A Comparative Electrochemical and Morphological Investigation on the Behavior of NiCr and CoCr Dental Alloys at Various Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Electrochemical Procedures
2.3. Surface Analysis and Ion Release
3. Results and Discussion
3.1. Electrochemichal Characterization
3.1.1. Open Circuit Potential Tests
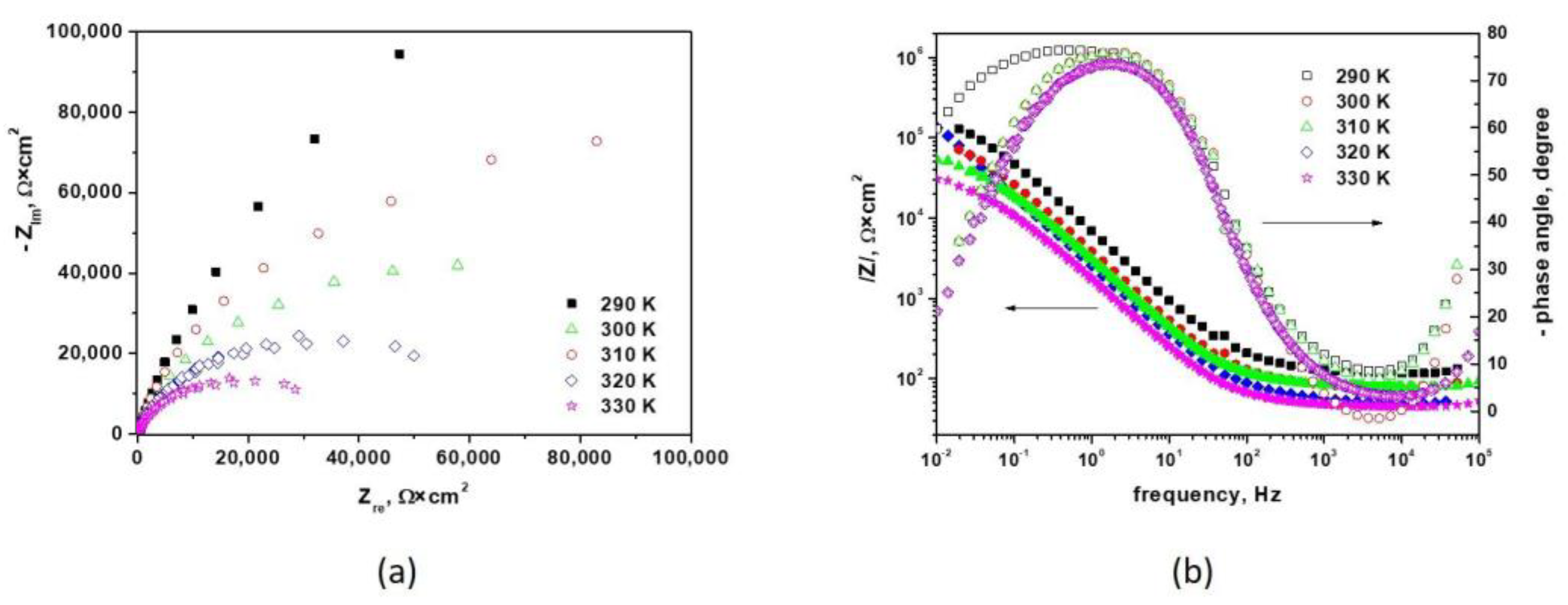
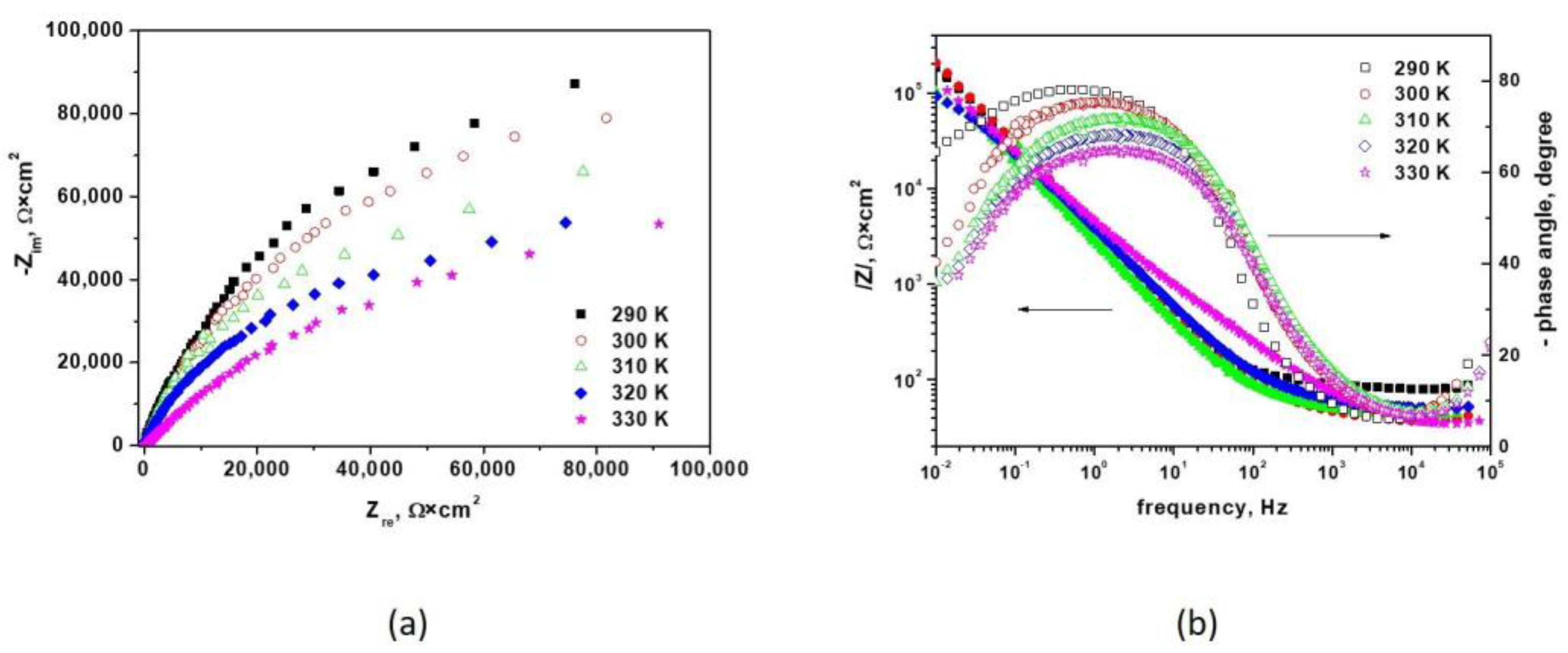
3.1.2. Electrochemical Impedance Spectroscopy Measurements
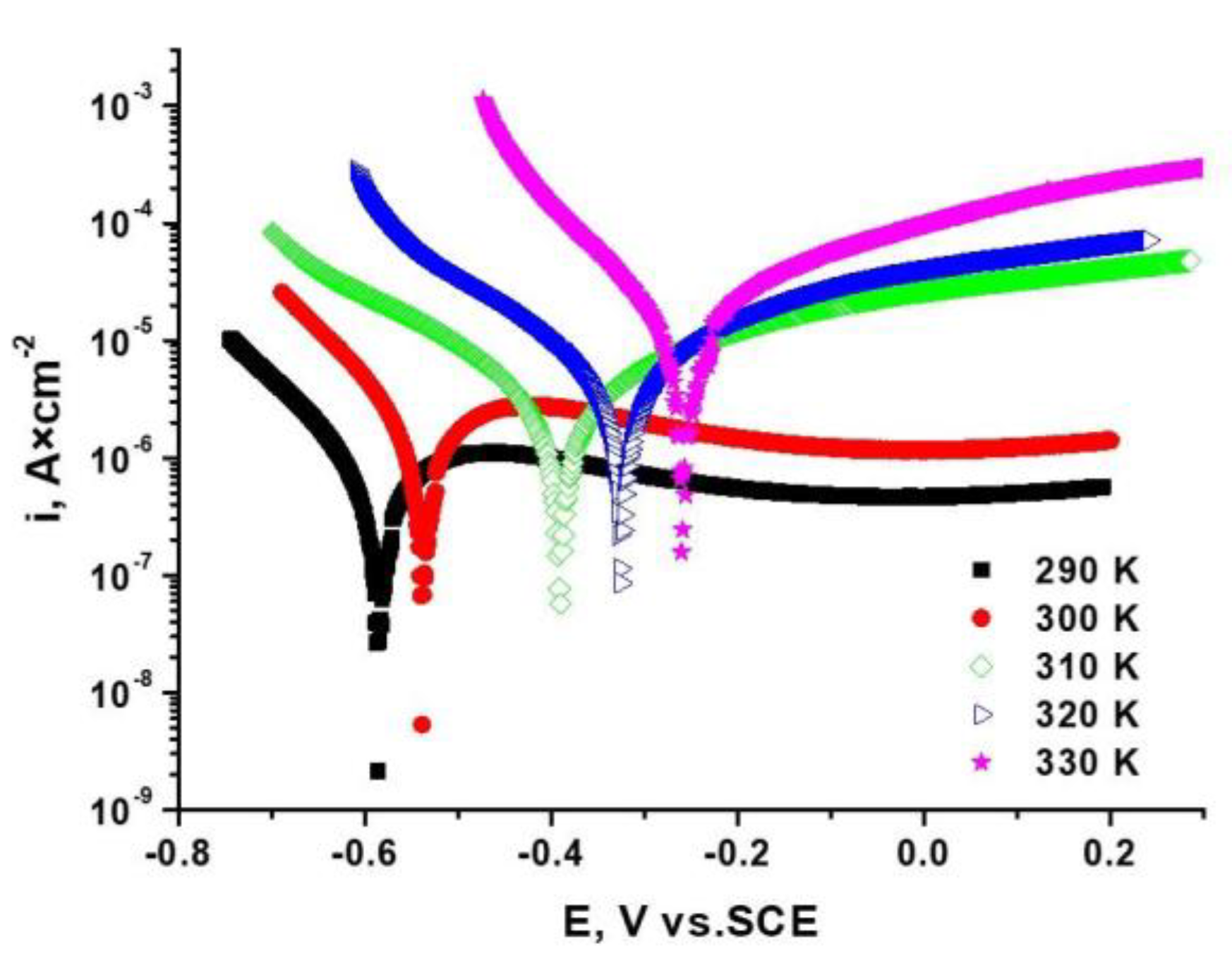
3.1.3. Potentiodynamic Tests
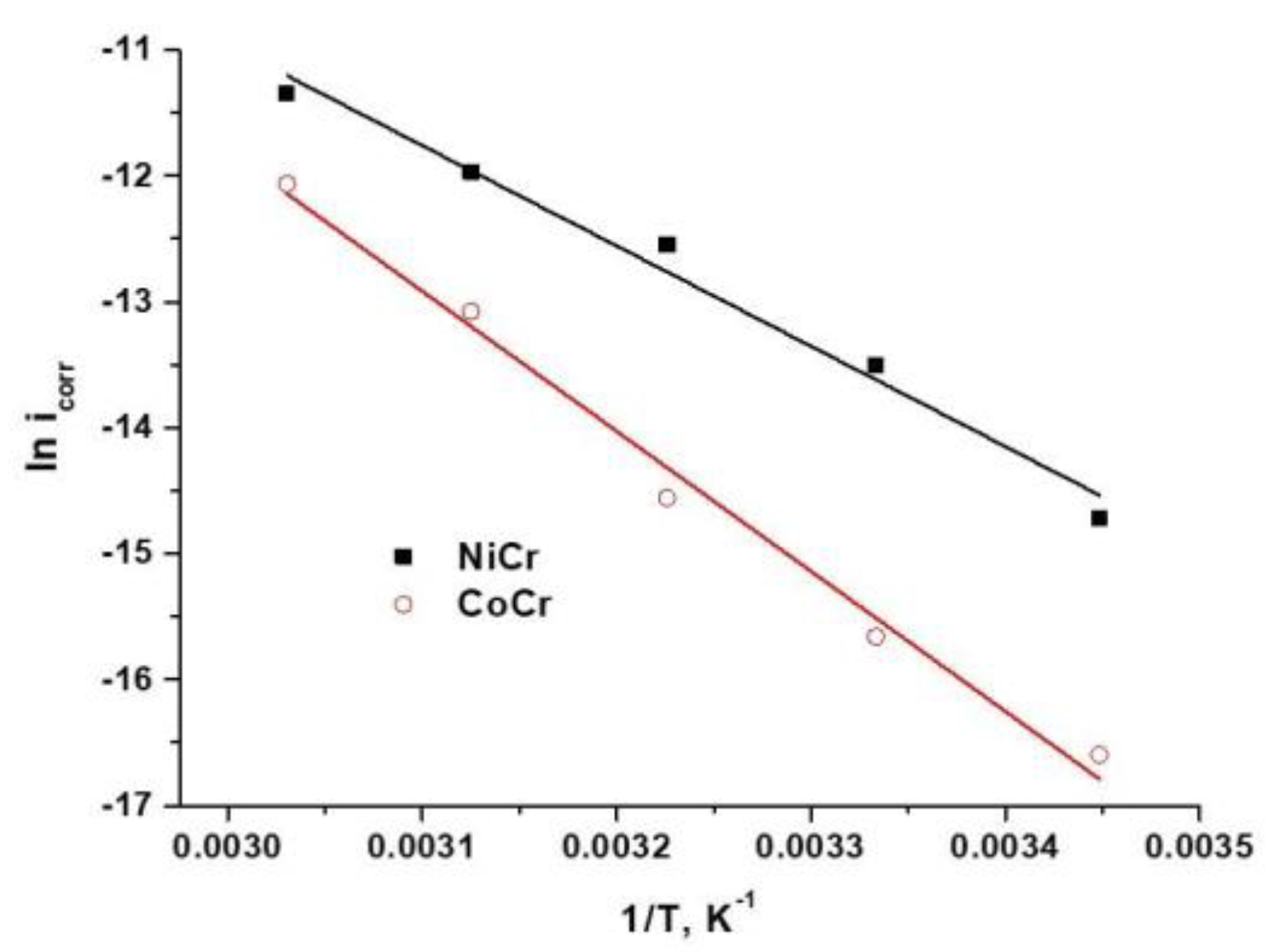
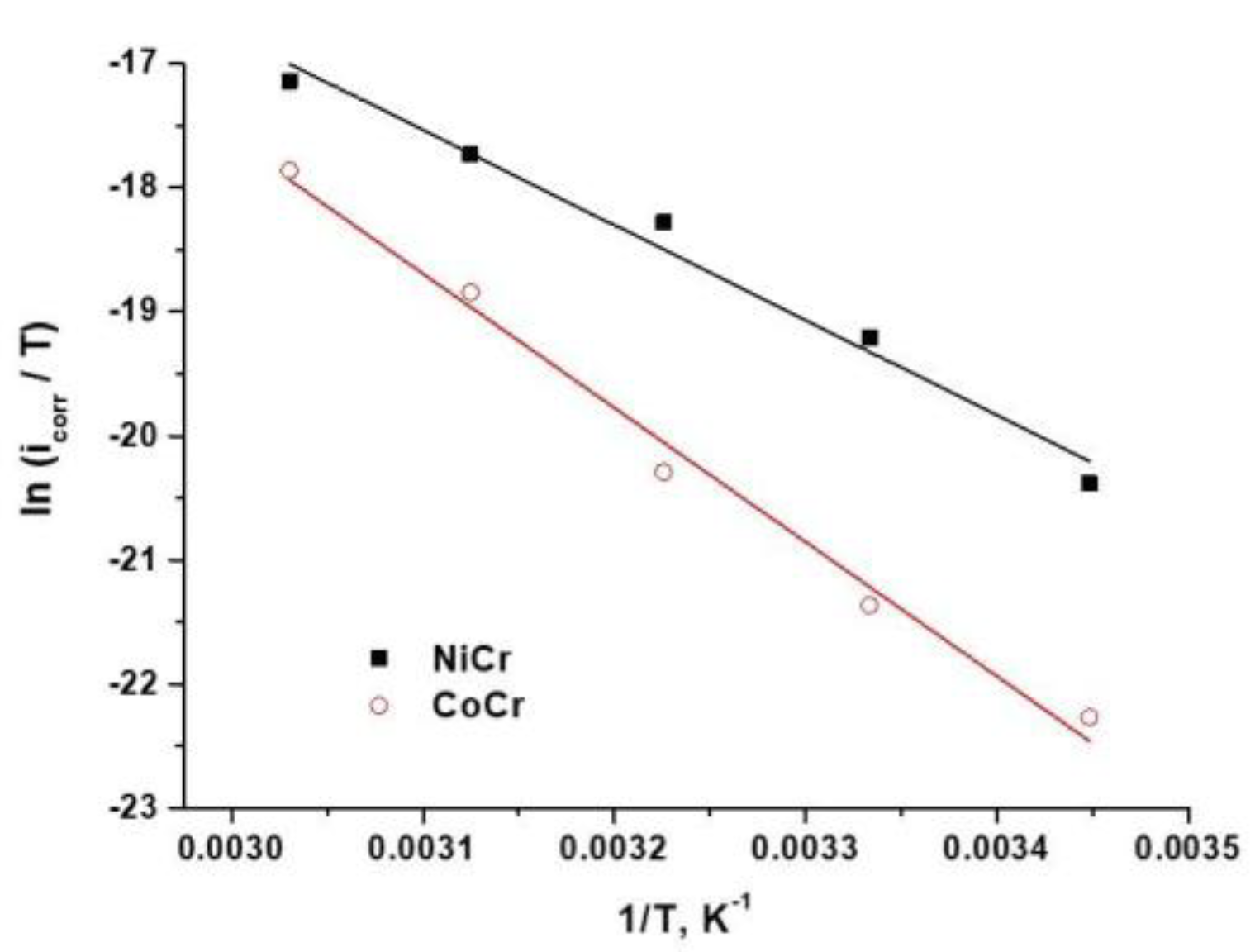
3.1.4. Calculation of the Kinetic Corrosion Parameters from Activation Energies
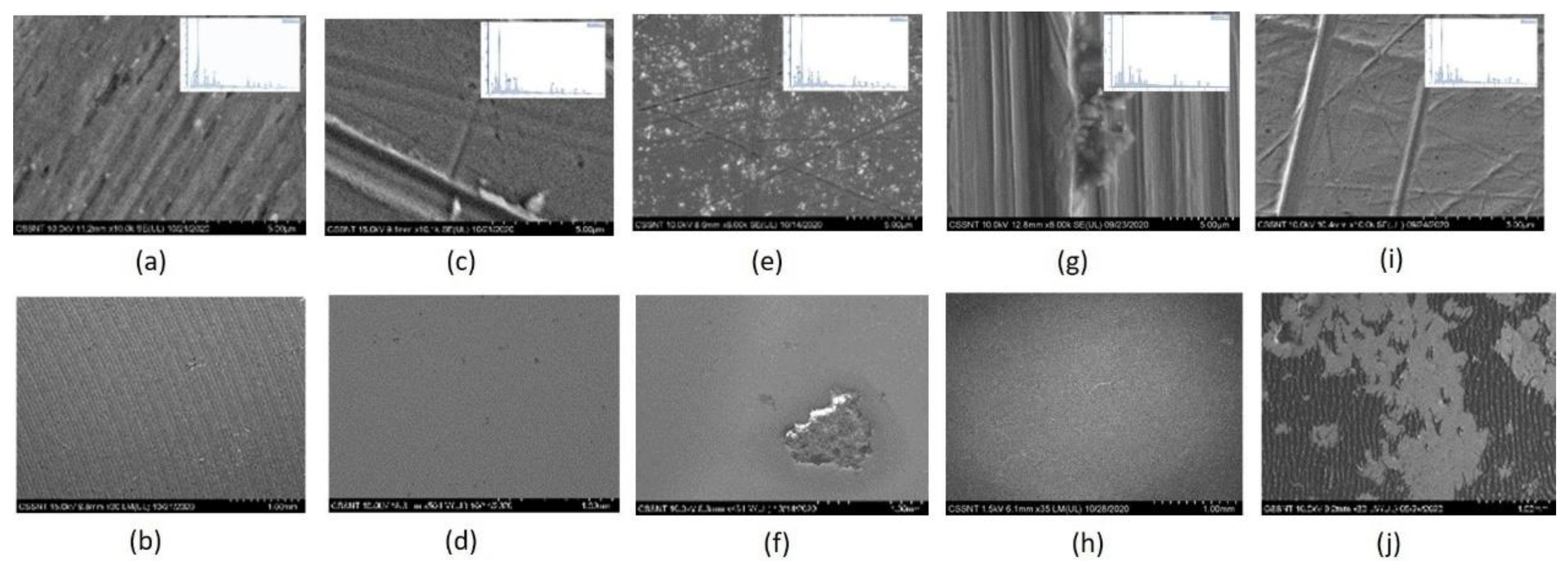
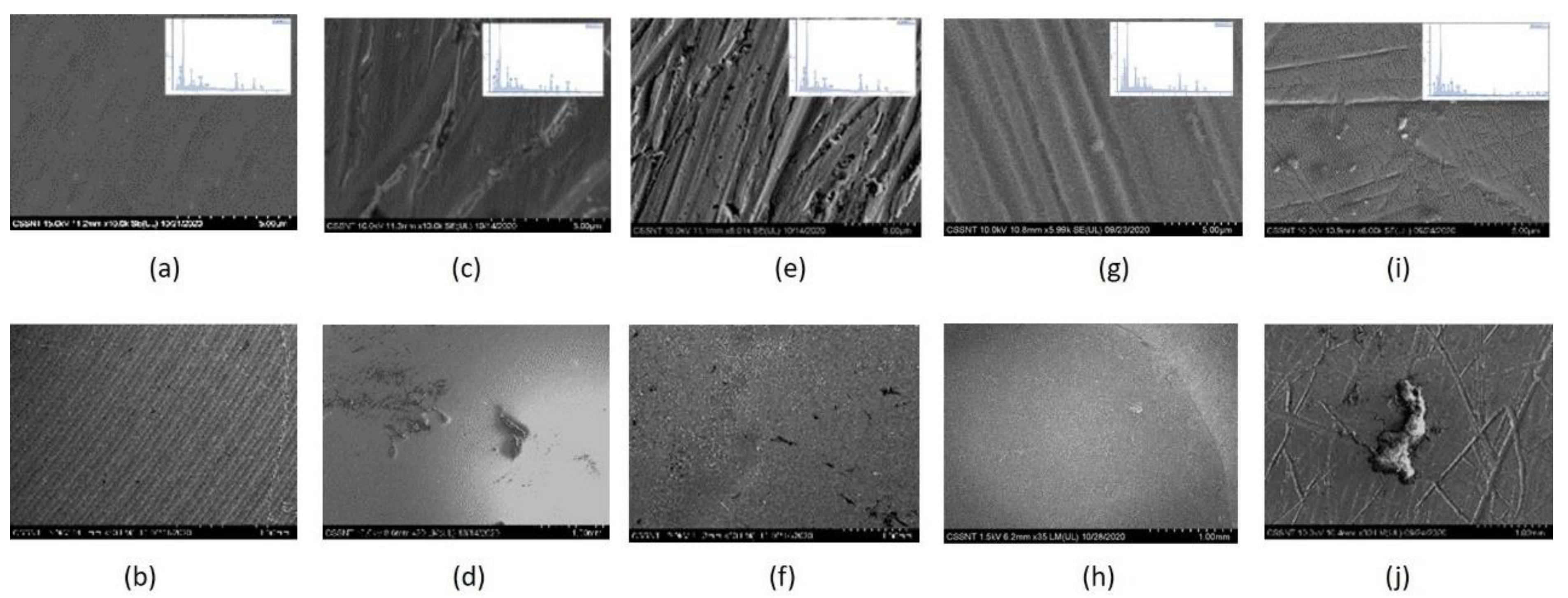
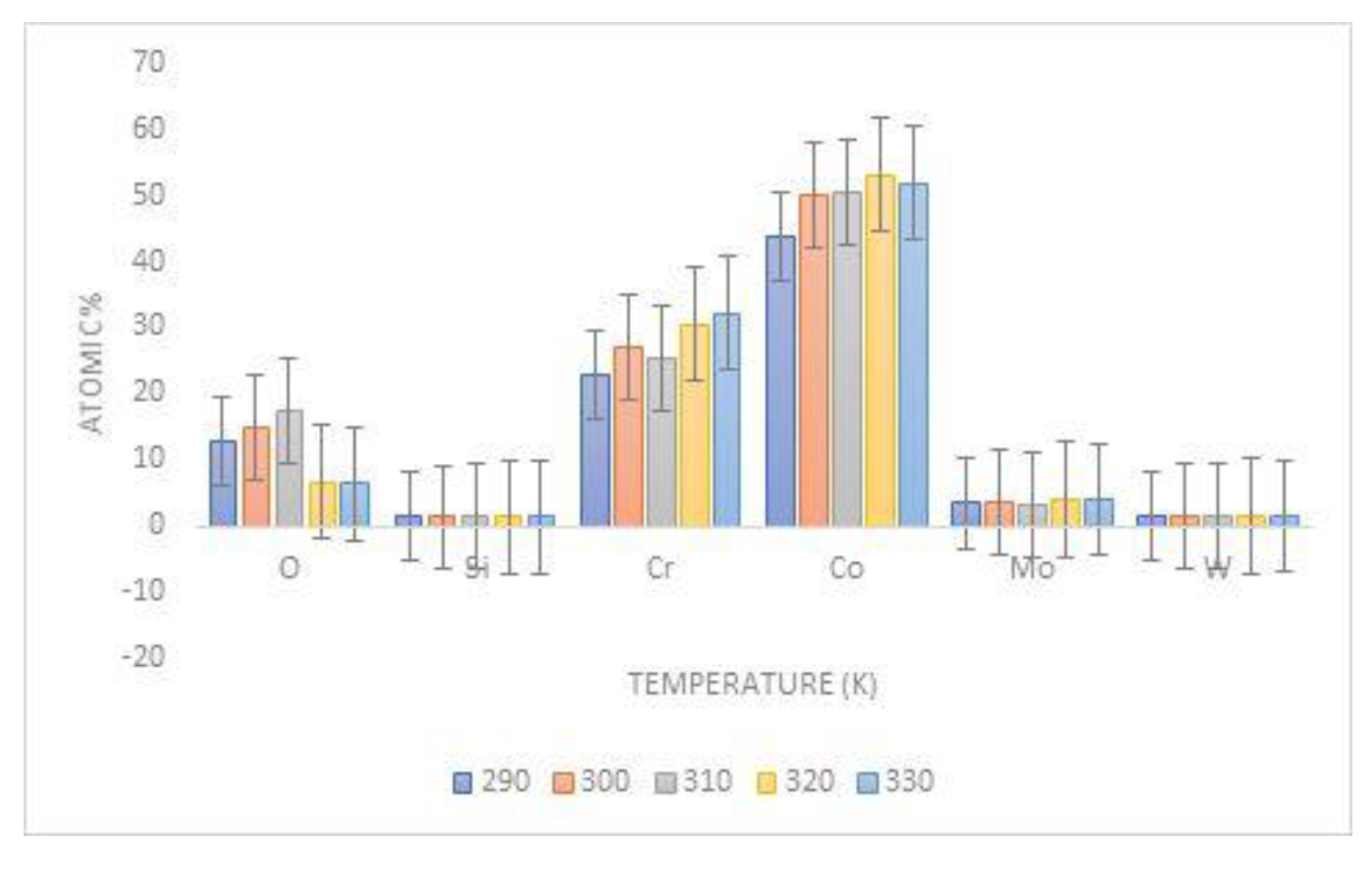
3.2. Surface Characterization
3.3. Contact Angle Evaluation
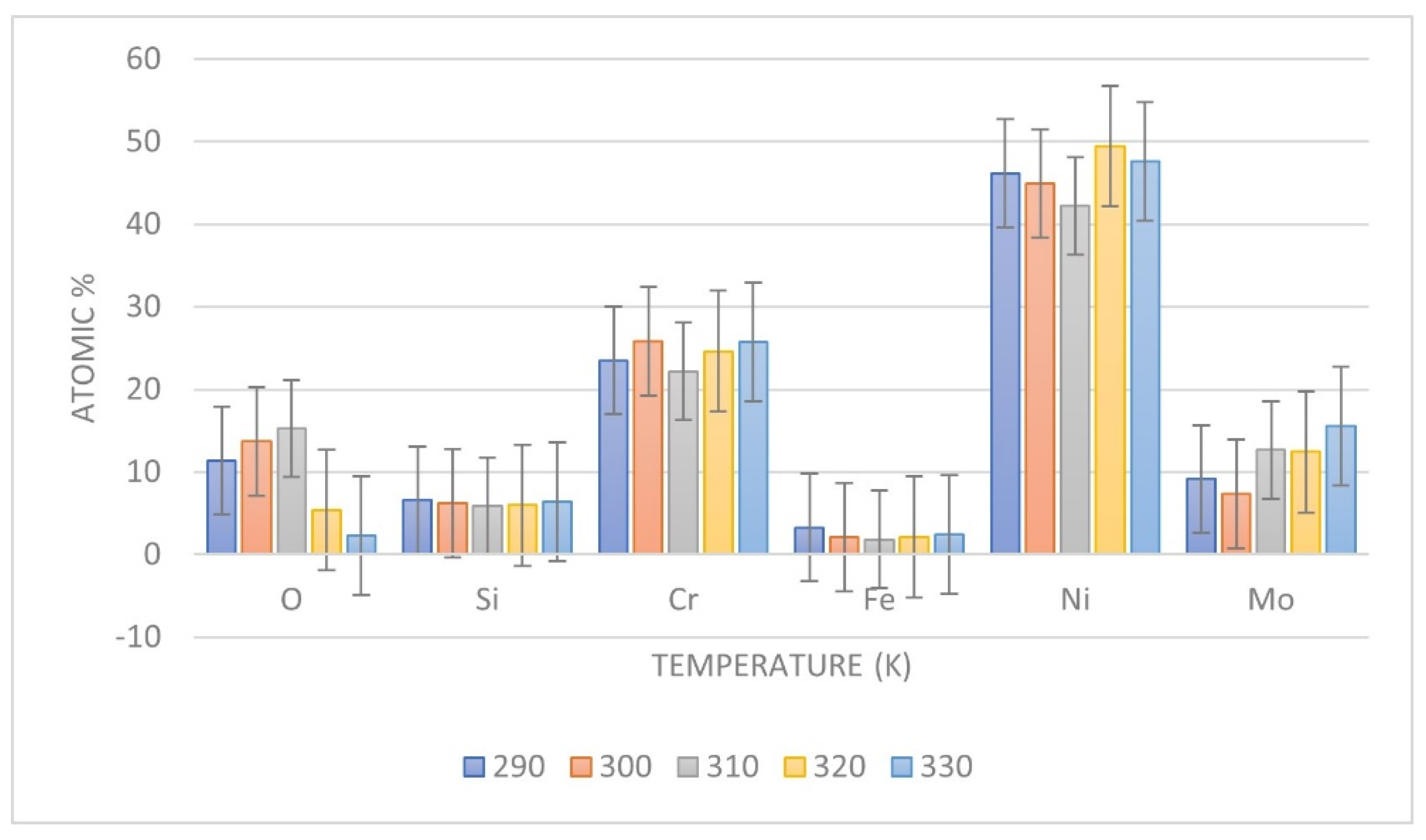
3.4. Ion Release by ICP-MS Measurenments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Liu, H.; Niinomi, M.; Horita, Z.; Fujii, H.; Hanawa, T. Fatigue property and cytocompatibility of a biomedical Co-Cr-Mo alloy subjected to a high-pressure torsion and a subsequent short time annealing. Mater. Trans. 