Modulation of the Serotonergic Receptosome in the Treatment of Anxiety and Depression: A Narrative Review of the Experimental Evidence
Abstract
:1. Introduction
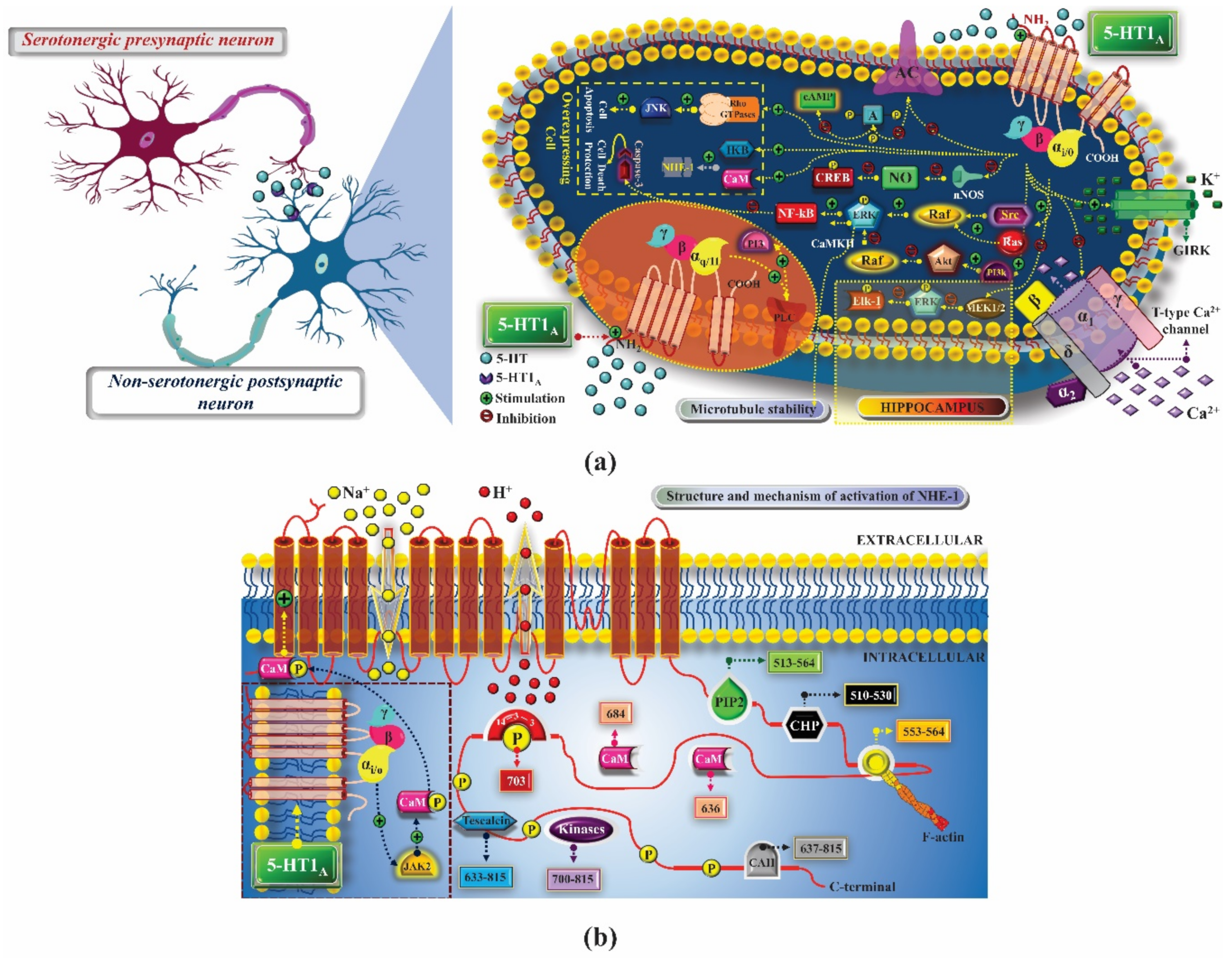
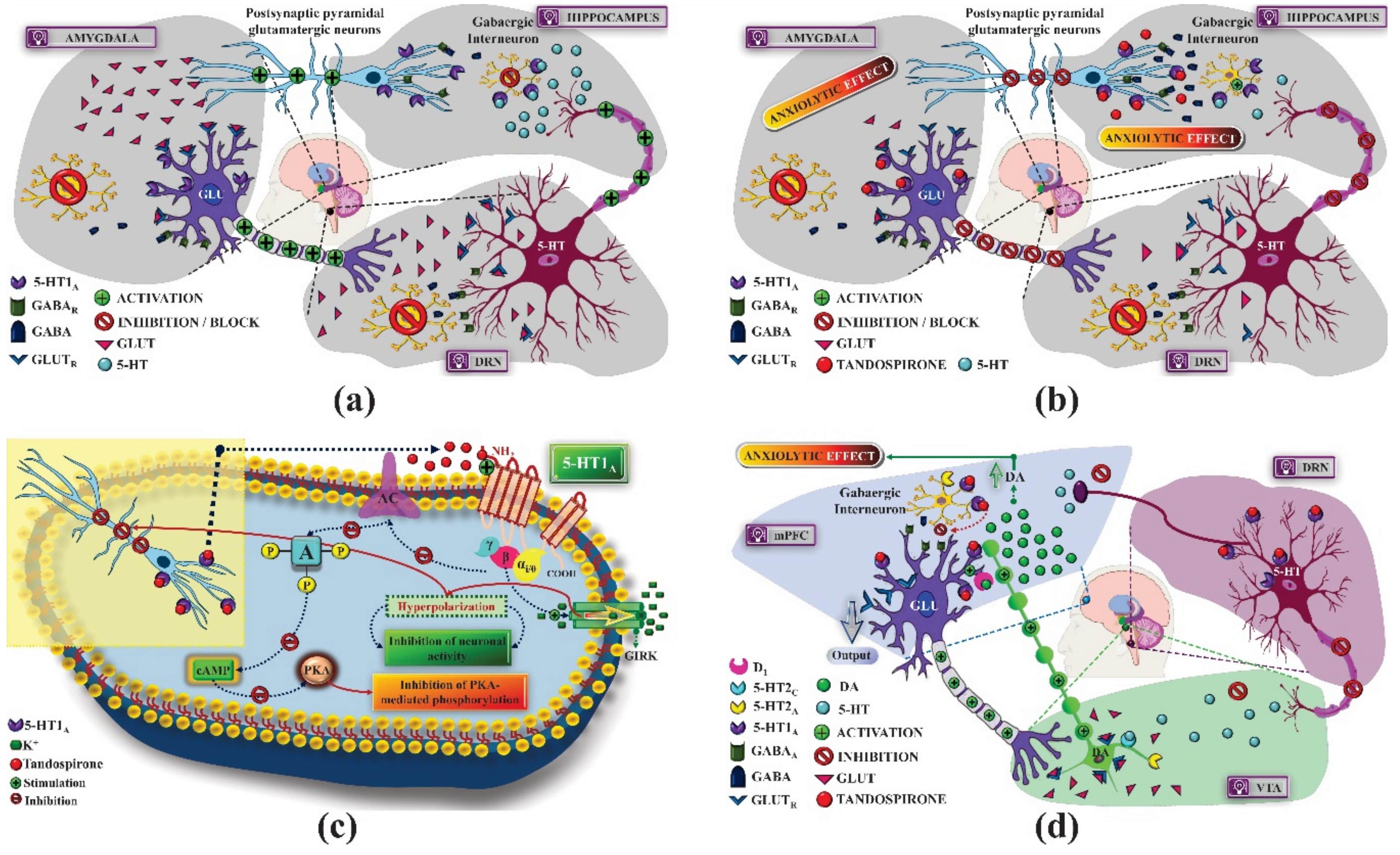
2. 5-HT1 Receptors
2.1. Mechanism of Pharmacological Action
2.1.1. Treatment of Anxiety
2.1.2. Treatment of Depression
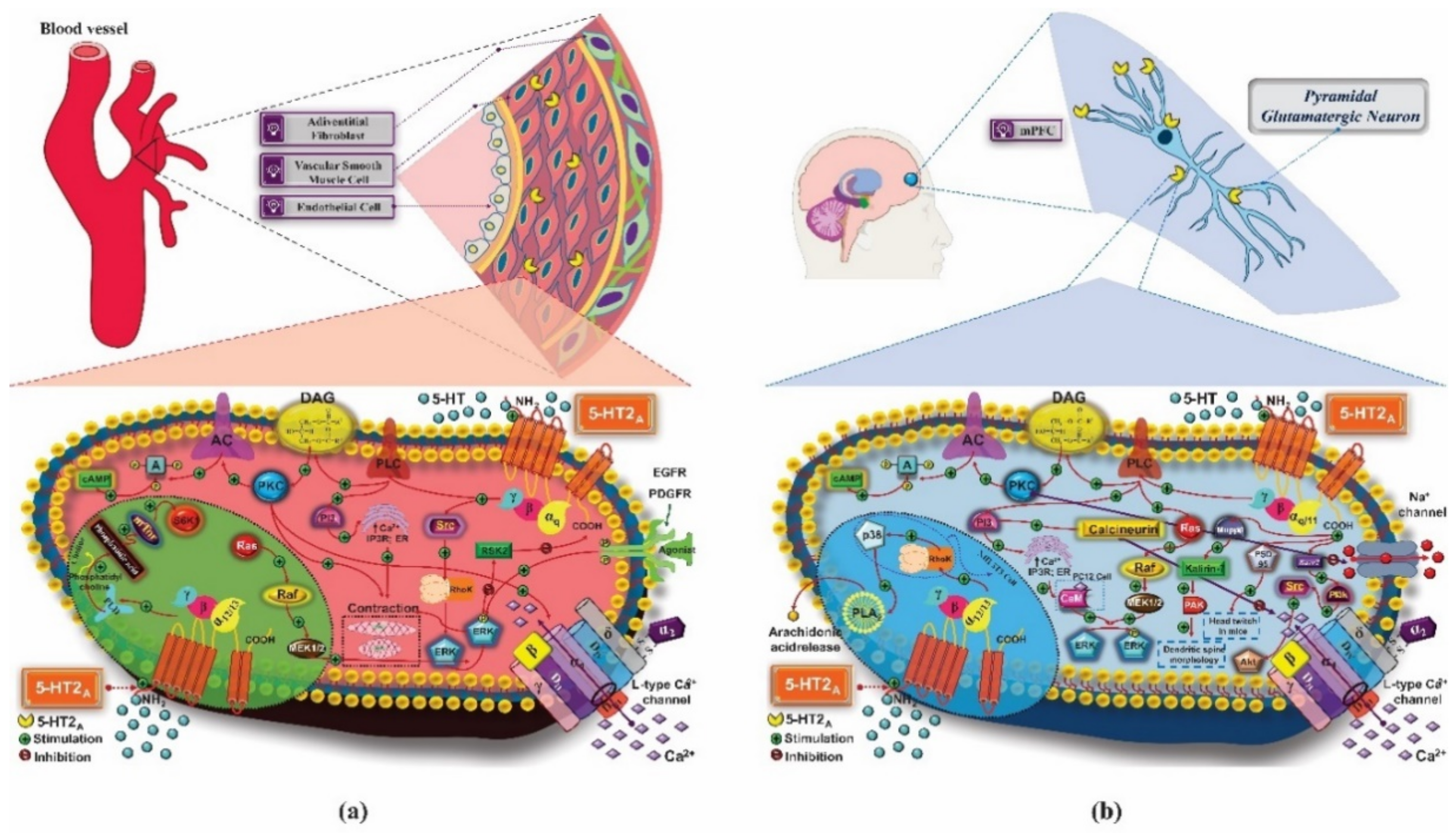
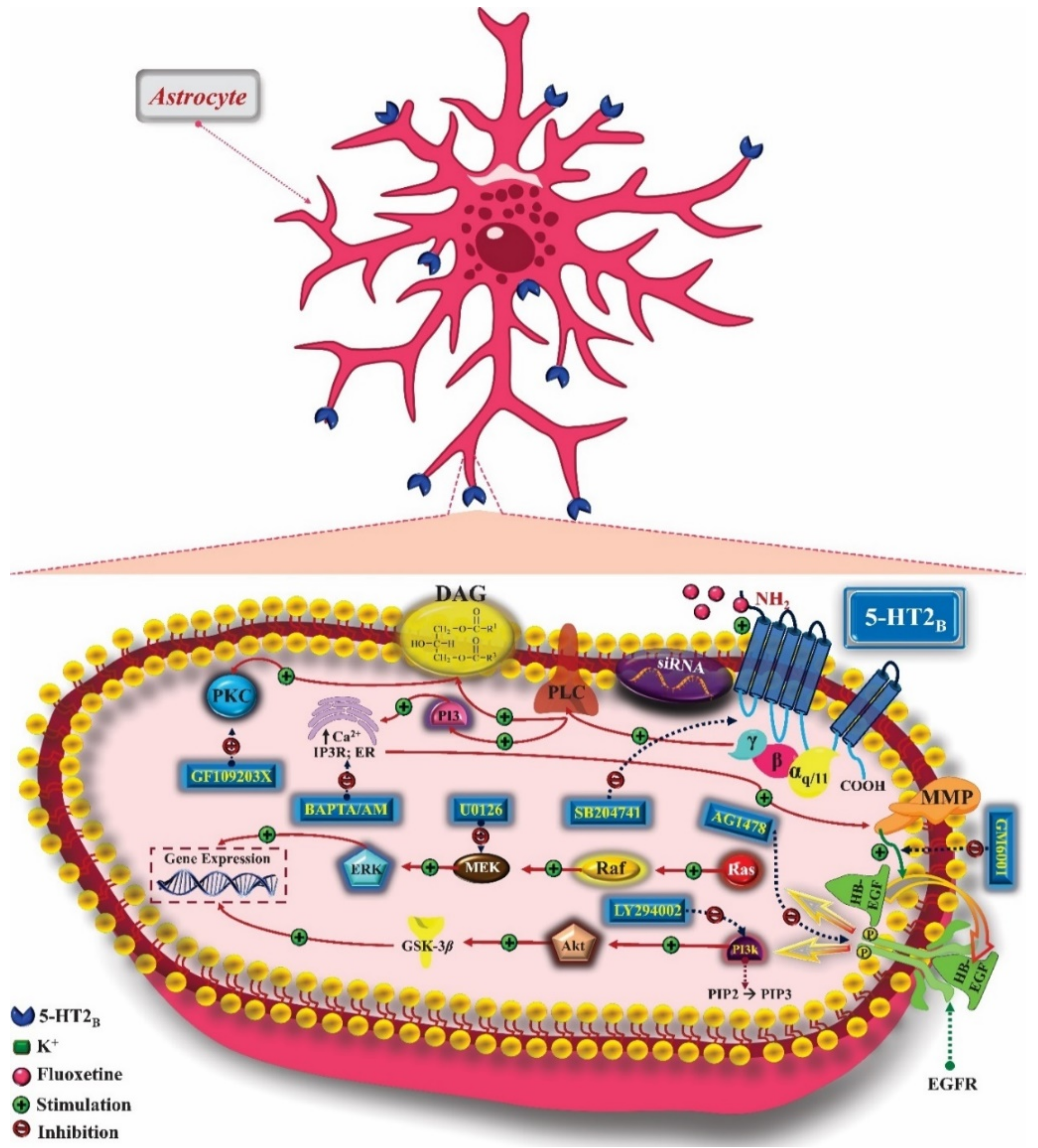
3. 5-HT2 Receptors
3.1. Mechanism of Pharmacological Action
3.1.1. Treatment of Anxiety
3.1.2. Treatment of Depression
4. 5-HT3 Receptors
4.1. Mechanism of Pharmacological Action
4.1.1. Treatment of Anxiety
4.1.2. Treatment of Depression
5. 5-HT4 Receptors
5.1. Mechanism of Pharmacological Action
5.1.1. Treatment of Anxiety
5.1.2. Treatment of Depression
6. 5-HT5 Receptors
6.1. Mechanism of Pharmacological Action
6.1.1. Treatment of Anxiety
6.1.2. Treatment of Depression
7. 5-HT6 Receptors
7.1. Mechanism of Pharmacological Action
7.1.1. Treatment of Anxiety
7.1.2. Treatment of Depression
8. 5-HT7 Receptors
8.1. Mechanism of Pharmacological Action
8.1.1. Treatment of Anxiety
8.1.2. Treatment of Depression
9. Phytochemical Compounds of Natural Origin Acting via 5-HT Receptors: Preclinical and Clinical Research and Future Perspectives in the Treatment of Anxiety and Depression
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaddum, J.H.; Picarelli, Z.P. Two kinds of tryptamine receptor. Br. J. Pharmacol. Chemother. 1957, 12, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Sharp, T.; Barnes, N.M. Central 5-HT receptors and their function; present and future. Neuropharmacology 2020, 177, 108–155. [Google Scholar] [CrossRef]
- Giulietti, M.; Vivenzio, V.; Piva, F.; Principato, G.; Bellantuono, C.; Nardi, B. How much do we know about the coupling of G-proteins to serotonin receptors? Mol. Brain 2014, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbano, A.; Corradetti, R.; Mlinar, B. Pharmacological Characterization of 5-HT1A Autoreceptor-Coupled GIRK Channels in Rat Dorsal Raphe 5-HT Neurons. PLoS ONE 2015, 10, e0140369. [Google Scholar] [CrossRef] [Green Version]
- Niedzielak, T.; Ravenelle, R.; Joseph, M.; Calhoun, C.; Plotkin, B.; Jones, R.; Herrera, M.; Tiffany Donaldson, S. 5-HT1A and α2 adrenergic receptor levels are associated with high anxiety-like patterns and impulsivity in selectively bred Long Evans rats. Behav. Brain Res. 2020, 383, 112522. [Google Scholar] [CrossRef]
- Da Silva, N.R.; Gomes, F.V.; Sonego, A.B.; da Silva, N.R.; Guimarães, F.S. Cannabidiol attenuates behavioral changes in a rodent model of schizophrenia through 5-HT1A, but not CB1 and CB2 receptors. Pharmacol. Res. 2020, 156, 104749. [Google Scholar] [CrossRef]
- Chilmonczyk, Z.; Bojarski, A.; Pilc, A.; Sylte, I. Functional Selectivity and Antidepressant Activity of Serotonin 1A Receptor Ligands. Int. J. Mol. Sci. 2015, 16, 18474–18506. [Google Scholar] [CrossRef] [Green Version]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Xie, F.; Fan, Q.; Li, B.X.; Xiao, X. Discovery of a Synergistic Inhibitor of cAMP-Response Element Binding Protein (CREB)-Mediated Gene Transcription with 666-15. J. Med. Chem. 2019, 62, 11423–11429. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, Y.; Wang, X.; Wang, C.; Xia, Z. Role of the hippocampal 5-HT1A receptor-mediated cAMP/PKA signalling pathway in sevoflurane-induced cognitivedysfunction in aged rats. J. Int. Med. Res. 2018, 46, 1073–1085. [Google Scholar] [CrossRef]
- Rojas, P.S.; Aguayo, F.; Neira, D.; Tejos, M.; Aliaga, E.; Muñoz, J.P.; Parra, C.S.; Fiedler, J.L. Dual effect of serotonin on the dendritic growth of cultured hippocampal neurons: Involvement of 5-HT1A and 5-HT7 receptors. Mol. Cell. Neurosci. 2017, 85, 148–161. [Google Scholar] [CrossRef]
- Masson, J.; Emerit, M.B.; Hamon, M.; Darmon, M. Serotonergic signaling: Multiple effectors and pleiotropic effects. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 685–713. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Bharatiya, R.; Chagraoui, A.; Di Giovanni, G. Constitutive activity of 5-HT receptors: Factual analysis. Neuropharmacology 2020, 168, 107967. [Google Scholar] [CrossRef]
- Cui, W.-Q.; Sun, W.-S.; Xu, F.; Hu, X.-M.; Yang, W.; Zhou, Y.; Du, L.-X.; Zhang, W.-W.; Mao-Ying, Q.-L.; Mi, W.-L.; et al. Spinal Serotonin 1A Receptor Contributes to the Analgesia of Acupoint Catgut Embedding by Inhibiting Phosphorylation of the N-Methyl-d-Aspartate Receptor GluN1 Subunit in Complete Freund’s Adjuvant-Induced Inflammatory Pain in Rats. J. Pain 2019, 19, 16.e1-16.e16. [Google Scholar] [CrossRef]
- Nishijo, T.; Momiyama, T. Serotonin 5-HT 1B receptor-mediated calcium influx-independent presynaptic inhibition of GABA release onto rat basal forebrain cholinergic neurons. Eur. J. Neurosci. 2016, 44, 1747–1760. [Google Scholar] [CrossRef]
- Polter, A.M.; Li, X. Glycogen Synthase Kinase-3 is an Intermediate Modulator of Serotonin Neurotransmission. Front. Mol. Neurosci. 2011, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goddard, A.W.; Shekhar, A.; Whiteman, A.F.; McDougle, C.J. Serotoninergic mechanisms in the treatment of obsessive–compulsive disorder. Drug Discov. Today 2008, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- McGowan, O.O.; Reynolds, G.P. Functional pharmacogenetics of serotonin receptors in psychiatric drug action. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 941–957. ISBN 9780444641250. [Google Scholar]
- Marin, P.; Bécamel, C.; Chaumont-Dubel, S.; Vandermoere, F.; Bockaert, J.; Claeysen, S. Classification and signaling characteristics of 5-HT receptors: Toward the concept of 5-HT receptosomes. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 91–120. ISBN 9780444641250. [Google Scholar]
