Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link?
Abstract
:1. Introduction
2. HHCy as a Risk Factor in CVD
3. H2S as a Gasotransmitter
3.1. H2S in Pathophysiological Conditions
3.2. H2S in Immune Cells
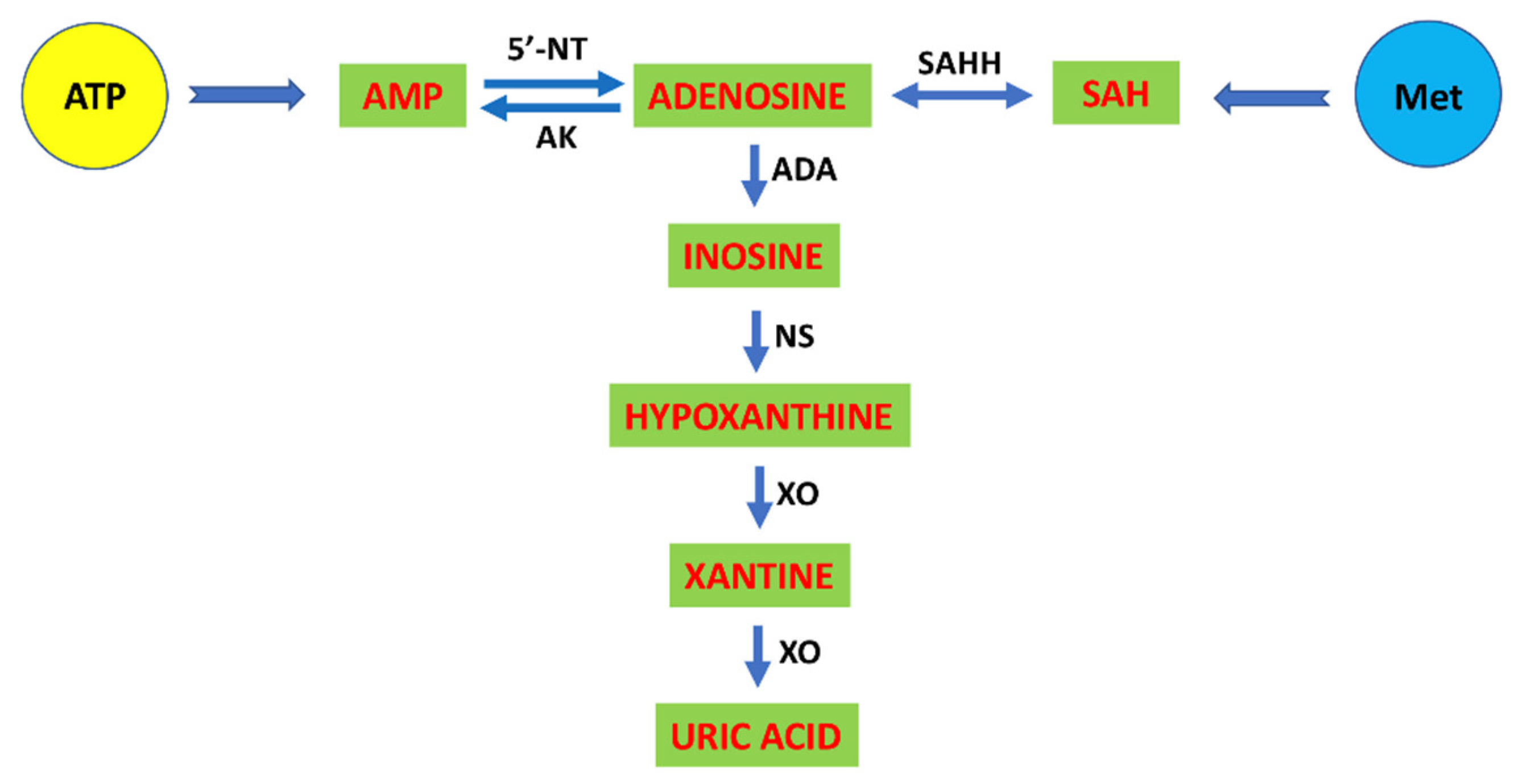
4. Adenosine as a Purinergic Modulator of Cardiovascular and Immune Systems
4.1. Adenosine Receptors in the Immune System
4.2. The Adenosinergic and Cardiovascular Systems
4.3. Adenosine and Its Receptors in Coronary Artery Disease (CAD)
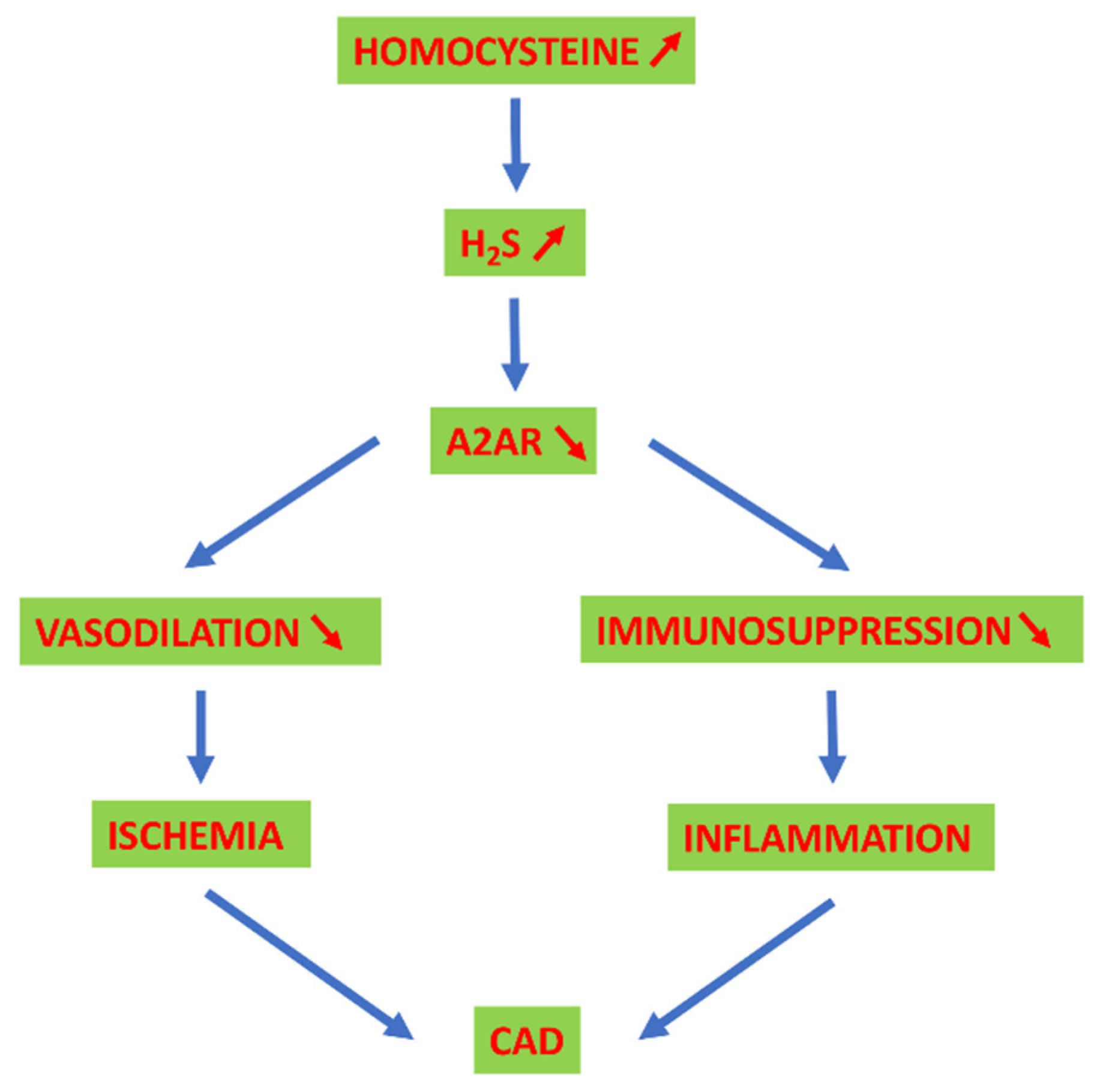
5. Adenosinergic System, HCy and H2S in CAD
6. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazurek, R.; Dave, J.M.; Chandran, R.R.; Misra, A.; Sheikh, A.Q.; Greif, D.M. Vascular cells in blood vessel wall development and disease. Adv. Pharmacol. 2017, 78, 323–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redon, J. Global cardiovascular risk assessment: Strengths and limitations. High Blood Press. Cardiovasc. Prev. 2016, 23, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Krasi, G.; Precone, V.; Paolacci, S.; Stuppia, L.; Nodari, S.; Romeo, F.; Perrone, M.; Bushati, V.; Dautaj, A.; Bertelli, M. Genetics and pharmacogenetics in the diagnosis and therapy of cardiovascular diseases. Acta Biomed. 2019, 90, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Doughty, K.N.; Del Pilar, N.X.; Audette, A.; Katz, D.L. Lifestyle medicine and the management of cardiovascular disease. Curr. Cardiol. Rep. 2017, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Zhang, B.; Yang, Y.; Qi, L.; Meng, L.; Zhang, Y.; Huo, Y. Correlating the relationship between interarm systolic blood pressure and cardiovascular disease risk factors. J. Clin. Hypertens. 2017, 19, 466–471. [Google Scholar] [CrossRef] [Green Version]
- Van Guldener, C.; Nanayakkara, P.W.B.; Stehouwer, C.D.A. Homocysteine and blood pressure. Curr. Hypertens. Rep. 2003, 5, 26–31. [Google Scholar] [CrossRef]
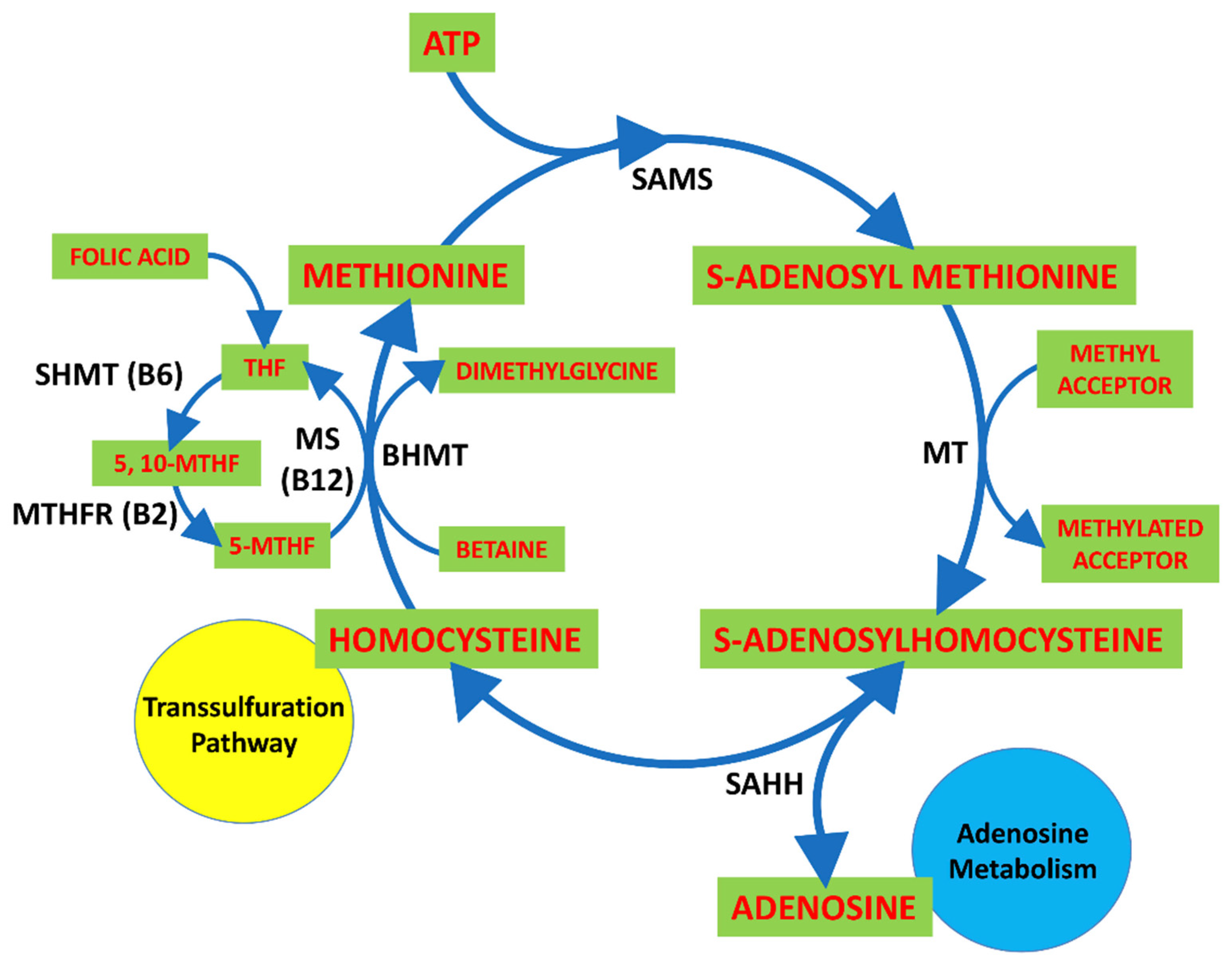
- Schalinske, K.L.; Smazal, A.L. Homocysteine imbalance: A pathological metabolic marker. Adv. Nutr. 2012, 3, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Skovierova, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [Green Version]
- Ntaios, G.; Savopoulos, C.; Grekas, D.; Hatzitolios, A. The controversial role of B-vitamins in cardiovascular risk: An update. Arch. Cardiovasc. Dis. 2009, 102, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.S.; Wong, P.W.K.; Malinow, M.R. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Perla-Kaján, J.; Twardowski, T.; Jakubowski, H. Mechanisms of homocysteine toxicity in humans. Amino Acids 2007, 32, 561–572. [Google Scholar] [CrossRef]
- Friedman, A.N.; Bostom, A.G.; Selhub, J.; Levey, A.S.; Rosenberg, I.H. The kidney and homocysteine metabolism. J. Am. Soc. Nephrol. 2001, 12, 2181–2189. [Google Scholar]
- Liaugaudas, G.; Jacques, P.F.; Selhub, J.; Rosenberg, I.H.; Bostom, A.G. Renal insufficiency, vitamin B12 status, and population attributable risk for mild hyperhomocysteinemia among coronary artery disease patients in the era of folic acid–fortified cereal grain flour. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 849–851. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; He, G.W. Imbalance of homocysteine and H(2)S: Significance, mechanisms, and therapeutic promise in vascular injury. Oxidative Med. Cell. Longev. 2019, 2019, 7629673. [Google Scholar] [CrossRef] [Green Version]
- Skeete, J.; Di Pette, D.J. Relationship between homocysteine and hypertension: New data add to the debate. J. Clin. Hypertens. 2017, 19, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Xu, R.; Li, G.; Yao, Y.; Li, J.; Cong, D.; Xu, X.; Zhang, L. Homocysteine and the C677T gene polymorphism of its key metabolic enzyme MTHFR are risk factors of early renal damage in hypertension in a chinese han population. Medicine (Baltimore) 2015, 94, e2389. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Adams, R.; Albers, G.; Alberts, M.J.; Benavente, O.; Furie, K.; Goldstein, L.B.; Gorelick, P.; Halperin, J.; Harbaugh, R.; et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American heart association/American stroke association council on stroke: Co-sponsored by the council on cardiovascular radiology and intervention: The American academy of neurology affirms the value of this guideline. Circulation 2006, 113, e409–e449. [Google Scholar] [CrossRef] [PubMed]
- Kundi, H.; Kiziltunc, E.; Ates, I.; Cetin, M.; Barca, A.N.; Ozkayar, N.; Ornek, E. Association between plasma homocysteine levels and end-organ damage in newly diagnosed type 2 diabetes mellitus patients. Endocr. Res. 2017, 42, 36–41. [Google Scholar] [CrossRef]
- Yang, B.; Fan, S.; Zhi, X.; Wang, Y.; Wang, Y.; Zheng, Q.; Sun, G. Prevalence of hyperhomocysteinemia in China: A systematic review and meta-analysis. Nutrients 2014, 7, 74–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wu, Q.; Zhang, L.; Hao, Y.; Fan, R.; Peng, X.; Liu, S.; Chen, Z.; Zhang, T.; Chen, S.; et al. Elevated total plasma homocysteine levels are associated with type 2 diabetes in women with hypertension. Asia Pac. J. Clin. Nutr. 2015, 24, 683–691. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Homocysteine and atherothrombosis: Diagnosis and treatment. Curr. Atheroscler. Rep. 2003, 5, 276–283. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Nait, F.; Capobianco, F.; Sechi, L.A. Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. Am. J. Hypertens. 2014, 28, 943–950. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Url–Michitsch, M.; Nait, F.; Sechi, L.A. Subclinical carotid artery disease and plasma homocysteine levels in patients with hypertension. J. Am. Soc. Hypertens. 2015, 9, 167–175. [Google Scholar] [CrossRef]
