Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application
Abstract
:1. Introduction
2. Limitations of Plant Proteins as Techno-Functional Food Ingredients
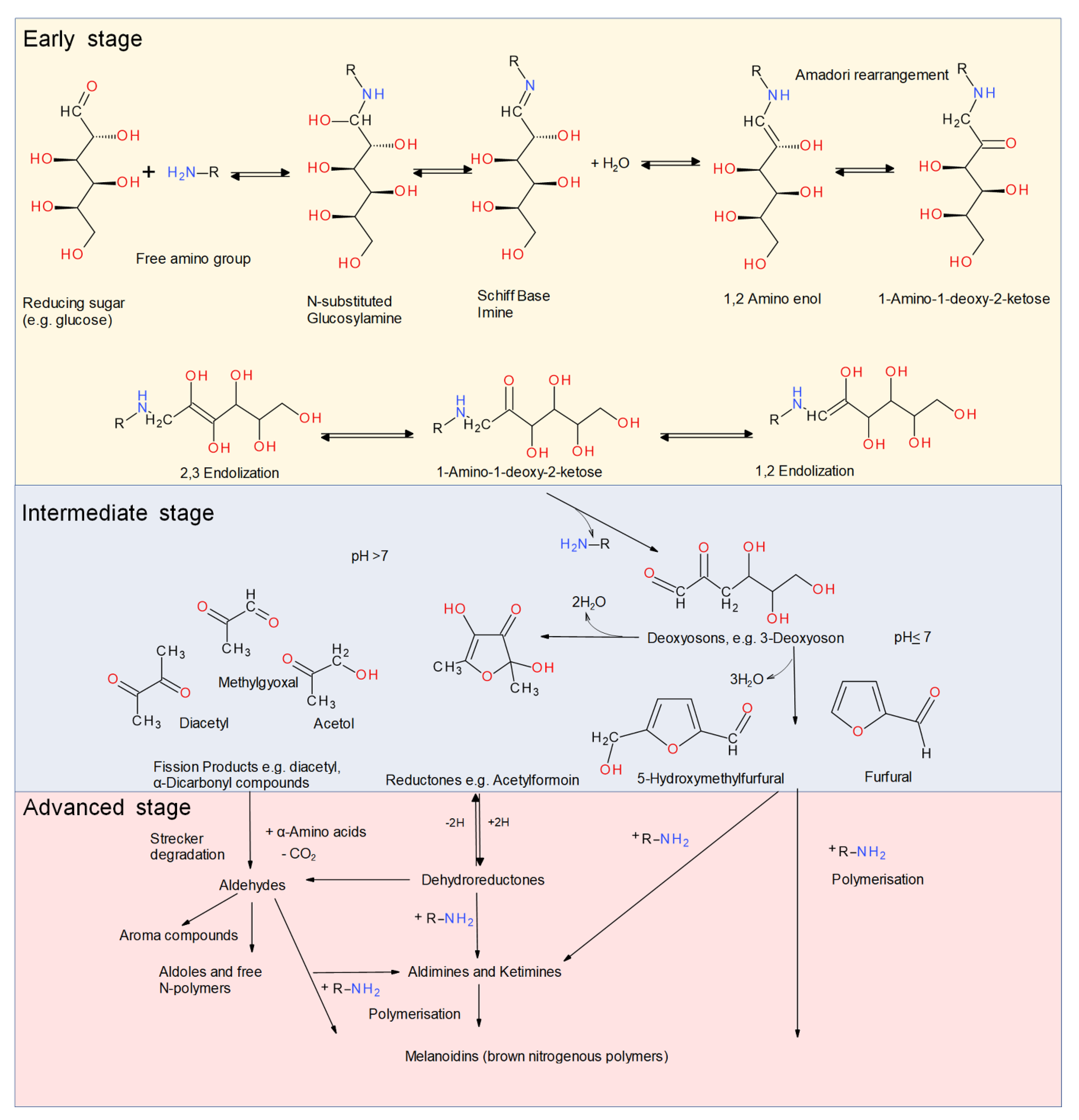
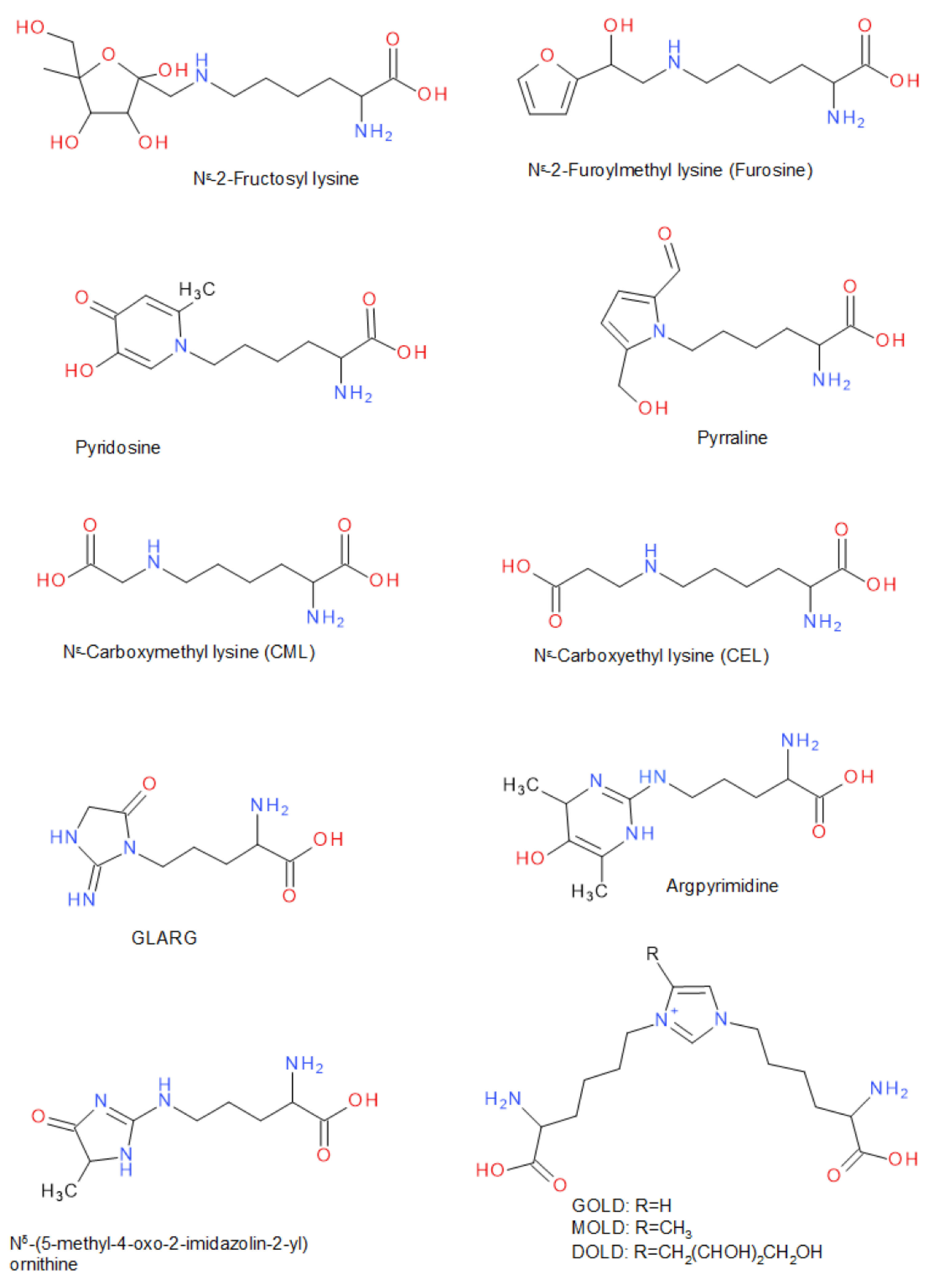
3. Reaction Chemistry of Food Protein Glycation Via the Maillard Reaction
3.1. Chemistry of Conjugation Reaction
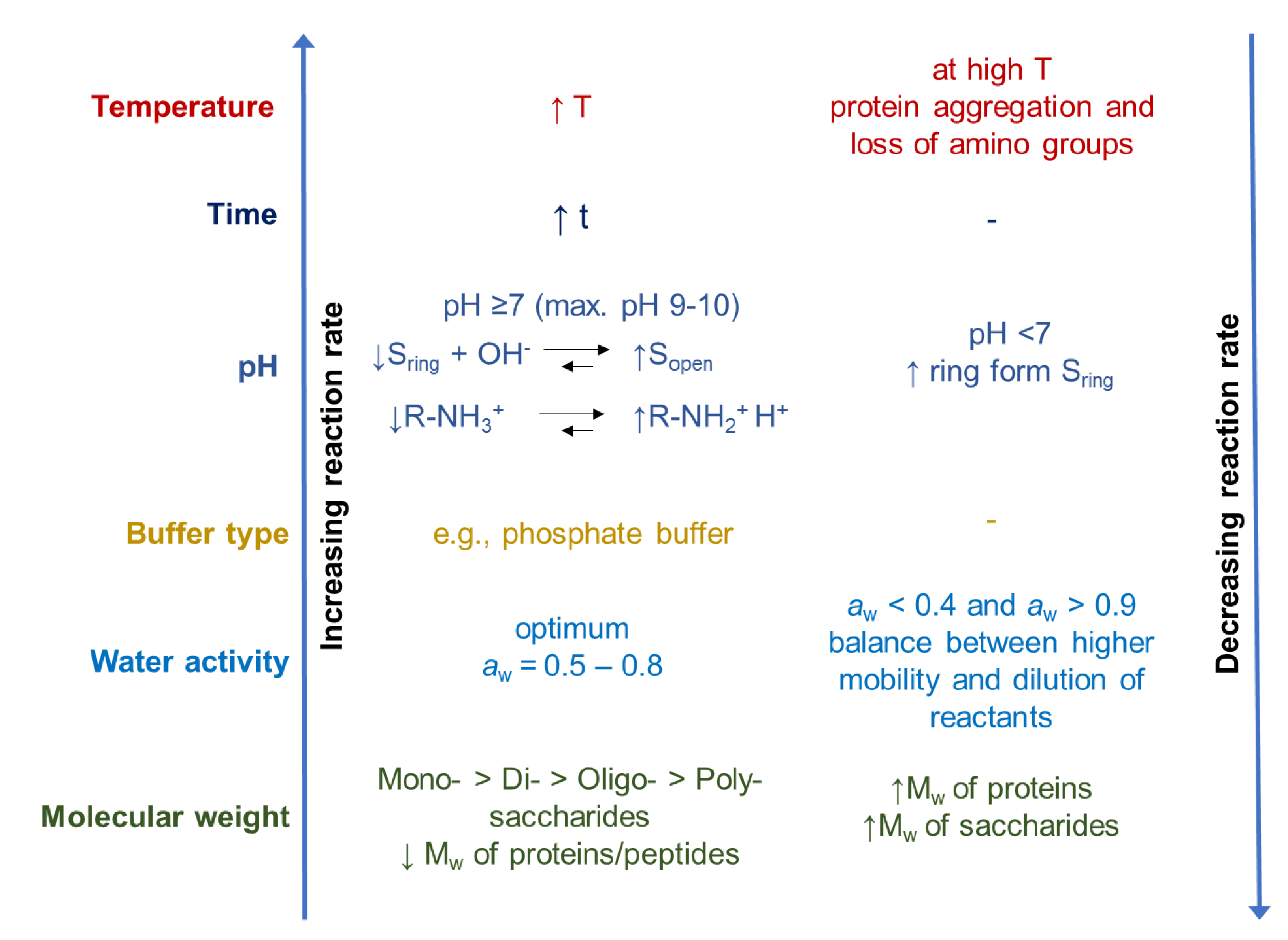
3.2. Influencing Factors
3.3. Manufacturing Techniques
3.3.1. Dry-State Heating
3.3.2. Wet-State Heating
3.3.3. Novel Approaches
4. Glycation of Major Plant Proteins
4.1. Grain Legumes
4.2. Cereal Grains and Pseudocereals
4.3. Oilseeds
4.4. Other
5. Functional Properties and Potential Applications of Glycated Plant Proteins
5.1. Emulsifiers
5.2. Foaming
5.3. Films
5.4. Encapsulation
6. Challenges Related to the Application of Glycated Plant Proteins
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Clean Label Ingredient Market-Growth, Trends, and Forecast (2018–2032); Mordor Intelligence LLP: Hyderabad, India, 2018.
