Two-Step Triethylamine-Based Synthesis of MgO Nanoparticles and Their Antibacterial Effect against Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
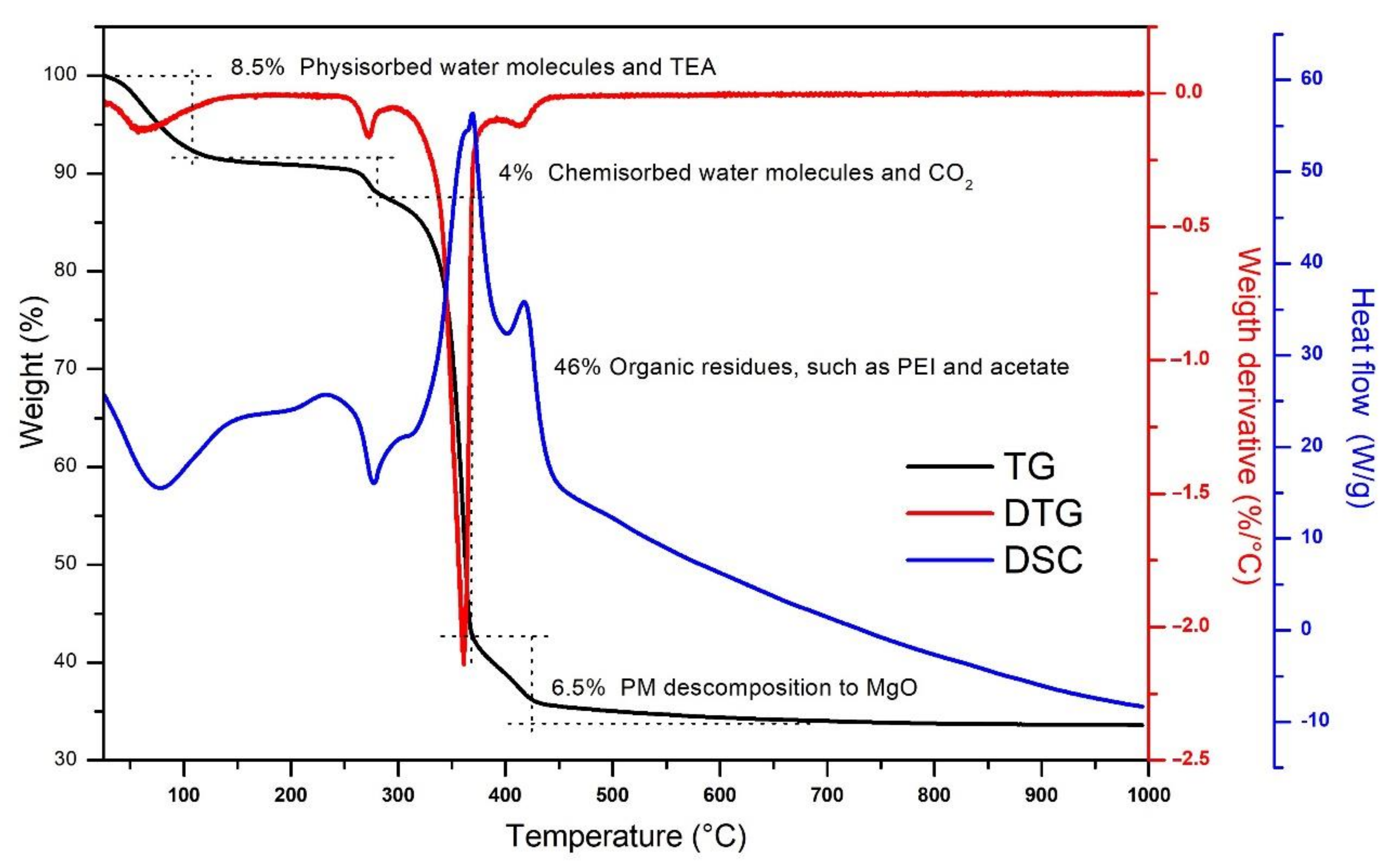
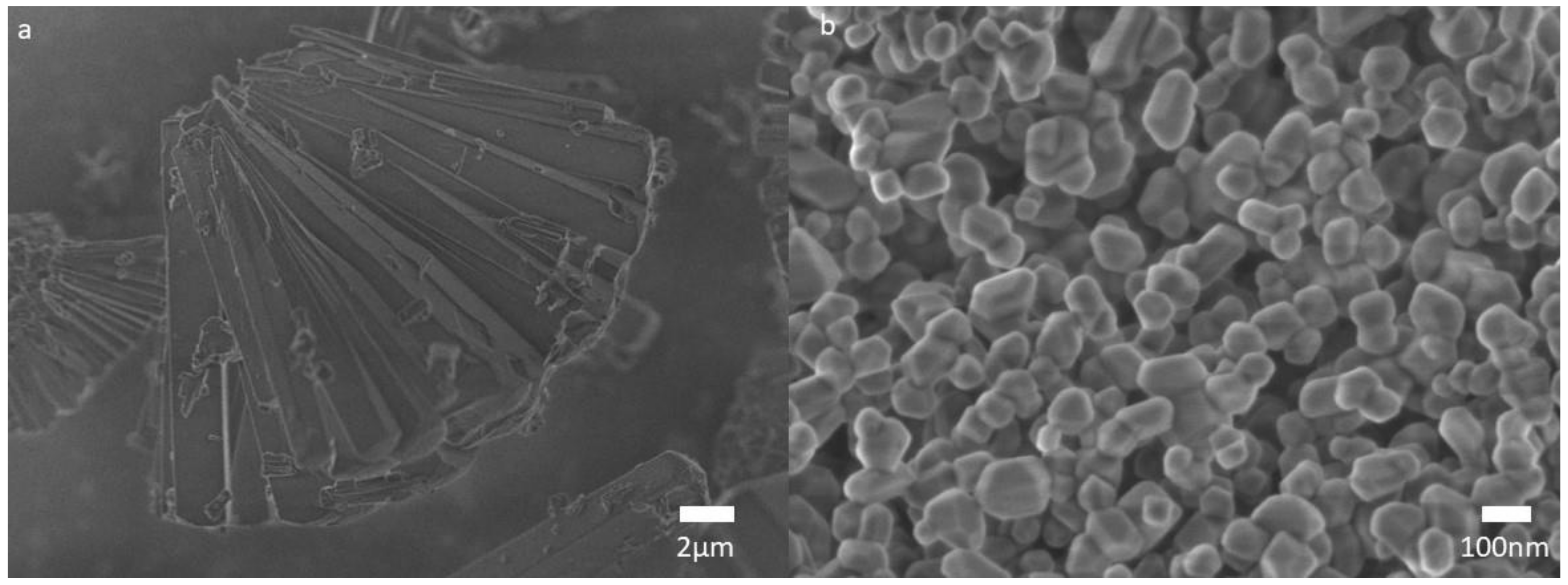
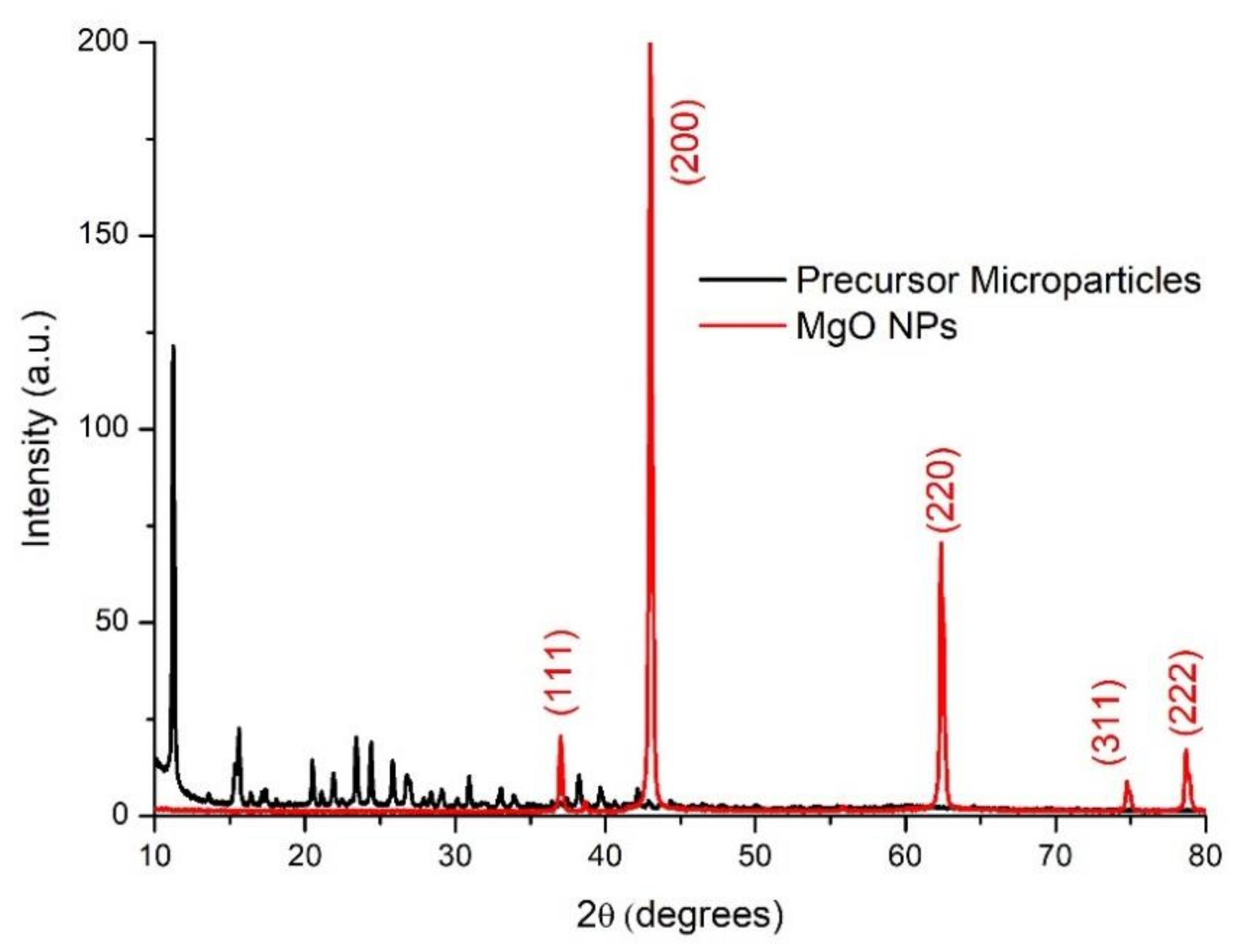
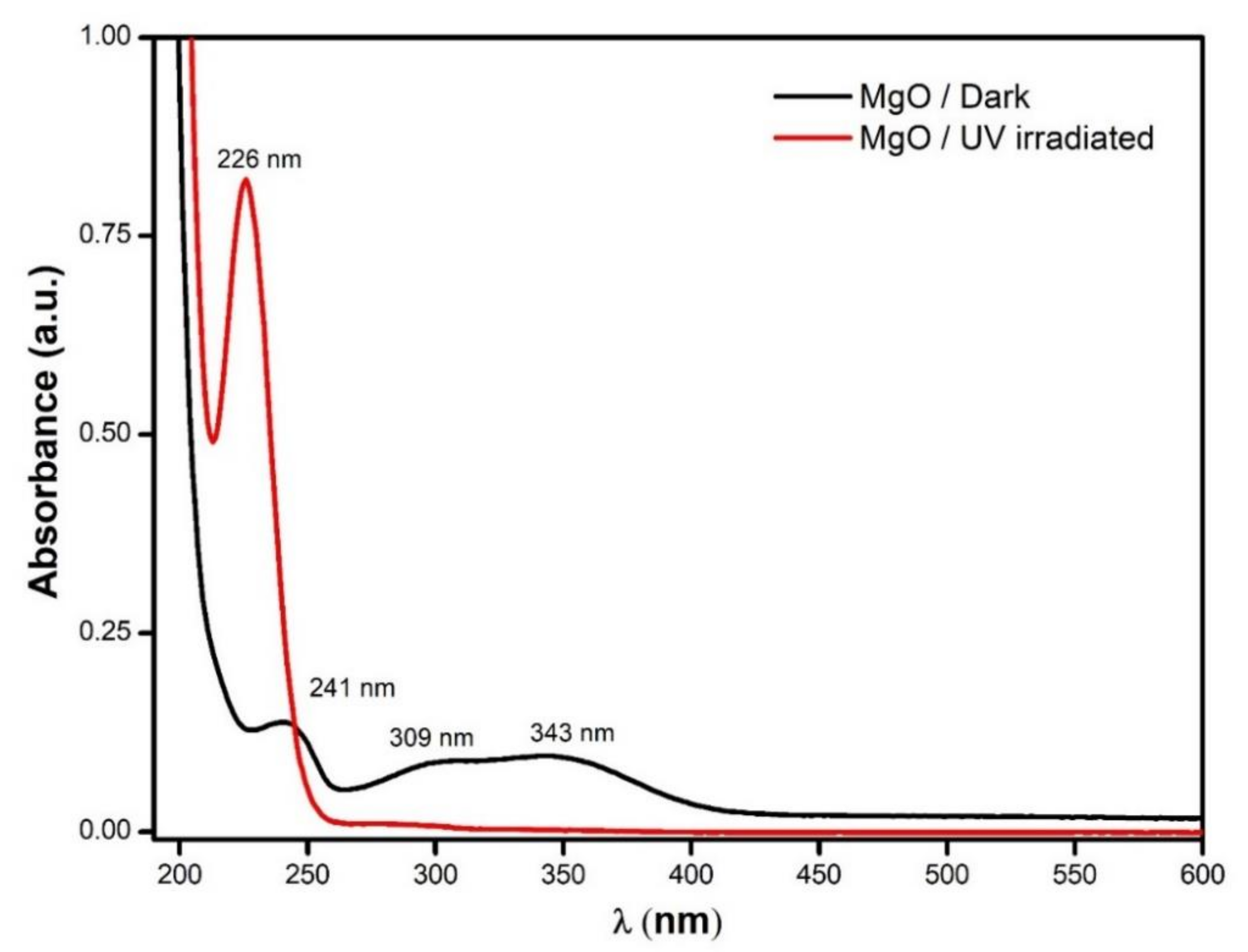
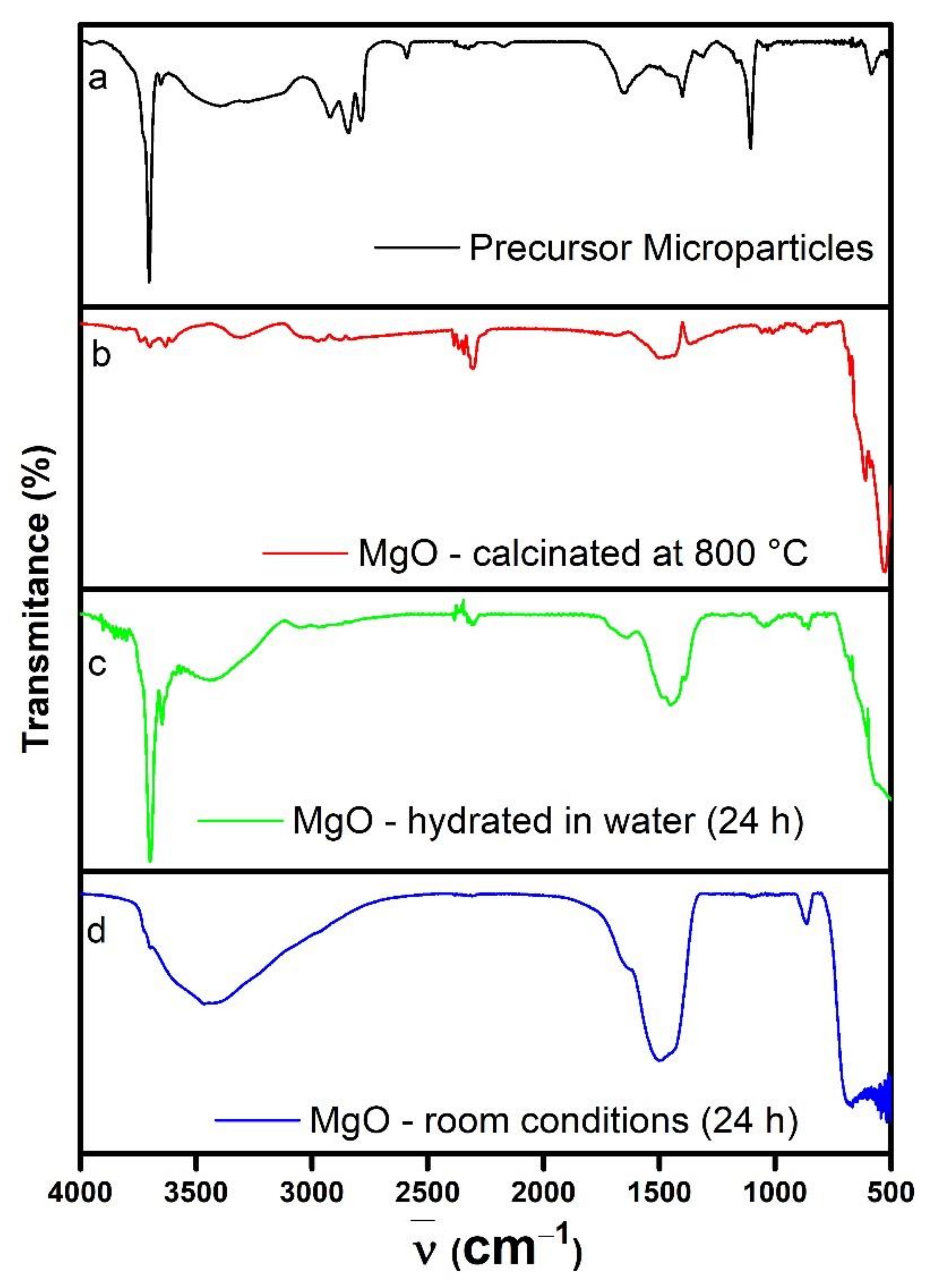
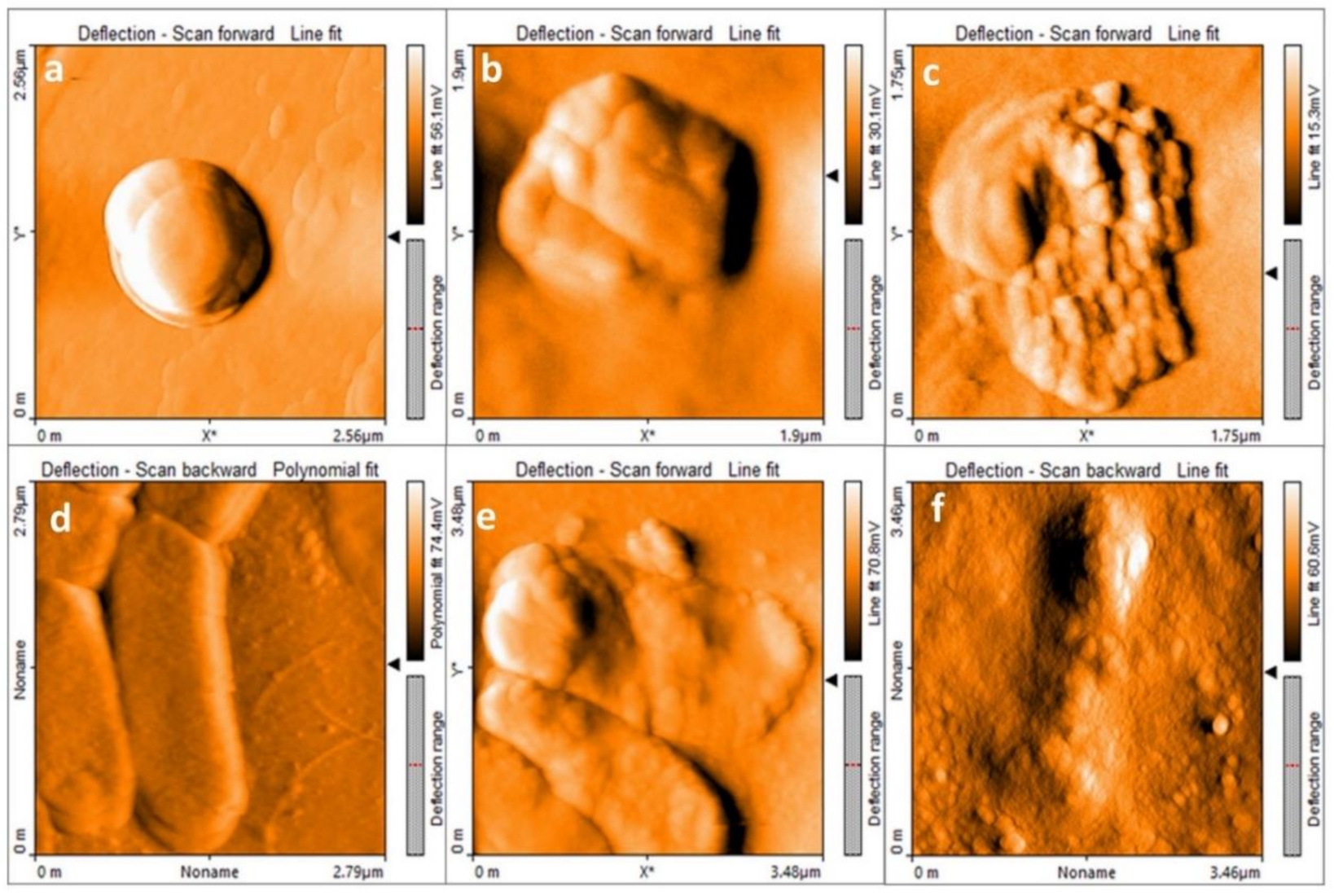
3.1. MgO Nanoparticles Characterization
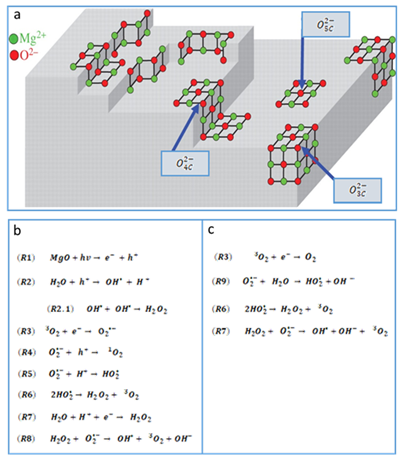
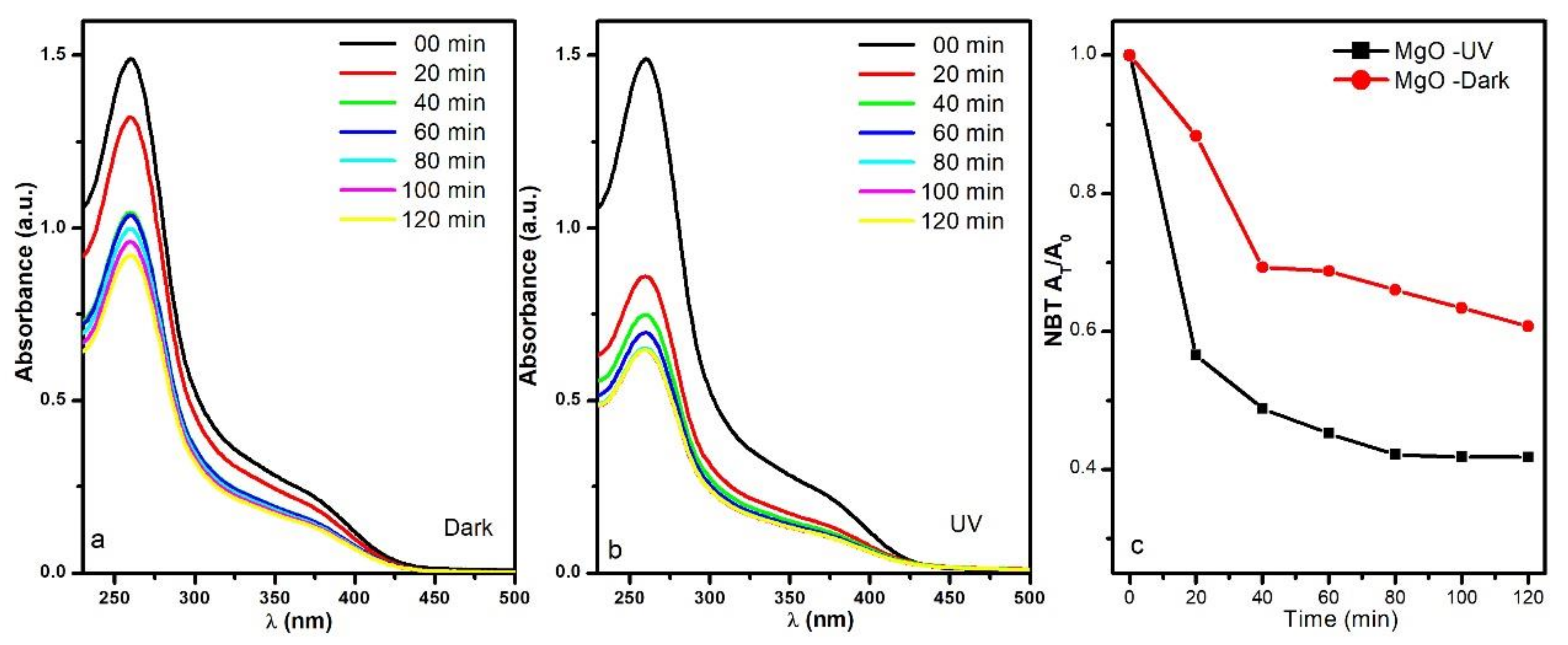
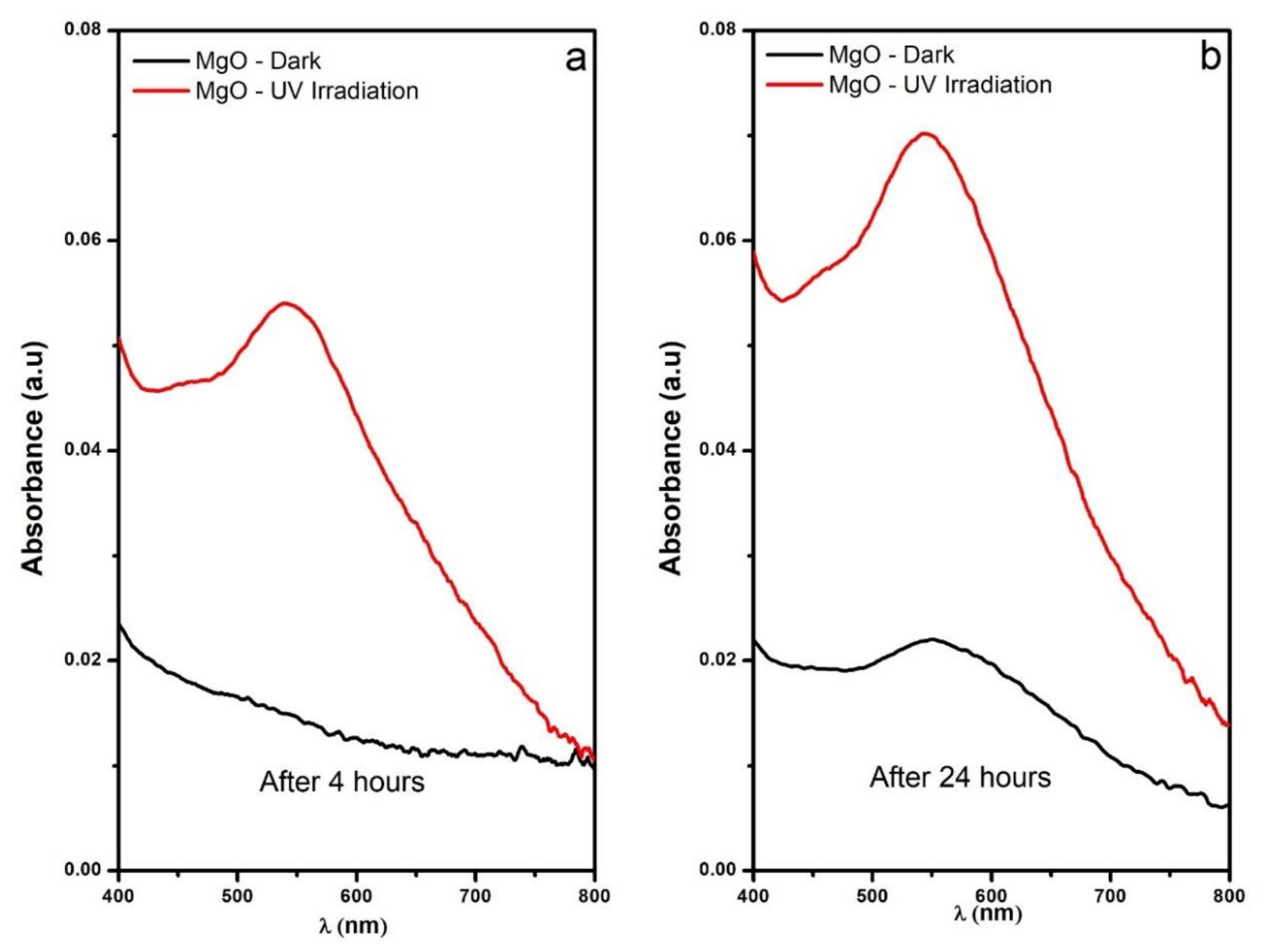
3.2. Reactive Oxygen Species Production by the MgO NPs
3.3. MgO NPs Antibacterial Effect Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawlinson, L.A.B.; Ryan, S.M.; Mantovani, G.; Syrett, J.A.; Haddleton, D.M.; Brayden, D.J. Antibacterial Effects of Poly(2-(dimethylaminoethyl)methacrylate) against Selected Gram-positive and Gram-negative Bacteria. Biomacromolecules 2010, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Vatsha, B.; Tetyana, P.; Shumbula, P.M.; Ngila, J.C.; Sikhwivhilu, L.M.; Moutloali, R.M. Effects of Precipitation Temperature on Nanoparticle Surface Area and Antibacterial Behaviour of Mg(OH)2 and MgO Nanoparticles. JBNB 2013, 4, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Foster, H.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Sizochenko, N.; Rasulev, B.; Gajewicz, A.; Kuz’Min, V.; Puzyn, T.; Leszczynski, J. From basic physics to mechanisms of toxicity: The “liquid drop” approach applied to develop predictive classification models for toxicity of metal oxide nanoparticles. Small 2014, 6, 13986–13993. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Nasibulin, A.G.; Sun, L.; Hämäläinen, S.; Shandakov, S.D.; Banhart FKauppinen, E.I. In Situ TEM Observation of MgO Nanorod Growth. Cryst. Growth Des. 2010, 10, 414–417. [Google Scholar] [CrossRef]
- Cha, S.-H.; Hong, J.; McGuffie, M.; Yeom, B.; Vanepps, J.S.; Kotov, N.A. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano 2015, 9, 9097–9105. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: A promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Bindhu, M.; Umadevi, M.; Micheal, M.K.; Arasu, M.V.; Al-Dhabi, N.A. Structural, morphological and optical properties of MgO nanoparticles for antibacterial applications. Mater. Lett. 2016, 166, 19–22. [Google Scholar] [CrossRef]
