase (ChrR) in Stenotrophomonas maltophilia. Molecules 2018, 23, 406. [Google Scholar] [CrossRef]
- Holt, J.C.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergeys Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Gadd, G.M. Heavy metal accumulation by bacteria and other microorganisms. Experientia 1990, 46, 834–840. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Karthik, C.; Barathi, S.; Pugazhendhi, A.; Ramkumar, V.S.; Thi, N.B.D.; Arulselvi, P.I. Evaluation of Cr(VI) reduction mechanism and removal by Cellulosimicrobium funkei strain AR8, a novel haloalkaliphilic bacterium. J. Hazard. Mater. 2017, 333, 42–53. [Google Scholar] [CrossRef]
- Libbert, E.; Kaiser, W.; Kunert, R. Interactions between Plants and Epiphytic Bacteria Regarding Their Auxin Metabolism VI. The Influence of the Epiphytic Bacteria on the Content of Extractable Auxin in the Plant. Physiol. Plant. 1969, 22, 432–439. [Google Scholar] [CrossRef]
- Cappuccino, J.G.; Welsh, C.T. Microbiology: A Laboratory Manual; Pearson Education: London, UK, 2017. [Google Scholar]
- Mody, B.; Bindra, M.; Modi, V. Extracellular polysaccharides of cowpea rhizobia: Compositional and functional studies. Arch. Microbiol. 1989, 153, 38–42. [Google Scholar] [CrossRef]
- Onyia, C.E.; Anyawu, C.U.; Ikegbunam, M.N. Ability of Fungi, Isolated from Nsukka Peppers and Garden-Egg Plant Rhizospheres, to Solubilize Phosphate and Tolerate Cadmium. J. Adv. Microbiol. 2015, 5, 500. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Seifi, S.; Patel, P.R.; Shaikh, S.S.; Jadhav, H.P.; Enshasy, H.E. Siderophore production in groundnut rhizosphere isolate, Achromobacter sp. RZS2 influenced by physicochemical factors and metal ions. Environ. Sustain. 2019, 2, 117–124. [Google Scholar] [CrossRef]
- Smibert, R.M.; Krieg. Phenotypic Characterization. In Methods for General and Molecular Bacteriology; Gerhardt, P., Murray, R.G.E., Wood, W.A., Krieg, N.R., Eds.; American Society for Microbiology: Washington, DC, USA, 1994; pp. 607–654. [Google Scholar]
- Mesa, J.; Mateos-Naranjo, E.; Caviedes, M.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D. Endophytic Cultivable Bacteria of the Metal Bioaccumulator Spartina maritima Improve Plant Growth but Not Metal Uptake in Polluted Marshes Soils. Front. Microbiol. 2015, 6, 1450. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, L.; Nagar, D.S.; Raina, C.; Parshad, R.; Gupta, V. Screening, isolation and production of lipase/esterase producing Bacillus sp. Strain DVL2 and its potential evaluation in esteritication and resolution reactions. Arch. Appl. Sci. Res. 2012, 4, 1763–1770. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- RoyChoudhury, A.; Roy, C.; Sengupta, D.N. Transgenic tobacco plants overexpressing the heterologous lea gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress. Plant Cell Rep. 2007, 26, 1839–1859. [Google Scholar] [CrossRef]
- Esfandiari, E.; Fariborz, S.; Shekari, F.; Manouchehr, E. The effect of salt stress on antioxidant enzymes’ activity and lipid peroxidation on the wheat seedling. Not. Bot. Horti Agrobot. Cluj Napoca 2007, 35, 48. [Google Scholar] [CrossRef]
- Dhindsa, R.; Plumb-Dhindsa, P.; Thorpe, T. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: London, UK, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Castillo, F.J.; Penel, C.; Greppin, H. Peroxidase Release Induced by Ozone in Sedum album Leaves: Involvement of Ca. Plant Physiol. 1984, 74, 846–851. [Google Scholar] [CrossRef]