- Alting, A.C.; van de Velde, F. Proteins as clean label ingredients in foods and beverages. In Natural Food Additives, Ingredients and Flavourings, 1st ed.; Baines, D., Seal, R., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 197–210. [Google Scholar]
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Lajolo, F.; Genovese, M.I. Nutritional significance of lectins and enzyme inhibitors from legumes. J. Agric. Food Chem. 2002, 50, 6592–6598. [Google Scholar] [CrossRef]
- Arntfield, S.D.; Murray, E.D. The influence of processing parameters on food protein functionality I. Differential scanning calorimetry as an indicator of protein denaturation. Can. Inst. Food Technol. J. 1981, 14, 289–294. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions—Principles, Practices, and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zhao, C.-B.; Zhang, H.; Xu, X.-Y.; Cao, Y.; Zheng, M.-Z.; Liu, J.-S.; Wu, F. Effect of acetylation and succinylation on physicochemical properties and structural characteristics of oat protein isolate. Process Biochem. 2017, 57, 117–123. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Mirmoghtadaie, L.; Shojaee Aliabadi, S.; Hosseini, S.M. Recent approaches in physical modification of protein functionality. Food Chem. 2016, 199, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating proteins with novel functionality via the Maillard reaction: A review. Crit. Rev. Food. Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef]
- Maillard, L.C. Action des acides aminés sur les sucres; formation des méla-noidines par voie methodique. C. R. Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Dunlap, C.A.; Côté, G.L. β-lactoglobulin-dextran conjugates: Effect of polysaccharide size on emulsion stability. J. Agric. Food Chem. 2005, 53, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Liu, G.; Zheng, W.; He, J.; Zhu, Z.; Lamikanra, O. Heat stability improvement of whey protein isolate via glycation with maltodextrin without control of the relative humidity. RSC Adv. 2016, 6, 41785–41792. [Google Scholar] [CrossRef]
- Wang, Q.; Ismail, B. Effect of Maillard-induced glycosylation on the nutritional quality, solubility, thermal stability and molecular configuration of whey proteinv [sic]. Int. Dairy J. 2012, 25, 112–122. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Physicochemical and emulsifying properties of whey protein isolate (WPI)-dextran conjugates produced in aqueous solution. J. Agric. Food Chem. 2010, 58, 2988–2994. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, J.A.; Drapala, K.P.; Mulcahy, E.M.; Mulvihill, D.M. Controlled glycation of milk proteins and peptides: Functional properties. Int. Dairy J. 2017, 67, 16–34. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Preparation and potential applications of casein-polysaccharide conjugates: A review. J. Sci. Food Agric. 2020, 100, 1852–1859. [Google Scholar] [CrossRef]
- Foegeding, E.A. Food protein functionality—A new model. J. Food Sci. 2015, 80, C2670–C2677. [Google Scholar] [CrossRef]
- Schwenke, K.D. Reflections about the functional potential of legume proteins A review. Food/Nahrung 2001, 45, 377–381. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocoll. 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Fukushima, D. Structures of plant storage proteins and their functions. Food Rev. Int. 1991, 7, 353–381. [Google Scholar] [CrossRef]
- Loveday, S.M. Plant protein ingredients with food functionality potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar] [CrossRef]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Cultivation and downstream processing of microalgae and cyanobacteria to generate protein-based technofunctional food ingredients. Crit. Rev. Food Sci. Nutr. 2019, 60, 2961–2989. [Google Scholar] [CrossRef]
- Kinsella, J.E.; Melachouris, N. Functional properties of proteins in foods: A survey. Crit. Rev. Food Sci. Nutr. 1976, 7, 219–280. [Google Scholar] [CrossRef]
- Walstra, P. Proteins. In Physical Chemistry of Foods, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Zayas, J.F. Functionality of Proteins in Food; Springer: New York, NY, USA, 1997. [Google Scholar]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a molecular understanding of protein solubility: Increased negative surface charge correlates with increased solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef] [Green Version]
- Trevino, S.R.; Scholtz, J.M.; Pace, C.N. Amino acid contribution to protein solubility: Asp, Glu, and Ser contribute more favorably than the other hydrophilic amino acids in RNase Sa. J. Mol. Biol. 2007, 366, 449–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. Food allergen labeling and consumer protection act of 2004 (FALCPA). In Public Law 108-282, Title II; USC 301 Note; FDA: Silver Spring, MD, USA, 2004; Volume 21. [Google Scholar]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836 e822. [Google Scholar] [CrossRef] [Green Version]
- Mills, E.N.; Sancho, A.I.; Rigby, N.M.; Jenkins, J.A.; Mackie, A.R. Impact of food processing on the structural and allergenic properties of food allergens. Mol. Nutr. Food Res. 2009, 53, 963–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Lamsal, B.P.; Jung, S.; Johnson, L.A. Rheological properties of soy protein hydrolysates obtained from limited enzymatic hydrolysis. LWT 2007, 40, 1215–1223. [Google Scholar] [CrossRef]
- Wouters, A.G.B.; Rombouts, I.; Fierens, E.; Brijs, K.; Delcour, J.A. Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Compr. Rev. Food Sci. Food Saf. 2016, 15, 786–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gan, J.; Zhou, Y.; Cheng, Y.; Nirasawa, S. Improving solubility and emulsifying property of wheat gluten by deamidation with four different acids: Effect of replacement of folded conformation by extended structure. Food Hydrocoll. 2017, 72, 105–114. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; O’Cuinn, G. Enzymatic debittering of food protein hydrolysates. Biotechnol. Adv. 2006, 24, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Hamada, J.S. Deamidation of food proteins to improve functionality. Crit. Rev. Food Sci. Nutr. 1994, 34, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-q.; Sontag-Strohm, T.; Salovaara, H.; Sibakov, J.; Kanerva, P.; Loponen, J. Oat protein solubility and emulsion properties improved by enzymatic deamidation. J. Cereal Sci. 2015, 64, 126–132. [Google Scholar] [CrossRef]
- Haque, Z.; Kito, M. Lipophilization of αs1-casein. 2. Conformational and functional effects. J. Agric. Food Chem. 1983, 31, 1231–1237. [Google Scholar] [CrossRef]
- Kito, M. Chemical and physical lipophilization of proteins. JAOCS 1987, 64, 1676–1681. [Google Scholar] [CrossRef]
- Chevalier, F.; Chobert, J.M.; Popineau, Y.; Nicolas, M.G.; Haertlé, T. Improvement of functional properties of β-lactoglobulin glycated through the Maillard reaction is related to the nature of the sugar. Int. Dairy J. 2001, 11, 145–152. [Google Scholar] [CrossRef]
- de Oliveira, F.C.; Coimbra, J.S.; de Oliveira, E.B.; Zuniga, A.D.; Rojas, E.E. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ru, Q.; Ding, Y. Glycation a promising method for food protein modification: Physicochemical properties and structure, a review. Food Res. Int. 2012, 49, 170–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Food browning and its prevention: An overview. J. Agric. Food Chem. 1996, 44, 631–653. [Google Scholar] [CrossRef]
- Jiménez-Castaño, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Kato, A. Industrial applications of Maillard-type protein-polysaccharide conjugates. Food Sci. Technol. Res. 2002, 8, 193–199. [Google Scholar] [CrossRef] [Green Version]
- van Boekel, M.A.J.S. Kinetic aspects of the Maillard reaction: A critical review. Food/Nahrung 2001, 45, 150–159. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; Van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Ames, J.M. The Maillard reaction. In Biochemistry of Food Proteins; Hudson, B.J.F., Ed.; Springer: Boston, MA, USA, 1992; pp. 99–153. [Google Scholar]
- Wrodnigg, T.M.; Eder, B. The Amadori and Heyns rearrangements: Landmarks in the history of carbohydrate chemistry or unrecognized synthetic opportunities. In Glycoscience; Stütz, A.E., Ed.; Springer: Berlin, Germany, 2001; pp. 115–152. [Google Scholar]
- Zhang, Y.; Zhang, Y. Formation and reduction of acrylamide in Maillard reaction: A review based on the current state of knowledge. Crit. Rev. Food Sci. Nutr. 2007, 47, 521–542. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Henle, T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids 2005, 29, 313–322. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, S.J. Review of pentosidine and pyrraline in food and chemical models: Formation, potential risks and determination. J. Sci. Food Agric. 2018, 98, 3225–3233. [Google Scholar] [CrossRef] [PubMed]
- Beksan, E.; Schieberle, P.; Robert, F.; Blank, I.; Fay, L.B.; Schlichtherle-Cerny, H.; Hofmann, T. Synthesis and sensory characterization of novel umami-tasting glutamate glycoconjugates. J. Agric. Food Chem. 2003, 51, 5428–5436. [Google Scholar] [CrossRef]
- Henle, T.; Walter, H.; Klostermeyer, H. Evaluation of the extent of the early Maillard-reaction in milk products by direct measurement of the Amadori-product lactuloselysine. ZLUF 1991, 193, 119–122. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Thorpe, S.R.; Baynes, J.W. Identification of NƐ-carboxymethyllysine as a degradation product of fructoselysine in glycated protein. J. Biol. Chem. 1986, 261, 4889–4894. [Google Scholar] [CrossRef]
- Schaafsma, G. The protein digestibility-corrected amino acid score (PDCAAS)—A concept for describing protein quality in foods and food ingredients: A critical review. J. AOAC Int. 2005, 88, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbersdobler, H.F.; Somoza, V. Forty years of furosine - forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol. Nutr. Food Res. 2007, 51, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Knoll, K.; Henle, T. Studies on the formation of furosine and pyridosine during acid hydrolysis of different Amadori products of lysine. Eur. Food Res. Technol. 2003, 216, 277–283. [Google Scholar] [CrossRef]
- Arena, S.; Salzano, A.M.; Renzone, G.; D’Ambrosio, C.; Scaloni, A. Non-enzymatic glycation and glycoxidation protein products in foods and diseases: An interconnected, complex scenario fully open to innovative proteomic studies. Mass Spectrom. Rev. 2014, 33, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Kiessling, M.; Rother, S.; Henle, T. Quantification of the glycation compound 6-(3-hydroxy-4-oxo-2-methyl-4(1H)-pyridin-1-yl)-l-norleucine (maltosine) in model systems and food samples. Eur. Food Res. Technol. 2016, 242, 547–557. [Google Scholar] [CrossRef]
- Kutzli, I.; Griener, D.; Gibis, M.; Schmid, C.; Dawid, C.; Baier, S.K.; Hofmann, T.; Weiss, J. Influence of Maillard reaction conditions on the formation and solubility of pea protein isolate-maltodextrin conjugates in electrospun fibers. Food Hydrocoll. 2020, 101, 105535. [Google Scholar] [CrossRef]
- Beck, J.; Ledl, F.; Severin, T. Formation of glucosyl-deoxyosones from Amadori compounds of maltose. Z. Lebensm.-Unters. Forsch. 1989, 188, 118–121. [Google Scholar] [CrossRef]
- Nedvidek, W.; Ledl, F.; Fischer, P. Detection of 5-hydroxymethyl-2-methyl-3 (2H)-furanone and of α-dicarbonyl compounds in reaction mixtures of hexoses and pentoses with different amines. Z. Lebensm.-Unters. Forsch. 1992, 194, 222–228. [Google Scholar] [CrossRef]