- Clemow, D.B.; Johnson, K.W.; Hochstetler, H.M.; Ossipov, M.H.; Hake, A.M.; Blumenfeld, A.M. Lasmiditan mechanism of action–review of a selective 5-HT1F agonist. J. Headache Pain 2020, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Soria-Fregozo, C.; Perez-Vega, M.I.; Rodríguez-Landa, J.F.; Germán-Ponciano, L.J.; García-Ríos, R.I.; Mora-Perez, A. Association of 5-HT1A Receptors with Affective Disorders. In Serotonin—A Chemical Messenger Between All Types of Living Cells; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Balaj, K.; Nowinski, L.; Walsh, B.; Mullett, J.; Palumbo, M.L.; Thibert, R.L.; McDougle, C.J.; Keary, C.J. Buspirone for the treatment of anxiety-related symptoms in Angelman syndrome. Psychiatr. Genet. 2019, 29, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.K.; Bansal, Y.; Bhandari, R.; Kaur, S.; Kaur, J.; Singh, R.; Kuhad, A.; Kuhad, A. Gepirone hydrochloride: A novel antidepressant with 5-HT1A agonistic properties. Drugs Today 2019, 55, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, J.; Yang, S.; Cao, S.; Qin, D.; Zhou, Y.; Li, X.; Ye, Y.; Wu, J. Role of tandospirone, a 5-HT1A receptor partial agonist, in the treatment of central nervous system disorders and the underlying mechanisms. Oncotarget 2017, 8, 102705–102720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Matsumoto, M.; Yanagawa, Y.; Hiraide, S.; Inoue, S.; Kubo, Y.; Shimamura, K.I.; Togashi, H. Facilitation of fear extinction by the 5-HT1A receptor agonist tandospirone: Possible involvement of dopaminergic modulation. Synapse 2013, 67, 161–170. [Google Scholar] [CrossRef]
- Li, X. Using the conditioned fear stress (CFS) animal model to understand the neurobiological mechanisms and pharmacological treatment of anxiety. Shanghai Arch. Psychiatry 2012, 24, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Spiacci, A.; Pobbe, R.L.H.; Matthiesen, M.; Zangrossi, H. 5-HT1A receptors of the rat dorsal raphe lateral wings and dorsomedial subnuclei differentially control anxiety- and panic-related defensive responses. Neuropharmacology 2016, 107, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.L.; Canetta, S.; Stujenske, J.M.; Burghardt, N.S.; Ansorge, M.S.; Dranovsky, A.; Leonardo, E.D. Serotonin inputs to the dorsal BNST modulate anxiety in a 5-HT1A receptor-dependent manner. Mol. Psychiatry 2018, 23, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Turcotte-Cardin, V.; Vahid-Ansari, F.; Luckhart, C.; Daigle, M.; Geddes, S.D.; Tanaka, K.F.; Hen, R.; James, J.; Merali, Z.; Béïque, J.; et al. Loss of Adult 5-HT1A Autoreceptors Results in a Paradoxical Anxiogenic Response to Antidepressant Treatment. J. Neurosci. 2019, 39, 1334–1346. [Google Scholar] [CrossRef] [Green Version]
- Marcinkiewcz, C.A.; Bierlein-De La Rosa, G.; Dorrier, C.E.; McKnight, M.; DiBerto, J.F.; Pati, D.; Gianessi, C.A.; Hon, O.J.; Tipton, G.; McElligott, Z.A.; et al. Sex-Dependent Modulation of Anxiety and Fear by 5-HT 1A Receptors in the Bed Nucleus of the Stria Terminalis. ACS Chem. Neurosci. 2019, 10, 3154–3166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, C.; Ren, J.; Chang, L.; Zhu, X.; Sun, N.; Meng, G.; Liu, M.; Zhang, J.; Li, Y.; et al. Dentate nNOS accounts for stress-induced 5-HT 1A receptor deficiency: Implication in anxiety behaviors. CNS Neurosci. Ther. 2020, 26, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Nautiyal, K.M.; Tritschler, L.; Ahmari, S.E.; David, D.J.; Gardier, A.M.; Hen, R. A Lack of Serotonin 1B Autoreceptors Results in Decreased Anxiety and Depression-Related Behaviors. Neuropsychopharmacology 2016, 41, 2941–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.K.; Purvis, E.M.; Ayala, K.; Collins, L.; Krug, J.T.; Mayes, M.S.; Ettenberg, A. Activation of 5-HT1B receptors in the Lateral Habenula attenuates the anxiogenic effects of cocaine. Behav. Brain Res. 2019, 357–358, 1–8. [Google Scholar] [CrossRef]
- Azevedo, H.; Ferreira, M.; Costa, R.W.; Russo, V.; Russo, E.; Mascarello, A.; Guimarães, C.R.W. Preclinical characterization of ACH-000029, a novel anxiolytic compound acting on serotonergic and alpha-adrenergic receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109707. [Google Scholar] [CrossRef]
- Hatherall, L.; Sánchez, C.; Morilak, D.A. Chronic Vortioxetine Treatment Reduces Exaggerated Expression of Conditioned Fear Memory and Restores Active Coping Behavior in Chronically Stressed Rats. Int. J. Neuropsychopharmacol. 2016, 20, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Doods, H.; Treede, R.-D.; Ceci, A. Duloxetine and 8-OH-DPAT, but not fluoxetine, reduce depression-like behaviour in an animal model of chronic neuropathic pain. Neurosci. Lett. 2016, 619, 162–167. [Google Scholar] [CrossRef]
- Haleem, D.J. Targeting Serotonin1A Receptors for Treating Chronic Pain and Depression. Curr. Neuropharmacol. 2019, 17, 1098–1108. [Google Scholar] [CrossRef]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef]
- Lattimore, K.A.; Donn, S.M.; Kaciroti, N.; Kemper, A.R.; Neal, C.R.; Vazquez, D.M. Selective Serotonin Reuptake Inhibitor (SSRI) Use during Pregnancy and Effects on the Fetus and Newborn: A Meta-Analysis. J. Perinatol. 2005, 25, 595–604. [Google Scholar] [CrossRef]
- Stuivenga, M.; Giltay, E.J.; Cools, O.; Roosens, L.; Neels, H.; Sabbe, B. Evaluation of vilazodone for the treatment of depressive and anxiety disorders. Expert Opin. Pharmacother. 2019, 20, 251–260. [Google Scholar] [CrossRef]
- McKeage, K. Adjunctive Brexpiprazole: A Review in Major Depressive Disorder. CNS Drugs 2016, 30, 91–99. [Google Scholar] [CrossRef]
- Stahl, S.M. Mechanism of action of brexpiprazole: Comparison with aripiprazole. CNS Spectr. 2016, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cha, D.S.; Luo, X.; Ahmed, J.; Becirovic, L.; Cha, R.H.; McIntyre, R.S. Brexpiprazole as an augmentation agent to antidepressants in treatment resistant major depressive disorder. Expert Rev. Neurother. 2019, 19, 777–783. [Google Scholar] [CrossRef]
- Gardier, A.M.; Trillat, A.-C.; Malagié, I.; David, D.; Hascoët, M.; Colombel, M.-C.; Jolliet, P.; Jacquot, C.; Hen, R.; Bourin, M. Récepteurs 5-HT1B de la sérotonine et effets antidépresseurs des inhibiteurs de recapture sélectifs de la sérotonine. Comptes Rendus l’Académie Sci.-Ser. III-Sci. Vie 2001, 324, 433–441. [Google Scholar] [CrossRef]
- David, D.J.; Gardier, A.M. Les bases de pharmacologie fondamentale du système sérotoninergique: Application à la réponse antidépressive. Encephale. 2016, 42, 255–263. [Google Scholar] [CrossRef]
- Orsolini, L.; Tomasetti, C.; Valchera, A.; Iasevoli, F.; Buonaguro, E.F.; Vellante, F.; Fornaro, M.; Fiengo, A.; Mazza, M.; Vecchiotti, R.; et al. New advances in the treatment of generalized anxiety disorder: The multimodal antidepressant vortioxetine. Expert Rev. Neurother. 2016, 16, 483–495. [Google Scholar] [CrossRef]