- Freitas, H.; Prasad, M.N.; Pratas, J. Analysis of serpentinophytes from north-east of Portugal for trace metal accumulation--relevance to the management of mine environment. Chemosphere 2004, 54, 1625–1642. [Google Scholar] [CrossRef]
- Banerjee, S.; Misra, A.; Chaudhury, S.; Dam, B. A Bacillus strain TCL isolated from Jharia coalmine with remarkable stress responses, chromium reduction capability and bioremediation potential. J. Hazard Mater. 2019, 367, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Sobol, Z.; Schiestl, R.H. Intracellular and extracellular factors influencing Cr(VI) and Cr(III) genotoxicity. Environ. Mol. Mutagen. 2012, 53, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Arivalagan, P.; Singaraj, D.; Haridass, V.; Kaliannan, T. Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecol. Eng. 2014, 71, 728–735. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Rajasekar, A.; Chang, Y.-C. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech 2016, 6, 242. [Google Scholar] [CrossRef]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium inhibition in the anaerobic digestion process: Antagonism and adaptation phenomena. Enzym. Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Yamina, B.; Tahar, B.; Lila, M.; Hocine, H.; Laure, F.M. Study on Cadmium Resistant-Bacteria Isolated from Hospital Wastewaters. J. Adv. Biosci. Biotechnol. 2014, 5, 47951. [Google Scholar] [CrossRef]
- Rajaram, R. Multiple Heavy Metal and Antibiotic Tolerance Bacteria Isolated from Equatorial Indian Ocean. Int. J. Microbiol. Res. 2013, 4, 212–218. [Google Scholar]
- Hossan, S.; Hossain, S.; Islam, M.R.; Kabir, M.H.; Ali, S.; Islam, M.S.; Imran, K.M.; Moniruzzaman, M.; Mou, T.J.; Parvez, A.K.; et al. Bioremediation of Hexavalent Chromium by Chromium Resistant Bacteria Reduces Phytotoxicity. Int. J. Environ. Res. Public Health 2020, 17, 6013. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Gupta, A.; Gupte, A.; Desai, N. Biotransformation of chromium by root nodule bacteria Sinorhizobium sp. SAR1. PLoS ONE 2019, 14, e0219387. [Google Scholar] [CrossRef]
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, A. Biosorption of chromium(VI) ions from aqueous solution by the bacterium Bacillus thuringiensis. Process Biochem. 2005, 40, 1895–1901. [Google Scholar] [CrossRef]
- Gilmore, M.E.; Bandyopadhyay, D.; Dean, A.M.; Linnstaedt, S.D.; Popham, D.L. Production of muramic delta-lactam in Bacillus subtilis spore peptidoglycan. J. Bacteriol. 2004, 186, 80–89. [Google Scholar] [CrossRef]
- Tan, H.; Wang, C.; Zeng, G.; Luo, Y.; Li, H.; Xu, H. Bioreduction and biosorption of Cr(VI) by a novel Bacillus sp. CRB-B1 strain. J. Hazard. Mater. 2020, 386, 121628. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, J.; Xia, L.; Zhang, X.; Luo, L. Mechanisms of Cr(VI) reduction by Bacillus sp. CRB-1, a novel Cr(VI)-reducing bacterium isolated from tannery activated sludge. Ecotoxicol. Environ. Saf. 2019, 186, 109792. [Google Scholar] [CrossRef]
- Cheung, K.H.; Gu, J.-D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. Int. Biodeterior. Biodegrad. 2007, 59, 8–15. [Google Scholar] [CrossRef]
- Raman, N.M.; Asokan, S.; Shobana Sundari, N.; Ramasamy, S. Bioremediation of chromium(VI) by Stenotrophomonas maltophilia isolated from tannery effluent. Int. J. Environ. Sci. Technol. 2018, 15, 207–216. [Google Scholar] [CrossRef]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef]
- Enbaia, S.; Eswayah, A.; Hondow, N.; Gardiner, P.H.E.; Smith, T.J. Detoxification, Active Uptake, and Intracellular Accumulation of Chromium Species by a Methane-Oxidizing Bacterium. Appl. Environ. Microbiol. 2021, 87, e00947-20. [Google Scholar] [CrossRef]