- Gobert, J.; Glomb, M.A. Degradation of glucose: Reinvestigation of reactive α-dicarbonyl compounds. J. Agric. Food Chem. 2009, 57, 8591–8597. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, Y.V.; Haase, P.T.; Kroh, L.W. Reactivity of thermally treated α-dicarbonyl compounds. J. Agric. Food Chem. 2013, 61, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, T.; Sun, D.-W. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, Y.; Huang, S.; Yao, M.; Lei, Y.; Khan, I.A.; Huang, M.; Zhou, X. Formation of Nϵ-carboxymethyllysine and Nϵ-carboxyethyllysine in prepared chicken breast by pan frying. J. Food Prot. 2019, 82, 2154–2160. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef]
- Soboleva, A.; Vikhnina, M.; Grishina, T.; Frolov, A. Probing protein glycation by chromatography and mass spectrometry: Analysis of glycation adducts. Int. J. Mol. Sci. 2017, 18, 2557. [Google Scholar] [CrossRef] [Green Version]
- Hellwig, M.; Henle, T. Quantification of the Maillard reaction product 6-(2-formyl-1-pyrrolyl)-l-norleucine (formyline) in food. Eur. Food Res. Technol. 2012, 235, 99–106. [Google Scholar] [CrossRef]
- Rabbani, N.; Al-Motawa, M.; Thornalley, P.J. Protein glycation in plants - An under-researched field with much still to discover. Int. J. Mol. Sci. 2020, 21, 3942. [Google Scholar] [CrossRef]
- Gruber, P.; Hofmann, T. Chemoselective synthesis of peptides containing major advanced glycation end-products of lysine and arginine. J. Pept. Res. 2005, 66, 111–124. [Google Scholar] [CrossRef]
- Chobert, J.M.; Gaudin, J.C.; Dalgalarrondo, M.; Haertlé, T. Impact of Maillard type glycation on properties of beta-lactoglobulin. Biotechnol. Adv. 2006, 24, 629–632. [Google Scholar] [CrossRef]
- Chevalier, F.; Chobert, J.-M.; Dalgalarrondo, M.; Choiset, Y.; Haertlé, T. Maillard glycation of β-lactoglobulin induces conformation changes. Food/Nahrung 2002, 46, 58–63. [Google Scholar] [CrossRef]
- Rombouts, I.; Lagrain, B.; Brunnbauer, M.; Koehler, P.; Brijs, K.; Delcour, J.A. Identification of isopeptide bonds in heat-treated wheat gluten peptides. J. Agric. Food Chem. 2011, 59, 1236–1243. [Google Scholar] [CrossRef]
- Gerrard, J.A. Protein–protein crosslinking in food: Methods, consequences, applications. Trends Food Sci Technol 2002, 13, 391–399. [Google Scholar] [CrossRef]
- O’Mahony, J.A.; Drapala, K.P.; Mulcahy, E.M.; Mulvihill, D.M. Whey protein-carbohydrate conjugates. In Whey Proteins, 1st ed.; Deeth, H.C., Bansal, N., Eds.; Academic Press: London, UK, 2019; pp. 249–280. [Google Scholar]
- Mulcahy, E.M.; Park, C.W.; Drake, M.; Mulvihill, D.M.; O’Mahony, J.A. Enhancement of the functional properties of whey protein by conjugation with maltodextrin under dry-heating conditions. Int. J. Dairy Technol. 2018, 71, 216–225. [Google Scholar] [CrossRef]
- Damodaran, S. Amino acids, peptides, and proteins. In Fennema’s Food Chemistry, 5th ed.; Damodaran, S., Parkin, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 235–356. [Google Scholar]
- Bell, L.N. Maillard reaction as influenced by buffer type and concentration. Food Chem. 1997, 59, 143–147. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Moreno, F.J.; Olano, A.; Villamiel, M. Structural characterization of bovine β-lactoglobulin-galactose/tagatose Maillard complexes by electrophoretic, chromatographic, and spectroscopic methods. J. Agric. Food Chem. 2008, 56, 4244–4252. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, R.; Schols, H.A.; Gruppen, H. Effect of saccharide structure and size on the degree of substitution and product dispersity of a-lactalbumin glycated via the Maillard reaction. J. Agric. Food Chem. 2011, 59, 9378–9385. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, E.M.; Mulvihill, D.M.; O’Mahony, J.A. Physicochemical properties of whey protein conjugated with starch hydrolysis products of different dextrose equivalent values. Int. Dairy J. 2016, 53, 20–28. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, J.; Ge, K.; Zhang, H.; Fang, B.; Jiang, L.; Guo, H.; Ding, Q.; Ren, F. Glycation of α-lactalbumin with different size saccharides: Effect on protein structure and antigenicity. Int. Dairy J. 2014, 34, 220–228. [Google Scholar] [CrossRef]
- Martinez-Alvarenga, M.S.; Martinez-Rodriguez, E.Y.; Garcia-Amezquita, L.E.; Olivas, G.I.; Zamudio-Flores, P.B.; Acosta-Muniz, C.H.; Sepulveda, D.R. Effect of Maillard reaction conditions on the degree of glycation and functional properties of whey protein isolate-maltodextrin conjugates. Food Hydrocoll. 2014, 38, 110–118. [Google Scholar] [CrossRef]
- Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Wu, J.; A’Yun, Q.; Van der Meeren, P. Maillard conjugation as an approach to improve whey proteins functionality: A review of conventional and novel preparation techniques. Trends Food Sci. Technol. 2019, 91, 1–11. [Google Scholar] [CrossRef]
- Akhtar, M.; Ding, R. Covalently cross-linked proteins & polysaccharides: Formation, characterisation and potential applications. Curr. Opin. Colloid Interface Sci. 2017, 28, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Sivapratha, S.; Sarkar, P. Multiple layers and conjugate materials for food emulsion stabilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 877–892. [Google Scholar] [CrossRef]
- Dickinson, E.; Galazka, V.B. Emulsion stabilization by ionic and covalent complexes of β-lactoglobulin with polysaccharides. Food Hydrocoll. 1991, 5, 281–296. [Google Scholar] [CrossRef]
- Wooster, T.J.; Augustin, M.A. β-lactoglobulin-dextran Maillard conjugates: Their effect on interfacial thickness and emulsion stability. J. Colloid Interface Sci. 2006, 303, 564–572. [Google Scholar] [CrossRef]
- Xue, J.; Davidson, P.M.; Zhong, Q. Thymol nanoemulsified by whey protein-maltodextrin conjugates: The enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J. Agric. Food Chem. 2013, 61, 12720–12726. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef]
- Hall, D.; Minton, A.P. Macromolecular crowding: Qualitative and semiquantitative successes, quantitative challenges. Biochim. Biophys. Acta Proteins Proteom. 2003, 1649, 127–139. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, J.-R.; Li, K.-K.; Yin, S.-W.; Wang, J.-M.; Zhu, J.-H.; Yang, X.-Q. Characterization of soy β-conglycinin–dextran conjugate prepared by Maillard reaction in crowded liquid system. Food Res. Int. 2012, 49, 648–654. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Wang, W.; Lv, R.; Guo, M.; Ding, T.; Ye, X.; Miao, S.; Liu, D. Preparation of modified whey protein isolate with gum acacia by ultrasound Maillard reaction. Food Hydrocoll. 2019, 95, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.X.; Tu, Z.C.; Yang, W.; Wang, H.; Zhang, L.; Ma, D.; Huang, T.; Liu, J.; Li, X. Investigation into allergenicity reduction and glycation sites of glycated beta-lactoglobulin with ultrasound pretreatment by high-resolution mass spectrometry. Food Chem. 2018, 252, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-W.; Yu, S.-J.; Zeng, X.-A.; Yang, X.-Q.; Jia, X. Properties of whey protein isolate-dextran conjugate prepared using pulsed electric field. Food Res. Int. 2011, 44, 1052–1058. [Google Scholar] [CrossRef]
- Guan, J.-J.; Qiu, A.-Y.; Liu, X.-Y.; Hua, Y.-F.; Ma, Y.-H. Microwave improvement of soy protein isolate-saccharide graft reactions. Food Chem. 2006, 97, 577–585. [Google Scholar] [CrossRef]
- Avila Ruiz, G.; Xi, B.; Minor, M.; Sala, G.; van Boekel, M.; Fogliano, V.; Stieger, M. High-pressure-high-temperature processing reduces Maillard reaction and viscosity in whey protein-sugar solutions. J. Agric. Food Chem. 2016, 64, 7208–7215. [Google Scholar] [CrossRef]
- Koch, L.; Emin, M.A.; Schuchmann, H.P. Influence of processing conditions on the formation of whey protein-citrus pectin conjugates in extrusion. J. Food Eng. 2017, 193, 1–9. [Google Scholar] [CrossRef]
- Koch, L.; Hummel, L.; Schuchmann, H.P.; Emin, M.A. Improving the emulsifying properties of whey protein isolate-citrus pectin blends by a novel reactive extrusion approach. J. Food Eng. 2018, 223, 175–188. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Formation of whey protein isolate (WPI)-maltodextrin conjugates in fibers produced by needleless electrospinning. J. Agric. Food Chem. 2018, 66, 10283–10291. [Google Scholar] [CrossRef]
- Turan, D.; Gibis, M.; Gunes, G.; Baier, S.K.; Weiss, J. The impact of the molecular weight of dextran on formation of whey protein isolate (WPI)–dextran conjugates in fibers produced by needleless electrospinning after annealing. Food Funct. 2018, 9, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Baier, S.; Given, P.; Kanjanapongkul, K.; Weiss, J. Formation of Conjugated Protein by Electrospinning. U.S. Patent 20130264731 A1, 21 June 2016. [Google Scholar]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- L’hocine, L.; Boye, J.I.; Arcand, Y. Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J. Food Sci. 2006, 71, C137–C145. [Google Scholar] [CrossRef]
- Sorgentini, D.A.; Wagner, J.R.; Anon, M.C. Effects of thermal treatment of soy protein isolate on the characteristics and structure-function relationship of soluble and insoluble fractions. J. Agric. Food Chem. 1995, 43, 2471–2479. [Google Scholar] [CrossRef]
- Xu, C.-H.; Yang, X.-Q.; Yu, S.-J.; Qi, J.-R.; Guo, R.; Sun, W.-W.; Yao, Y.-J.; Zhao, M.-M. The effect of glycosylation with dextran chains of differing lengths on the thermal aggregation of β-conglycinin and glycinin. Food Res. Int. 2010, 43, 2270–2276. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. B 2016, 138, 70–77. [Google Scholar] [CrossRef]
- Diftis, N.; Kiosseoglou, V. Stability against heat-induced aggregation of emulsions prepared with a dry-heated soy protein isolate–dextran mixture. Food Hydrocoll. 2006, 20, 787–792. [Google Scholar] [CrossRef]
- Wang, L.; Wu, M.; Liu, H.M. Emulsifying and physicochemical properties of soy hull hemicelluloses-soy protein isolate conjugates. Carbohydr. Polym. 2017, 163, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.-R.; Liao, J.-S.; Yin, S.-W.; Zhu, J.; Yang, X.-Q. Formation of acid-precipitated soy protein-dextran conjugates by Maillard reaction in liquid systems. Int. J. Food Sci. Technol. 2010, 45, 2573–2580. [Google Scholar] [CrossRef]
- Tian, S.; Chen, J.I.E.; Small, D.M. Enhancement of solubility and emulsifying properties of soy protein isolates by glucose conjugation. J. Food Process. Preserv. 2011, 35, 80–95. [Google Scholar] [CrossRef]
- Xu, K.; Yao, P. Stable oil-in-water emulsions prepared from soy protein-dextran conjugates. Langmuir 2009, 25, 9714–9720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J.; Xu, N.; Wang, G.; Wang, X. Influence of protein hydrolysis on the freeze-thaw stability of emulsions prepared with soy protein-dextran conjugates. J. Oleo Sci. 2019, 68, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, S.; Cui, Q.; Li, R.; Wang, X.; Jiang, L. Effect of pH on freeze-thaw stability of glycated soy protein isolate. J. Oleo Sci. 2019, 68, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, A.; Wang, Y.; Wang, X.; Xu, N.; Jiang, L. Effects of irradiation on the structure and properties of glycosylated soybean proteins. Food Funct. 2020, 11, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Diftis, N.; Kiosseoglou, V. Competitive adsorption between a dry-heated soy protein–dextran mixture and surface-active materials in oil-in-water emulsions. Food Hydrocoll. 2004, 18, 639–646. [Google Scholar] [CrossRef]
- Usui, M.; Tamura, H.; Nakamura, K.; Ogawa, T.; Muroshita, M.; Azakami, H.; Kanuma, S.; Kato, A. Enhanced bactericidal action and masking of allergen structure of soy protein by attachment of chitosan through maillard-type protein-polysaccharide conjugation. Food/Nahrung 2004, 48, 69–72. [Google Scholar] [CrossRef]
- Diftis, N.G.; Biliaderis, C.G.; Kiosseoglou, V.D. Rheological properties and stability of model salad dressing emulsions prepared with a dry-heated soybean protein isolate–dextran mixture. Food Hydrocoll. 2005, 19, 1025–1031. [Google Scholar] [CrossRef]