- Araldi, D.; Ferrari, L.F.; Levine, J.D. Gi-protein–coupled 5-HT1B/D receptor agonist sumatriptan induces type I hyperalgesic priming. Pain 2016, 157, 1773–1782. [Google Scholar] [CrossRef] [Green Version]
- Kurrasch-Orbaugh, D.M.; Parrish, J.C.; Watts, V.J.; Nichols, D.E. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: The involvement of MAP kinases. J. Neurochem. 2003, 86, 980–991. [Google Scholar] [CrossRef]
- Bécamel, C.; Berthoux, C.; Barre, A.; Marin, P. Growing Evidence for Heterogeneous Synaptic Localization of 5-HT2A Receptors. ACS Chem. Neurosci. 2017, 8, 897–899. [Google Scholar] [CrossRef] [Green Version]
- Fuxe, K.; Tarakanov, A.; Romero Fernandez, W.; Ferraro, L.; Tanganelli, S.; Filip, M.; Agnati, L.F.; Garriga, P.; Diaz-Cabiale, Z.; Borroto-Escuela, D.O. Diversity and bias through receptor–receptor interactions in GPCR heteroreceptor complexes. Focus on examples from dopamine D2 receptor heteromerization. Front. Endocrinol. 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiard, B.P.; Di Giovanni, G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: The missing link? Front. Pharmacol. 2015, 6, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wischhof, L.; Koch, M. 5-HT2A and mGlu2/3 receptor interactions. Behav. Pharmacol. 2016, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hámor, P.U.; Šírová, J.; Páleníček, T.; Zaniewska, M.; Bubeníková-Valešová, V.; Schwendt, M. Chronic methamphetamine self-administration dysregulates 5-HT2A and mGlu2 receptor expression in the rat prefrontal and perirhinal cortex: Comparison to chronic phencyclidine and MK-801. Pharmacol. Biochem. Behav. 2018, 175, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G.; Launay, J.-M.; Hickel, P.; Tournois, C.; Maroteaux, L. 5-Hydroxytryptamine 2B receptor regulates cell-cycle progression: Cross-talk with tyrosine kinase pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 2591–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz, L.; Rothman, D.L.; Li, B.; Peng, L. Chronic SSRI stimulation of astrocytic 5-HT2B receptors change multiple gene expressions/editings and metabolism of glutamate, glucose and glycogen: A potential paradigm shift. Front. Behav. Neurosci. 2015, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nebigil, C.G.; Maroteaux, L. Functional Consequence of Serotonin/5-HT 2B Receptor Signaling in Heart. Circulation 2003, 108, 902–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doly, S.; Diaz, S.L.; Belmer, A.; Roumier, A.; Maroteaux, L.; Becamel, C.; Marin, P.; Bockaert, J. 5-Hydroxytryptamine Receptor 2B. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: New York, NY, USA, 2012; pp. 2–12. ISBN 978-1-4419-0461-4. [Google Scholar]
- Chagraoui, A.; Thibaut, F.; Skiba, M.; Thuillez, C.; Bourin, M. 5-HT2C receptors in psychiatric disorders: A review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Jiang, Y.; Hu, Z.; Yang, Y.; Du, X.; Botchway, B.O.; Fang, M. Serotonin receptors 2A and 1A modulate anxiety-like behavior in post-traumatic stress disordered mice. Am. J. Transl. Res. 2019, 11, 2288–2303. [Google Scholar]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain. Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Boroda, S.; Niccum, M.; Raje, V.; Purow, B.W.; Harris, T.E. Dual activities of ritanserin and R59022 as DGKα inhibitors and serotonin receptor antagonists. Biochem. Pharmacol. 2017, 123, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Masse, F.; Petit-Demouliere, B.; Dubois, I.; Hascoët, M.; Bourin, M. Anxiolytic-like effects of DOI microinjections into the hippocampus (but not the amygdala nor the PAG) in the mice four plates test. Behav. Brain Res. 2007, 188, 291–297. [Google Scholar] [CrossRef]
- Petit-Demouliere, B.; Bourin, M. Temporal parameters of one-trial tolerance to benzodiazepines in four-plate test–retest. Behav. Brain Res. 2007, 183, 222–225. [Google Scholar] [CrossRef]
- Ponzoni, L.; Sala, M.; Braida, D. Ritanserin-sensitive receptors modulate the prosocial and the anxiolytic effect of MDMA derivatives, DOB and PMA, in zebrafish. Behav. Brain Res. 2016, 314, 181–189. [Google Scholar] [CrossRef]
- Clinard, C.T.; Bader, L.R.; Sullivan, M.A.; Cooper, M.A. Activation of 5-HT2a receptors in the basolateral amygdala promotes defeat-induced anxiety and the acquisition of conditioned defeat in Syrian hamsters. Neuropharmacology 2015, 90, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Vermeire, S.T.; Audenaert, K.R.; Dobbeleir, A.A.; De Meester, R.H.; De Vos, F.J.; Peremans, K.Y. Evaluation of the Brain 5-HT2A Receptor Binding Index in Dogs with Anxiety Disorders, Measured with 123I-5I-R91150 and SPECT. J. Nucl. Med. 2009, 50, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Nunes-de-Souza, V.; Nunes-de-Souza, R.L.; Rodgers, R.J.; Canto-de-Souza, A. 5-HT2 receptor activation in the midbrain periaqueductal grey (PAG) reduces anxiety-like behaviour in mice. Behav. Brain Res. 2008, 187, 72–79. [Google Scholar] [CrossRef]
- Kennett, G.; Trail, B.; Bright, F. Anxiolytic-like actions of BW 723C86 in the rat Vogel conflict test are 5-HT2B receptor mediated. Neuropharmacology 1998, 37, 1603–1610. [Google Scholar] [CrossRef]
- Kennett, G.A.; Bright, F.; Trail, B.; Baxter, G.S.; Blackburn, T.P. Effects of the 5-HT2B receptor agonist, BW 723C86, on three rat models of anxiety. Br. J. Pharmacol. 1996, 117, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef]
- Sant’Ana, A.B.; Vilela-Costa, H.H.; Vicente, M.A.; Hernandes, P.M.; de Andrade, T.G.C.S.; Zangrossi, H. Role of 5-HT2C receptors of the dorsal hippocampus in the modulation of anxiety- and panic-related defensive responses in rats. Neuropharmacology 2019, 148, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Mei, Q.; Shiwalkar, N.; Zuo, W.; Zhang, H.; Gregor, D.; Patel, S.; Ye, J.-H. Anxiety during alcohol withdrawal involves 5-HT2C receptors and M-channels in the lateral habenula. Neuropharmacology 2020, 163, 107863. [Google Scholar] [CrossRef]
- Buoli, M.; Grassi, S.; Serati, M.; Altamura, A.C. Agomelatine for the treatment of generalized anxiety disorder. Expert Opin. Pharmacother. 2017, 18, 1373–1379. [Google Scholar] [CrossRef]