- Villagrasa, E.; Bonet-Garcia, N.; Solé, A. Ultrastructural evidences for chromium(III) immobilization by Escherichia coli K-12 depending on metal concentration and exposure time. Chemosphere 2021, 285, 131500. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Z.; Hu, Y.; Hu, L.; Zhong, H. Both cell envelope and cytoplasm were the locations for chromium(VI) reduction by Bacillus sp. M6. Bioresour. Technol. 2019, 273, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, S.H.; Khaine, I.; Kwak, M.J.; Lee, H.K.; Lee, T.Y.; Lee, W.Y.; Woo, S.Y. Physiological changes and growth promotion induced in poplar seedlings by the plant growth-promoting rhizobacteria Bacillus subtilis JS. Photosynthetica 2018, 56, 1188–1203. [Google Scholar] [CrossRef]
- Thorgersen, M.P.; Lancaster, W.A.; Ge, X.; Zane, G.M.; Wetmore, K.M.; Vaccaro, B.J.; Poole, F.L.; Younkin, A.D.; Deutschbauer, A.M.; Arkin, A.P.; et al. Mechanisms of Chromium and Uranium Toxicity in Pseudomonas stutzeri RCH2 Grown under Anaerobic Nitrate-Reducing Conditions. Front. Microbiol. 2017, 8, 1529. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Chen, M.; Zhang, X.; Tang, C.Y.; Wu, Z. Acute Responses of Microorganisms from Membrane Bioreactors in the Presence of NaOCl: Protective Mechanisms of Extracellular Polymeric Substances. Environ. Sci. Technol. 2017, 51, 3233–3241. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Nagaraju, G.P.; Kariali, E. Current Perspectives on Rhizobacterial-EPS interactions in Alleviation of Stress Responses: Novel Strategies for Sustainable Agricultural Productivity. In Role of Rhizospheric Microbes in Soil; Volume 1: Stress Management and Agricultural, Sustainability; Meena, V.S., Ed.; Springer: Singapore, 2018; pp. 33–55. [Google Scholar]
- Rafique, M.; Haque, K.; Hussain, T.; Amna; Chaudhary, H.J. Biochemical Talk in the Rhizospheric Microbial Community for Phytoremediation; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 113–164. [Google Scholar]
- Ahemad, M. Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: A review. 3 Biotech 2015, 5, 111–121. [Google Scholar] [CrossRef]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef]
- Tirry, N.; Tahri Joutey, N.; Sayel, H.; Kouchou, A.; Bahafid, W.; Asri, M.; El Ghachtouli, N. Screening of plant growth promoting traits in heavy metals resistant bacteria: Prospects in phytoremediation. J. Genet. Eng. Biotechnol. 2018, 16, 613–619. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Svatoš, A.; Dabrowska, P.; Schmidt, A.; Boland, W.; Kothe, E. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 2008, 74, 19–25. [Google Scholar] [CrossRef]
- Tripathi, M.; Munot, H.P.; Shouche, Y.; Meyer, J.M.; Goel, R. Isolation and Functional Characterization of Siderophore-Producing Lead- and Cadmium-Resistant Pseudomonas putida KNP9. Curr. Microbiol. 2005, 50, 233–237. [Google Scholar] [CrossRef]
- Braud, A.; Jézéquel, K.; Bazot, S.; Lebeau, T. Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 2009, 74, 280–286. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Isolation of a novel rhizobacteria having multiple plant growth promoting traits and antifungal activity against certain phytopathogens. Microbiol. Res. 2020, 240, 126516. [Google Scholar] [CrossRef]
- Galabova, D.; Sotirova, A.; Karpenko, E.; Karpenko, O. Chapter 3—Role of Microbial Surface-Active Compounds in Environmental Protection. In The Role of Colloidal Systems in Environmental Protection; Fanun, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 41–83. [Google Scholar]