- Zhong, C.; Xu, T.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Geng, D.; Zhang, D.; et al. Plasma homocysteine and prognosis of acute ischemic stroke: A gender-specific analysis from CATIS randomized clinical trial. Mol. Neurobiol. 2017, 54, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Ankri, A.; Chadefaux-Vekemans, B.; Blacher, J.; Philippe, F.; Drobinski, G.; Benzidia, R.; Kamoun, P.; Thomas, D. Plasma homocysteine and the extent of atherosclerosis in patients with coronary artery disease. Int. J. Cardiol. 1997, 60, 295–300. [Google Scholar] [CrossRef]
- Nygard, O.; Nordrehaug, J.E.; Refsum, H.; Ueland, P.M.; Farstad, M.; Vollset, S.E. Plasma homocysteine levels and mortality in patients with coronary artery disease. N. Engl. J. Med. 1997, 337, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tan, H.M.; Wang, H. Hyperhomocysteinemia and atherosclerosis. Sheng Li Xue Bao 2005, 57, 103–114. [Google Scholar] [PubMed]
- Ungvari, Z.; Csiszar, A.; Edwards, J.G.; Kaminski, P.M.; Wolin, M.S.; Kaley, G.; Koller, A. Increased superoxide production in coronary arteries in hyperhomocysteinemia. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 418–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, J.C.; Wang, H.; Perrella, M.A.; Yoshizumi, M.; Sibinga, N.E.; Tan, L.C.; Haber, E.; Chang, T.H.; Schlegel, R.; Lee, M.E. Induction of cyclin A gene expression by homocysteine in vascular smooth muscle cells. J. Clin. Investig. 1996, 97, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, D.; Tan, H.; Hui, R.; Li, Z.; Jiang, X.; Gaubatz, J.; Yang, F.; Durante, W.; Chan, L.; Schafer, A.I.; et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ. Res. 2006, 99, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowski, H. The pathophysiological hypothesis of homocysteine thiolactone-mediated vascular disease. J. Physiol. Pharmacol. 2008, 9, 155–167. [Google Scholar]
- Undas, A.; Brożek, J.; Jankowski, M.; Siudak, Z.; Szczeklik, A.; Jakubowski, H. Plasma homocysteine affects fibrin clot permeability and resistance to lysis in human subjects. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- Elsherbiny, N.M.; Sharma, I.; Kira, D.; Alhusban, S.; Samra, Y.A.; Jadeja, R.; Martin, P.; Al-Shabrawey, M.; Tawfik, A. Homocysteine induces inflammation in retina and brain. Biomolecules 2020, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- Wald, D.S.; Wald, N.J.; Morris, J.K.; Law, M. Folic acid, homocysteine, and cardiovascular disease: Judging causality in the face of inconclusive trial evidence. BMJ 2006, 333, 1114–1117. [Google Scholar] [CrossRef] [Green Version]
- Faverzani, J.L.; Hammerschmidt, T.G.; Sitta, A.; Deon, M.; Wajner, M.; Vargas, C.R. Oxidative stress in homocystinuria due to cystathionine ß-synthase deficiency: Findings in patients and in animal models. Cell. Mol. Neurobiol. 2017, 37, 1477–1485. [Google Scholar] [CrossRef]
- Stuhlinger, M.C.; Tsao, P.S.; Her, J.H.; Kimoto, M.; Balint, R.F.; Cooke, J.P. Homocysteine impairs the nitric oxide synthase pathway: Role of asymmetric dimethylarginine. Circulation 2001, 104, 2569–2575. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial dysfunction: The link between homocysteine and hydrogen sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef]
- Castro, R.; Rivera, I.; Struys, E.A.; Jansen, E.E.W.; Ravasco, P.; Camilo, M.E.; Blom, H.J.; Jakobs, C.; Tavaresde Almeida, I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003, 49, 1292–1296. [Google Scholar] [CrossRef]
- Choumenkovitch, S.F.; Selhub, J.; Bagley, P.J.; Maeda, N.; Nadeau, M.R.; Smith, D.E.; Choi, S.-W. In the cystathionine beta-synthase knockout mouse, elevations in total plasma homocysteine increase tissue S-adenosylhomocysteine, but responses of S-adenosylmethionine and DNA methylation are tissue specific. J. Nutr. 2002, 132, 2157–2160. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.W. Homocysteine and vitamins in cardiovascular disease. Clin. Chem. 1998, 44, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Büdy, B.; O’Neill, R.; Di Bello, P.M.; Sengupta, S.; Jacobsen, D.W. Homocysteine transport by human aortic endothelial cells: Identification and properties of import systems. Arch. Biochem. Biophys. 2006, 446, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2010, 41, 113–121. [Google Scholar] [CrossRef]
- Giuffrè, A.; Vicente, J.B. Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxidative Med. Cell. Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef]
- Chen, X.; Jhee, K.H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef] [Green Version]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Ishii, K.; Kimura, H.; Togawa, T. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Banerjee, R. PLP-dependent H(2)S biogenesis. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2011, 1814, 1518–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Poddar, R.; Tipa, E.V.; Dibello, P.M.; Moravec, C.D.; Robinson, K.; Green, R.; Kruger, W.D.; Garrow, T.A.; Jacobsen, D.W. Homocysteine metabolism in cardiovascular cells and tissues: Implications for hyperhomocysteinemia and cardiovascular disease. Adv. Enzyme Regul. 1999, 39, 93–109. [Google Scholar] [CrossRef]
- Tipa, E.V.; Chen, P.; Majors, A.K.; Robinson, K.; Jacobsen, D.W. Failure of homocysteine to restore the glutathione (GHS) pool in cysteine-starved human aortic endothelial cells (HAEC). FASEB J. 2000, 14, A460. [Google Scholar]
- Sørensen, J.T.; Gaustadnes, M.; Stabler, S.P.; Allen, R.H.; Mudd, S.H.; Hvas, A.M. Molecular and biochemical investigations of patients with intermediate or severe hyperhomocysteinemia. Mol. Genet. Metab. 2016, 117, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Chertok, V.M.; Kotsyuba, A.E. Distribution of H2S synthesis enzymes in the walls of cerebral arteries in rats. Bull. Exp. Biol. Med. 2012, 154, 104–107. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Masi, A.d.; Ascenzi, P. H2S: A “Double face” molecule in health and disease. Biofactors 2012, 39, 186–196. [Google Scholar] [CrossRef]