- Harvey, B.H.; Regenass, W.; Dreyer, W.; Möller, M. Social isolation rearing-induced anxiety and response to agomelatine in male and female rats: Role of corticosterone, oxytocin, and vasopressin. J. Psychopharmacol. 2019, 33, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Papp, N.; Koncz, S.; Kostyalik, D.; Kitka, T.; Petschner, P.; Vas, S.; Bagdy, G. Acute 5-HT2C Receptor Antagonist SB-242084 Treatment Affects EEG Gamma Band Activity Similarly to Chronic Escitalopram. Front. Pharmacol. 2020, 10, 1636. [Google Scholar] [CrossRef]
- Heisler, L.K.; Zhou, L.; Bajwa, P.; Hsu, J.; Tecott, L.H. Serotonin 5-HT 2C receptors regulate anxiety-like behavior. Genes, Brain Behav. 2007, 6, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, L.J.; Underwood, M.D.; Bakalian, M.J.; Kassir, S.A.; Mann, J.J.; Arango, V. 5-HT1A receptor, 5-HT2A receptor and serotonin transporter binding in the human auditory cortex in depression. J. Psychiatry Neurosci. 2019, 44, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, J.F. A preliminary open trial of nefazodone added to mood stabilizers for bipolar depression. J. Affect. Disord. 2013, 144, 176–178. [Google Scholar] [CrossRef]
- Krajnc, E.; Visentin, M.; Gai, Z.; Stieger, B.; Samodelov, S.L.; Häusler, S.; Kullak-Ublick, G.A. Untargeted metabolomics reveals anaerobic glycolysis as a novel target of the hepatotoxic antidepressant nefazodone. J. Pharmacol. Exp. Ther. 2020. [Google Scholar] [CrossRef]
- Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] - Nefazodone. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548179/ (accessed on 11 February 2020).
- Davis, R.; Whittington, R.; Bryson, H.M. Nefazodone: A Review of its Pharmacology and Clinical Efficacy in the Management of Major Depression. Drugs 1997, 53, 608–636. [Google Scholar] [CrossRef]
- Watanabe, N.; Omori, I.; Nakagawa, A.; Cipriani, A.; Barbui, C.; McGuire, H.; Churchill, R.; Furukawa, T.A. Mirtazapine versus other anti-depressive agents for depression. In Cochrane Database of Systematic Reviews; Watanabe, N., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2007. [Google Scholar]
- Kessler, D.S.; MacNeill, S.J.; Tallon, D.; Lewis, G.; Peters, T.J.; Hollingworth, W.; Round, J.; Burns, A.; Chew-Graham, C.A.; Anderson, I.M.; et al. Mirtazapine added to SSRIs or SNRIs for treatment resistant depression in primary care: Phase III randomised placebo controlled trial (MIR). BMJ 2018, 363, k4218. [Google Scholar] [CrossRef] [Green Version]
- Tatara, A.; Shimizu, S.; Shin, N.; Sato, M.; Sugiuchi, T.; Imaki, J.; Ohno, Y. Modulation of antipsychotic-induced extrapyramidal side effects by medications for mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 252–259. [Google Scholar] [CrossRef]
- Xu, C.; Ma, X.-M.; Chen, H.-B.; Zhou, M.-H.; Qiao, H.; An, S.-C. Orbitofrontal cortex 5-HT2A receptor mediates chronic stress-induced depressive-like behaviors and alterations of spine density and Kalirin7. Neuropharmacology 2016, 109, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Villas Boas, G.R.; Boerngen de Lacerda, R.; Paes, M.M.; Gubert, P.; Almeida, W.L.d.C.; Rescia, V.C.; de Carvalho, P.M.G.; de Carvalho, A.A.V.; Oesterreich, S.A. Molecular aspects of depression: A review from neurobiology to treatment. Eur. J. Pharmacol. 2019, 851, 99–121. [Google Scholar] [CrossRef]
- Zhang, X.; Song, D.; Gu, L.; Ren, Y.; Verkhratsky, A.; Peng, L. Decrease of gene expression of astrocytic 5-HT2B receptors parallels development of depressive phenotype in a mouse model of Parkinson’s disease. Front. Cell. Neurosci. 2015, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Li, B.; Lovatt, D.; Xu, J.; Song, D.; Goldman, S.A.; Nedergaard, M.; Hertz, L.; Peng, L. 5-HT 2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol. 2010, 6, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gu, L.; Li, B.; Hertz, L. Fluoxetine and all other SSRIs are 5-HT2B Agonists-Importance for their Therapeutic Effects. Curr. Neuropharmacol. 2014, 12, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Diaz, S.L.; Narboux-Nême, N.; Boutourlinsky, K.; Doly, S.; Maroteaux, L. Mice lacking the serotonin 5-HT 2B receptor as an animal model of resistance to selective serotonin reuptake inhibitors antidepressants. Eur. Neuropsychopharmacol. 2016, 26, 265–279. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liang, S.; Li, Z.; Li, S.; Xia, M.; Verkhratsky, A.; Li, B. Leptin Increases Expression of 5-HT2B Receptors in Astrocytes Thus Enhancing Action of Fluoxetine on the Depressive Behavior Induced by Sleep Deprivation. Front. Psychiatry 2019, 9, 734. [Google Scholar] [CrossRef] [Green Version]
- Wold, E.A.; Wild, C.T.; Cunningham, K.A.; Zhou, J. Targeting the 5-HT2C Receptor in Biological Context and the Current State of 5-HT2C Receptor Ligand Development. Curr. Top. Med. Chem. 2019, 19, 1381–1398. [Google Scholar] [CrossRef]
- Kamal, M.; Gbahou, F.; Guillaume, J.-L.; Daulat, A.M.; Benleulmi-Chaachoua, A.; Luka, M.; Chen, P.; Kalbasi Anaraki, D.; Baroncini, M.; Mannoury la Cour, C.; et al. Convergence of Melatonin and Serotonin (5-HT) Signaling at MT 2 /5-HT 2C Receptor Heteromers. J. Biol. Chem. 2015, 290, 11537–11546. [Google Scholar] [CrossRef] [Green Version]
- Palacios, J.M.; Pazos, A.; Hoyer, D. A short history of the 5-HT2C receptor: From the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology 2017, 234, 1395–1418. [Google Scholar] [CrossRef] [Green Version]
- Cremers, T.I.F.H.; Giorgetti, M.; Bosker, F.J.; Hogg, S.; Arnt, J.; Mørk, A.; Honig, G.; Bøgesø, K.-P.; Westerink, B.H.C.; den Boer, H.; et al. Inactivation of 5-HT2C Receptors Potentiates Consequences of Serotonin Reuptake Blockade. Neuropsychopharmacology 2004, 29, 1782–1789. [Google Scholar] [CrossRef] [Green Version]
- Visser, A.K.D.; Kleijn, J.; van Faassen, M.H.J.R.; Dremencov, E.; Flik, G.; Kema, I.P.; Den Boer, J.A.; van Waarde, A.; Dierckx, R.A.J.O.; Bosker, F.J. Serotonin-2C antagonism augments the effect of citalopram on serotonin and dopamine levels in the ventral tegmental area and nucleus accumbens. Neurochem. Int. 2015, 81, 10–15. [Google Scholar] [CrossRef]
- Jilani, T.N.; Gibbons, J.R.; Faizy, R.M.; Saadabadi, A. Mirtazapine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519059/ (accessed on 11 February 2020).