- van de Lagemaat, J.; Manuel Silván, J.; Javier Moreno, F.; Olano, A.; Dolores del Castillo, M. In vitro glycation and antigenicity of soy proteins. Food Res. Int. 2007, 40, 153–160. [Google Scholar] [CrossRef]
- Mesa, M.D.; Silván, J.M.; Olza, J.; Gil, Á.; del Castillo, M.D. Antioxidant properties of soy protein–fructooligosaccharide glycation systems and its hydrolyzates. Food Res. Int. 2008, 41, 606–615. [Google Scholar] [CrossRef]
- Matemu, A.O.; Kayahara, H.; Murasawa, H.; Nakamura, S. Importance of size and charge of carbohydrate chains in the preparation of functional glycoproteins with excellent emulsifying properties from tofu whey. Food Chem. 2009, 114, 1328–1334. [Google Scholar] [CrossRef]
- Qi, J.-R.; Yang, X.-Q.; Liao, J.-S. Improvement of functional properties of acid-precipitated soy protein by the attachment of dextran through Maillard reaction. Int. J. Food Sci. Technol. 2009, 44, 2296–2302. [Google Scholar] [CrossRef]
- Chiu, T.-h.; Chen, M.-L.; Chang, H.-C. Comparisons of emulsifying properties of Maillard reaction products conjugated by green, red seaweeds and various commercial proteins. Food Hydrocoll. 2009, 23, 2270–2277. [Google Scholar] [CrossRef]
- Liu, P.; Huang, M.; Song, S.; Hayat, K.; Zhang, X.; Xia, S.; Jia, C. Sensory characteristics and antioxidant activities of Maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food Bioprocess Technol. 2010, 5, 1775–1789. [Google Scholar] [CrossRef]
- Dou, C.R.; Yu, G.P.; Jiang, L.Z.; Song, Y.; An, S.M. Optimization of glycosylating condition for soy protein isolates and maltodextrin by Maillard reaction. Adv. Mater. Res. 2012, 554–556, 1262–1267. [Google Scholar] [CrossRef]
- Kasran, M.; Cui, S.W.; Goff, H.D. Covalent attachment of fenugreek gum to soy whey protein isolate through natural Maillard reaction for improved emulsion stability. Food Hydrocoll. 2013, 30, 552–558. [Google Scholar] [CrossRef]
- Li, X.R.; Tang, C.H. Influence of glycation on microencapsulating properties of soy protein isolate-lactose blends. J. Sci. Food Agric. 2013, 93, 2715–2722. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Zhu, X.; Wang, L.; Pan, S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Res. Int. 2013, 51, 490–495. [Google Scholar] [CrossRef]
- Zhuo, X.Y.; Qi, J.R.; Yin, S.W.; Yang, X.Q.; Zhu, J.H.; Huang, L.X. Formation of soy protein isolate-dextran conjugates by moderate Maillard reaction in macromolecular crowding conditions. J. Sci. Food Agric. 2013, 93, 316–323. [Google Scholar] [CrossRef]
- Li, B.; Bao, Z.; Xu, W.; Chi, Y. Influence of glycation extent on the physicochemical and gelling properties of soybean β-conglycinin. Eur. Food Res. Technol. 2014, 240, 399–411. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Zhang, X.; Xia, S.; Jia, C.; Eric, K.; Abbas, S.; Feng, B.; Zhong, F. Effects of maltodextrin glycosylation following limited enzymatic hydrolysis on the functional and conformational properties of soybean protein isolate. Eur. Food Res. Technol. 2014, 238, 957–968. [Google Scholar] [CrossRef]
- Bu, G.; Zhu, T.; Chen, F.; Zhang, N.; Liu, K.; Zhang, L.; Yang, H. Effects of saccharide on the structure and antigenicity of β-conglycinin in soybean protein isolate by glycation. Eur. Food Res. Technol. 2014, 240, 285–293. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, N.; Lan, T.; Yang, X. Improvement in emulsifying properties of soy protein isolate by conjugation with maltodextrin using high-temperature, short-time dry-heating Maillard reaction. Int. J. Food Sci. Technol. 2014, 49, 460–467. [Google Scholar] [CrossRef]
- Li, R.; Hettiarachchy, N.; Rayaprolu, S.; Davis, M.; Eswaranandam, S.; Jha, A.; Chen, P. Improved functional properties of glycosylated soy protein isolate using D-glucose and xanthan gum. J. Food Sci. Technol. 2015, 52, 6067–6072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.; Gong, J.; Miller, S.S.; Wang, Q.; Hua, Y. Stability of citral in oil-in-water emulsions protected by a soy protein–polysaccharide Maillard reaction product. Food Res. Int. 2015, 69, 357–363. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Shi, J.; Huang, X.; Peng, Q.; Xue, F. Encapsulation of tomato oleoresin using soy protein isolate-gum aracia conjugates as emulsifier and coating materials. Food Hydrocoll. 2015, 45, 301–308. [Google Scholar] [CrossRef]
- Zhao, C.B.; Zhou, L.Y.; Liu, J.Y.; Zhang, Y.; Chen, Y.; Wu, F. Effect of ultrasonic pretreatment on physicochemical characteristics and rheological properties of soy protein/sugar Maillard reaction products. J. Food Sci. Technol. 2016, 53, 2342–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, G.; Zhu, T.; Chen, F. The structural properties and antigenicity of soybean glycinin by glycation with xylose. J. Sci. Food Agric. 2017, 97, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Shekarforoush, E.; Mirhosseini, H.; Sarker, M.Z.I.; Kostadinović, S.; Ghazali, H.M.; Muhamad, K.; Samaram, S. Soy protein-gum karaya conjugate: Emulsifying activity and rheological behavior in aqueous system and oil in water emulsion. J. Am. Oil Chem. Soc. 2015, 93, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xiong, Y.L. Oxidative polyaldehyde production: A novel approach to the conjugation of dextran with soy peptides for improved emulsifying properties. J. Food Sci. Technol. 2016, 53, 3215–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.; Greenberg, Y.; Sriramarao, P.; Ismail, B.P. Limited hydrolysis combined with controlled Maillard-induced glycation does not reduce immunoreactivity of soy protein for all sera tested. Food Chem. 2016, 213, 742–752. [Google Scholar] [CrossRef]
- Boostani, S.; Aminlari, M.; Moosavi-Nasab, M.; Niakosari, M.; Mesbahi, G. Fabrication and characterisation of soy protein isolate-grafted dextran biopolymer: A novel ingredient in spray-dried soy beverage formulation. Int. J. Biol. Macromol. 2017, 102, 297–307. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Yu, J.; Chen, S.; Ge, H.; Jiang, L. Freeze-thaw stability of oil-in-water emulsions stabilized by soy protein isolate-dextran conjugates. LWT 2017, 78, 241–249. [Google Scholar] [CrossRef]
- Mao, L.; Pan, Q.; Hou, Z.; Yuan, F.; Gao, Y. Development of soy protein isolate-carrageenan conjugates through Maillard reaction for the microencapsulation of Bifidobacterium longum. Food Hydrocoll. 2018, 84, 489–497. [Google Scholar] [CrossRef]
- Wang, L.-H.; Sun, X.; Huang, G.-Q.; Xiao, J.-X. Conjugation of soybean protein isolate with xylose/fructose through wet-heating Maillard reaction. J. Food Meas. Charact. 2018, 12, 2718–2724. [Google Scholar] [CrossRef]
- Yu, J.; Wang, G.; Wang, X.; Xu, Y.; Chen, S.; Wang, X.; Jiang, L. Improving the freeze-thaw stability of soy protein emulsions via combing limited hydrolysis and Maillard-induced glycation. LWT 2018, 91, 63–69. [Google Scholar] [CrossRef]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Huang, G.Q.; Xu, T.C.; Liu, L.N.; Xiao, J.X. Comparative study on the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int. J. Biol. Macromol. 2019, 131, 601–607. [Google Scholar] [CrossRef]
- Zhang, A.; Yu, J.; Wang, G.; Wang, X.; Zhang, L. Improving the emulsion freeze-thaw stability of soy protein hydrolysate-dextran conjugates. LWT 2019, 116, 108506. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, X.H. Structure and property changes of the soy protein isolate glycated with maltose in an ionic liquid through the Maillard reaction. Food Funct. 2019, 10, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Liu, J.; Cui, Q.; Wang, X.; Chen, S.; Jiang, L. Relationship between molecular flexibility and emulsifying properties of soy protein isolate-glucose conjugates. J. Agric. Food Chem. 2019, 67, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hou, F.; Zhao, H.; Wang, D.; Chen, W.; Miao, S.; Liu, D. Conjugation of soy protein isolate (SPI) with pectin by ultrasound treatment. Food Hydrocoll. 2020, 108, 106056. [Google Scholar] [CrossRef]
- Ma, X.; Chen, W.; Yan, T.; Wang, D.; Hou, F.; Miao, S.; Liu, D. Comparison of citrus pectin and apple pectin in conjugation with soy protein isolate (SPI) under controlled dry-heating conditions. Food Chem. 2020, 309, 125501. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, L.; Wang, Y.; Li, Z.; Wang, Z.; Han, J. Effect of high hydrostatic pressure on solubility and conformation changes of soybean protein isolate glycated with flaxseed gum. Food Chem. 2020, 333, 127530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Teng, F.; Tian, T.; Sami, R.; Wu, C.; Zhu, Y.; Zheng, L.; Jiang, L.; Wang, Z.; Li, Y. The impact of soy protein isolate-dextran conjugation on capsicum oleoresin (Capsicum annuum L.) nanoemulsions. Food Hydrocoll. 2020, 108, 105818. [Google Scholar] [CrossRef]
- Palupi, N.S.; Prangdimurti, E.; Faridah, D.N.; Asyhari, M.H. Reducing allergenicity of soy protein isolate from several varieties of soybean through glycation with lactose. Curr. Res. Nutr. Food Sci. 2020, 8, 268–280. [Google Scholar] [CrossRef]
- Feng, J.; Berton-Carabin, C.C.; Ataç Mogol, B.; Schroën, K.; Fogliano, V. Glycation of soy proteins leads to a range of fractions with various supramolecular assemblies and surface activities. Food Chem. 2020, 343, 128556. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Wang, X.; Xu, N.; Jiang, L. The radiation assisted-Maillard reaction comprehensively improves the freeze-thaw stability of soy protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2020, 103, 105684. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Zhao, G. Fabricating a Pickering stabilizer from okara dietary fibre particulates by conjugating with soy protein isolate via Maillard reaction. Foods 2020, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Yin, H.; Yan, J.; Niu, X.; Qi, B.; Liu, J. Structure and acid-induced gelation properties of soy protein isolate–maltodextrin glycation conjugates with ultrasonic pretreatment. Food Hydrocoll. 2021, 112, 106278. [Google Scholar] [CrossRef]
- Marciniak-Darmochwal, K.; Kostyra, H. Influence of nonenzymatic glycosylation (glycation) of pea proteins (Pisum sativum) on their susceptibility to enzymatic hydrolysis. J. Food Biochem. 2009, 33, 506–521. [Google Scholar] [CrossRef]
- Swiatecka, D.; Narbad, A.; Ridgway, K.P.; Kostyra, H. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. Pea protein isolate-gum arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef]
- Zha, F.; Yang, Z.; Rao, J.; Chen, B. Gum arabic-mediated synthesis of glyco-pea protein hydrolysate via Maillard reaction improves solubility, flavor profile, and functionality of plant protein. J. Agric. Food Chem. 2019, 67, 10195–10206. [Google Scholar] [CrossRef]
- Kutzli, I.; Griener, D.; Gibis, M.; Grossmann, L.; Baier, S.K.; Weiss, J. Improvement of emulsifying behavior of pea proteins as plant-based emulsifiers via Maillard-induced glycation in electrospun pea protein-maltodextrin fibers. Food Funct. 2020, 11, 4049–4056. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The structural modification of pea protein concentrate with gum arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Zha, F.; Yang, Z.; Rao, J.; Chen, B. Conjugation of pea protein isolate via Maillard-driven chemistry with saccharide of diverse molecular mass: Molecular interactions leading to aggregation or glycation. J. Agric. Food Chem. 2020, 68, 10157–10166. [Google Scholar] [CrossRef]
- Caballero, S.; Davidov-Pardo, G. Comparison of legume and dairy proteins for the impact of Maillard conjugation on nanoemulsion formation, stability, and lutein color retention. Food Chem. 2021, 338, 128083. [Google Scholar] [CrossRef]
- Tang, C.H.; Sun, X.; Foegeding, E.A. Modulation of physicochemical and conformational properties of kidney bean vicilin (phaseolin) by glycation with glucose: Implications for structure-function relationships of legume vicilins. J. Agric. Food Chem. 2011, 59, 10114–10123. [Google Scholar] [CrossRef]