- Saleem, M.U.; Asghar, H.N.; Zahir, Z.A.; Shahid, M. Integrated Effect of Compost and Cr Reducing Bacteria on Antioxidant System and Plant Physiology of Alfalfa. Int. J. Agric. Biol. 2018, 20, 2745–2752. [Google Scholar]
- Shahid, M.; Ameen, F.; Maheshwari, H.S.; Ahmed, B.; AlNadhari, S.; Khan, M.S. Colonization of Vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, stressor metabolites, antioxidant status and yield under NaCl stress. Appl. Soil Ecol. 2021, 158, 103809. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, R.; Kumari, M.; Sharma, S. Nontarget effects of chemical pesticides and biological pesticide on rhizospheric microbial community structure and function in Vigna radiata. Environ. Sci. Pollut. Res. 2015, 22, 11290–11300. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, N.L.; Singh, C.K.; Yerramilli, V.; Narayan, R.; Sarkar, S.K.; Singh, I. Chromium (VI)-Induced Alterations in Physio-Chemical Parameters, Yield, and Yield Characteristics in Two Cultivars of Mungbean (Vigna radiata L.). Front. Plant Sci. 2021, 12, 2059. [Google Scholar] [CrossRef]
- Karthik, C.; Kadirvelu, K.; Bruno, B.; Maharajan, K.; Rajkumar, M.; Manoj, S.R.; Arulselvi, P.I. Cellulosimicrobium funkei strain AR6 alleviate Cr(VI) toxicity in Lycopersicon esculentum by regulating the expression of growth responsible, stress tolerant and metal transporter genes. Rhizosphere 2021, 18, 100351. [Google Scholar] [CrossRef]
- Pattnaik, S.; Dash, D.; Mohapatra, S.; Pattnaik, M.; Marandi, A.K.; Das, S.; Samantaray, D.P. Improvement of rice plant productivity by native Cr(VI) reducing and plant growth promoting soil bacteria Enterobacter cloacae. Chemosphere 2020, 240, 124895. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tokas, J.; Singal, H.R. Amelioration of Chromium VI Toxicity in Sorghum (Sorghum bicolor L.) using Glycine Betaine. Sci. Rep. 2019, 9, 16020. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Oves, M.; Thangabalu, R.; Sharma, R.; Santhosh, S.B.; Indra Arulselvi, P. Cellulosimicrobium funkei-like enhances the growth of Phaseolus vulgaris by modulating oxidative damage under Chromium(VI) toxicity. J. Adv. Res. 2016, 7, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Das, S.; Banerjee, S.; Mukherjee, S.; Ganguli, A.; Mondal, S. Heavy metals bio-removal potential of the isolated Klebsiella sp TIU20 strain which improves growth of economic crop plant (Vigna radiata L.) under heavy metals stress by exhibiting plant growth promoting and protecting traits. Biocatal. Agric. Biotechnol. 2021, 38, 102204. [Google Scholar] [CrossRef]
- Subrahmanyam, G.; Sharma, R.K.; Kumar, G.N.; Archana, G. Vigna radiata var. GM4 Plant Growth Enhancement and Root Colonization by a Multi-Metal-Resistant Plant Growth-Promoting Bacterium Enterobacter sp. C1D in Cr(VI)-Amended Soils. Pedosphere 2018, 28, 144–156. [Google Scholar] [CrossRef]
- El-Meihy, R.M.; Abou-Aly, H.E.; Youssef, A.M.; Tewfike, T.A.; El-Alkshar, E.A. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ. Exp. Bot. 2019, 162, 295–301. [Google Scholar] [CrossRef]
- Saif, S.; Khan, M.S. Assessment of toxic impact of metals on proline, antioxidant enzymes, and biological characteristics of Pseudomonas aeruginosa inoculated Cicer arietinum grown in chromium and nickel-stressed sandy clay loam soils. Environ. Monit. Assess. 2018, 190, 290. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Din, B.U.; Javed, M.T.; Afridi, M.S.; Mukhtar, T.; Kamran, M.A.; Ain, Q.U.; Khan, A.A.; Ali, J.; et al. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol. Biochem. 2020, 152, 90–99. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Bhagyawant, S.S.; Narvekar, D.T.; Gupta, N.; Bhadkaria, A.; Koul, K.K.; Srivastava, N. Variations in the antioxidant and free radical scavenging under induced heavy metal stress expressed as proline content in chickpea. Physiol. Mol. Biol. Plants 2019, 25, 683–696. [Google Scholar] [CrossRef]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing Adverse Effects of Pb on Maize Plants by Combined Treatment with Jasmonic, Salicylic Acids and Proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Cuypers, A.; Hendrix, S.; Amaral dos Reis, R.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen Peroxide, Signaling in Disguise during Metal Phytotoxicity. Front. Plant Sci. 2016, 7, 470. [Google Scholar] [CrossRef]
- Christou, A.; Georgiadou, E.C.; Zissimos, A.M.; Christoforou, I.C.; Christofi, C.; Neocleous, D.; Dalias, P.; Torrado, S.O.C.A.; Argyraki, A.; Fotopoulos, V. Hexavalent chromium leads to differential hormetic or damaging effects in alfalfa (Medicago sativa L.) plants in a concentration-dependent manner by regulating nitro-oxidative and proline metabolism. Environ. Pollut. 2020, 267, 115379. [Google Scholar] [CrossRef]
- Nemat, H.; Shah, A.A.; Akram, W.; Ramzan, M.; Yasin, N.A. Ameliorative effect of co-application of Bradyrhizobium japonicum EI09 and Se to mitigate chromium stress in Capsicum annum L. Int. J. Phytoremediation 2020, 22, 1396–1407. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Khan, I.; Tanveer, M.; Shahid, M.; Shakoor, A.; Wang, L. Phyto-Toxicity of Chromium in Maize: Oxidative Damage, Osmolyte Accumulation, Anti-Oxidative Defense and Chromium Uptake. Pedosphere 2017, 27, 262–273. [Google Scholar] [CrossRef]
- Vellosillo, T.; Vicente, J.; Kulasekaran, S.; Hamberg, M.; Castresana, C. Emerging Complexity in Reactive Oxygen Species Production and Signaling during the Response of Plants to Pathogens. Plant Physiol. 2010, 154, 444–448. [Google Scholar] [CrossRef]
- Arif, N.; Yadav, V.; Singh, S.; Kushwaha, B.K.; Singh, S.; Tripathi, D.K.; Vishwakarma, K.; Sharma, S.; Dubey, N.K.; Chauhan, D.K. Assessment of antioxidant potential of plants in response to heavy metals. In Plant Responses to Xenobiotics; Singh, A., Prasad, S.M., Singh, R.P., Eds.; Springer: Singapore, 2016; pp. 97–125. [Google Scholar]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant& Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 5855. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J. Hazard. Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef]

| Properties | Garden Soil | Tannery Effluent Contaminated Soil |
|---|---|---|
| Soil texture | Soil mixed with clay and sand | Grainy sand |
| pH | 7.5 | 8.2 |
| Moisture content | 52.10 | 39.5 |
| Electro conductivity (dsm−1) | 1.2 | 6.8 |
| Macro nutrients (kg/ha) | ||
| Nitrogen (N) | 224 | 156 |
| Phosphorous (P) | 24 | 3.5 |
| Potassium (K) | 760 | 48 |
| Heavy metals (mg/kg) | ||
| Chromium (Cr) | BDL | 21.2 |
| Arsenic (As) | BDL | 3.2 |
| Cadmium (Cd) | 0.004 | 1.6 |
| Nickel (Ni) | 0.002 | 1.1 |
| Heavy Metals | Metal Concentration (µg/mL) | MIC (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 750 | 1000 | 1250 | 1500 | 2000 | ||||
| Cr | + | + | + | + | + | + | + | 3000 * | ||
| Pb | + | + | + | - | - | - | - | 1000 | ||
| Cd | + | + | + | + | - | - | - | 1250 | ||
| Ni | + | + | + | + | + | + | - | 2000 | ||
| Cu | + | + | + | + | - | - | - | 1250 | ||
| Mn | + | + | + | + | - | - | - | 1250 | ||
| Zn | + | + | + | + | - | - | - | 1250 | ||
| Antibiotic Tolerance of the Rhizobacterial Strain M2 | ||||||||||
| Antibiotic | Concentration (mcg) | Resistance/Sensitive | ||||||||