- Naguy, A. Mirtazapine for Major Depression Developed After Hyperemesis Gravidarum. Am. J. Ther. 2019, 26, e661–e662. [Google Scholar] [CrossRef]
- Dekeyne, A.; Mannoury la Cour, C.; Gobert, A.; Brocco, M.; Lejeune, F.; Serres, F.; Sharp, T.; Daszuta, A.; Soumier, A.; Papp, M.; et al. S32006, a novel 5-HT2C receptor antagonist displaying broad-based antidepressant and anxiolytic properties in rodent models. Psychopharmacology 2008, 199, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Dekeyne, A.; Brocco, M.; Loiseau, F.; Gobert, A.; Rivet, J.-M.; Di Cara, B.; Cremers, T.I.; Flik, G.; Fone, K.C.F.; Watson, D.J.G.; et al. S32212, a Novel Serotonin Type 2C Receptor Inverse Agonist/α 2 -Adrenoceptor Antagonist and Potential Antidepressant: II. A Behavioral, Neurochemical, and Electrophysiological Characterization. J. Pharmacol. Exp. Ther. 2012, 340, 765–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenzweig-Lipson, S.; Sabb, A.; Stack, G.; Mitchell, P.; Lucki, I.; Malberg, J.E.; Grauer, S.; Brennan, J.; Cryan, J.F.; Sukoff Rizzo, S.J.; et al. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology 2007, 192, 159–170. [Google Scholar] [CrossRef]
- Thompson, A.J.; Lummis, S.C.R. 5-HT3 receptors. Curr. Pharm. Des. 2006, 12, 3615–3630. [Google Scholar] [CrossRef]
- Faerber, L.; Drechsler, S.; Ladenburger, S.; Gschaidmeier, H.; Fischer, W. The neuronal 5-HT3 receptor network after 20 years of research—Evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007, 560, 1–8. [Google Scholar] [CrossRef]
- Bétry, C.; Etiévant, A.; Oosterhof, C.; Ebert, B.; Sanchez, C.; Haddjeri, N. Role of 5-HT3 receptors in the antidepressant response. Pharmaceuticals 2011, 4, 603–629. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, S.; Mahesh, R.; Devadoss, T.; Jindal, A. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (4-benzylpiperazin-1-yl)(3-methoxyquinoxalin-2-yl) methanone (6g) on lipopolysaccharide-induced anxiety models in mice. J. Basic Clin. Physiol. Pharmacol. 2016, 28, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Fakhfouri, G.; Rahimian, R.; Dyhrfjeld-Johnsen, J.; Zirak, M.R.; Beaulieu, J.-M. 5-HT 3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies beneath the Surface. Pharmacol. Rev. 2019, 71, 383–412. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, S.; Mahesh, R.; Jindal, A.; Devadoss, T. Neuropharmacological and neurochemical evaluation of N-n-propyl-3-ethoxyquinoxaline-2-carboxamide (6n): A novel serotonergic 5-HT3 receptor antagonist for co-morbid antidepressant- and anxiolytic-like potential using traumatic brain injury model in rats. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 93–100. [Google Scholar] [CrossRef]
- Amiri, S.; Amini-Khoei, H.; Haj-Mirzaian, A.; Rahimi-Balaei, M.; Naserzadeh, P.; Dehpour, A.; Mehr, S.E.; Hosseini, M.-J. Tropisetron attenuated the anxiogenic effects of social isolation by modulating nitrergic system and mitochondrial function. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 2464–2475. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhu, W.; Liang, Q.; LIU, J.; Yang, X.; Sun, G. Tropisetron attenuates lipopolysaccharide induced neuroinflammation by inhibiting NF-κB and SP/NK1R signaling pathway. J. Neuroimmunol. 2018, 320, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Bradesi, S. Alosetron and irritable bowel syndrome. Expert Opin. Pharmacother. 2003, 4, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Kurhe, Y.; Mahesh, R. Ondansetron attenuates co-morbid depression and anxiety associated with obesity by inhibiting the biochemical alterations and improving serotonergic neurotransmission. Pharmacol. Biochem. Behav. 2015, 136, 107–116. [Google Scholar] [CrossRef]
- Costall, B.; Domeney, A.M.; Gerrard, P.A.; Kelly, M.E.; Naylor, E.J. Zacopride: Anxiolytic profile in rodent and primate models of anxiety. J. Pharm. Pharmacol. 1988, 40, 302–305. [Google Scholar] [CrossRef]
- Gupta, D.; Radhakrishnan, M.; Thangaraj, D.; Kurhe, Y. Antidepressant and anti-anxiety like effects of 4i (N-(3-chloro-2-methylphenyl) quinoxalin-2-carboxamide), a novel 5-HT3 receptor antagonist in acute and chronic neurobehavioral rodent models. Eur. J. Pharmacol. 2014, 735, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Costescu, M.; Paunescu, H.; Coman, O.; Coman, L.; Fulga, I. Antidepressant effect of the interaction of fluoxetine with granisetron. Exp. Ther. Med. 2019, 5108–5111. [Google Scholar] [CrossRef] [Green Version]
- Fan, P. Mepacrine-induced inhibition of the inward current mediated by 5-HT3 receptors in rat nodose ganglion neurones. Br. J. Pharmacol. 1994, 112, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Bétry, C.; Overstreet, D.; Haddjeri, N.; Pehrson, A.L.; Bundgaard, C.; Sanchez, C.; Mørk, A. A 5-HT3 receptor antagonist potentiates the behavioral, neurochemical and electrophysiological actions of an SSRI antidepressant. Pharmacol. Biochem. Behav. 2015, 131, 136–142. [Google Scholar] [CrossRef]
- Gupta, D.; Radhakrishnan, M.; Kurhe, Y. Ondansetron, a 5HT3 receptor antagonist reverses depression and anxiety-like behavior in streptozotocin-induced diabetic mice: Possible implication of serotonergic system. Eur. J. Pharmacol. 2014, 744, 59–66. [Google Scholar] [CrossRef]
- Gupta, D.; Radhakrishnan, M.; Kurhe, Y.; Thangaraj, D.; Prabhakar, V.; Kanade, P. Antidepressant-like effects of a novel 5-HT3 receptor antagonist 6z in acute and chronic murine models of depression. Acta Pharmacol. Sin. 2014, 35, 1493–1503. [Google Scholar] [CrossRef] [Green Version]
- Hao, R.; Qi, Y.; Hou, D.-N.; Ji, Y.-Y.; Zheng, C.-Y.; Li, C.-Y.; Yung, W.-H.; Lu, B.; Huang, Y. BDNF val66met Polymorphism Impairs Hippocampal Long-Term Depression by Down-Regulation of 5-HT3 Receptors. Front. Cell. Neurosci. 2017, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pytka, K.; Głuch-Lutwin, M.; Kotańska, M.; Waszkielewicz, A.; Kij, A.; Walczak, M. Single Administration of HBK-15—a Triple 5-HT1A, 5-HT7, and 5-HT3 Receptor Antagonist—Reverses Depressive-Like Behaviors in Mouse Model of Depression Induced by Corticosterone. Mol. Neurobiol. 2017, 55, 3931–3945. [Google Scholar] [CrossRef] [Green Version]
- Kurhe, Y.; Mahesh, R. Mechanisms linking depression co-morbid with obesity: An approach for serotonergic type 3 receptor antagonist as novel therapeutic intervention. Asian J. Psychiatr. 2015, 17, 3–9. [Google Scholar] [CrossRef]
- Riga, M.S.; Sánchez, C.; Celada, P.; Artigas, F. Involvement of 5-HT 3 receptors in the action of vortioxetine in rat brain: Focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology 2016, 108, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Vieta, E.; Florea, I.; Schmidt, S.N.; Areberg, J.; Ettrup, A. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder. Int. Clin. Psychopharmacol. 2019, 34, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Haj-Mirzaian, A.; Amiri, S.; Amini-Khoei, H.; Rahimi-Balaei, M.; Kordjazy, N.; Olson, C.O.; Rastegar, M.; Naserzadeh, P.; Marzban, H.; Dehpour, A.R.; et al. Attenuation of oxidative and nitrosative stress in cortical area associates with antidepressant-like effects of tropisetron in male mice following social isolation stress. Brain Res. Bull. 2016, 124, 150–163. [Google Scholar] [CrossRef]
- Dhar, A.K.; Mahesh, R.; Jindal, A.; Bhatt, S. Piperazine Analogs of Naphthyridine-3-carboxamides and Indole-2-carboxamides: Novel 5-HT 3 Receptor Antagonists with Antidepressant-Like Activity. Arch. Pharm. 2015, 348, 34–45. [Google Scholar] [CrossRef]
- Kurhe, Y.; Mahesh, R.; Devadoss, T. Novel 5-HT3 receptor antagonist QCM-4 attenuates depressive-like phenotype associated with obesity in high-fat-diet-fed mice. Psychopharmacology 2017, 234, 1165–1179. [Google Scholar] [CrossRef]
- Kurhe, Y.; Mahesh, R.; Gupta, D.; Thangaraj, D. Effect of (4a) a novel 5-HT3 receptor antagonist on chronic unpredictable mild stress induced depressive-like behavior in mice: An approach using behavioral tests battery. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 25–33. [Google Scholar] [CrossRef]
- Richter, D.W.; Manzke, T.; Wilken, B.; Ponimaskin, E. Serotonin receptors: Guardians of stable breathing. Trends Mol. Med. 2003, 9, 542–548. [Google Scholar] [CrossRef]
- Hilenski, L.L.; Griendling, K.K. Vascular Smooth Muscle. In Vascular Medicine: A Companion to Braunwald’s Heart Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 25–42. ISBN 9781437729306. [Google Scholar]
- Ahmad, I.; Nirogi, R. 5-HT4 Receptor Agonists for the Treatment of Alzheimer’s Disease. Neurosci. Med. 2011, 2, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Wohlfarth, C.; Schmitteckert, S.; Härtle, J.D.; Houghton, L.A.; Dweep, H.; Fortea, M.; Assadi, G.; Braun, A.; Mederer, T.; Pöhner, S.; et al. miR-16 and miR-103 impact 5-HT4 receptor signalling and correlate with symptom profile in irritable bowel syndrome. Sci. Rep. 2017, 7, 14680. [Google Scholar] [CrossRef] [Green Version]
- Mendez-David, I.; David, D.J.; Darcet, F.; Wu, M.V.; Kerdine-Römer, S.; Gardier, A.M.; Hen, R. Rapid Anxiolytic Effects of a 5-HT4 Receptor Agonist Are Mediated by a Neurogenesis-Independent Mechanism. Neuropsychopharmacology 2014, 39, 1366–1378. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.K.; Mendez-David, I.; Luna, V.M.; Faye, C.; Gardier, A.M.; David, D.J.; Denny, C.A. Prophylactic efficacy of 5-HT4R agonists against stress. Neuropsychopharmacology 2020, 45, 542–552. [Google Scholar] [CrossRef]
- Bell, R.; Duke, A.A.; Gilmore, P.E.; Page, D.; Bègue, L. Anxiolytic-like effects observed in rats exposed to the elevated zero-maze following treatment with 5-HT2/5-HT3/5-HT4 ligands. Sci. Rep. 2015, 4, 3881. [Google Scholar] [CrossRef]
- Silvestre, J.S.; Fernández, A.G.; Palacios, J. Effects of 5-HT4 receptor antagonists on rat behaviour in the elevated plus-maze test. Eur. J. Pharmacol. 1996, 309, 219–222. [Google Scholar] [CrossRef]