- Arogundade, L.A.; Eromosele, C.O.; Eromosele, I.C.; Ademuyiwa, O. Rheological characterization of acylated and dextran conjugated African yam bean (Sphenostylis stenocarpa Hochst. Ex A. Rich.) proteins. LWT 2012, 45, 58–64. [Google Scholar] [CrossRef]
- Campbell, L.; Euston, S.R.; Ahmed, M.A. Effect of addition of thermally modified cowpea protein on sensory acceptability and textural properties of wheat bread and sponge cake. Food Chem. 2016, 194, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, F.; Sui, X.; Qi, B.; Yang, Y.; Zhang, H.; Wang, R.; Li, Y.; Jiang, L. Effect of ultrasound treatment on the wet heating Maillard reaction between mung bean [Vigna radiate (L.)] protein isolates and glucose and on structural and physico-chemical properties of conjugates. J. Sci. Food Agric. 2016, 96, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wu, F.; Zhang, X.; Wang, Z. Structural and functional properties of Maillard reaction products of protein isolate (mung bean, Vigna radiate (L.)) with dextran. Int. J. Food Prop. 2017, 20, 1246–1258. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Pitkanen, L.; Maina, N.H.; Coda, R.; Katina, K.; Tenkanen, M. Interactions between fava bean protein and dextrans produced by Leuconostoc pseudomesenteroides DSM 20193 and Weissella cibaria Sj 1b. Carbohydr. Polym. 2018, 190, 315–323. [Google Scholar] [CrossRef]
- Xu, J.; Han, D.; Chen, Z.; Li, M.; Jin, H. Effect of glucose glycosylation following limited enzymatic hydrolysis on functional and conformational properties of black bean protein isolate. Eur. Food Res. Technol. 2018, 244, 1111–1120. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, Q.; Feng, H.; Wang, Y.; Wang, J.; Liu, Y.; Han, D.; Xu, J. Changes on the structural and physicochemical properties of conjugates prepared by the Maillard reaction of black bean protein isolates and glucose with ultrasound pretreatment. Polymers 2019, 11, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavi, F.; Chen, L.; Wang, Z.; Emam-Djomeh, Z. Consequences of heating under alkaline pH alone or in the presence of maltodextrin on solubility, emulsifying and foaming properties of faba bean protein. Food Hydrocoll. 2021, 112, 106335. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Champagne, T. Allergenicity of Maillard reaction products from peanut proteins. J. Agric. Food Chem. 1999, 47, 5227–5231. [Google Scholar] [CrossRef] [PubMed]
- Wellner, A.; Nußpickel, L.; Henle, T. Glycation compounds in peanuts. Eur. Food Res. Technol. 2011, 234, 423–429. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Zhao, M.; Ren, J.; Yang, B. Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem. 2012, 131, 901–906. [Google Scholar] [CrossRef]
- Li, C.; Zhu, B.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Physicochemical properties of dry-heated peanut protein isolate conjugated with cextran or gum arabic. J. Am. Oil Chem. Soc. 2013, 90, 1801–1807. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Peng, Q.; Shan, Y.; Xue, F. Physicochemical properties of peanut protein isolate-glucomannan conjugates prepared by ultrasonic treatment. Ultrason. Sonochem. 2014, 21, 1722–1727. [Google Scholar] [CrossRef]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Lin, W.J.; Liu, H.Z.; Shi, A.M.; Liu, L.; Wang, Q.; Adhikari, B. Effect of glycosylation with xylose on the mechanical properties and water solubility of peanut protein films. J. Food Sci. Technol. 2015, 52, 6242–6253. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, J.; Wu, K.; Yu, L. Improved low pH emulsification properties of glycated peanut protein isolate by ultrasound Maillard reaction. J. Agric. Food Chem. 2016, 64, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lin, W.-J.; Liu, H.-Z.; Shi, A.-M.; Hu, H.; Nasir, M.N.; Deleu, M.; Wang, Q. Effect of xylose on the structural and physicochemical properties of peanut isolated protein based films. RSC Adv. 2017, 7, 52357–52365. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sun-Waterhouse, D.; Feng, Y.; Su, G.; Zhao, M.; Lin, L. The umami intensity enhancement of peanut protein isolate hydrolysate and its derived factions and peptides by Maillard reaction and the analysis of peptide (EP) Maillard products. Food Res. Int. 2019, 120, 895–903. [Google Scholar] [CrossRef]
- Ji, H.; Tang, X.; Li, L.; Peng, S.; Gao, C.; Chen, Y. Improved physicochemical properties of peanut protein isolate glycated by atmospheric pressure cold plasma (ACP) treatment. Food Hydrocoll. 2020, 109, 106124. [Google Scholar] [CrossRef]
- Yu, J.-J.; Ji, H.; Chen, Y.; Yi-Fu, Z.; Zhen, X.-C.; Shu-Hong, L.; Chen, Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Int. J. Biol. Macromol. 2020, 158, 1194–1203. [Google Scholar] [CrossRef]
- Gupta, R.K.; Raghav, A.; Sharma, A.; Gupta, K.; Neelabh; Mandal, P.; Tripathi, A.; Ansari, I.A.; Das, M.; Dwivedi, P.D. Glycation of clinically relevant chickpea allergen attenuates its allergic immune response in Balb/c mice. Food Chem. 2017, 235, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Legumes. In Encyclopedia of Human Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 3, pp. 74–79. [Google Scholar]
- Gharsallaoui, A.; Saurel, R.; Chambin, O.; Voilley, A. Pea (Pisum sativum, L.) protein isolate stabilized emulsions: A novel system for microencapsulation of lipophilic ingredients by spray drying. Food Bioprocess Technol. 2011, 5, 2211–2221. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Singh, B.; Singh, U. Peanut as a source of protein for human foods. Plant Foods Hum. Nutr. 1991, 41, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Shi, A.; Liu, L.; Wu, H.; Ma, T.; He, X.; Lin, W.; Feng, X.; Liu, Y. Peanut processing technology. In Peanuts: Processing Technology and Product Development; Wang, Q., Ed.; Academic Press: Beijing, China, 2016; pp. 84–207. [Google Scholar]
- Wu, H.; Wang, Q.; Ma, T.; Ren, J. Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res. Int. 2009, 42, 343–348. [Google Scholar] [CrossRef]
- Ahmed, E.M.; Schmidt, R.H. Functional properties of peanut and soybean proteins as influenced by processing method. Peanut Sci. 1979, 6, 1–6. [Google Scholar] [CrossRef]
- Gruber, P.; Becker, W.-M.; Hofmann, T. Influence of the Maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. J. Agric. Food Chem. 2005, 53, 2289–2296. [Google Scholar] [CrossRef]
- Burks, A.W.; Williams, L.W.; Thresher, W.; Connaughton, C.; Corckrell, G.; Helm, R.M. Allergenicity of peanut and soybean extracts altered by chemical or thermal denaturation in patients with atopic dermatitis and positive food challenges. J. Allergy Clin. Immunol. 1992, 90, 889–897. [Google Scholar] [CrossRef]
- Gil, A.M. Techniques for analysing wheat proteins. In Breadmaking, 2nd ed.; Cauvain, S.P., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 77–99. [Google Scholar]
- Price, R.K.; Welch, R.W. Cereal grains. In Encyclopedia of Human Nutrition, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 307–316. [Google Scholar]
- Shewry, P.R.; Casey, R. Seed proteins. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 1–10. [Google Scholar]
- Capouchová, I.; Petr, J.; Tlaskalová-Hogenová, H.; Michalík, I.; Faměra, O.; Urminská, D.; Tučková, L.; Knoblochová, H.; Borovská, D. Protein fractions of oats and possibilities of oat utilisation for patients with coeliac disease. Czech J. Food Sci. 2004, 22, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Juhász, A.; Békés, F.; Wrigley, C.W. Wheat proteins. In Applied Food Protein Chemistry, 1st ed.; Ustunol, Z., Ed.; Wiley-Blackwell: West Sussex, UK, 2014; pp. 219–304. [Google Scholar]
- Mejri, M.; Roge, B.; Bensouissi, A.; Michels, F.; Mathlouthi, M. Effects of some additives on wheat gluten solubility: A structural approach. Food Chem. 2005, 92, 7–15. [Google Scholar] [CrossRef]
- Malalgoda, M.; Simsek, S. Celiac disease and cereal proteins. Food Hydrocoll. 2017, 68, 108–113. [Google Scholar] [CrossRef]
- Jianghe, L.I.; Ruiqi, Q.I.N.; Yongling, S.; Ruolan, W.; Shaoming, Y. Improvement of foaming and emulsifying properties of gluten by conjugation with fructose through Maillard reaction. Grain Oil Sci. Technol. 2019, 1, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.-Y.; Jiang, S.-T.; Pan, L.-J.; Zhai, Y.-S. Characteristics and functional properties of wheat germ protein glycated with saccharides through Maillard reaction. Int. J. Food Sci. Technol. 2011, 46, 2197–2203. [Google Scholar] [CrossRef]
- Wong, B.T.; Day, L.; Augustin, M.A. Deamidated wheat protein–dextran Maillard conjugates: Effect of size and location of polysaccharide conjugated on steric stabilization of emulsions at acidic pH. Food Hydrocoll. 2011, 25, 1424–1432. [Google Scholar] [CrossRef]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food proteins from plants and fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Tang, W.-J.; Xu, Z.; Wen, L.; Chen, M.-L. Structure and functional properties of rice protein-dextran conjugates prepared by the Maillard reaction. Int. J. Food Sci. Technol. 2018, 53, 372–380. [Google Scholar] [CrossRef]
- Du, Y.; Shi, S.; Jiang, Y.; Xiong, H.; Woo, M.W.; Zhao, Q.; Bai, C.; Zhou, Q.; Sun, W. Physicochemical properties and emulsion stabilization of rice dreg glutelin conjugated with kappa-carrageenan through Maillard reaction. J. Sci. Food Agric. 2013, 93, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Wong, B.T.; Day, L.; McNaughton, D.; Augustin, M.A. The effect of Maillard conjugation of deamidated wheat proteins with low molecular weight carbohydrates on the secondary structure of the protein. Food Biophys. 2008, 4, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, J.; Li, Y.; Nirasawa, S.; Cheng, Y. Conformation and emulsifying properties of deamidated wheat gluten-maltodextrin/citrus pectin conjugates and their abilities to stabilize β-carotene emulsions. Food Hydrocoll. 2019, 87, 129–141. [Google Scholar] [CrossRef]
- Paraman, I.; Hettiarachchy, N.S.; Schaefer, C. Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chem. 2007, 84, 593–599. [Google Scholar] [CrossRef]
- Li, Y.; Lu, F.; Luo, C.; Chen, Z.; Mao, J.; Shoemaker, C.; Zhong, F. Functional properties of the Maillard reaction products of rice protein with sugar. Food Chem. 2009, 117, 69–74. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.; Zhu, K.; Peng, W.; Zhou, H. Improvement of emulsifying properties of oat protein isolate-dextran conjugates by glycation. Carbohydr. Polym. 2015, 127, 168–175. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food Hydrocoll. 2019, 87, 459–469. [Google Scholar] [CrossRef]
- Hassan, A.B.; Osman, G.A.; Babiker, E.E. Effect of chymotrypsin digestion followed by polysaccharide conjugation or transglutaminase treatment on functional properties of millet proteins. Food Chem. 2007, 102, 257–262. [Google Scholar] [CrossRef]
- Guo, X.; Xiong, Y.L. Characteristics and functional properties of buckwheat protein–sugar Schiff base complexes. LWT 2013, 51, 397–404. [Google Scholar] [CrossRef]
- Vaštag, Ž.; Popović, L.; Popović, S.; Krimer, V.; Peričin, D. Production of enzymatic hydrolysates with antioxidant and angiotensin-I converting enzyme inhibitory activity from pumpkin oil cake protein isolate. Food Chem. 2011, 124, 1316–1321. [Google Scholar] [CrossRef]
- Prakash, V.; Rao, M.S. Physicochemical properties of oilseed proteins. Crit. Rev. Biochem. Mol. Biol. 1986, 20, 265–363. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond oil: Press cakes and meals supplying global protein requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Canola proteins for human consumption: Extraction, profile, and functional properties. J. Food Sci. 2011, 76, R16–R28. [Google Scholar] [CrossRef] [Green Version]