| Penicillin | 10 | + | ||||||||
| Erythromycin | 10 | + | ||||||||
| Methicillin | 30 | + | ||||||||
| Chloramphenicol | 25 | - | ||||||||
| Gentamicin | 30 | - | ||||||||
| Ciprofloxacin | 30 | - | ||||||||
| Vancomycin | 10 | + | ||||||||
| Ampicillin | 25 | + | ||||||||
| Amoxicillin | 30 | - | ||||||||
| Cr(VI) Conc. (mg/L) | P a Solubilization | Siderophore | Catalase | Protease | Amylase | Lipase | IAA | Ammonia (µg/mL) | EPS (µg/mL) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TBA b | T20 c | 25T (µg/mL) | 50T (µg/mL) | ||||||||
| 0 | +++ | ++ | +++ | +++ | +++ | +++ | +++ | 29.85 ± 1.27 b | 38.82 ± 1.07 a | 59.3 ± 3.64 a | 21.24 ± 2.10 e |
| 100 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | 19.85 ± 1.26 e | 36.63 ± 1.30 a | 45.7 ± 1.71 b | 38.22 ± 2.91 c |
| 200 | ++ | +++ | ++ | ++ | ++ | + | ++ | 18.53 ± 1.55 e | 21.10 ± 1.13 d | 36.59 ± 2.18 c | 56.75 ± 3.18 a |
| 300 | + | + | + | ++ | + | + | + | 7.21 ± 1.50 e | 9.26 ± 1.04 e | 29.81 ± 1.94 c | 49.75 ± 3.70 b |
| Treatment | Seed Germination (%) | Root Length (cm) | Shoot Length (cm) | Pigments (mg/g F.W) | |||
|---|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoids | ||||
| Control | 95 | 8.61 ± 1.15 a | 27.18 ± 1.44 a | 51.15 ± 1.29 a | 23.11 ± 1.44 a | 74.26 ± 2.73 a | 10.45 ± 1.28 a |
| Cr(VI) | 28 | 2.78 ± 1.38 b | 16.21 ± 1.18 b | 24.34 ± 1.48 b | 10.28 ± 1.45 b | 34.62 ± 2.93 b | 3.86 ± 1.35 b |
| M2 | 97 | 14.62 ± 1.50 c | 31.45 ± 1.60 c | 55.14 ± 1.68 c | 26.74 ± 1.60 c | 86.88 ± 3.28 c | 17.51 ± 1.39 c |
| M2+ Cr(VI) | 90 | 12.44 ± 1.29 c | 25.85 ± 1.35 a | 48.75 ± 2.05 a | 23.05 ± 1.65 a | 71.80 ± 3.70 a | 9.29 ± 1.52 a |
| Treatment | Chromium Accumulation (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st Week | 2nd Week | 3rd Week | 4th Week | |||||
| Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | |
| Control | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Cr(VI) | 80.15 d | 22.73 e | 104.25 c | 42.17 e | 185.14 b | 59.52 e | 230.19 a | 65.82 d |
| M2 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| M2+ Cr(VI) | 5.95 e | 3.89 e | 17.08 e | 8.25 e | 48.69 e | 23.11 e | 80.48 d | 38.94 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivas Ravi, M.; Karthik, C.; Padikasan, I.A.; Ma, Y. Alleviation of Cr(VI) Toxicity and Improve Phytostabilization Potential of Vigna radiata Using a Novel Cr(VI) Reducing Multi-Stress-Tolerant Plant Growth Promoting Rhizobacterial Strain Bacillus flexus M2. Agronomy 2022, 12, 3079. https://doi.org/10.3390/agronomy12123079
Srinivas Ravi M, Karthik C, Padikasan IA, Ma Y. Alleviation of Cr(VI) Toxicity and Improve Phytostabilization Potential of Vigna radiata Using a Novel Cr(VI) Reducing Multi-Stress-Tolerant Plant Growth Promoting Rhizobacterial Strain Bacillus flexus M2. Agronomy. 2022; 12(12):3079. https://doi.org/10.3390/agronomy12123079
Chicago/Turabian StyleSrinivas Ravi, Manoj, Chinnannan Karthik, Indra Arulselvi Padikasan, and Ying Ma. 2022. "Alleviation of Cr(VI) Toxicity and Improve Phytostabilization Potential of Vigna radiata Using a Novel Cr(VI) Reducing Multi-Stress-Tolerant Plant Growth Promoting Rhizobacterial Strain Bacillus flexus M2" Agronomy 12, no. 12: 3079. https://doi.org/10.3390/agronomy12123079
APA StyleSrinivas Ravi, M., Karthik, C., Padikasan, I. A., & Ma, Y. (2022). Alleviation of Cr(VI) Toxicity and Improve Phytostabilization Potential of Vigna radiata Using a Novel Cr(VI) Reducing Multi-Stress-Tolerant Plant Growth Promoting Rhizobacterial Strain Bacillus flexus M2. Agronomy, 12(12), 3079. https://doi.org/10.3390/agronomy12123079