- Faye, C.; Hen, R.; Guiard, B.P.; Denny, C.A.; Gardier, A.M.; Mendez-David, I.; David, D.J. Rapid Anxiolytic Effects of RS67333, a Serotonin Type 4 Receptor Agonist, and Diazepam, a Benzodiazepine, Are Mediated by Projections From the Prefrontal Cortex to the Dorsal Raphe Nucleus. Biol. Psychiatry 2020, 87, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Amigó, J.; Díaz, A.; Pilar-Cuéllar, F.; Vidal, R.; Martín, A.; Compan, V.; Pazos, A.; Castro, E. The absence of 5-HT4 receptors modulates depression- and anxiety-like responses and influences the response of fluoxetine in olfactory bulbectomised mice: Adaptive changes in hippocampal neuroplasticity markers and 5-HT1A autoreceptor. Neuropharmacology 2016, 111, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castello, J.; LeFrancois, B.; Flajolet, M.; Greengard, P.; Friedman, E.; Rebholz, H. CK2 regulates 5-HT4 receptor signaling and modulates depressive-like behavior. Mol. Psychiatry 2018, 23, 872–882. [Google Scholar] [CrossRef]
- Murphy, S.E.; de Cates, A.N.; Gillespie, A.L.; Godlewska, B.R.; Scaife, J.C.; Wright, L.C.; Cowen, P.J.; Harmer, C.J. Translating the promise of 5HT 4 receptor agonists for the treatment of depression. Psychol. Med. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, G.; Rymar, V.V.; Du, J.; Mnie-Filali, O.; Bisgaard, C.; Manta, S.; Lambas-Senas, L.; Wiborg, O.; Haddjeri, N.; Piñeyro, G.; et al. Serotonin4 (5-HT4) Receptor Agonists Are Putative Antidepressants with a Rapid Onset of Action. Neuron 2007, 55, 712–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascual-Brazo, J.; Castro, E.; Díaz, Á.; Valdizán, E.M.; Pilar-Cuéllar, F.; Vidal, R.; Treceño, B.; Pazos, Á. Modulation of neuroplasticity pathways and antidepressant-like behavioural responses following the short-term (3 and 7 days) administration of the 5-HT4 receptor agonist RS67333. Int. J. Neuropsychopharmacol. 2012, 15, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Lucas, G.; Du, J.; Romeas, T.; Mnie-Filali, O.; Haddjeri, N.; Piñeyro, G.; Debonnel, G. Selective Serotonin Reuptake Inhibitors Potentiate the Rapid Antidepressant-Like Effects of Serotonin4 Receptor Agonists in the Rat. PLoS ONE 2010, 5, e9253. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Cryan, J.F. The Behavioral Genetics of Serotonin: Relevance to Anxiety and Depression. In Handbook of Behavioral Neuroscience; Elsevier B.V.: Amsterdam, The Netherlands, 2010; Volume 21, pp. 749–789. ISBN 9780123746344. [Google Scholar]
- Blackburn, T.P. Serotonin (5-Hydroxytryptamine; 5-HT): Receptors. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 701–714. ISBN 9780080450469. [Google Scholar]
- Goodfellow, N.M.; Bailey, C.D.C.; Lambe, E.K. The Native Serotonin 5-HT5A Receptor: Electrophysiological Characterization in Rodent Cortex and 5-HT1A-Mediated Compensatory Plasticity in the Knock-Out Mouse. J. Neurosci. 2012, 32, 5804–5809. [Google Scholar] [CrossRef]
- Noda, M.; Yasuda, S.; Okada, M.; Higashida, H.; Shimada, A.; Iwata, N.; Ozaki, N.; Nishikawa, K.; Shirasawa, S.; Uchida, M.; et al. Recombinant human serotonin 5A receptors stably expressed in C6 glioma cells couple to multiple signal transduction pathways. J. Neurochem. 2003, 84, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Hofer, A.M. Signal Transduction and Second Messengers. In Cell Physiology Source Book; Elsevier: Amsterdam, The Netherlands, 2012; pp. 85–98. ISBN 9780123877383. [Google Scholar]
- Cuthbert, B.N.; Lang, P.J.; Strauss, C.; Drobes, D.; Patrick, C.J.; Bradley, M.M. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology 2003, 40, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Brasil, E.S.d.A.; Furini, C.R.G.; Rodrigues, F.d.S.; Nachtigall, E.G.; Behling, J.A.K.; Saenger, B.F.; Farias, C.P.; Myskiw, J.d.C.; Izquierdo, I. The blockade of the serotoninergic receptors 5-HT5A, 5-HT6 and 5-HT7 in the basolateral amygdala, but not in the hippocampus facilitate the extinction of fear memory. Behav. Brain Res. 2019, 372, 112055. [Google Scholar] [CrossRef]
- Kassai, F.; Schlumberger, C.; Kedves, R.; Pietraszek, M.; Jatzke, C.; Lendvai, B.; Gyertyán, I.; Danysz, W. Effect of 5-HT5A antagonists in animal models of schizophrenia, anxiety and depression. Behav. Pharmacol. 2012, 23, 397–406. [Google Scholar] [CrossRef]
- Sagi, Y.; Medrihan, L.; George, K.; Barney, M.; McCabe, K.A.; Greengard, P. Emergence of 5-HT5A signaling in parvalbumin neurons mediates delayed antidepressant action. Mol. Psychiatry 2020, 25, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D. 5-ht5A receptors as a therapeutic target. Pharmacol. Ther. 2006, 111, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.J. 5-HT6 receptors and Alzheimer’s disease. Alzheimers. Res. Ther. 2013, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meffre, J.; Chaumont-Dubel, S.; Mannoury la Cour, C.; Loiseau, F.; Watson, D.J.G.; Dekeyne, A.; Séveno, M.; Rivet, J.; Gaven, F.; Déléris, P.; et al. 5-HT 6 receptor recruitment of mTOR as a mechanism for perturbed cognition in schizophrenia. EMBO Mol. Med. 2012, 4, 1043–1056. [Google Scholar] [CrossRef]
- Dawson, L.A. The Central Role of 5-HT6 Receptors in Modulating Brain Neurochemistry. In International Review of Neurobiology; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 96, pp. 1–26. ISBN 9780123859020. [Google Scholar]
- Geng, F.; Tian, J.; Wu, J.-L.; Luo, Y.; Zou, W.-J.; Peng, C.; Lu, G.-F. Dorsomedial prefrontal cortex 5-HT6 receptors regulate anxiety-like behavior. Cogn. Affect. Behav. Neurosci. 2018, 18, 58–67. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Kos, T.; Wesołowska, A. The 5-HT6 receptor agonist EMD 386088 produces antidepressant and anxiolytic effects in rats after intrahippocampal administration. Psychopharmacology 2011, 217, 411–418. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A. Effects of the brain-penetrant and selective 5-HT6 receptor antagonist SB-399885 in animal models of anxiety and depression. Neuropharmacology 2007, 52, 1274–1283. [Google Scholar] [CrossRef]
- Partyka, A.; Wasik, A.; Jastrzębska-Więsek, M.; Mierzejewski, P.; Bieńkowski, P.; Kołaczkowski, M.; Wesołowska, A. ADN-1184, a monoaminergic ligand with 5-HT6/7 receptor antagonist action, exhibits activity in animal models of anxiety. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Abraham, R.; Nirogi, R.; Shinde, A.; Benade, V. Therapeutic Potential of 5-HT6 Antagonist SB399885 in Traumatic Stress Disorder. Drug Res. 2014, 65, 442–445. [Google Scholar] [CrossRef]
- Carr, G.V.; Schechter, L.E.; Lucki, I. Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology 2011, 213, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Wesolowska, A.; Nikiforuk, A.; Stachowicz, K. Anxiolytic-like and antidepressant-like effects produced by the selective 5-HT6 receptor antagonist SB-258585 after intrahippocampal administration to rats. Behav. Pharmacol. 2007, 18, 439–446. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Zhang, L.; Wang, Y.; Sun, Y.-N.; Guo, Y.; Du, C.-X.; Zhang, J.; Yao, L.; Yu, S.-Q.; Liu, J. Activation and blockade of prelimbic 5-HT6 receptors produce different effects on depressive-like behaviors in unilateral 6-hydroxydopamine-induced Parkinson’s rats. Neuropharmacology 2016, 110, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska-Więsek, M.; Siwek, A.; Partyka, A.; Szewczyk, B.; Sowa-Kućma, M.; Wasik, A.; Kołaczkowski, M.; Wesołowska, A. Antidepressant-like activity of EMD 386088, a 5-HT6 receptor partial agonist, following systemic acute and chronic administration to rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015, 388, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska-Więsek, M.; Siwek, A.; Partyka, A.; Antkiewicz-Michaluk, L.; Michaluk, J.; Romańska, I.; Kołaczkowski, M.; Wesołowska, A. Study of a mechanism responsible for potential antidepressant activity of EMD 386088, a 5-HT6 partial agonist in rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 839–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suárez-Santiago, J.E.; Briones-Aranda, A.; Espinosa-Raya, J.; Picazo, O. Agonist E-6837 and antagonist SB-271046 of 5-HT6 receptors both reverse the depressive-like effect induced in mice by subchronic ketamine administration. Behav. Pharmacol. 2017, 28, 582–585. [Google Scholar] [CrossRef]
- Volpicelli, F.; Speranza, L.; di Porzio, U.; Crispino, M.; Perrone-Capano, C. The serotonin receptor 7 and the structural plasticity of brain circuits. Front. Behav. Neurosci. 2014, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Guseva, D.; Wirth, A.; Ponimaskin, E. Cellular mechanisms of the 5-HT 7 receptor-mediated signaling. Front. Behav. Neurosci. 2014, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Speranza, L.; Labus, J.; Volpicelli, F.; Guseva, D.; Lacivita, E.; Leopoldo, M.; Bellenchi, G.C.; di Porzio, U.; Bijata, M.; Perrone-Capano, C.; et al. Serotonin 5-HT7 receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J. Neurochem. 2017, 141, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Du, C.X.; Guo, Y.; Zhang, Q.J.; Zhang, J.; Lv, S.X.; Liu, J. Involvement of prelimbic 5-HT 7 receptors in the regulation of anxiety-like behaviors in hemiparkinsonian rats. Neurol. Res. 2018, 40, 847–855. [Google Scholar] [CrossRef]
- Canale, V.; Kurczab, R.; Partyka, A.; Satała, G.; Lenda, T.; Jastrzębska-Więsek, M.; Wesołowska, A.; Bojarski, A.J.; Zajdel, P. Towards new 5-HT 7 antagonists among arylsulfonamide derivatives of (aryloxy)ethyl-alkyl amines: Multiobjective based design, synthesis, and antidepressant and anxiolytic properties. Eur. J. Med. Chem. 2016, 108, 334–346. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Stachowicz, K.; Tatarczyńska, E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology 2006, 51, 578–586. [Google Scholar] [CrossRef]