2020, 61, 361–367. [Google Scholar] [CrossRef]
- German, R.M. Precious-metal dental casting alloys. Int. Met. Rev. 1982, 27, 260–288. [Google Scholar] [CrossRef]
- Iacoban, S.; Bolat, G.; Munteanu, C.; Cailean, D.; Trinca, L.; Mareci, D. A comparative study on the corrosion behaviour of CoCr and NiCr dental alloys in saline medium. Rev. Roum. Chim. 2015, 60, 949–955. [Google Scholar]
- Mareci, D.; Nemtoi, G.H.; Aelenei, N.; Bocanu, C. The electrochemical behavior of various non-precious Ni and Co Based Alloys in Artificial Saliva. Eur. Cell. Mater. 2005, 10, 1–7. [Google Scholar] [PubMed]
- Ramírez-Ledesma, L.; Roncagliolo, P.; Alvarez-Perez, M.A.; Lopez, H.F.; Juárez-Islas, J.A. Corrosion assessment of an implantable dental Co-Cr alloy in artificial saliva and biocompatibility behavior. J. Mater. Eng. Perform. 2020, 29, 1657–1670. [Google Scholar] [CrossRef]
- Turdean, G.L.; Craciun, A.; Popa, D.; Constantiniuc, M. Study of electrochemical corrosion of biocompatible Co–Cr and Ni–Cr dental alloys in artificial saliva. Influence of pH of the solution. Mat. Chem. Phys. 2019, 233, 390–398. [Google Scholar] [CrossRef]
- Galateanu, B.; Golgovici, F.; Hudita, A.; Stan, M.; Dinescu, S.; Costache, M.; Demetrescu, I.; Popescu, A. About electrochemical stability and biocompatibility of two types of CoCr commercial dental alloys. Mater. Corros. 2016, 67, 1096–1104. [Google Scholar] [CrossRef]
- Han, W.; Fang, F. Investigation of electrochemical properties of electropolishing Co–Cr dental alloy. J. Appl Electrochem. 2020, 50, 367–381. [Google Scholar] [CrossRef]
- Kajima, N.Y.; Takaichi, A.; Kittikundecha, N.; Nakamoto, T.; Kimura, T.; Nomura, N.; Kawasaki, A.; Hanawa, T.; Takahashi, H.; Wakabayashi, N. Effect of Heat-Treatment Temperature on Microstructures and Mechanical Properties of Co-Cr–Mo Alloys and Fabricated by Selective Laser Melting. Mat. Sci. Eng. A 2018, 726, 21–31. [Google Scholar] [CrossRef]
- Standard Specification for Wrought Titanium 6Al 4V ELI Alloy for Surgical Implants; ASTM Designation F136-82; ASTM: Philadelphia, PA, USA, 1994; pp. 19–20.