- Lo Faro, M.L.; Fox, B.; Whatmore, J.L.; Winyard, P.G.; Whiteman, M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide 2014, 41, 38–47. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The reactive species interactome: Evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [Green Version]
- Kevil, C.; Cortese-Krott, M.M.; Nagy, P.; Papapetropoulos, A.; Feelisch, M.; Szabo, C. Cooperative interactions between NO and H2S: Chemistry, biology, physiology, pathophysiology. In Nitric Oxide; Ignarro, L., Freeman, B., Eds.; Elsevier: London, UK, 2017; pp. 57–83. [Google Scholar]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef] [PubMed]
- Korbut, E.; Brzozowski, T.; Magierowski, M. Carbon monoxide being hydrogen sulfide and nitric oxide molecular sibling, as endogenous and exogenous modulator of oxidative stress and antioxidative mechanisms in the digestive system. Oxidative Med. Cell. Longev. 2020, 2020, 5083876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowicka, E.; Beltowski, J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacol. Rep. 2007, 59, 4–24. [Google Scholar] [PubMed]
- Andreadou, I.; Schulz, R.; Papapetropoulos, A.; Turan, B.; Ytrehus, K.; Ferdinandy, P.; Daiber, A.; Di Lisa, F. The role of mitochondrial reactive oxygen species, NO and H(2)S in ischaemia/reperfusion injury and cardioprotection. J. Cell. Mol. Med. 2020, 24, 6510–6522. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Papapetropoulos, A. International union of basic and clinical pharmacology. CII: Pharmacological modulation of H(2)S levels: H(2)S donors and H(2)S biosynthesis inhibitors. Pharmacol. Rev. 2017, 69, 497–564. [Google Scholar] [CrossRef] [Green Version]
- Polhemus, D.J.; Lefer, D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef]
- Nicholls, P.; Marshall, D.C.; Cooper, C.E.; Wilson, M.T. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 2013, 41, 1312–1316. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.J.; Wu, Z.Y.; Cao, L.; Zhu, M.Y.; Liu, T.T.; Guo, L.; Lin, Y.; Nie, X.W.; Bian, J.S. Hydrogen sulfide: Recent progression and perspectives for the treatment of diabetic nephropathy. Molecules 2019, 24, 2857. [Google Scholar] [CrossRef] [Green Version]
- Giuffrè, A.; Tomé, C.S.; Fernandes, D.G.F.; Zuhra, K.; Vicente, J.B. Hydrogen sulfide metabolism and signaling in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1219, 335–353. [Google Scholar] [CrossRef]
- Augsburger, F.; Szabo, C. Potential role of the 3-mercaptopyruvate sulfurtransferase (3-MST)—hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol. Res. 2020, 154, 104083. [Google Scholar] [CrossRef]
- Li, H.; Xu, F.; Gao, G.; Gao, X.; Wu, B.; Zheng, C.; Wang, P.; Li, Z.; Hua, H.; Li, D. Hydrogen sulfide and its donors: Novel antitumor and antimetastatic therapies for triple-negative breast cancer. Redox Biol. 2020, 34, 101564. [Google Scholar] [CrossRef]
- Sun, H.J.; Wu, Z.Y.; Nie, X.W.; Bian, J.S. Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef] [Green Version]
- Sunzini, F.; De Stefano, S.; Chimenti, M.S.; Melino, S. Hydrogen sulfide as potential regulatory gasotransmitter in arthritic diseases. Int. J. Mol. Sci. 2020, 21, E1180. [Google Scholar] [CrossRef] [Green Version]
- Szabo, C. The re-emerging pathophysiological role of the cystathionine-β-synthase—hydrogen sulfide system in Down syndrome. FEBS J. 2020, 287, 3150–3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, R.; Jeong, N.Y.; Jung, J. Therapeutic importance of hydrogen sulfide in age-associated neurodegenerative diseases. Neural Regen. Res. 2020, 15, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y.; Li, T.; Tuo, Q. Role of hydrogen sulfide in chronic diseases. DNA Cell Biol. 2020, 39, 187–196. [Google Scholar] [CrossRef]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possomato-Vieira, J.S.; Gonçalves-Rizzi, V.H.; do Nascimento, R.A.; Wandekin, R.R.; Caldeira-Dias, M.; Chimini, J.S.; da Silva, M.L.S.; Dias-Junior, C.A. Clinical and experimental evidences of hydrogen sulfide involvement in lead-induced hypertension. BioMed Res. Int. 2018, 2018, 4627391. [Google Scholar] [CrossRef] [PubMed]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X.; Kong, W. Hyperhomocysteinaemia and vascular injury: Advances in mechanisms and drug targets. Br. J. Pharmacol. 2018, 175, 1173–1189. [Google Scholar] [CrossRef]
- Li, J.J.; Li, Q.; Du, H.P.; Wang, Y.L.; You, S.J.; Wang, F.; Xu, X.S.; Cheng, J.; Cao, Y.J.; Liu, C.F.; et al. Homocysteine triggers inflammatory responses in macrophages through inhibiting CSE-H2S signaling via DNA hypermethylation of CSE promoter. Int. J. Mol. Sci. 2015, 16, 12560–12577. [Google Scholar] [CrossRef]
- Li, M.H.; Tang, J.P.; Zhang, P.; Li, X.; Wang, C.Y.; Wei, H.J.; Yang, X.F.; Zou, W.; Tang, X.Q. Disturbance of endogenous hydrogen sulfide generation and endoplasmic reticulum stress in hippocampus are involved in homocysteine-induced defect in learning and memory of rats. Behav. Brain Res. 2014, 262, 35–41. [Google Scholar] [CrossRef]
- Tang, X.Q.; Shen, X.T.; Huang, Y.E.; Chen, R.Q.; Ren, Y.K.; Fang, H.R.; Zhuang, Y.Y.; Wang, C.Y. Inhibition of endogenous hydrogen sulfide generation is associated with homocysteine-induced neurotoxicity: Role of ERK1/2 activation. J. Mol. Neurosci. 2010, 45, 60–67. [Google Scholar] [CrossRef]