- González-Pérez, S.; Vereijken, J.M. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S.; Jasniewski, J. Effect of glycosylation with gum arabic by Maillard reaction in a liquid system on the emulsifying properties of canola protein isolate. Carbohydr. Polym. 2017, 157, 1620–1627. [Google Scholar] [CrossRef]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S.; Jasniewski, J. Structural properties of canola protein isolate-gum arabic Maillard conjugate in an aqueous model system. Food Hydrocoll. 2018, 79, 228–234. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Chen, W.; Wang, Z.; He, R.; Ma, H. Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason. Sonochem. 2018, 42, 250–259. [Google Scholar] [CrossRef]
- Qin, Z.; Han, Y.F.; Wang, N.N.; Liu, H.M.; Zheng, Y.Z.; Wang, X.D. Improvement of the oxidative stability of cold-pressed sesame oil using products from the Maillard reaction of sesame enzymatically hydrolyzed protein and reducing sugars. J. Sci. Food Agric. 2020, 100, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Eric, K.; Raymond, L.V.; Huang, M.; Cheserek, M.J.; Hayat, K.; Savio, N.D.; Amédée, M.; Zhang, X. Sensory attributes and antioxidant capacity of Maillard reaction products derived from xylose, cysteine and sunflower protein hydrolysate model system. Food Res. Int. 2013, 54, 1437–1447. [Google Scholar] [CrossRef]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S. Preparation of chemically modified canola protein isolate with gum arabic by means of Maillard reaction under wet-heating conditions. Carbohydr. Polym. 2017, 155, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zhang, X.; Han, X.; Wang, Z.; He, R.; Ma, H. Structure and functional characteristics of rapeseed protein isolate-dextran conjugates. Food Hydrocoll. 2018, 82, 329–337. [Google Scholar] [CrossRef]
- Saatchi, A.; Kiani, H.; Labbafi, M. A new functional proteinpolysaccharide conjugate based on protein concentrate from sesame processing by-products: Functional and physico-chemical properties. Int. J. Biol. Macromol. 2019, 122, 659–666. [Google Scholar] [CrossRef]
- Seo, S.; Karboune, S.; Archelas, A. Production and characterisation of potato patatin-galactose, galactooligosaccharides, and galactan conjugates of great potential as functional ingredients. Food Chem. 2014, 158, 480–489. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.; Chen, P. Characteristics and functionality enhancement by glycosylation of bitter melon (Momordica charantia) seed protein. J. Food Sci. 2014, 79, C2215–C2221. [Google Scholar] [CrossRef]
- Ullah, S.F.; Khan, N.M.; Ali, F.; Ahmad, S.; Khan, Z.U.; Rehman, N.; Jan, A.K.; Muhammad, N. Effects of Maillard reaction on physicochemical and functional properties of walnut protein isolate. Food Sci. Biotechnol. 2019, 28, 1391–1399. [Google Scholar] [CrossRef]
- Seo, S.; L’Hocine, L.; Karboune, S. Allergenicity of potato proteins and of their conjugates with galactose, galactooligosaccharides, and galactan in native, heated, and digested forms. J. Agric. Food Chem. 2014, 62, 3591–3598. [Google Scholar] [CrossRef]
- Seo, S.; Karboune, S. Investigation of the use of Maillard reaction inhibitors for the production of patatin-carbohydrate conjugates. J. Agric. Food Chem. 2014, 62, 12235–12243. [Google Scholar] [CrossRef]
- Han, M.M.; Yi, Y.; Wang, H.X.; Huang, F. Investigation of the Maillard reaction between polysaccharides and proteins from longan pulp and the improvement in activities. Molecules 2017, 22, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahsiri, Z.; Mirzaei, H.; Hosseini, S.M.H.; Khalesi, M. Gum arabic improves the mechanical properties of wild almond protein film. Carbohydr. Polym. 2019, 222, 114994. [Google Scholar] [CrossRef] [PubMed]
- Kasran, M.; Cui, S.W.; Goff, H.D. Emulsifying properties of soy whey protein isolate-fenugreek gum conjugates in oil-in-water emulsion model system. Food Hydrocoll. 2013, 30, 691–697. [Google Scholar] [CrossRef]
- Delahaije, R.J.; Gruppen, H.; van Nieuwenhuijzen, N.H.; Giuseppin, M.L.; Wierenga, P.A. Effect of glycation on the flocculation behavior of protein-stabilized oil-in-water emulsions. Langmuir 2013, 29, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Dickinson, E. Emulsifying properties of whey protein-dextran conjugates at low pH and different salt concentrations. Colloids Surf. B 2003, 31, 125–132. [Google Scholar] [CrossRef]
- Fechner, A.; Knoth, A.; Scherze, I.; Muschiolik, G. Stability and release properties of double-emulsions stabilised by caseinate-dextran conjugates. Food Hydrocoll. 2007, 21, 943–952. [Google Scholar] [CrossRef]
- Sagis, L.M.C.; Scholten, E. Complex interfaces in food: Structure and mechanical properties. Trends Food Sci. Technol. 2014, 37, 59–71. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Q. Properties of whey protein-maltodextrin conjugates as impacted by powder acidity during the Maillard reaction. Food Hydrocoll. 2014, 38, 85–94. [Google Scholar] [CrossRef]
- Halling, P.J. Protein-stabilized foams and emulsions. Crit. Rev. Food Sci. Nutr. 1981, 15, 155–203. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Miñones Conde, J.; Rodríguez Patino, J.M. The effect of enzymatic treatment of a sunflower protein isolate on the rate of adsorption at the air–water interface. J. Food Eng. 2007, 78, 1001–1009. [Google Scholar] [CrossRef]
- Benbettaieb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.E.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: Bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Jiang, Q.; Yang, Y. Preparation and properties of peanut protein films crosslinked with citric acid. Ind. Crops Prod. 2012, 39, 26–30. [Google Scholar] [CrossRef]
- Li, C.; Zhu, W.; Xue, H.; Chen, Z.; Chen, Y.; Wang, X. Physical and structural properties of peanut protein isolate-gum arabic films prepared by various glycation time. Food Hydrocoll. 2015, 43, 322–328. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Zheng, X.; Jiang, L.; Lv, X.; Shi, Y.; Li, L. Effect of glycosylation on the mechanical properties of edible soy protein packaging film. Eur. Food Res. Technol. 2014, 238, 1049–1055. [Google Scholar] [CrossRef]
- Trojanowska, A.; Nogalska, A.; Valls, R.G.; Giamberini, M.; Tylkowski, B. Technological solutions for encapsulation. Phys. Sci. Rev. 2017, 2. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
| Protein | Carbohydrate | Ratio w/w (If Not Stated Otherwise) | Solvent | Manufacturing Technique | Heating Parameters | Functionality | Ref. |
|---|---|---|---|---|---|---|---|
| Soy | |||||||
| soy protein isolate | dextran (144 kD) | 1:1 | water | dry | 60 °C, 1 week, over KBr solution | Increased emulsion stability | [131] |
| acid precipitated soy protein | chitosan (3–30 kDa) | 1:1 | water | dry | 60 °C, 0–14 days, 65% RH | Increased antimicrobial activity and emulsifying properties; decreased allergenicity | [132] |
| soy protein isolate | dextran (144 kDa) | 1:1 | distilled water | dry | 60 °C, 1 and 3 weeks, 81% RH | Increased gel-like rheological behavior and emulsion stability against coalescence and creaming | [133] |
| soy protein isolate | dextran (188 kDa) | 1:1 | water | dry | 60 °C, 1 week, over KBr solution | Increased emulsion stability after prolonged heating | [121] |
| soy protein isolate | fructooligosaccharides (180–1260 kDa) | dry: 1:1; wet: 1:4/14/30/52/74 molar ratio of NH2 to carbohydrate | dry: demineralized water, pH 7.4; wet: 0.5 M phosphate buffer, pH 7.4 | dry and wet | dry: 60 °C, 0–19 days, 65% RH; wet: 95 °C, 0–5 h | Decreased antigenicity | [134] |
| soy protein isolate | fructooligosaccharides | 1:52 molar ratio of NH2 to carbohydrate | 0.5 M phosphate buffer, pH 7.4 | wet | 95 °C, 1 h | Glycation did not change antioxidant activity of soy protein | [135] |
| tofu whey, acid precipitated soy protein, glycinin, β-conglycinin | galactomannan, okara polysaccharides, xyloglucan, chitin, chitosan oligosaccharides | 1:1 | 0.5 mM phosphate buffer and distilled water, pH 7 | dry | 60 °C, 7 days, 65% RH | Increased emulsification activity and emulsion stability; high or low oil/water binding capacities depending on carbohydrate | [136] |
| acid soluble soy protein | dextran (62 kDa) | 1:0.5/1/2/3/6/9/12 | water, pH 8.5 | dry | 60 °C, 3–144 h, 79% RH | Increased hydrophilicity and emulsifying properties (against pH changes, heat treatment, and long-term storage) | [125] |
| acid precipitated soy protein | dextran (60–90 kDa) | 1:1 | water | dry | 50–90 °C, 1–7 days, 79% RH | Increased heat stability and emulsifying properties; no change of solubility but maintained after heat treatment | [137] |
| soy protein isolate and concentrate | green and red seaweed polysaccharides | 1:1 | water | wet | 60 °C, 24 h, over KBr solution, afterwards 55 °C, 6 h in oven | Increased water absorption capacities, emulsifying properties, and foaming properties; decreased oil absorption capacities | [138] |
| glycinin, β-conglycinin | dextran (67, 150, 500 kDa) | 1:1 molar ratio | distilled water | dry | 60 °C, 1 week, over KBr solution | Increased stability against thermal aggregation at various pH or ionic strength values | [119] |
| acid precipitated soy protein | dextran (60–90 kDa) | 1:1 | distilled water, ethanol addition for wet heating | dry and wet | dry: 60 °C, 1–7 days, 79% RH; wet: 50 and 60 °C, 6 and 24 h | Increased solubility, heat stability and emulsifying properties (against heat treatment and ionic stress), ethanol enhanced glycation | [123] |
| soy protein isolate | glucose | 4/2/1/0.5:1 | distilled water, pH 8 | dry | 50 °C, 6 h, 1–14 days, 65% | Increased solubility (at various pH, heat treatment, and ionic stress), and emulsifying properties (against various pH, ionic stress, and heat treatment) | [124] |
| soy protein hydrolysate peptide fractions | xylose | 1:1.6 | distilled water, pH 7.4 | wet | 120 °C, 2 h | Increased antioxidant activities and flavor (increased caramel-like, soy sauce-like, umami, and mouthful taste, reduced bitterness) | [139] |
| β-conglycinin | dextran (67 kDa) | 1:0.5/1/2/3/4 | deionized water, pH 7 | wet | 95 °C, 0–6 h | Increased solubility, macromolecular crowding conditions prompt glycation and prevent thermal aggregation | [102] |
| soy protein isolate | maltodextrin (DE-7, 9–12, 13–17) | 1:0.5/1/2/3 | distilled water | wet | 70–100 °C, 1–6 h | Optimization of reaction conditions to achieve maximum degree of glycation | [140] |
| soy whey protein isolate | fenugreek gum, partially hydrolyzed fenugreek gum | 1:1/3/5 | distilled water | dry | 60 °C, 12 h, 1–3 days, 75% RH | Increased emulsifying properties | [141] |
| soy protein isolate | lactose | 1:2 | distilled water, pH 7 | wet | 65–75 °C, 0–8 h | Increased emulsifying properties, encapsulation efficiency of oil in spray-dried emulsions, and redispersion and dissolution properties; decreased apparent viscosity of emulsions and storage stability of spray-dried emulsions | [142] |
| soy protein isolate | maltodextrin (DE 10), gum acacia | 1:1 | distilled water, pH 7 | dry | 60 °C, 3 days for maltodextrin, 1 week for gum acacia, over KBr solution | Increased solubility and emulsifying properties; decreased surface hydrophobicity | [143] |
| soy protein isolate | dextran (67 kDa) | 1/0.5/1/2/3 | 1 mM phosphate buffer, pH 6.5 | wet | 50–65 °C, 18–36 h | Increased heat stability, structural flexibility, and emulsifying properties; macromolecular crowding conditions enhanced glycation | [144] |
| β-conglycinin | glucose, maltose, dextran (10 kDa) | 1:1 | 0.1 M phosphate buffer, pH 7 | dry | 60 °C, 5 days, 75% RH | Increased solubility, heat stability; decreased surface hydrophobicity; denser gels with higher elastic modulus | [145] |
| hydrolyzed soy protein isolate | maltodextrin (DE 8–10) | 6:1 | distilled water, pH 7 | wet | 80 °C, 120–300 min | Increased heat stability, antioxidant activities, and emulsifying properties | [146] |