- Yousefi, S.; Ghasemi, B.; Tajally, M.; Asghari, A. Optical properties of MgO and Mg(OH)2 nanostructures synthesized by a chemical precipitation method using impure brine. J. Alloys Compd. 2017, 711, 521–529. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, L.; Wang, C.; Ma, X.; Li, Y.; Liu, Z. Polymer encapsulated upconversion nanoparticle/iron oxide nanocomposites for multimodal imaging and magnetic targeted drug delivery. Biomaterials 2011, 32, 9364–9373. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: Current status. Colloids Surf. B Biointerfaces 2016, 146, 70–83. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, M.-T.; Ou-Yang, H.D.; Walker, S.G.; Wang, H.Z.; Gordon, C.R.; Guterman, S.; Zawacki, E.; Applebaum, E.; Brink, P.R.; et al. Exposure to TiO2 nanoparticles increases Staphylococcus aureus infection of HeLa cells. J. Nanobiotechnol. 2016, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Dianqing, L.; Yanjun, L.; Evans, D.G.; Xue, D. Influence of nano-MgO particle size on bactericidal action against Bacillus subtilis var. niger. Chin. Sci. Bull. 2005, 50, 514–519. [Google Scholar]
- Saleh, N.B.; Milliron, D.J.; Aich, N.; Katz, L.E.; Liljestrand, H.M.; Kirisits, M.J. Importance of doping, dopant distribution, and defects on electronic band structure alteration of metal oxide nanoparticles: Implications for reactive oxygen species. Sci. Total Environ. 2016, 568, 926–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, T.; Nosaka, Y. Properties of O2•− and OH• Formed in TiO2 Aqueous Suspensions by Photocatalytic Reaction and the Influence of H2O2 and Some Ions. Langmuir 2002, 18, 3247–3254. [Google Scholar] [CrossRef]
- Pathak, N.; Ghosh, P.S.; Gupta, S.K.; Kadam, R.M.; Arya, A. Defects induced changes in the electronic structures of MgO and their correlation with the optical properties: A special case of electron–hole recombination from the conduction band. RSC Adv. 2016, 6, 96398–96415. [Google Scholar] [CrossRef]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Moon, J.Y.; Hyun, H.B.; Cho, S.K.; Kim, S.-J. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J. Mater. Chem. 2012, 22, 24610–24617. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Manivannan, G.; Kim, S.-J.; Jeyasubramanian, K.; Premanathan, M. Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Allegri, M.; Bianchi, M.G.; Chiu, M.; Varet, J.; Costa, A.L.; Ortelli, S.; Blosi, M.; Bussolati, O.; Poland, C.A.; Bergamaschi, E. Shape-Related Toxicity of Titanium Dioxide Nanofibres. PLoS ONE 2016, 11, e0151365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef]