- d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Di Minno, M.N.D.; Kirkby, N.S.; Warner, T.D.; Di Minno, G.; Cirino, G.; Sorrentino, R. Hydrogen sulphide pathway contributes to the enhanced human platelet aggregation in hyperhomocysteinemia. Proc. Natl. Acad. Sci. USA 2013, 110, 15812–15817. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Navneet, S.; Wang, J.; Roon, P.; Chen, W.; Xian, M.; Smith, S.B. Analysis of MTHFR, CBS, glutathione, taurine, and hydrogen sulfide levels in retinas of hyperhomocysteinemic mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1954–1963. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Ingrid, S.; Ding, Y.G.; Liu, Y.; Qi, J.G.; Tang, C.S.; Du, J.B. Imbalance of endogenous homocysteine and hydrogen sulfide metabolic pathway in essential hypertensive children. Chin. Med. J. 2007, 120, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, S.; Lietal, Y. Potential biomarkers predicting risk of pulmonary hypertension in congenital heart disease: The role of homocysteine and hydrogen sulfide. Chin. Med. J. 2014, 127, 893–899. [Google Scholar] [PubMed]
- He, Y.; Liu, S.; Zhang, Z.; Liao, C.; Lin, F.; Yao, W.; Chen, Y. Imbalance of endogenous hydrogen sulfide and homocysteine in chronic obstructive pulmonary disease combined with cardiovascular disease. Front. Pharmacol. 2017, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [Green Version]
- Wagner, C.A. Hydrogen sulfide: A new gaseous signal molecule and blood pressure regulator. J. Nephrol. 2009, 22, 173–176. [Google Scholar]
- Deplancke, B.; Gaskins, H.R. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J. 2003, 17, 1310–1312. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Hui, Y.; Cheung, Y.; Bin, G.; Jiang, H.; Chen, X.; Tang, C. The possible role of hydrogen sulfide as a smooth muscle cell proliferation inhibitor in rat cultured cells. Heart Vessels 2004, 19, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Cao, K.; Wu, L.; Wang, R. Cystathionine γ-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J. Biol. Chem. 2004, 279, 49199–49205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Sun, X.; Wang, R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004, 18, 1782–1784. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Wang, R. Pro-apoptotic effect of endogenous H2S on human aorta smooth muscle cells. FASEB J. 2006, 20, 553–555. [Google Scholar] [CrossRef]
- Zanardo, R.C.O.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L.; Zanardo, R.C.O.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Bhatia, M.; Sidhapuriwala, J.; Moochhala, S.M.; Moore, P.K. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br. J. Pharmacol. 2005, 145, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, L.; Gobbi, G.; Pambianco, M.; Micheloni, C.; Mirandola, P.; Vitale, M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab. Investig. 2006, 86, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.; Rauh-Pfeiffer, A.; Ming-Yu, Y.; Lu, X.M.; Zurakowski, D.; Curley, M.; Collier, S.; Duggan, C.; Nurko, S.; Thompson, J.; et al. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Crit. Care Med. 2001, 29, 870–877. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.; Wu, L.; Yang, G. Hydrogen sulfide signaling in regulation of cell behaviors. Nitric Oxide 2020, 103, 9–19. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Liu, S.J.; Liu, Y.J.; Wang, S.; Ni, X. Glucocorticoids suppress cystathionine gamma-lyase expression and H2S production in lipopolysaccharide-treated macrophages. Cell. Mol. Life Sci. 2010, 67, 1119–1132. [Google Scholar] [CrossRef]
- George, L.; Ramasamy, T.; Sirajudeen, K.N.S.; Manickam, V. LPS-induced apoptosis is partially mediated by hydrogen sulphide in RAW 264.7 murine macrophages. Immunol. Investig. 2019, 48, 451–465. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, F.; You, S.J.; Cao, Y.J.; Cao, L.D.; Han, Q.; Liu, C.F.; Hu, L.F. Dysregulation of cystathionine γ-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cell. Signal. 2013, 25, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Liu, F.; Cui, S.; Jiang, N.; Yu, H.; Zhou, Y.; Liu, Y.; Kou, X. Mechanical load-induced H(2)S production by periodontal ligament stem cells activates M1 macrophages to promote bone remodeling and tooth movement via STAT1. Stem Cell Res. Ther. 2020, 11, 112. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Luo, N.; Mu, D.; Jiang, P.; Liu, R.; Sun, H.; Xiong, S.; Liu, X.; Wang, L.; Chu, Y. Lipopolysaccharide regulates biosynthesis of cystathionine γ-lyase and hydrogen sulfide through toll-like receptor-4/p38 and toll-like receptor-4/NF-κB pathways in macrophages. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Badiei, A.; Gieseg, S.; Davies, S.; Othman, M.I.; Bhatia, M. LPS up-regulates cystathionine γ -lyase gene expression in primary human macrophages via NF-κB/ERK pathway. Inflamm. Allergy Drug Targets 2015, 14, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Wang, E.A.; Gould, S.; Stein, E.V.; Kaur, S.; Lim, L.; Amarnath, S.; Fowler, D.H.; Roberts, D.D. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012, 287, 4211–4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kida, K.; Ichinose, F. Hydrogen sulfide and neuroinflammation. Handb. Exp. Pharmacol. 2015, 230, 181–189. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ding, Y.P.; Wang, Z.; Kong, Y.; Gao, R.; Chen, G. Hydrogen sulfide therapy in brain diseases: From bench to bedside. Med. Gas Res. 2017, 7, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Rivers, J.R.; Badiei, A.; Bhatia, M. Hydrogen sulfide as a therapeutic target for inflammation. Expert Opin. Ther. Targets 2012, 16, 439–449. [Google Scholar] [CrossRef]
- Bhatia, M. H2S and substance P in inflammation. Methods Enzymol. 2015, 555, 195–205. [Google Scholar] [CrossRef]