| soy protein isolate | dextran (20 kDa, 40 kDa), maltose, lactose, glucose, galactose | 1:1 | - | dry (direct) | 60 °C, 12 h, 79% RH | Decreased antigenicity | [147] |
| soy protein isolate | maltodextrin (DE 13–17) | 1:2 | 0.01 M phosphate buffer, pH 6.8 | dry | 90–140 °C, 2 h, 79% RH | Increased emulsifying properties (against pH changes, thermal treatment, ionic stress, and storage stability) | [148] |
| soy protein isolate | glucose, xanthan | glucose: 1:0.5/1/2; xanthan: 100/10:1 | deionized water, pH 8 | dry | 50 °C, 6–24 h, 65% RH | Increased emulsifying properties and foaming properties | [149] |
| soy protein isolate | soy soluble polysaccharide (54.2 kDa) | 2:1, 5:3, 5:4, 8:3 | water, pH 7 | dry | 55–65 °C, 36–96 h, 75% RH | Increased emulsifying properties for citral-loaded emulsions (enhanced stability during storage, after heat treatment or under simulated gastrointestinal conditions) | [150] |
| soy protein isolate | soy soluble polysaccharide (54.2 kDa) | 5:3 | water, pH 7 | dry | 60 °C, 72 h, 75% RH | Increased encapsulation properties for citral-loaded emulsions (protection and targeted delivery) | [151] |
| soy protein isolate | gum acacia | 1:1 | deionized water, pH 7 | dry | 60 °C, 3–9 days, 79% RH | Increased emulsifying properties; encapsulation of tomato oleoresin in spray-dried emulsions; protection of lycopene in particles against light, humidity, and temperature | [152] |
| soy protein isolate | glucose, maltose | 8:2/4/8/16 | 0.1 M phosphate buffer, pH 7 | wet with ultrasonic pretreatment | 95 °C, 15 min, ultrasonication at 200 W (138.26 W/cm2) for 20 min | Ultrasonication enhances glycation and eliminates the weakening effect of glycation on gel network of acid-induced protein gel | [153] |
| soybean glycinin | xylose | 3:1 | distilled water | dry | 55 °C, 3–12 h, 79% RH | Decreased antigenicity | [154] |
| soy protein isolate | gum karaya | 1:1/2/3 | water, pH 7 | dry | 60 °C, 3 days, 75% RH | Increased emulsion viscosity/shear thinning and emulsifying properties | [155] |
| soy protein hydrolysate | glucose, maltose, maltodextrin (DE 20), dextran (40 kDa) | 1:1 | distilled water, pH 7 | wet | 60 °C, 3 days | Increased surface properties of conjugates as result of strong membrane formed by closely packed molecular and multilayer adsorption at interface, and emulsifying properties | [120] |
| soy protein peptides | dextran (40 kDa), polyaldehyde dextran | 1:10 | 10 mM phosphate buffer, pH 6.5 | wet | 60 °C, 48 h | Increased emulsifying properties | [156] |
| enzymatically hydrolyzed soy protein isolate | dextran (10 kDa) | 1:4 | 0.1 M phosphate buffer, pH 7 | dry | 60 °C, 24–120 h, aw = 0.43 | Increased or decreased immunoreactivity of glycated protein depending on the blood serum used | [157] |
| soy protein isolate | dextran (67–76 kDa) | 1:4 | 0.1 M phosphate buffer, pH 7 and 8.5 | dry | 40–80 °C, 1 h-12 days, 79% RH | Increased heat stability, solubility, water holding capacity antioxidant properties, and emulsifying properties; spray-dried conjugate powders had better reconstitution properties | [158] |
| soy protein isolate | soy hull hemicelluloses | 1:9, 2:8, 3:7, 4:6, 5:5, 6:4 | water, pH 7 | dry | 60 °C, 7 days, over NaCl solution | Increased emulsifying properties (against heat treatment, over prolonged storage) | [122] |
| soy protein isolate | dextran (40 kDa) | 2:3 | 10 mM phosphate buffer, pH 8 | wet with ultrasound or microwave assistance | ultrasound: 80 °C, 25 kHz, 500 W, 40 min; microwave: 2450 MHz, 800 W, 2 min | Increased freeze–thaw stability of emulsions | [159] |
| soy protein isolate | ι-carrageenan | 1:3/2/1, 2:1, 3:1 | deionized water, pH 8 | dry, spray drying as pretreatment | 60 °C, 0–48 h, 79% RH | Increased encapsulation properties for B. Longum in freeze-dried or spray-dried microcapsules; protection against pasteurization and simulated gastrointestinal digestion | [160] |
| soy protein isolate | xylose, fructose | 4:1 | deionized water, pH 9 | wet | 80 °C, 2–10 h | Increased solubility; decreased emulsifying activity | [161] |
| enzymatically hydrolyzed soy protein isolate | dextran (40 kDa) | 1:1 | 10 mM phosphate buffer, pH 7 | wet | 95 °C, 1.5 h | Increased freeze–thaw stability of emulsions | [162] |
| soybean peptide fractions | xylose, cysteine | 1.5:0.6 + 0.3 cysteine | deionized water, pH 7.4 | wet | 120 °C, 120 min | Increased antioxidant properties and sensory characteristics (increased umami taste, decreased bitterness) | [163] |
| soy protein isolate | glucose, chitosan oligosaccharide | 4:1 | distilled water, pH 8 | dry | 80 °C, 3–48 h, 80.3% RH | Increased emulsifying properties with chitosan oligosaccharide | [164] |
| enzymatically hydrolyzed soy protein isolate | dextran (40 kDa) | 2:1, 1:1, 2:3, 1:2, 2:5 | 10 mM phosphate buffer, pH 7–9 | wet | 85–125 °C, 1.5–2.5 h, in some cases pressure application | Increased freeze–thaw stability of emulsions | [165] |
| soy protein isolate | maltose | 1:0.5/1/1.5 | ionic liquid 1-butyl-3-methylimidazolium chloride | wet | 90–120 °C, 0.5–2.5 h | Increased water/oil binding capacities and emulsifying properties; decreased surface hydrophobicity, in vitro digestibility, and thermal stability | [166] |
| enzymatically hydrolyzed soy protein isolate | dextran (40 kDa) | 2:3 | 10 mM phosphate buffer, pH 8 | wet | 95 °C, 1.5 h | Increased freeze–thaw stability of emulsions | [126] |
| soy protein isolate and enzymatically hydrolyzed soy protein isolate | dextran (40 kDa) | 2:3/6 | 10 mM PBS, pH 8 | wet | isolate: 95 °C, 4 h; hydrolysate: 85 °C, 1 h | Increased emulsifying properties (against pH changes), freeze–thaw stability of emulsions | [127] |
| soy protein isolate | glucose | 2:3 | 20 mM phosphate buffer, pH 7 | wet | 60 °C, 2–6 h | Increased foaming properties and emulsifying properties; changes were positively correlated with molecular flexibility | [128] |
| soy protein isolate | glucose | 2:3 | 20 mM phosphate buffer, pH 7 | wet | 50–90 °C, 5 h | Increased emulsifying properties; molecular flexibility can be indicator for emulsifying properties | [167] |
| soy protein isolate | citrus pectin, apple pectin | 1:1 | wet: water, pH 6–12; ultrasound: water, pH 10 | wet with and without ultrasound assistance | wet: 50–90 °C; ultrasound: 270–630 W, 15–120 min, 50–90 min | Increased molecular flexibility, surface hydrophobicity, and emulsifying properties; ultrasound treatment accelerated glycation | [168] |
| soy protein isolate | citrus pectin, apple pectin | 1:1 | deionized water, pH 7 | dry | 60 °C, 1–7 days, 79% RH | Increased solubility and emulsifying properties; decreased surface hydrophobicity | [169] |
| soy protein isolate | lentinan | wet: 1:1; ultrasound: 4/2/1:1, 1:2/4 | wet: water, pH 10; ultrasound: water, pH 7–12 | wet and slit divergent ultrasonic-assisted wet heating | wet: 90 °C; ultrasound: 50–90 °C, 100–300 W, 20–60 min | Increased surface hydrophobicity, solubility, thermal stability, viscosity, foaming properties, and emulsifying properties (against pH changes, thermal treatment, and ionic stress); ultrasonic treatment enhanced glycation | [129] |
| soy protein isolate | flaxseed gum | 1:1 | 10 mM phosphate buffer, pH 8 | wet with high hydrostatic pressure | 60 °C, 3 days, 0.1–300 MPa | Increased solubility; moderate pressure promotes glycation | [170] |
| soy protein isolate | dextran (10, 40, 70, 150 kDa) | 1:1 | 10 mM PBS, pH 8 | dry | 60 °C, 24 h, 79% RH | Encapsulation of capsaicin in nanoemulsions; increased pH/thermal/storage stability of emulsions; decreased mean particle diameter | [171] |
| soy protein isolate | lactose | 4:1 | 0.5 M carbonate-bicarbonate buffer, pH 9.5 | wet | 95 °C, 30–90 min | Decreased allergenicity | [172] |
| soy protein isolate | glucose, dextran (70 kDa) | 10/2/1:1 | water | dry | 60 °C, 1 day, 79% RH | Findings suggest fractionating the complex reaction mixture for analyzing and different functionalities | [173] |
| soy protein isolate | maltose | 4:1 | 10 mM phosphate buffer, pH 7 | wet with irradiation | 2.5–12.5 kGy | Increased freeze–thaw stability of emulsions | [174] |
| soy protein isolate | okara dietary fiber | 1:1 | deionized water, pH 7 | dry | 60 °C, 6–72 h, aw = 0.78 | Increased thermal stability and Pickering emulsion stabilization | [175] |
| soy protein isolate | maltose | 4:1 | 10 mM phosphate buffer, pH 7 | wet with irradiation | 2.5–12.5 kGy | Increased solubility, thermal stability, water/fat absorption capacity, foaming properties, and emulsifying properties; irradiation is highly efficient and affordable | [130] |
| soy protein isolate | maltodextrin | 2:1 | 0.1 M phosphate buffer, pH 7 | wet with ultrasound pretreatment | 200 W (20 kHz) for 5–25 min pretreatment, 95 °C, 30 min | Ultrasonic treatment promotes glycation; increased surface hydrophobicity; decreased acid-induced gelation properties; gel quality of ultrasonicated conjugates better | [176] |
| Pea | |||||||
| pea protein | glucose, fructose, lactose, glucosamine | 1:2 | 0.2 M phosphate buffer, pH 7.4 | wet | 37 °C, 7 days | Increased susceptibility to pepsin hydrolysis | [177] |
| pea protein | glucose | 1:2 | 0.2 M phosphate buffer, pH 7.4 | wet | 37 °C, 7 days | Positive effect on growth of gut commensal bacteria (lactobacilli and bifidobacteria) | [178] |
| pea protein isolate | gum arabic | 1:4 | deionized water, pH 7 | dry | 60 °C, 0–5 days, 79% RH | Increased solubility and emulsifying properties (physical stability against pH changes, temperature and ionic stress and chemical stability against lipid oxidation) | [179] |
| pea protein hydrolysate | gum arabic | 1:4 | deionized water, pH 7 | dry | 60 °C, 0–5 days, 79% RH | Increased solubility, surface hydrophilicity, and emulsifying properties (physical stability against pH changes and chemical stability against lipid oxidation); decrease of beany flavor markers | [180] |
| pea protein isolate | maltodextrin (DE 2 and 21) | 1:16:2 | demineralized water | dry after electrospinning | 65 and 70 °C, 12 and 24 h, 75% RH | Increased solubility | [69] |
| pea protein isolate | maltodextrin (DE 2 and 21) | 1:16:2 | demineralized water | dry after electrospinning | 70 °C, 24 h, 75% RH | Increased interfacial tension and emulsifying properties (against pH changes) | [181] |
| pea protein concentrate | gum arabic | 1:4 | deionized water, pH 7 | dry | 60 °C, 0–5 days, 79% RH | Increased solubility and emulsifying properties (against pH changes, ionic stress, and heat treatment); decrease of beany flavor markers | [182] |
| pea protein isolate | glucose, lactose, maltodextrin (DE 5, 10, 18) | 5:1 | 10 mM carbonate buffer, pH 10 | wet | 80 °C, 12 and 24 h | Increased solubility and surface hydrophilicity; decreased thermal stability and beany flavor | [183] |
| soluble fraction of pea protein isolate | dextran (40 kDa) | 1:1 | water | dry | 60 °C, 48 h, 76.5% RH | Increased solubility and emulsifying properties (against pH changes, ionic stress, and storage at elevated temperatures); decreased lutein color degradation in emulsions | [184] |
| Beans | |||||||
| kidney bean vicilin (phaseolin) | glucose | 1:50/100 | 10 mM phosphate buffer, pH 7 | dry | 60 °C, 2.5–10 h, 79% RH | Increased surface hydrophobicity, molecular flexibility, emulsification activity, and emulsion stability; decreased solubility | [185] |
| African yam bean protein | dextran | 2:1 | distilled water, pH 3.5 | dry | 80 °C, 2 h, 79% RH | Increased apparent viscosity, shear thinning, and yield stress | [186] |
| defatted cowpea flour | - | - | distilled water, pH 10 | wet | 85 °C, 30–120 min | Decreased solubility; good properties when used in bread/cake dough | [187] |
| mung bean protein isolate | glucose | 1:1 | 0.2 M phosphate buffer, pH 7.8 | ultrasound-assisted wet | 80 °C, 10 and 20 min, 20 kHz, 150–450 W | Ultrasonication enhanced glycation; increased solubility, emulsification activity, and emulsion stability; decreased surface hydrophobicity | [188] |