- Horie, M.; Fujita, K.; Kato, H.; Endoh, S.; Nishio, K.; Komaba, L.K.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Hagihara, Y.; et al. Association of the physical and chemical properties and the cytotoxicity of metal oxide nanoparticles: Metal ion release, adsorption ability and specific surface area. Metallomics 2012, 4, 350–360. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L. Calcium and Magnesium Ions Are Membrane-Active against Stationary-Phase Staphylococcus aureus with High Specificity. Sci. Rep. 2016, 6, 20628. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Joyce, L.; Ellicott, H.; Jiang, Y. Surfactant controlled magnesium oxide synthesis for base catalysis. Catal. Sci. Technol. 2016, 6, 1903–1912. [Google Scholar] [CrossRef]
- Liu, J.; Cai, J.; Son, Y.-C.; Gao, Q.; Suib, S.L.; Aindow, M. Magnesium Manganese Oxide Nanoribbons: Synthesis, Characterization, and Catalytic Application. J. Phys. Chem. B 2002, 106, 9761–9768. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Microstructural, optical and magnetic properties study of nanocrystalline MgO. Mater. Res. Express 2014, 1, 025026. [Google Scholar] [CrossRef]
- Vahedi, J.; Karimi-Maleh, H.; Baghayeri, M.; Sanati, A.L.; Khalilzadeh, M.A.; Bahrami, M. A fast and sensitive nanosensor based on MgO nanoparticle room-temperature ionic liquid carbon paste electrode for determination of methyldopa in pharmaceutical and patient human urine samples. Ionics 2013, 19, 1907–1914. [Google Scholar] [CrossRef]
- Purwajanti, S.; Zhou, L.; Nor, Y.A.; Zhang, J.; Zhang, H.; Huang, X.; Yu, C. Synthesis of Magnesium Oxide Hierarchical Microspheres: A Dual-Functional Material for Water Remediation. ACS Appl. Mater. Interfaces 2015, 7, 21278–21286. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Ma, J.; Jo, S.; Lee, S.; Kim, C.S. Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment. Nanomaterials 2020, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Bertinetti, L.; Drouet, C.; Combes, C.; Rey, C.; Tampieri, A.; Coluccia, S.; Martra, G. Surface Characteristics of Nanocrystalline Apatites: Effect of Mg Surface Enrichment on Morphology, Surface Hydration Species, and Cationic Environments. Langmuir 2009, 25, 5647–5654. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Marwaha, N.; Verma, R.; Gupta, B.K.; Srivastava, A.K. Facile synthesis of defect-induced highly-luminescent pristine MgO nanostructures for promising solid-state lighting applications. RSC Adv. 2016, 6, 4960–4968. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of Antibacterial Activity of MgO: Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-Assisted Synthesis of Nanocrystalline MgO and Its Use as a Bacteriocide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanopart. Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, D.; Wang, W.; Tan, F.; Wong, P.K.; Wang, X.; Qiu, X.; Qiao, X. Highly effective antibacterial activity and synergistic effect of Ag-MgO nanocomposite against Escherichia coli. J. Alloys Compd. 2016, 684, 282–290. [Google Scholar] [CrossRef]
- Khalid, A.; Norello, R.; Abraham, A.N.; Tetienne, J.-P.; Karle, T.J.; Lui, E.W.; Xia, K.; Tran, P.A.; O’Connor, A.J.; Mann, G.B.; et al. Biocompatible and Biodegradable Magnesium Oxide Nanoparticles with In Vitro Photostable Near-Infrared Emission: Short-Term Fluorescent Markers. Nanomaterials 2019, 9, 1360. [Google Scholar] [CrossRef] [Green Version]
- Sawai, J.; Kojima, H.; Igarashi, H.; Hashimoto, A.; Shoji, S.; Sawaki, T.; Hakoda, A.; Kawada, E.; Kokugan, T.; Shimizu, M. Antibacterial characteristics of magnesium oxide powder. World J. Microbiol. Biotechnol. 2000, 16, 187–194. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Samodi, A.; Rashidi, A.; Marjani, K.; Ketabi, S. Effects of surfactants, solvents and time on the morphology of MgO nanoparticles prepared by the wet chemical method. Mater. Lett. 2013, 109, 269–274. [Google Scholar] [CrossRef]
- Niu, H.; Yang, Q.; Tang, K.; Xie, Y. Large-scale synthesis of single-crystalline MgO with bone-like nanostructures. J. Nanopart. Res. 2006, 8, 881–888. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Pahari, S.K.; Pal, P.; Bajaj, H.C.; Mukhopadhyay, I.; Panda, A.B. Controlled Synthesis of Different Morphologies of MgO and Their Use as Solid Base Catalysts. J. Phys. Chem. C 2011, 115, 12308–12316. [Google Scholar] [CrossRef]