- Guo, F.F.; Yu, T.C.; Hong, J.; Fang, J.Y. Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front. Physiol. 2016, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Burguera, E.F.; Meijide-Failde, R.; Blanco, F.J. Hydrogen sulfide and inflammatory joint diseases. Curr. Drug Targets 2017, 18, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Feliers, D.; Lee, H.J.; Kasinath, B.S. Hydrogen sulfide in renal physiology and disease. Antioxid. Redox Signal. 2016, 25, 720–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Bian, J.S. The role of hydrogen sulfide in renal system. Front. Pharmacol. 2016, 7, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.L.; Qin, M.; Liu, X.H.; Zhu, Y.Z. The role of hydrogen sulfide on cardiovascular homeostasis: An overview with update on immunomodulation. Front. Pharmacol. 2017, 8, 686. [Google Scholar] [CrossRef] [Green Version]
- Shefa, U.; Yeo, S.G.; Kim, M.S.; Song, I.O.; Jung, J.; Jeong, N.Y.; Huh, Y. Role of gasotransmitters in oxidative stresses, neuroinflammation, and neuronal repair. BioMed Res. Int. 2017, 2017, 1689341. [Google Scholar] [CrossRef]
- Han, Y.; Shang, Q.; Yao, J.; Ji, Y. Hydrogen sulfide: A gaseous signaling molecule modulates tissue homeostasis: Implications in ophthalmic diseases. Cell Death Dis. 2019, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- He, J.T.; Li, H.; Yang, L.; Mao, C.Y. Role of hydrogen sulfide in cognitive deficits: Evidences and mechanisms. Eur. J. Pharmacol. 2019, 849, 146–153. [Google Scholar] [CrossRef]
- Coavoy-Sánchez, S.A.; Costa, S.K.P.; Muscará, M.N. Hydrogen sulfide and dermatological diseases. Br. J. Pharmacol. 2020, 177, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Bruzzese, L.; Fromonot, J.; By, Y.; Durand-Gorde, J.M.; Condo, J.; Kipson, N.; Guieu, R.; Fenouillet, E.; Ruf, J. NF-κB enhances hypoxia-driven T-cell immunosuppression via upregulation of adenosine A2A receptors. Cell. Signal. 2014, 26, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling-an overview. In Novartis Foundation Symposia; Burnstock, G., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 26–48. [Google Scholar]
- Paganelli, F.; Gaudry, M.; Ruf, J.; Guieu, R. Recent advances in the role of the adenosinergic system in coronary artery disease. Cardiovasc. Res. 2020, cvaa275. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of adenosine receptors: The state of the art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Queiroz, G.; Talaia, C.; Gonçalves, J. Adenosine A2A receptor-mediated facilitation of noradrenaline release involves protein kinase C activation and attenuation of presynaptic inhibitory receptor-mediated effects in the rat vas deferens. J. Neurochem. 2003, 85, 740–748. [Google Scholar] [CrossRef]
- Ruf, J.; Paganelli, F.; Bonello, L.; Kipson, N.; Mottola, G.; Fromonot, J.; Condo, J.; Boussuges, A.; Bruzzese, L.; Kerbaul, F.; et al. Spare adenosine A2a receptors are associated with positive exercise stress test in coronary artery disease. Mol. Med. 2016, 22, 530–536. [Google Scholar] [CrossRef]
- Conlon, B.A. Advances in understanding adenosine as a plurisystem modulator in sepsis and the systemic inflammatory response syndrome (SIRS). Front. Biosci. 2005, 10, 2548–2565. [Google Scholar] [CrossRef] [Green Version]
- Fredholm, B.B. Adenosine receptors as targets for drug development. Drug News Perspect. 2003, 16, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; Yao, S.Y.M.; Hyde, R.J.; Ng, A.M.L.; Foppolo, S.; Barnes, K.; Ritzel, M.W.L.; Cass, C.E.; Young, J.D. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J. Biol. Chem. 2005, 280, 15880–15887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, S.A.; Beal, P.R.; Yao, S.Y.; King, A.E.; Cass, C.E.; Young, J.D. The equilibrative nucleoside transporter family, SLC29. Pflug. Arch. Eur. J. Physiol. 2004, 447, 735–743. [Google Scholar] [CrossRef]
- Young, J.D.; Yao, S.Y.M.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547. [Google Scholar] [CrossRef]
- Peleli, M.; Carlstrom, M. Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications. Mol. Asp. Med. 2017, 55, 62–74. [Google Scholar] [CrossRef]
- Fenouillet, E.; Mottola, G.; Kipson, N.; Paganelli, F.; Guieu, R.; Ruf, J. Adenosine receptor profiling reveals an association between the presence of spare receptors and cardiovascular disorders. Int. J. Mol. Sci. 2019, 20, 5964. [Google Scholar] [CrossRef] [Green Version]
- Gaudry, M.; Vairo, D.; Marlinge, M.; Gaubert, M.; Guiol, C.; Mottola, G.; Gariboldi, V.; Deharo, P.; Sadrin, S.; Maixent, J.M.; et al. Adenosine and its receptors: An expected tool for the diagnosis and treatment of coronary artery and ischemic heart diseases. Int. J. Mol. Sci. 2020, 21, 5321. [Google Scholar] [CrossRef]
- Haskó, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Bowser, J.L.; Lee, J.W.; Yuan, X.; Eltzschig, H.K. The hypoxia-adenosine link during inflammation. J. Appl. Physiol. 2017, 123, 1303–1320. [Google Scholar] [CrossRef] [Green Version]
- Singh, L.; Kulshrestha, R.; Singh, N.; Jaggi, A.S. Mechanisms involved in adenosine pharmacological preconditioning-induced cardioprotection. Korean J. Physiol. Pharmacol. 2018, 22, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredholm, B.B.; Yang, J.; Wang, Y. Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol. Asp. Med. 2017, 55, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Varani, K.; Portaluppi, F.; Gessi, S.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: Functional and biochemical aspects. Circulation 2000, 102, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.S.; Huang, L.; Ye, H.; Duong, E.T.A.; Bolton, W.K.; Linden, J.; Okusa, M.D. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2006, 290, F828–F837. [Google Scholar] [CrossRef] [Green Version]