- Mazare, A.; Dilea, M.; Ionita, D.; Demetrescu, I. Electrochemical behavior in simulated body fluid of TiO2 nanotubes on TiAlNb alloy elaborated in various anodizing electrolyte. Surf. Interface Anal. 2014, 46, 186–192. [Google Scholar] [CrossRef]
- Ionita, D.; Pirvu, C.; Stoian, A.B.; Demetrescu, I. The trends of TiZr alloy research as a viable alternative for Ti and Ti16 Zr roxolid dental implants. Coatings 2020, 10, 422. [Google Scholar] [CrossRef]
- Reclaru, L.; Unger, R.E.; Kirkpatrick, C.J.; Susz, C.; Eschler, P.-Y.; Zuercher, M.-H.; Antoniac, I.; Lüthy, H. Ni–Cr based dental alloys; Ni release, corrosion and biological evaluation. Mater. Sci. Eng. C 2012, 32, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Nuoh, T.; Andre Mars, J.; Thovhogi, N.; Gihwala, D.; Baleg, A.A.; Maaza, M. Influence of temperature and pH on corrosion resistance of Ni-Cr and Co-Cr dental alloys on oral environmen. J. Dent. Oral Health 2015, 22, 1–9. [Google Scholar]
- Souza, J.; Apaza-Bedoya, K.; Benfatti, C.; Silva, F.; Henriques, B. A comprehensive review on the corrosion pathways of titanium dental implants and their biological adverse effects. Metals 2020, 10, 1272. [Google Scholar] [CrossRef]