- Hadia, N.; Mohamed, H.A.-H. Characteristics and optical properties of MgO nanowires synthesized by solvothermal method. Mater. Sci. Semicond. Process. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Esmaeili, E.; Khodadadi, A.; Mortazavi, Y.; Khodadadi, A.A. Microwave-induced combustion process variables for MgO nanoparticle synthesis using polyethylene glycol and sorbitol. J. Eur. Ceram. Soc. 2009, 29, 1061–1068. [Google Scholar] [CrossRef]
- Gandhi, S.; Abiramipriya, P.; Pooja, N.; Jeyakumari, J.J.L.; Arasi, A.Y.; Dhanalakshmi, V.; Nair, M.G.; Anbarasan, R. Synthesis and characterizations of nano sized MgO and its nano composite with poly(vinyl alcohol). J. Non-Cryst. Solids 2011, 357, 181–185. [Google Scholar] [CrossRef]
- Alamdari, S.; Tafreshi, M.J.; Ghamsari, M.S. Preparation and characterization of gallium-doped zinc oxide/polystyrene nanocomposite scintillator for alpha particles detection. Appl. Phys. A 2019, 125, 450. [Google Scholar] [CrossRef]
- Bian, S.-W.; Baltrusaitis, J.; Galhotra, P.; Grassian, V.H. A template-free, thermal decomposition method to synthesize mesoporous MgO with a nanocrystalline framework and its application in carbon dioxide adsorption. J. Mater. Chem. 2010, 20, 8705–8710. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, D.; Zhu, X.; Wang, W.; Tan, F.; Chen, J.; Qiao, X.; Qiu, X. Sol-gel preparation of Ag-doped MgO nanoparticles with high efficiency for bacterial inactivation. Ceram. Int. 2017, 43, 1066–1072. [Google Scholar] [CrossRef]
- Obregón, S.; Ruíz-Gómez, M.A.; Hernández-Uresti, D.B. Direct evidence of the photocatalytic generation of reactive oxygen species (ROS) in a Bi2W2O9 layered-structure. J. Colloid Interface Sci. 2017, 506, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, D.; Yi, Z.; Jiang, M.; Wang, L.; Zhou, Z.; Fan, X.; Wang, Y.; Hui, D. Antimicrobial Mechanism Based on H2O2 Generation at Oxygen Vacancies in ZnO Crystals. Langmuir 2013, 29, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.; Bula, K.; Myszka, K.; Rozmanowski, T.; Szwarc-Rzepka, K.; Pilarski, K.; Chrzanowski, Ł.; Czaczyk, K.; Jesionowski, T. Functional polypropylene composites filled with ultra-fine magnesium hydroxide. Open Chem. 2015, 13, 161–171. [Google Scholar] [CrossRef]