- Persson, P.; Friederich-Persson, M.; Fasching, A.; Hansell, P.; Inagi, R.; Palm, F. Adenosine A2a receptor stimulation prevents proteinuria in diabetic rats by promoting an anti-inflammatory phenotype without affecting oxidative stress. Acta Physiol. 2015, 214, 311–318. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Csóka, B.; Németh, Z.H.; Virág, L.; Gergely, P.; Leibovich, S.J.; Pacher, P.; Sun, C.X.; Blackburn, M.R.; Vizi, E.S.; Deitch, E.A.; et al. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 2007, 110, 2685–2695. [Google Scholar] [CrossRef] [Green Version]
- Koscsó, B.; Csóka, B.; Kókai, E.; Németh, Z.H.; Pacher, P.; Virág, L.; Leibovich, S.J.; Haskó, G. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J. Leukoc. Biol. 2013, 94, 1309–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akash, M.S.H.; Rehman, K.; Chen, S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2013, 114, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Paquot, N.; Scheen, A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 2014, 24, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zollbrecht, C.; Winerdal, M.E.; Zhuge, Z.; Zhang, X.M.; Terrando, N.; Checa, A.; Sällström, J.; Wheelock, C.E.; Winqvist, O.; et al. Genetic abrogation of adenosine A3 receptor prevents uninephrectomy and high salt-induced hypertension. J. Am. Heart Assoc. 2016, 5, e003868. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Boeynaems, J.M. Purinergic signalling and immune cells. Purinergic Signal. 2014, 10, 529–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiel, M.; Caldwell, C.C.; Sitkovsky, M.V. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 2003, 5, 515–526. [Google Scholar] [CrossRef]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of neutrophil function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [Green Version]
- Cronstein, B.N.; Levin, R.I.; Philips, M.; Hirschhorn, R.; Abramson, S.B.; Weissmann, G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J. Immunol. 1992, 148, 2201–2206. [Google Scholar]
- Sullivan, G.W.; Lee, D.D.; Ross, W.G.; DiVietro, J.A.; Lappas, C.M.; Lawrence, M.B.; Linden, J. Activation of A2Aadenosine receptors inhibits expression of α4/β1 integrin (very late antigen-4) on stimulated human neutrophils. J. Leukoc. Biol. 2003, 75, 127–134. [Google Scholar] [CrossRef]
- Thiel, M.; Chambers, J.D.; Chouker, A.; Fischer, S.; Zourelidis, C.; Bardenheuer, H.J.; Arfors, K.E.; Peter, K. Effect of adenosine on the expression of β2 integrins and L-selectin of human polymorphonuclear leukocytes in vitro. J. Leukoc. Biol. 1996, 59, 671–682. [Google Scholar] [CrossRef]
- Sevigny, C.P.; Li, L.; Awad, A.S.; Huang, L.; McDuffie, M.; Linden, J.; Lobo, P.I.; Okusa, M.D. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J. Immunol. 2007, 178, 4240–4249. [Google Scholar] [CrossRef] [Green Version]
- Day, Y.J.; Li, Y.; Rieger, J.M.; Ramos, S.I.; Okusa, M.D.; Linden, J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J. Immunol. 2005, 174, 5040–5046. [Google Scholar] [CrossRef] [Green Version]
- Reutershan, J.; Cagnina, R.E.; Chang, D.; Linden, J.; Ley, K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J. Immunol. 2007, 179, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Montesinos, M.C.; Desai, A.; Delano, D.; Chen, J.F.; Fink, J.S.; Jacobson, M.A.; Cronstein, B.N. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003, 48, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.C.; Desai, A.; Chen, J.F.; Yee, H.; Schwarzschild, M.A.; Fink, J.S.; Cronstein, B.N. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am. J. Pathol. 2002, 160, 2009–2018. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, W.; Tang, R.; Zhu, C.; Bucher, C.; Blazar, B.R.; Geng, J.G.; Zhang, C.; Linden, J.; Wu, C.; et al. Adenosine receptor A2A deficiency in leukocytes increases arterial neointima formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.W.; Linden, J.; Buster, B.L.; Scheld, W.M. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: Synergy with the type IV phosphodiesterase inhibitor, rolipram. J. Infect. Dis. 1999, 180, 1550–1560. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, G.W.; Rieger, J.M.; Scheld, W.M.; Macdonald, T.L.; Linden, J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br. J. Pharmacol. 2001, 132, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Steingold, J.M.; Hatfield, S.M. Targeting hypoxia-A2A adenosinergic immunosuppression of antitumor T cells during cancer immunotherapy. Front. Immunol. 2020, 11, 570041. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signaling in the cardiovascular system. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Peleli, M.; Fredholm, B.B.; Sobrevia, L.; Carlström, M. Pharmacological targeting of adenosine receptor signaling. Mol. Asp. Med. 2017, 55, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Paganelli, F.; Saadjian, A.; Sampol, J.J.; Maixent, J.; Levy, S.; Guieu, R. Effects of percutaneous transluminal coronary angioplasty on coronary adenosine concentrations in humans. Eur. J. Clin. Investig. 2000, 30, 105–110. [Google Scholar] [CrossRef]
- Gariboldi, V.; Vairo, D.; Guieu, R.; Marlingue, M.; Ravis, E.; Lagier, D.; Mari, A.; Thery, E.; Collart, F.; Gaudry, M.; et al. Expressions of adenosine A2A receptors in coronary arteries and peripheral blood mononuclear cells are correlated in coronary artery disease patients. Int. J. Cardiol. 2017, 230, 427–431. [Google Scholar] [CrossRef]
- Shenoy, V.; Mehendale, V.; Prabhu, K.; Shetty, R.; Rao, P. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J. Clin. Biochem. 2014, 29, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headrick, J.P.; Ashton, K.J.; Rose’Meyer, R.B.; Peart, J.N. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol. Ther. 2013, 140, 92–111. [Google Scholar] [CrossRef]
- Shryock, J.C.; Belardinelli, L. Adenosine and adenosine receptors in the cardiovascular system: Biochemistry, physiology, and pharmacology. Am. J. Cardiol. 1997, 79, 2–10. [Google Scholar] [CrossRef]