- Ammer, M.A.; Khamis, E.; Al-Motlag, A. Electrochemical behavior of recasting Ni-Cr and Co-Cr non-precious dental alloys. Corros. Sci. 2004, 46, 2825–2836. [Google Scholar] [CrossRef]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.R.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2015, 4, 5729. [Google Scholar] [CrossRef] [PubMed]
- Mercieca, S.; Caligari Conti, M.; Buhagiar, J.; Camilleri, J. Assessment of corrosion resistance of cast cobalt- and nickel-chromium dental alloys in acidic environments. J. Appl. Biomater. Funct. Mater. 2018, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiyasat, A.; Darmani, H. The effects of recasting on the cytotoxicity of base metal alloys. J. Prosthet. Dent. 2005, 93, 158–163. [Google Scholar] [CrossRef]
- Syed, M.; Chopra, R.; Sachdev, V. Allergic reactions to dental materials-A systematic review. J. Clin. Diagn. Res. 2015, 9, ZE04–ZE09. [Google Scholar] [CrossRef]
- Olms, C.; Yahiaoui-Doktor, M.; Remmerbach, T.W. Contact allergies to dental materials. Swiss Dent. J. 2019, 129, 571–579. [Google Scholar]
- Al-Imam, H.; Benetti, A.R.; Øzhayat, E.B.; Pedersen, A.M.L.; Johansen, J.D.; Thyssen, J.; Jellesen, M.S.; Gotfredsen, K. Cobalt release and complications resulting from the use of dental prostheses. Contact Dermat. 2016, 75, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Ahing, S.I.; Karaiskos, N.E.; Wiltshire, W.A. Nickel allergy and orthodontics, a review and report of two cases. Br. Dent. J. 2008, 204, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Zigante, M.; Rincic Mlinaric, M.; Kastelan, M.; Perkovic, V.; Trinajstic Zrinski, M.; Spalj, S. Symptoms of titanium and nickel allergic sensitization in orthodontic treatment. Prog. Orthod. 2020, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Hostýnek, J.; Reagan, K.; Maibach, H. Nickel allergic hypersensitivity: Prevalence and Incidence by country, gender, age, and occupation. In Nickel and the Skin; Taylor & Fancisc: Oxfordshire, UK, 2019; pp. 39–82. [Google Scholar]
- Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices. Available online: http://data.europa.eu/eli/reg/2017/745/ (accessed on 6 January 2021).