- Fricker, K.J.; Park, A.-H.A. Effect of H2O on Mg(OH)2 carbonation pathways for combined CO2 capture and storage. Chem. Eng. Sci. 2013, 100, 332–341. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Hemalatha, P.; Ganesh, M.; Mei, P.M.; Jang, H.T. A direct synthesis of mesoporous carbon supported MgO sorbent for CO2 capture. Fuel 2011, 90, 1662–1667. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Y.; Li, B.; Wang, H.; Zeng, G. Adsorption mechanism of polyethyleneimine modified magnetic core–shell Fe3O4@SiO2 nanoparticles for anionic dye removal. RSC Adv. 2019, 9, 32462–32471. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Unluer, C.; Yang, E.-H.; Al-Tabbaa, A. Recovery of reactive MgO from reject brine via the addition of NaOH. Desalination 2018, 429, 88–95. [Google Scholar] [CrossRef]
- Askeland, D.R. The Science and Engineering of Materials; PWS Publishing Company: Boston, MA, USA, 1994. [Google Scholar]
- Rodenbough, P.P.; Zheng, C.; Liu, Y.; Hui, C.; Xia, Y.; Ran, Z.; Hu, Y.; Chan, S.-W. Lattice Expansion in Metal Oxide Nanoparticles: MgO, Co3O4, & Fe3O4. J. Am. Ceram. Soc. 2017, 100, 384–392. [Google Scholar] [CrossRef]
- Illas, F.; Pacchioni, G. Optical properties of surface and bulk F centers in MgO from ab initio cluster model calculations. J. Chem. Phys. 1998, 108, 7835–7841. [Google Scholar] [CrossRef]
- Sterrer, M.; Diwald, O.; Knözinger, E. Vacancies and Electron Deficient Surface Anions on the Surface of MgO Nanoparticles. J. Phys. Chem. B 2000, 104, 3601–3607. [Google Scholar] [CrossRef]
- Popov, A.; Monge, M.; González, R.; Chen, Y.; Kotomin, E. Dynamics of F-center annihilation in thermochemically reduced MgO single crystals. Solid State Commun. 2001, 118, 163–167. [Google Scholar] [CrossRef]
- Popov, A.I.; Shirmane, L.; Pankratov, V.; Lushchik, A.; Kotlov, A.; Serga, V.; Kulikova, L.; Chikvaidze, G.; Zimmermann, J. Comparative study of the luminescence properties of macro- and nanocrystalline MgO using synchrotron radiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 310, 23–26. [Google Scholar] [CrossRef]
- Berger, T.; Sterrer, M.; Stankic, S.; Bernardi, J.; Diwald, O.; Knözinger, E. Trapping of photogenerated charges in oxide nanoparticles. Mater. Sci. Eng. C 2005, 25, 664–668. [Google Scholar] [CrossRef]
- Badar, N.; Chayed, N.F.; Roshidah, R.; Kamarudin, N.; Kamarulzaman, N.; Rusdi, R. Band Gap Energies of Magnesium Oxide Nanomaterials Synthesized by the Sol-Gel Method. Adv. Mater. Res. 2012, 545, 157–160. [Google Scholar] [CrossRef]