- Musser, B.; Morgan, M.E.; Leid, M.; Murray, T.F.; Linden, J.; Vestal, R.E. Species comparison of adenosine and beta-adrenoceptors in mammalian atrial and ventricular myocardium. Eur. J. Pharmacol. 1993, 246, 105–111. [Google Scholar] [CrossRef]
- Hussain, T.; Mustafa, S.J. Binding of A1Adenosine receptor ligand [3H]8-cyclopentyl-1,3-dipropylxanthine in coronary smooth muscle. Circ. Res. 1995, 77, 194–198. [Google Scholar] [CrossRef]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of adenosine A2 receptor-cAMP system in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 1994, 199, 905–910. [Google Scholar] [CrossRef]
- Marala, R.B.; Mustafa, J.S. Immunological characterization of adenosine A2A receptors in human and porcine cardiovascular tissues. J. Pharmacol. Exp. Ther. 1998, 286, 1051–1057. [Google Scholar]
- Cushing, D.J.; Brown, G.L.; Sabouni, M.H.; Mustafa, S.J. Adenosine receptor-mediated coronary artery relaxation and cyclic nucleotide production. Am. J. Physiol. 1991, 261, H343–H348. [Google Scholar] [CrossRef]
- Ledent, C.; Vaugeois, J.M.; Schiffmann, S.N.; Pedrazzini, T.; Yacoubi, M.E.; Vanderhaeghen, J.J.; Costentin, J.; Heath, J.K.; Vassart, G.; Parmentier, M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 1997, 388, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Shryock, J.C.; Snowdy, S.; Baraldi, P.G.; Cacciari, B.; Spalluto, G.; Monopoli, A.; Ongini, E.; Baker, S.P.; Belardinelli, L. A2A-adenosine receptor reserve for coronary vasodilation. Circulation 1998, 98, 711–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berwick, Z.C.; Payne, G.A.; Lynch, B.; Dick, G.M.; Sturek, M.; Tune, J.D. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: Role of K(V) and K(ATP) channels. Microcirculation 2010, 17, 600–607. [Google Scholar] [CrossRef]
- Sanjani, M.S.; Teng, B.; Krahn, T.; Tilley, S.; Ledent, C.; Mustafa, S.J. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double-knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2322–H2333. [Google Scholar] [CrossRef] [Green Version]
- Monahan, T.S.; Sawmiller, D.R.; Fenton, R.A.; Dobson, J.G. Adenosine A2a-receptor activation increases contractility in isolated perfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1472–H1481. [Google Scholar] [CrossRef] [Green Version]
- Dobson, J. Adenosine A2 receptor function in rat ventricular myocytes. Cardiovasc. Res. 1997, 34, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Morrison, R.R.; Talukder, M.A.H.; Ledent, C.; Mustafa, S.J. Cardiac effects of adenosine in A2A receptor knockout hearts: Uncovering A2B receptors. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H437–H444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, Y.K.; Park, W.S.; Ko, J.H.; Han, J.; Kim, N.; Earm, Y.E. Protein kinase A-dependent activation of inward rectifier potassium channels by adenosine in rabbit coronary smooth muscle cells. Biochem. Biophys. Res. Commun. 2005, 337, 1145–1152. [Google Scholar] [CrossRef]
- Zhao, Z.; Francis, C.E.; Ravid, K. An A3-subtype adenosine receptor is highly expressed in rat vascular smooth muscle cells: Its role in attenuating adenosine-induced increase in cAMP. Microvasc. Res. 1997, 54, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Makaritsis, K.; Francis, C.E.; Gavras, H.; Ravid, K. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: Studies in knock-out mice. Biochim. Biophys. Acta 2000, 1500, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J. Am. Coll. Cardiol. 2018, 72, e91–e220. [Google Scholar] [CrossRef] [Green Version]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Headrick, J.P.; Lasley, R.D. Adenosine receptors and reperfusion injury of the heart. Handb. Exp. Pharmacol. 2009, 193, 189–214. [Google Scholar] [CrossRef] [Green Version]
- Haskó, G.; Cronstein, B.N. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef]
- Grenz, A.; Homann, D.; Eltzschig, H.K. Extracellular adenosine: A safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid. Redox Signal. 2011, 15, 2221–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, M.L.; Gorzolla, I.C.; Schittenhelm, J.; Robson, S.C.; Eltzschig, H.K. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J. Immunol. 2010, 184, 4017–4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenz, A.; Zhang, H.; Hermes, M.; Eckle, T.; Klingel, K.; Huang, D.Y.; Müller, C.E.; Robson, S.C.; Osswald, H.; Eltzschig, H.K. Contribution of E-NTPDasel (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007, 21, 2863–2873. [Google Scholar] [CrossRef]
- Köhler, D.; Eckle, T.; Faigle, M.; Grenz, A.; Mittelbronn, M.; Laucher, S.; Hart, M.L.; Robson, S.C.; Müller, C.E.; Eltzschig, H.K. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 2007, 116, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.L.; Much, C.; Gorzolla, I.C.; Schittenhelm, J.; Kloor, D.; Stahl, G.L.; Eltzschig, H.K. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology 2008, 135, 1739–1750.e3. [Google Scholar] [CrossRef] [PubMed]
- Eckle, T.; Krahn, T.; Grenz, A.; Köhler, D.; Mittelbronn, M.; Ledent, C.; Jacobson, M.A.; Osswald, H.; Thompson, L.F.; Unertl, K.; et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 2007, 115, 1581–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenz, A.; Zhang, H.; Eckle, T.; Mittelbronn, M.; Wehrmann, M.; Köhle, C.; Kloor, D.; Thompson, L.F.; Osswald, H.; Eltzschig, H.K. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J. Am. Soc. Nephrol. 2007, 18, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.L.; Henn, M.; Köhler, D.; Kloor, D.; Mittelbronn, M.; Gorzolla, I.C.; Stahl, G.L.; Eltzschig, H.K. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008, 22, 2784–2797. [Google Scholar] [CrossRef] [Green Version]