- Sowa, J.; Hess, G. Prenatal stress-related alterations in synaptic transmission and 5-HT 7 receptor-mediated effects in the rat dorsal raphe nucleus are ameliorated by the 5-HT 7 receptor antagonist SB 269970. Eur. J. Neurosci. 2020, ejn.14778. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Huitron-Resendiz, S.; Henriksen, S.J.; Sutcliffe, J.G. 5-HT7 Receptor Inhibition and Inactivation Induce Antidepressantlike Behavior and Sleep Pattern. Biol. Psychiatry 2005, 58, 831–837. [Google Scholar] [CrossRef]
- Bonaventure, P.; Kelly, L.; Aluisio, L.; Shelton, J.; Lord, B.; Galici, R.; Miller, K.; Atack, J.; Lovenberg, T.W.; Dugovic, C. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J. Pharmacol. Exp. Ther. 2007, 321, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Wesołowska, A.; Kowalska, M. Influence of serotonin 5-HT(7) receptor blockade on the behavioral and neurochemical effects of imipramine in rats. Pharmacol. Rep. 2008, 60, 464–474. [Google Scholar] [PubMed]
- Stroth, N.; Svenningsson, P. S100B interacts with the serotonin 5-HT7 receptor to regulate a depressive-like behavior. Eur. Neuropsychopharmacol. 2015, 25, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, P.; Dugovic, C.; Kramer, M.; De Boer, P.; Singh, J.; Wilson, S.; Bertelsen, K.; Di, J.; Shelton, J.; Aluisio, L.; et al. Translational Evaluation of JNJ-18038683, a 5-Hydroxytryptamine Type 7 Receptor Antagonist, on Rapid Eye Movement Sleep and in Major Depressive Disorder. J. Pharmacol. Exp. Ther. 2012, 342, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- White, C.M. The Pharmacology, Pharmacokinetics, Efficacy, and Adverse Events Associated With Kava. J. Clin. Pharmacol. 2018, 58, 1396–1405. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Campos, A.C.; Coelho, L.D.; Duman, R.S.; Guimarães, F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 2018, 135. [Google Scholar] [CrossRef]
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, C.; Xue, C.; Liu, C.; Wang, Y.; Chen, L.; Deng, Y.; Huang, J.; Zhai, H. Anxiolytic effect of a novel 9,10-dihydrophenanthrene, juncuenin H, is associated with metabolic changes in cortical serotonin/dopamine levels in mice. Fitoterapia 2019, 134, 165–171. [Google Scholar] [CrossRef]
- Qiu, Z.-K.; He, J.-L.; Liu, X.; Zeng, J.; Xiao, W.; Fan, Q.-H.; Chai, X.-M.; Ye, W.-H.; Chen, J.-S. Anxiolytic-like effects of paeoniflorin in an animal model of post traumatic stress disorder. Metab. Brain Dis. 2018, 33, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.-H.; Sheen, L.-Y.; Chang, S.-T. Evaluation of anxiolytic potency of essential oil and S-(+)-linalool from Cinnamomum osmophloeum ct. linalool leaves in mice. J. Tradit. Complement. Med. 2015, 5, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannan, M.A.; Abir, A.B.; Rahman, M.R.; Khatun, A.; Datta, B. Analgesic, antidepressant and anxiolytic effects of the methanolic extract of Achyranthes aspera leaves in mice. Stamford J. Pharm. Sci. 2019, 9, 11–35. [Google Scholar]
- Jiménez-Ferrer, E.; Santillán-Urquiza, M.A.; Alegría-Herrera, E.; Zamilpa, A.; Noguerón-Merino, C.; Tortoriello, J.; Navarro-García, V.; Avilés-Flores, M.; Fuentes-Mata, M.; Herrera-Ruiz, M. Anxiolytic effect of fatty acids and terpenes fraction from Aloysia triphylla: Serotoninergic, GABAergic and glutamatergic implications. Biomed. Pharmacother. 2017, 96, 320–327. [Google Scholar] [CrossRef]
- Diniz, T.C.; de Oliveira Júnior, R.G.; Miranda Bezerra Medeiros, M.A.; Gama e Silva, M.; de Andrade Teles, R.B.; dos Passos Menezes, P.; de Sousa, B.M.H.; Abrahão Frank, L.; de Souza Araújo, A.A.; Russo Serafini, M.; et al. Anticonvulsant, sedative, anxiolytic and antidepressant activities of the essential oil of Annona vepretorum in mice: Involvement of GABAergic and serotonergic systems. Biomed. Pharmacother. 2019, 111, 1074–1087. [Google Scholar] [CrossRef]
- He, D.; Wang, X.; Zhang, P.; Luo, X.; Li, X.; Wang, L.; Li, S.; Xu, Y. Evaluation of the Anxiolytic and Antidepressant Activities of the Aqueous Extract from Camellia euphlebia Merr. ex Sealy in Mice. Evidence-Based Complement. Altern. Med. 2015, 2015, 618409. [Google Scholar] [CrossRef] [Green Version]
- Kurauchi, Y.; Devkota, H.P.; Hori, K.; Nishihara, Y.; Hisatsune, A.; Seki, T.; Katsuki, H. Anxiolytic activities of Matcha tea powder, extracts, and fractions in mice: Contribution of dopamine D1 receptor- and serotonin 5-HT1A receptor-mediated mechanisms. J. Funct. Foods 2019, 59, 301–308. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. The anxiolytic effect of essential oil of Cananga odorata exposure on mice and determination of its major active constituents. Phytomedicine 2016, 23, 1727–1734. [Google Scholar] [CrossRef]
- Ishola, I.; Olayemi, S.; Yemitan, O.; Umeh, E. Antidepressant and Anxiolytic Effects of the Methanol Root Extract of Capparis thonningii: Involvement of Monoaminergic, Cholinergic and GABAergic Systems. Drug Res. 2014, 65, 205–213. [Google Scholar] [CrossRef]
- Qazi, N.; Khan, R.A.; Rizwani, G.H. Evaluation of antianxiety and antidepressant properties of Carthamus tinctorius L. (Safflower) petal extract. Pak. J. Pharm. Sci. 2015, 28, 991–995. [Google Scholar] [PubMed]
- Lima, E.B.C.; de Sousa, C.N.S.; Meneses, L.N.; e Silva Pereira, Y.F.; Matos, N.C.B.; de Freitas, R.B.; Lima, N.B.C.; Patrocínio, M.C.A.; Leal, L.K.A.M.; Viana, G.S.B.; et al. Involvement of monoaminergic systems in anxiolytic and antidepressive activities of the standardized extract of Cocos nucifera L. J. Nat. Med. 2017, 71, 227–237. [Google Scholar] [CrossRef]
- Sahoo, S.; Brijesh, S. Anxiolytic activity of Coriandrum sativum seeds aqueous extract on chronic restraint stressed mice and effect on brain neurotransmitters. J. Funct. Foods 2020, 68, 103884. [Google Scholar] [CrossRef]
- Thomas, S.; Shrikumar, S.; Velmurugan, C.; Kumar, B.S.A. Evaluation of anxiolytic effect of whole plant of Cuscuta reflexa. World J. Pharm. Pharm. Sci. 2015, 4, 1245–1253. [Google Scholar]
- Citó, M.C.O.; Silva, M.I.G.; Santos, L.K.X.; Fernandes, M.L.; Melo, F.H.C.; Aguiar, J.A.C.; Lopes, I.S.; Sousa, P.B.; Vasconcelos, S.M.M.; Macêdo, D.S.; et al. Antidepressant-like effect of Hoodia gordonii in a forced swimming test in mice: Evidence for involvement of the monoaminergic system. Brazilian J. Med. Biol. Res. 2015, 48, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benneh, C.K.; Biney, R.P.; Mante, P.K.; Tandoh, A.; Adongo, D.W.; Woode, E. Maerua angolensis stem bark extract reverses anxiety and related behaviours in zebrafish—Involvement of GABAergic and 5-HT systems. J. Ethnopharmacol. 2017, 207, 129–145. [Google Scholar] [CrossRef]
- Narasingam, M.; Vijeepallam, K.; Mohamed, Z.; Pandy, V. Anxiolytic- and antidepressant-like activities of a methanolic extract of Morinda citrifolia Linn. (noni) fruit in mice: Involvement of benzodiazepine-GABAAergic, serotonergic and adrenergic systems. Biomed. Pharmacother. 2017, 96, 944–952. [Google Scholar] [CrossRef]
- Murtala, A.A.; Akindele, A.J. Anxiolytic- and antidepressant-like activities of hydroethanol leaf extract of Newbouldia laevis (P.Beauv.) Seem. (Bignoniaceae) in mice. J. Ethnopharmacol. 2020, 249, 112420. [Google Scholar] [CrossRef]
- Billah, M.M.; Hasan, M.R.; Nawrin, K.; Mohiuddin, M.; Habib, M.R. Evaluation of Analgesic and Sedative-anxiolytic Potential of Paderia foetida Leaf Extract. Am. J. Biomed. Sci. 2015, 7, 98–104. [Google Scholar] [CrossRef]
- Shahamat, Z.; Abbasi-Maleki, S.; Motamed, S.M. Evaluation of antidepressant-like effects of aqueous and ethanolic extracts of Pimpinella anisum fruit in mice. Avicenna J. Phytomedicine 2016, 6, 322–328. [Google Scholar]
- Liu, A.D.; Cai, G.H.; Wei, Y.Y.; Yu, J.P.; Chen, J.; Yang, J.; Wang, X.; Che, Y.W.; Chen, J.Z.; Wu, S.X. Anxiolytic effect of essential oils of Salvia miltiorrhiza in rats. Int. J. Clin. Exp. Med. 2015, 8, 12756–12764. [Google Scholar]
- Jawaid, T.; Tiwari, N.; Kamal, M.; Alkhamees, O.A.; Alaseem, A.M.; Al Shagha, W.M.; Alsanad, S.M. Evaluation of Antidepressant and Anxiolytic Activity of Solanum melongena L. Fruits Aqueous Extract via Monoaminergic and GABAergic Pathway. J. Pharm. Res. Int. 2020. [Google Scholar] [CrossRef]
- Pérez-Ortega, G.; Angeles-López, G.E.; Argueta-Villamar, A.; González-Trujano, M.E. Preclinical evidence of the anxiolytic and sedative-like activities of Tagetes erecta L. reinforces its ethnobotanical approach. Biomed. Pharmacother. 2017, 93, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Jaime, H.; Guadarrama-Cruz, G.; Alarcon-Aguilar, F.J.; Limón-Morales, O.; Vazquez-Palacios, G. Antidepressant-like activity of Tagetes lucida Cav. is mediated by 5-HT1A and 5-HT2A receptors. J. Nat. Med. 2015, 69, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, J.; Reyes-Pérez, V.; Hernández-Navarro, M.D.; Dorantes-Barrón, A.M.; Almazán, S.; Estrada-Reyes, R. Anxiolytic- and antidepressant-like effects of an aqueous extract of Tanacetum parthenium L. Schultz-Bip (Asteraceae) in mice. J. Ethnopharmacol. 2017, 200, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Hernández, E.; González-Trujano, M.E.; Terrazas, T.; Herrera, J.; Guevara-Fefer, P. Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation. Salud Ment. 2016, 39, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Keugong Wado, E.; Kubicki, M.; Ngatanko, A.H.H.; Léa Blondelle, K.D.; Jorelle linda, D.; Roland, R.N.; Balbine, K.; Lamshoeft, M.; Assongalem, A.E.; Foyet, H.S. Anxiolytic and antidepressant effects of Ziziphus mucronata hydromethanolic extract in male rats exposed to unpredictable chronic mild stress: Possible mechanisms of actions. J. Ethnopharmacol. 2020, 260, 112987. [Google Scholar] [CrossRef]
- Sarris, J. Herbal medicines in the treatment of psychiatric disorders: 10-year updated review. Phyther. Res. 2018, 32, 1147–1162. [Google Scholar] [CrossRef]



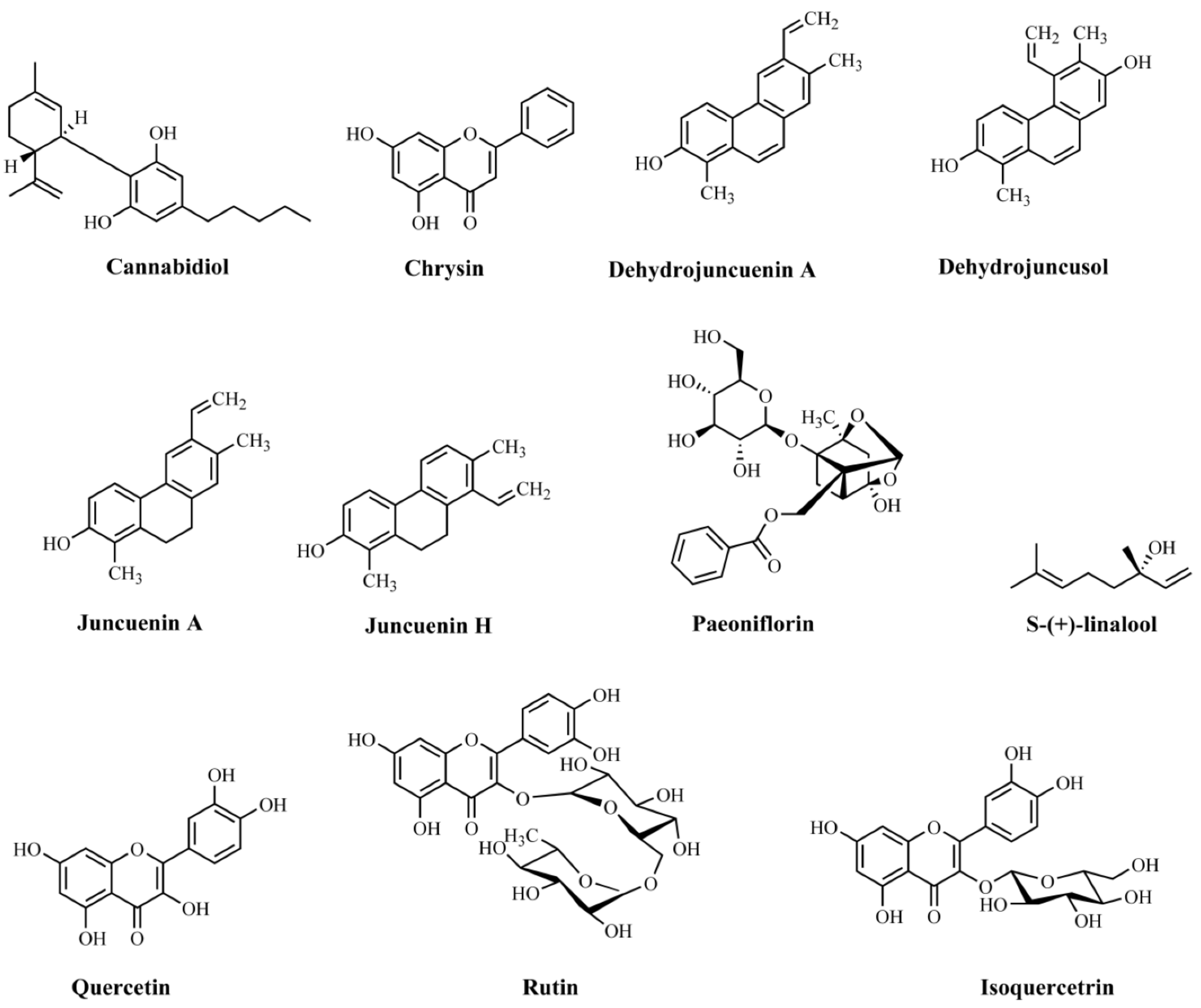
| Compound | Plant/Extract | Model/Test Used | Animal Species | Administration | Major Findings | Mechanisms of Action | Reference |