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- Moreto, J.A.; Costa, R.A.; Rodrigues da Silva, L.R.; Rossi, A.; da Silva, L.A.; Alves, A.V. Effect of temperature, electrolyte composition and immersion time on the electrochemical corrosion behavior of CoCrMo implant alloy exposed to physiological serum and Hank’s solution. Mat. Res. 2018, 21, 1–9. [Google Scholar] [CrossRef]
- Dong, H.; Ju, X.; Qian, L.; Zhou, Z. Effect of ceramic conversion treatments on the surface damage and nickel ion release of NiTi alloys under fretting corrosion conditions. J. Mater. Sci. Mater. Med. 2008, 19, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Sonofuchi, K.; Hagiwara, Y.; Koizumi, Y.; Chiba, A.; Kawano, M.; Nakayama, M.; Ogasawara, K.; Yabe, Y.; Itoi, E. Quantitative in vivo biocompatibility of new ultralow-nickel cobalt-chromium-molybdenum alloys. J. Orthop. Res. 2016, 34, 1505–1513. [Google Scholar] [CrossRef]
- Padrós, R.; Giner-Tarrid, L.; Herrero-Climent, M.; Punset, M.; Francisco Gil, F.J. Corrosion resistance and ion release of dental prosthesis of CoCr obtained by CAD-CAM Milling, Casting and Laser Sintering. Metals 2020, 10, 827. [Google Scholar] [CrossRef]
- Hamza, G.E.; Sallam, H.; Eldwakhly, E. Effect of surface treatment of milled cobalt–chromium alloy on shear bond strength to porcelain. J. Arab Soc. Med. Res. 2019, 14, 113–123. [Google Scholar] [CrossRef]
- Padrós, R.; Punset, M.; Molmeneu, M.; Velasco, A.B.; Herrero-Climent, M.; Rupérez, E.; Gil, F.J. Mechanical properties of CoCr dental-prosthesis restorations made by three manufacturing processes. Influence of the microstructure and topography. Metals 2020, 10, 788. [Google Scholar] [CrossRef]
- Sarangi, D.; Mohapatra, U. Alloys used in fixed prosthodontics: An overview. Indian J. Public Health Res. Dev. 2019, 10, 171. [Google Scholar] [CrossRef]
- Golgovici, F.; Ionascu, F.G. Electrochemical behavior of NiCr based alloys in human and artificial saliva. UPB Sci. Bull. Series B 2017, 79, 157–166. [Google Scholar]
- Bauer, J.; Costa, J.F.; Carvalho, C.N.; Grande, R.H.; Loguercio, A.D.; Reis, A. Characterization of two Ni-Cr dental alloys and the influence of casting mode on mechanical properties. J. Prosthodont. Res. 2012, 56, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Szala, M.; Beer-Lech, K.; Gancarczyk, K.; Baran Kilic, O.; Pędrak, P.; Ozer, A.; Skic, A. Microstructural characterisation of Co-Cr-Mo casting dental alloys. Adv. Sci. Technol. Res. J. 2017, 11, 76–82. [Google Scholar] [CrossRef][Green Version]
- Rao, S.; Chowdhary, R. Evaluation on the corrosion of the three Ni-Cr alloys with different composition. Int. J. Dent. 2011, 2011, 397029. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, A.; Li, Z.; Conaway, C.; McGinley, R.; Dhingra, S.; Vahdat, V.; Zhou, F.; D’Urso, B.; Liu, H.; Li, L. Study on the surface energy of graphene by contact angle measurements. Langmuir 2014, 30, 8598–8606. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zheng, Y.; Zhong, Q. Corrosion of dental alloys in artificial saliva with Streptococcus mutans. PLoS ONE 2017, 12, e0174440. [Google Scholar] [CrossRef] [PubMed]
- Yfantis, C.; Yfantis, D.; Anastassopoulou, J.; Theophanides, T. Analytical and electrochemical evaluation of the in vitro corrosion behavior of nickel-chrome and cobalt-chrome alloys for metal-ceramic restorations. Eur. J. Prosthodont. Rest. Dent. 2007, 15, 33–40. [Google Scholar]
- Lloyd, A.C.; Noel, J.J.; McIntyre, S.; Shoesmith, D.W. Cr, Mo and W alloying additions in Ni alloys and their effect on passivity. Electrochim. Acta 2004, 49, 3015–3027. [Google Scholar] [CrossRef]
- Silva, J.; Sousa, L.; Nakazato, R.; Codaro, E.; Felipe, H. Electrochemical and microstructural study of Ni-Cr-Mo alloys used in dental prostheses. Mater. Sci. Appl. 2011, 2, 42–48. [Google Scholar] [CrossRef]
- Musa, A.; Mohamad, A.B.; Kadhum, A.A.H.; Chee, E.P. Galvanic corrosion of aluminum alloy (Al2024) and copper in 1.0 m nitric acid. Int. J. Electrochem. Sci. 2011, 6, 5052–5065. [Google Scholar]
- Vallance, C. An introduction to Chemical Kinetics; Morgan & Claypool Publishers: Bristol, UK, 2017. [Google Scholar]