| mung bean protein isolate | dextran | 1:1 | 0.2 M phosphate buffer, pH 7.8 | wet | 80 and 90 °C, 0–6 h | Increased solubility, emulsification activity, and emulsion stability; decreased surface hydrophobicity | [189] |
| fava bean protein isolate | dextran | 1:1 | milli-Q water | dry | 60 °C, 6 days, 63% RH | No big influence on rheological properties and gel stability/stiffness | [190] |
| partially hydrolyzed black bean protein isolate | glucose | 2:1 | 0.1 M phosphate buffer, pH 7 | wet | 80 °C, 1–6 h | Increased solubility, antioxidant activity, emulsification activity, and emulsion stability; decreased surface hydrophobicity | [191] |
| black bean protein isolate, ultrasound pretreatment | glucose | 2:1 | 0.1 M phosphate buffer, pH 7 | wet | 80 °C, 1–6 h | Increased solubility, surface hydrophobicity, antioxidant activity, emulsification activity, and emulsion stability | [192] |
| fava bean protein isolate | maltodextrin (DE 13–17) | 2:1 | distilled water, pH 7 and 11 | wet | 90 °C, 2 h | Increased solubility, surface hydrophobicity, emulsifying properties (during storage and against ionic stress), and foaming properties | [193] |
| Peanut | |||||||
| peanut lectin | glucose, fructose | 5 mg protein + 150 mM sugar | 0.3 M phosphate buffer, pH 8 | wet | 50 °C, 0–5 weeks | Potential allergenicity of Maillard reaction products | [194] |
| fried/roasted peanuts | - | - | - | - | fried at 120 °C, 5 min; roasted at 170 °C, 20 min | Oxidative lipid degradation in peanuts may affect lysine derivatization | [195] |
| peanut protein isolate | dextran (35–45 kDa) | 1:1 | water | dry | 60 °C, 1–7 days, 79% RH | Increased thermal stability, solubility, emulsifying properties, and foaming properties | [196] |
| peanut protein isolate | dextran (40 kDa), gum arabic (240–580 kDa) | 1:1 | deionized water | dry | 60 °C, 7 days, 79% RH | Increased solubility and emulsifying properties; decreased surface hydrophobicity; conjugate structure more flexible and less compact | [197] |
| peanut protein isolate | glucomannan | 1:1 | 0.2 M phosphate buffer, pH 7.5 | wet with ultrasonication | 60–80 °C, 20–100 min, 302.55–786.62 W/cm2 | Ultrasound enhanced glycation; increased solubility and emulsifying properties | [198] |
| peanut protein isolate | dextran (40 kDa), gum arabic (240–580 kDa) | 1:1 | 0.2 M phosphate buffer, pH 7.5 | wet with ultrasonication | 80 °C, 40 min, 150.76 W/cm2 | Ultrasound enhanced glycation; increased solubility and emulsifying properties | [199] |
| peanut protein isolate | xylose | 10:1 | distilled water, pH 3–11 | wet | 30–90 °C, 30–180 min | Increased tensile strength, elongation, and water resistance; decreased solubility | [200] |
| peanut protein isolate | maltodextrin (DE 4.2, 8.1 kDa) | 1:1 | 0.2 M phosphate buffer, pH 7 | ultrasound-assisted wet heating | 70 °C, 10–100 min, 250 W/20 kHz | Ultrasound enhanced glycation; increased solubility and emulsifying properties | [201] |
| peanut protein isolate | xylose | 1:0.01/0.02/0.05/0.1/0.2 | water, pH 9 | wet | 90 °C, 90 min | Increased surface hydrophobicity, tensile strength, and elongation; decreased solubility | [202] |
| peanut protein isolate, hydrolysate and fractions thereof | glucose | 1:0.02 | deionized water, pH 6.5 | wet | 98 °C, 70 min | Increased umami taste and umami-enhancing properties | [203] |
| peanut protein isolate | dextran (50 kDa) | 1:1 | 0.1 M phosphate buffer, pH 7 | wet with cold plasma treatment | 60 °C, subsequent plasma treatment at 35 V and 2 A for 0–3 min | Plasma treatment enhanced glycation; increased solubility and emulsifying properties | [204] |
| peanut protein isolate | lactose | 1:1 | 10 mM phosphate buffer, pH 7 | wet with cold plasma treatment | 80 °C, 40 min, subsequent plasma treatment at 90 W for 0–5 min | Increased thermal stability; decreased surface hydrophobicity and protein enthalpy | [205] |
| Other | |||||||
| chickpea protein (albumin, 26 kDa) | glucose | 1:1 | water | dry | 55 °C, 72 h, 65% RH | Decreased allergenicity | [206] |
| Protein | Carbohydrate | Ratio w/w (If Not Stated Otherwise) | Solvent | Manufacturing Technique | Heating Parameters | Functionality | Ref. |
|---|---|---|---|---|---|---|---|
| Wheat | |||||||
| deamidated soluble wheat protein isolate | glucose, maltodextrins (1, 1.9, 4.3 kDa) | 1:2 molar ratio of NH2 to reducing groups | milli-Q water, pH 6.5 | dry | 60 °C, 60 min, 75% RH | Evaluation of protein secondary structure | [231] |
| deamidated soluble wheat protein isolate | dextran (6.4, 41 kDa) | 1:1 molar ratio of NH2 to reducing groups | deionized water | dry | 60 °C, 0–5 days, 75% RH | Increased emulsifying properties and interfacial layer thickness | [226] |
| wheat germ protein | xylose, glucose, lactose, dextran, maltodextrin | 1:1 | deionized water, pH 11 | wet | 90 °C, 0–50 min | Increased solubility and emulsifying properties | [225] |
| deamidated wheat gluten | maltodextrin (DE 8–10, 10–15, 16–20), citrus pectin | 1:1/2 | deionized water, pH 7 | dry | 80 °C, 3–24 h, 79% RH | Increased emulsifying properties (against pH changes and ionic stress); decreased surface hydrophobicity; successful encapsulation of β-carotene in emulsions | [232] |
| Rice | |||||||
| rice endosperm protein | glucose, xanthan | 1:0.46 glucose; 1:0.1 xanthan | deionized water | dry | 50 °C, 8 h for glucose, 20 h for xanthan, 65% RH | Increased solubility, emulsification activity, and emulsion stability | [233] |
| rice protein isolate | glucose, lactose, maltodextrin, dextran | 1:1 | deionized water, pH 11 | wet | 100 °C, 0–30 min | Increased solubility, emulsification activity, and emulsion stability | [234] |
| rice protein hydrolysate | glucose, lactose, maltodextrin (DE 20), dextran (0.18, 0.34, 1, 20 kDa) | 1:1 | water, pH 11 | wet | 100 °C, 0–40 min | Increased solubility, emulsification activity, and emulsion stability; decreased surface hydrophobicity | [230] |
| rice dreg glutelin | κ-carrageenan (200 kDa) | 1:2 | distilled water | dry | 60 °C, 0–96 h, 79% RH | Increased solubility and emulsion stability (against pH changes and ionic stress) | [229] |
| rice protein isolate | dextran (20 kDa) | 25/20/15/10/5/2/1:1 | deionized water, pH 12 | wet | 80–100 °C, 10–30 min | Increased solubility, emulsification activity, emulsion stability, foaming activity, and foam stability | [228] |
| Oat | |||||||
| oat protein isolate | dextran (40 kDa) | 1:1 | 20 mM PBS, pH 9 | wet | 90 °C, 0–100 min | Increased emulsifying properties (against pH changes and ionic stress) | [235] |
| oat protein isolate | P. ostreatus β-glucan | 1:1/2/3/4/5 | 50 mM phosphate buffer, pH 4/6/8/10/12 | dry | 60 °C, 1–9 days, 75% RH | Increased solubility, thermal stability, and emulsifying properties; decreased surface hydrophobicity | [236] |
| Millet | |||||||
| sorghum protein | dextran (19.6 kDa), galactomannan (15 kDa) | 1:5 | water | dry | 60 °C, 7 days, 79% RH | Increased solubility, thermal stability, and emulsifying properties | [12] |
| chymotrypsin-digested millet protein | galactomannan (15 kDa) | 1:4 | distilled water | dry | 60 °C, 7 days, 79% RH | Increased thermal stability and emulsifying properties | [237] |
| Pseudocereals | |||||||
| buckwheat protein | xylose, fructose, glucose, dextran, maltodextrin | 1:3.33 | 20 mM phosphate buffer, pH 6.5 | wet | 60 °C, 12–48 h | Increased thermal stability and emulsifying properties | [238] |
| Protein | Carbohydrate | Ratio w/w (If Not Stated Otherwise) | Solvent | Manufacturing Technique | Heating Parameters | Functionality | Ref. |
|---|---|---|---|---|---|---|---|
| Canola/sunflower | |||||||
| sunflower protein hydrolysate | xylose | 2:1 + 0.75 cysteine | distilled water, pH 7.4 | wet | 120 °C, 2 h | Increased antioxidant capacity and sensory properties (mouthfulness and continuity taste) | [248] |
| canola protein isolate | gum arabic | 1:0.5/1/2 | 0.2 M phosphate buffer, pH 7 | wet | 90 °C, 0–60 min | Increased solubility (especially at pI) | [249] |
| canola protein isolate | gum arabic | 1:0.5 | 0.2 M phosphate buffer, pH 7 | wet | 90 °C, 15 min | Increased viscosity, emulsifying activity, and emulsion stability at various pH values and after heat treatment | [244] |
| canola protein isolate | gum arabic | 1:0.5 | 0.2 M phosphate buffer, pH 7 | wet | 90 °C, 15 min | Increased thermal stability; decreased protein aggregation | [245] |
| Rapeseed | |||||||
| rapeseed protein isolate | dextran (20 kDa) | 1:1 | water, pH 10 | wet | 90 °C, 1–3 h | Increased surface hydrophilicity, solubility, emulsifying properties, and thermal stability | [250] |
| rapeseed protein isolate | dextran (20 kDa) | 1:1 | water, pH 10 | ultrasound-assisted wet | 70–90 °C, 0–60 min, 20–50 kHz | Ultrasound enhanced glycation; increased solubility, thermal stability, emulsifying activity, and emulsion stability; decreased digestibility | [246] |
| Sesame | |||||||
| sesame protein concentrate | maltodextrin (DE 19) | 1/2/3:1 | deionized water | dry | 80 °C, 24 h, 79% RH | Increased solubility, structural flexibility of the molecule, and emulsifying properties | [251] |
| sesame protein hydrolysate | xylose, fructose, glucose | 1/2/4/6/8/10:1 | water, pH 6.5/7/7.5/8/8.5 | wet | 110–140 °C, 60–180 min | Increased antioxidant activity and oxidative stability of sesame oil | [247] |
| Protein | Carbohydrate | Ratio w/w (If Not Stated Otherwise) | Solvent | Manufacturing Technique | Heating Parameters | Functionality | Ref. |
|---|---|---|---|---|---|---|---|
| Potato | |||||||
| Solanic 206P (75% patatin, 25% protease inhibitors | galactose, galactan, galactooligosaccharides from potato (1.9 kDa) | 1:9 molar ratio | 50 mM phosphate buffer, pH 7 | dry | 48 °C, 1–7 days, aw = 0.65 | Increased heat stability, pH stability, antioxidant activity, and emulsifying properties; galactose most reactive | [252] |
| patatin, protease inhibitors | galactose, galactan, galactooligosaccharides from potato (1.9 kDa) | 1:9 molar ratio | 50 mM phosphate buffer, pH 7 | dry | 48 °C, 1–3 days, aw = 0.65 | Decreased immunoreactivity after galactan conjugation to patatin | [255] |
| patatin | galactose, xylose, galactooligosaccharides, xylooligosaccharides, galactan, xylan | 1:7 molar ratio + :0.2 Maillard reaction inhibitor | 50 mM phosphate buffer, pH 7 | dry | 48 °C, 1–7 days, aw = 0.65 | Maillard reaction inhibitors limit protein cross-linking and increase digestibility | [256] |
| Other | |||||||
| bitter melon seed protein isolate | glucose | 10:1 | water, pH 7 | dry | 40–60 °C, 48 h, 50–80% RH | Increased emulsifying activity, emulsion stability, foaming capacity, and foaming stability; decreased surface hydrophobicity and solubility | [253] |
| longan pulp protein | longan pulp polysaccharides | - | distilled water, pH 5 | dry | 60 °C, 1–6 days, over KBr solution | Increased antioxidant, antitumor, and immuno-stimulating activities | [257] |
| wild almond protein isolate | gum arabic (240–580 kDa) | 9:1 | water, pH 7 | dry | 60 °C, 3–9 days, 79% RH | Increased film tensile strength and elongation; decreased water vapor permeability | [258] |
| walnut protein isolate | glucose | 1:1 | 50 mM phosphate buffer, pH 8 | dry | 95 °C, 1–3 h | Increased antioxidant activity, emulsifying activity, and emulsion stability; decreased hydrophobicity | [254] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods 2021, 10, 376. https://doi.org/10.3390/foods10020376
Kutzli I, Weiss J, Gibis M. Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods. 2021; 10(2):376. https://doi.org/10.3390/foods10020376
Chicago/Turabian StyleKutzli, Ines, Jochen Weiss, and Monika Gibis. 2021. "Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application" Foods 10, no. 2: 376. https://doi.org/10.3390/foods10020376