|---|---|---|---|---|---|---|---|
| Cannabidiol | Cannabis sativa/not informed | Elevated plus-maze and novelty suppressed feeding tests | Male C57BL/6 mice | Intraperitoneal administration (30 mg/kg) 2 h after daily stressor for 14 days | Anxiolytic effectiveness | Not assayed | [181] |
| Chrysin | Not informed | Splash, rota-rod, and tail suspension tests | C57B/6J mice | Oral administration (10 ml/kg) 30 min before tests | Antidepressant effectiveness | Decrease in the levels of 5-HT in the hippocampus | [182] |
| Dehydrojuncuenin A (4) | Juncus setchuenensis/ethanolic extract | Elevated plus maze | Male CD-1 mice | Oral administration (5, 10, or 20 mg/kg) 30 min before test | Anxiolytic effectiveness | Not assayed | [183] |
| Dehydrojuncusol (2) | Juncus setchuenensis/ethanolic extract | Elevated plus maze | Male CD-1 mice | Oral administration (2.5, 5, or 10 mg/kg) 30 min before the test | Anxiolytic effectiveness | Not assayed | [183] |
| Juncuenin A (3) | Juncus setchuenensis/ethanolic extract | Elevated plus maze | Male CD-1 mice | Oral administration (2.5, 5, 10, or 20 mg/kg) 30 min before test | Anxiolytic effectiveness | Not assayed | [183] |
| Juncuenin H (1) | Juncus setchuenensis/ethanolic extract | Elevated plus maze and locomotor test | Male CD-1 mice | Oral administration (5, 10, or 20 mg/kg) 30 min before tests | Anxiolytic effectiveness | Reductions in the levels of 5-HT and 5-HT/DA metabolites in the cerebral cortex and hippocampus. | [183] |
| Paeoniflorin | Not informed | Elevated plus maze test | Male Sprague–Dawley rats | Intraperitoneal administration (5, 10, and 20 mg/kg) 1 h before test | Anxiolytic effectiveness | Increased levels of 5-HT and 5-HIAA in the hippocampus | [184] |
| S-(+)-linalool | Cinnamomum osmophloeum/essential oil | Elevated plus maze test | Male ICR mice | Oral administration (250 and 500 mg/kg) 1 h before tests for 14 days | Anxiolytic effectiveness | Anxiolytic effect via modulation of 5-HT | [185] |
| Plant/Extract | Model/Test Used | Animal Species | Administration | Major Findings | Mechanisms of Action | Reference |
|---|---|---|---|---|---|---|
| Achyranthes aspera/methanolic extract | Hole cross, OF, forced swimming, tail suspension, elevated plus maze, and light/dark | Mice | Oral administration (50, 100, and 200 mg/kg) 30 min before tests | Anxiolytic and antidepressant effectiveness | Not assayed | [186] |
| Aloysia triphylla/methanolic, dicloromethane and hexanic extracts | Elevated plus maze test | Male ICR mice | Oral administration (125, 250, 500, and 750 mg/kg) 30 min before test | Anxiolytic effectiveness | Interaction with serotonergic transmission | [187] |
| Annona vepretorum/essential oil | Elevated plus-maze, hole-board, open-field, rota-rod, and tail suspension tests | Male albino Swiss mice | Intraperitoneal administration (25, 50, and 100 mg/kg) | Anxiolytic and antidepressant effectiveness | Not assayed | [188] |
| Camellia euphlebia/aqueous extract | Light/dark box, elevated plus maze, forced swimming, tail suspension, and open-field tests | Male Kunming mice | Intragrastrical administration (100, 200, or 400 mg/kg) 1 h before tests for 7 days | Anxiolytic and antidepressant effectiveness | Not assayed | [189] |
| Camellia sinensis/aqueous and ethanolic extracts | Elevated plus maze and OF tests | Male C57BL/6J mice | Oral administration (50 and 100 mg/kg) 1 hour before test | Anxiolytic effectiveness | Activation of serotonin 5-HT1A receptors | [190] |
| Cananga odorata/essential oil | Open field, elevated plus maze, and light/dark box tests | ICR mice | 10 mL inhalation 10 min before tests | Anxiolytic effectiveness | Increased 5-HT concentration in the hippocampus of male mice | [191] |
| Capparis thonningii/methanolic extract | Forced swimming, tail suspension, hole-board, light/dark, and elevated plus maze tests | Swiss albino mice | Oral administration (500–4000 mg/kg) 1 h before tests | Anxiolytic and antidepressant effectiveness | 5-HT2 receptor inhibition | [192] |
| Carthamus tinctorius/ethanolic extract | Elevated plus maze and forced swim tests | White albino rats | Oral administration (100 and 200 mg/kg) 1 h before tests | Anxiolytic and antidepressant effectiveness | Not assayed | [193] |
| Cocos nucifera/hydroalcoholic extract | Elevated plus maze, hole-board, forced swimming, tail suspension, and open-field tests | Swiss mice | Oral administration (50, 100, or 200 mg/kg) 1 h before tests | Anxiolytic and antidepressant effectiveness | Inhibition of the 5-HT system | [194] |
| Coriandrum sativum/aqueous extract | Elevated plus-maze test and light/dark transition | Male Swiss albino mice | Oral administration (100, 200, or 400 mg/kg) 2 hours/day for 14 days | Anxiolytic effectiveness | Decrease in levels of NE, DA, and 5-HT in cortex, hippocampus, cerebellum, and brain stem | [195] |
| Cuscuta reflexa/methanolic extract | Elevated plus maze and light/dark box tests | Swiss albino mice | Oral administration (200 and 400 mg/kg) 30 min before tests for 14 days | Anxiolytic effectiveness | Not assayed | [196] |
| Hoodia gordonii/aqueous extract | Forced swim and OF tests | Male Swiss mice | Oral administration (25 and 50 mg/kg) 1 h before tests | Antidepressant effectiveness | Results showed that only 5-HT monoamine was significantly increased after acute H. gordonii administration | [197] |
| Maerua angolensis/crude extract | Novel tank and light/dark box tests | Zebrafish | 1.0, 0.3, 0.1 mg/ml diluted in water 20 min before tests | Anxiolytic effectiveness | Direct or indirect effect on the activation of GABAA and 5-HT1–3 receptor | [198] |
| Morinda citrifolia/methanolic extract | Elevated plus maze, light/dark transition, and tail suspension tests | Male Swiss albino mice | Oral administration (0.5, 1, 3 g/kg) 1 h before tests | Anxiolytic and antidepressant effectiveness | Not assayed | [199] |
| Newbouldia laevis/hydroethanolic extract | Hole-board, open-field, elevated plus maze, light/dark box exploration, social interaction, forced swim, and tail suspension tests | Mice | Intraperitoneal administration (50, 100, 200, 400, and 800 mg/kg) | Anxiolytic and antidepressant effectiveness | Not assayed | [200] |
| Paederia foetida/aqueous, ethanolic, and ethyl acetate extracts | Hole cross, OF, and elevated plus maze tests | Albino mice | Oral administration (400 mg/kg) | Anxiolytic effectiveness | Not assayed | [201] |
| Pimpinella anisum/aqueous and ethanolic extracts | Forced swimming, tail suspension tests | Mice | Intraperitoneal administration (50, 100, and 200 mg/kg) | Antidepressant effectiveness | Not assayed | [202] |
| Salvia miltiorrhiza/essential oil | Elevated plus maze, social interaction, and rota-rod tests | Male Sprague–Dawley rats | Oral administration (50, 100, and 200 mg/kg) 1 h before tests | Anxiolytic effectiveness | Reduction of monoamine and 5-HT system levels in the cerebral cortex | [203] |
| Solanum melongena/aqueous extract | Elevated plus maze, forced swimming, and tail suspension tests | Male albino mice | Oral administration (100 and 200 mg/kg) | Anxiolytic and antidepressant effectiveness | Increase of 5-HT levels | [204] |
| Tagetes erecta/aqueous extract | Hole-board, open-field, and exploration cylinder tests | Male Swiss Webster mice | Intraperitoneal administration (10, 30, 100 mg/kg) 1 h before tests | Anxiolytic and sedative effectiveness | Not assayed | [205] |
| Tagetes lucida/aqueous extract | Forced swimming | Male Wistar rats | Intragastric administration (50, 100, and 200 mg/kg) 72, 48, 24, 18, and 1 h before test | Antidepressant effectiveness | Modulating the release/reuptake of serotoninby interaction with 5-HT1A and 5-HT2A receptors | [206] |
| Tanacetum parthenium/aqueous extract | Burying behavior, elevated plus maze, forced swimming, and OF tests | Male Swiss Webster mice | Oral administration (0.5, 1.0, 5, 10, 20, and 40 mg/kg) 30 min before tests | Anxiolytic and antidepressant effectiveness | Not assayed | [207] |
| Tilia americana/hexanic, ethyl acetate, and methanolic extracts | Elevated plus maze and hole-board tests | Male CD-1 mice | Intraperitoneal administration (100 mg/kg) 50 min before test | Anxiolytic and sedative effectiveness | Production of the anxiolytic effect reversed in the presence of 5-HT1A receptor antagonists | [208] |
| Ziziphus mucronata/hydromethanolic extract | Elevated plus maze, light/dark, and forced swim tests | Adult male Wistar rats | Oral administration (300 mg/kg) 30 min before tests | Anxiolytic and antidepressant effectiveness | Modulation of serotonergic and noradrenergic systems | [209] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villas-Boas, G.R.; Lavorato, S.N.; Paes, M.M.; de Carvalho, P.M.G.; Rescia, V.C.; Cunha, M.S.; de Magalhães-Filho, M.F.; Ponsoni, L.F.; de Carvalho, A.A.V.; de Lacerda, R.B.; et al. Modulation of the Serotonergic Receptosome in the Treatment of Anxiety and Depression: A Narrative Review of the Experimental Evidence. Pharmaceuticals 2021, 14, 148. https://doi.org/10.3390/ph14020148
Villas-Boas GR, Lavorato SN, Paes MM, de Carvalho PMG, Rescia VC, Cunha MS, de Magalhães-Filho MF, Ponsoni LF, de Carvalho AAV, de Lacerda RB, et al. Modulation of the Serotonergic Receptosome in the Treatment of Anxiety and Depression: A Narrative Review of the Experimental Evidence. Pharmaceuticals. 2021; 14(2):148. https://doi.org/10.3390/ph14020148
Chicago/Turabian StyleVillas-Boas, Gustavo R., Stefânia N. Lavorato, Marina M. Paes, Pablinny M. G. de Carvalho, Vanessa C. Rescia, Mila S. Cunha, Manoel F. de Magalhães-Filho, Luis F. Ponsoni, Adryano Augustto Valladao de Carvalho, Roseli B. de Lacerda, and et al. 2021. "Modulation of the Serotonergic Receptosome in the Treatment of Anxiety and Depression: A Narrative Review of the Experimental Evidence" Pharmaceuticals 14, no. 2: 148. https://doi.org/10.3390/ph14020148