- Cronstein, B.N.; Daguma, L.; Nichols, D.; Hutchison, A.J.; Williams, M. The adenosine/neutrophil paradox resolved: Human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J. Clin. Investig. 1990, 85, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.L.; Linden, J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood 2010, 116, 5010–5020. [Google Scholar] [CrossRef] [Green Version]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [Green Version]
- Grenz, A.; Osswald, H.; Eckle, T.; Yang, D.; Zhang, H.; Tran, Z.V.; Klingel, K.; Ravid, K.; Eltzschig, H.K. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008, 5, e137. [Google Scholar] [CrossRef] [Green Version]
- Eckle, T.; Faigle, M.; Grenz, A.; Laucher, S.; Thompson, L.F.; Eltzschig, H.K. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 2008, 111, 2024–2035. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.L.; Jacobi, B.; Schittenhelm, J.; Henn, M.; Eltzschig, H.K. A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J. Immunol. 2009, 182, 3965–3968. [Google Scholar] [CrossRef]
- Eckle, T.; Hartmann, K.; Bonney, S.; Reithel, S.; Mittelbronn, M.; Walker, L.A.; Lowes, B.D.; Han, J.; Borchers, C.H.; Buttrick, P.M.; et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat. Med. 2012, 18, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, P.; Linden, J.; Lobo, P.; Douglas Okusa, M.; Brayman, K.L. The immunosuppressive role of adenosine A2A receptors in ischemia reperfusion injury and islet transplantation. Curr. Diabetes Rev. 2012, 8, 419–433. [Google Scholar] [CrossRef] [Green Version]
- Guieu, R.; Kipson, N.; Ruf, J.; Fournier, N.; Laine, M.; Foucher, M.C.; Fromonot, J.; Mottola, G.; Bruzzese, L.; Boussuges, A.; et al. Low basal expression of A2A adenosine receptors and increase in adenosine plasma concentration are associated with positive exercise stress testing. Int. J. Cardiol. 2015, 180, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Laghi-Pasini, F.; Camurri, A.; Capecchi, P.L.; Maccherini, M.; Diciolla, F.; Ceccatelli, L.; Enea Lazzerini, P.; Ulouglu, C.; Cattabeni, F.; et al. Changes of peripheral A 2A adenosine receptors in chronic heart failure and cardiac transplantation. FASEB J. 2002, 17, 280–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudry, M.; Marlinge, M.; Deharo, P.; Vairo, D.; Bottone, S.; Mottola, G.; Kipson, N.; Criado, C.; Mace, P.; Chefrour, M.; et al. Pharmacological profile of adenosine A2A receptors in patients with lower extremity peripheral artery disease and associated coronary artery disease: A pilot study. Int. J. Cardiol. 2019, 285, 121–127. [Google Scholar] [CrossRef]
- Vairo, D.; Giacobbe, C.; Guiol, C.; Chaptal, M.C.; Di Taranto, M.D.; Bruzzese, L.; Ruf, J.; Guieu, R.; Fortunato, G.; Fenouillet, E.; et al. Correlation between low adenosine A2A receptor expression and hypercholesterolemia: A new component of the cardiovascular risk? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158850. [Google Scholar] [CrossRef]
- Bagheri, B.; Zargari, M.; Meshkini, F.; Dinarvand, K.; Mokhberi, V.; Azizi, S.; Rasouli, M. Uric acid and coronary artery disease, two sides of a single coin: A determinant of antioxidant system or a factor in metabolic syndrome. J. Clin. Diagn. Res. 2016, 10, OC27–OC31. [Google Scholar] [CrossRef]
- Gaubert, M.; Marlinge, M.; Alessandrini, M.; Laine, M.; Bonello, L.; Fromonot, J.; Cautela, J.; Thuny, F.; Barraud, J.; Mottola, G.; et al. Uric acid levels are associated with endothelial dysfunction and severity of coronary atherosclerosis during a first episode of acute coronary syndrome. Purinergic Signal. 2018, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, L.; Fenouillet, E.; Fromonot, J.; Durand-Gorde, J.M.; Condo, J.; Kipson, N.; Mottola, G.; Deharo, P.; Guieu, R.; Ruf, J. High homocysteine levels prevent via H2 S the CoCl2 -induced alteration of lymphocyte viability. J. Cell. Mol. Med. 2016, 20, 1411–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromonot, J.; Deharo, P.; Bruzzese, L.; Cuisset, T.; Quilici, J.; Bonatti, S.; Fenouillet, E.; Mottola, G.; Ruf, J.; Guieu, R. Adenosine plasma level correlates with homocysteine and uric acid concentrations in patients with coronary artery disease. Can. J. Physiol. Pharmacol. 2016, 94, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Deharo, P.; Marlinge, M.; Guiol, C.; Vairo, D.; Fromonot, J.; Mace, P.; Chefrour, M.; Gastaldi, M.; Bruzzese, L.; Gaubert, M.; et al. Homocysteine concentration and adenosine A(2A) receptor production by peripheral blood mononuclear cells in coronary artery disease patients. J. Cell. Mol. Med. 2020, 24, 8942–8949. [Google Scholar] [CrossRef] [PubMed]
- Ruf, J.; Vairo, D.; Paganelli, F.; Guieu, R. Extracellular vesicles with ubiquitinated adenosine A(2A) receptor in plasma of patients with coronary artery disease. J. Cell. Mol. Med. 2019, 23, 6805–6811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koupenova, M.; Ravid, K. Biology of platelet purinergic receptors and implications for platelet heterogeneity. Front. Pharmacol. 2018, 9, 37. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganelli, F.; Mottola, G.; Fromonot, J.; Marlinge, M.; Deharo, P.; Guieu, R.; Ruf, J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? Int. J. Mol. Sci. 2021, 22, 1690. https://doi.org/10.3390/ijms22041690
Paganelli F, Mottola G, Fromonot J, Marlinge M, Deharo P, Guieu R, Ruf J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? International Journal of Molecular Sciences. 2021; 22(4):1690. https://doi.org/10.3390/ijms22041690
Chicago/Turabian StylePaganelli, Franck, Giovanna Mottola, Julien Fromonot, Marion Marlinge, Pierre Deharo, Régis Guieu, and Jean Ruf. 2021. "Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link?" International Journal of Molecular Sciences 22, no. 4: 1690. https://doi.org/10.3390/ijms22041690