- Vijayalakshmi, B.; Lakshmipriya, V.; Radha, K.P.; Natarajan, B.; Ramadas, V.; Muthuselvi, C. Synthesis and optical characterization of MgO nanoparticles using chemical precipitation method. Int. Res. J. India 2016, 11, 1–6. [Google Scholar]
- Chizallet, C.; Costentin, G.; Che, M.; Delbecq, F.; Sautet, P. Infrared Characterization of Hydroxyl Groups on MgO: A Periodic and Cluster Density Functional Theory Study. J. Am. Chem. Soc. 2007, 129, 6442–6452. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Li, H.; Wei, D.; Qiu, G.; Liu, F.; Yang, B. Electrochemical properties of tadpole-like MgO nanobelts. Mater. Lett. 2013, 106, 45–48. [Google Scholar] [CrossRef]
- Somanathan, T.; Krishna, V.M.; Saravanan, V.; Kumar, R.; Kumar, R. MgO Nanoparticles for Effective Uptake and Release of Doxorubicin Drug: pH Sensitive Controlled Drug Release. J. Nanosci. Nanotechnol. 2016, 16, 9421–9431. [Google Scholar] [CrossRef]
- Layek, K.; Kantam, M.L.; Shirai, M.; Nishio-Hamane, D.; Sasaki, T.; Maheswaran, H. Gold nanoparticles stabilized on nanocrystalline magnesium oxide as an active catalyst for reduction of nitroarenes in aqueous medium at room temperature. Green Chem. 2012, 14, 3164. [Google Scholar] [CrossRef]
- Zahir, H.; Rahman, M.M.; Irshad, K.; Rahman, M.M.; Rahman, M.M.; Rahman, M. Shape-Stabilized Phase Change Materials for Solar Energy Storage: MgO and Mg(OH)2 Mixed with Polyethylene Glycol. Nanomaterials 2019, 9, 1773. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, K.; Lan, L.; Ma, J.; Zeng, Y.; Xu, L.; Wu, D. Double-layered hyaluronic acid/stearic acid-modified polyethyleneimine nanoparticles encapsulating (−)-gossypol: A nanocarrier for chiral anticancer drugs. J. Mater. Chem. B 2014, 2, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wu, R.; Mao, M.; Li, L.S.; Zhou, C.; Shen, H. The enhanced fluorescence properties & colloid stability of aqueous CdSe/ZnS QDs modified with N-alkylated poly(ethyleneimine). New J. Chem. 2015, 39, 4334–4342. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Zhang, H.; Zhang, Z.; Zhao, Y.; Liu, Y. Preparation of Calcium Magnesium Acetate Snow Melting Agent Using Raw Calcium Acetate-Rich Made from Eggshells. Waste Biomass. Valor. 2020, 11, 6757–6767. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J. On the synthesis and optical absorption studies of nano-size magnesium oxide poder. J. Phys. Chem. Solids 2008, 69, 2764–2772. [Google Scholar] [CrossRef]
- Elkhalifa, E.A.; Friedrich, H.B. Magnesium oxide as a catalyst for the dehydrogenation of n-octane. Arab. J. Chem. 2014, 11, 1154–1159. [Google Scholar] [CrossRef] [Green Version]
- Ojha, A.K.; Forster, S.; Kumar, S.; Vats, S.; Negi, S.; Fischer, I. Synthesis of well-dispersed silver nanorods of different aspect ratios and their antimicrobial properties against gram positive and negative bacterial strains. J. Nanobiotechnol. 2013, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tafur, J.D.; Torres, J.A.; Villegas, M.V. Mechanisms of antibiotic resistance in Gram negative bacteria. Infectio 2008, 12, 227–232. [Google Scholar]
- Troncoso, C.; Pavez, M.; Santos, A.; Salazar, R.; Barrientos Díaz, L. Structural and Physiological Implications of Bacterial Cell in Antibiotic Resistance Mechanisms. Int. J. Morphol. 2017, 35, 1214–1223. [Google Scholar] [CrossRef] [Green Version]
- Vázquez Olmos, A.R.; Vega Jiménez, A.L.; Paz Díaz, B. Mechanosynthesis and antimicrobial effect of nanostructured metal oxides. Mundo Nano 2018, 11, 29–44. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñiz Diaz, R.; Cardoso-Avila, P.E.; Pérez Tavares, J.A.; Patakfalvi, R.; Villa Cruz, V.; Pérez Ladrón de Guevara, H.; Gutiérrez Coronado, O.; Arteaga Garibay, R.I.; Saavedra Arroyo, Q.E.; Marañón-Ruiz, V.F.; et al. Two-Step Triethylamine-Based Synthesis of MgO Nanoparticles and Their Antibacterial Effect against Pathogenic Bacteria. Nanomaterials 2021, 11, 410. https://doi.org/10.3390/nano11020410
Muñiz Diaz R, Cardoso-Avila PE, Pérez Tavares JA, Patakfalvi R, Villa Cruz V, Pérez Ladrón de Guevara H, Gutiérrez Coronado O, Arteaga Garibay RI, Saavedra Arroyo QE, Marañón-Ruiz VF, et al. Two-Step Triethylamine-Based Synthesis of MgO Nanoparticles and Their Antibacterial Effect against Pathogenic Bacteria. Nanomaterials. 2021; 11(2):410. https://doi.org/10.3390/nano11020410
Chicago/Turabian StyleMuñiz Diaz, Ramiro, Pablo Eduardo Cardoso-Avila, José Antonio Pérez Tavares, Rita Patakfalvi, Virginia Villa Cruz, Héctor Pérez Ladrón de Guevara, Oscar Gutiérrez Coronado, Ramón Ignacio Arteaga Garibay, Quetzalcoatl Enrique Saavedra Arroyo, Virginia Francisca Marañón-Ruiz, and et al. 2021. "Two-Step Triethylamine-Based Synthesis of MgO Nanoparticles and Their Antibacterial Effect against Pathogenic Bacteria" Nanomaterials 11, no. 2: 410. https://doi.org/10.3390/nano11020410