- Viennot, S.; Dalard, F.; Lissac, M.; Grosgogeat, B. Corrosion resistance of cobalt-chromium and palladium-silver alloys used in fixed prosthetic restorations. Eur. J. Oral Sci. 2005, 113, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, F.C.; Giacomelli, C.; Spinelli, A. Behavior of a Co-Cr-Mo biomaterial in simulated body fluid solutions studied by electrochemical and surface analysis techniques. J. Braz. Chem. Soc. 2004, 15, 54–547. [Google Scholar] [CrossRef]
- Geurtsen, W. Biocompatibility of dental casting alloys. Crit. Rev. Oral Biol. Med. 2002, 13, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Paterlini, T.T.; Nogueira, L.F.B.; Tovani, C.B.; Cruz, M.A.E.; Derradi, R.; Ramos, A.P. The role played by modified bioinspired surfaces in interfacial properties of biomaterials. Biophys Rev. 2017, 9, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, T.P. Allergy related to dental implant and its clinical significance. Clin. Cosmet. Investig. Dent. 2013, 5, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Drob, S.I.; Vasilescu, C.; Andrei, M.; Calderon Moreno, J.M.; Demetrescu, I. Microstructural, mechanical and anticorrosion characterisation of new CoCrNbMoZr alloy. Mater. Corros. 2015, 67, 739–747. [Google Scholar] [CrossRef]
- Zhang, E. A new antibacterial Co-Cr-Mo-Cu alloy: Preparation, biocorrosion, mechanical and antibacterial property. Mat. Sci. Eng. C 2016, 69, 134–143. [Google Scholar] [CrossRef]



| Alloy | Co% | Ni% | Cr% | Mo% | W% | Ga% | Si% | Fe% | Mn% |
|---|---|---|---|---|---|---|---|---|---|
| CoCr | 60.2 | - | 25 | 4.8 | 6.2 | 2.9 | <1 | - | <1 |
| NiCr | - | 62 | 24.5 | 10.3 | - | - | 1.7 | 1.5 | - |
| Alloy | T, K | Tafel Method | Polarization Resistance Method | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecorr, mV | icorr, μA × cm−2 | Kg, G × m−2h−1 | P, mm × year−1 | Ba, mV | −Bc, mV | RP, KΩ | icorr, μA × cm−2 | ||
| NiCr | 290 | −578 | 0.407 | 0.0046 | 0.0049 | 120 | 98 | 57.88 | 0.405 |
| ±0.02 | ±0.01 | ±0.0001 | ±0.0001 | ±0.2 | ±0.1 | ±0.07 | ±0.01 | ||
| 300 | −528 | 1.364 | 0.0155 | 0.0164 | 123 | 91 | 17.61 | 1.289 | |
| ±0.02 | ±0.01 | ±0.0001 | ±0.0001 | ±0.2 | ±0.1 | ±0.001 | ±0.01 | ||
| 310 | −385 | 3.562 | 0.0407 | 0.0429 | 114 | 88 | 7.15 | 3.016 | |
| ±0.015 | ±0.01 | ±0.0002 | ±0.0003 | ±0.2 | ±0.1 | ±0.005 | ±0.01 | ||
| 320 | −326 | 6.351 | 0.0726 | 0.0766 | 116 | 89 | 3.64 | 6.011 | |
| ±0.01 | ±0.02 | ±0.0005 | ±0.0006 | ±0.1 | ±0.1 | ±0.005 | ±0.02 | ||
| 330 | −255 | 11.87 | 0.1357 | 0.1432 | 92 | 97 | 1.75 | 11.689 | |
| ±0.01 | ±0.04 | ±0.001 | ±0.001 | ±0.1 | ±0.1 | ±0.002 | ±0.03 | ||
| CoCr | 290 | −584 | 0.062 | 0.0008 | 0.0008 | 120 | 93 | 348.83 | 0.065 |
| ±0.02 | ±0.01 | ±0.0001 | ±0.0001 | ±0.2 | ±0.1 | ±0.06 | ±0.01 | ||
| 300 | −538 | 0.158 | 0.0019 | 0.0021 | 104 | 98 | 157.61 | 0.139 | |
| ±0.02 | ±0.01 | ±0.0001 | ±0.0002 | ±0.1 | ±0.1 | ±0.05 | ±0.01 | ||
| 310 | −476 | 0.477 | 0.0060 | 0.0062 | 115 | 110 | 54.52 | 0.448 | |
| ±0.01 | ±0.01 | ±0.0001 | ±0.0002 | ±0.1 | ±0.2 | ±0.004 | ±0.02 | ||
| 320 | −425 | 2.097 | 0.0264 | 0.0272 | 110 | 108 | 11.78 | 2.008 | |
| ±0.01 | ±0.02 | ±0.0005 | ±0.0005 | ±0.2 | ±0.1 | ±0.0008 | ±0.02 | ||
| 330 | −350 | 5.772 | 0.0727 | 0.0749 | 94 | 76 | 3.49 | 5.221 | |
| ±0.01 | ±0.03 | ±0.001 | ±0.0007 | ±0.1 | ±0.1 | ±0.001 | ±0.03 | ||
| Alloy | Arrhenius Equation | Transition State Equation | |
|---|---|---|---|
| Ea, kJ mol−1 | ΔH, kJ mol−1 | ΔS, J mol−1K−1 | |
| NiCr | 66.23 | 63.66 | −145.81 |
| CoCr | 92.45 | 89.88 | −74.13 |
| Ions Release (ppm) | Temperature (K) | ||||
|---|---|---|---|---|---|
| 290 | 300 | 310 | 320 | 330 | |
| Ni | 5.65 ± 0.02 | 6.25 ± 0.01 | 7.24 ± 0.02 | 7.39 ± 0.01 | 12.79 ± 0.01 |
| Cr | 5.25 ± 0.03 | 5.34 ± 0.02 | 9.31 ± 0.02 | 9.37 ± 0.02 | 9.74 ± 0.02 |
| Mo | 0 ± 0.01 | 0 ± 0.01 | 8.83 ± 0.01 | 9.11 ± 0.01 | 14.54 ± 0.01 |
| Si | 0.65 ± 0.01 | 0.76 ± 0.01 | 1.76 ± 0.02 | 2.15 ± 0.01 | 3.93 ± 0.01 |
| Fe | 6.4 ± 0.02 | 6.86 ± 0.01 | 7.25 ± 0.02 | 7.27 ± 0.02 | 7.77 ± 0.02 |
| Elements (ppm) | Temperature (K) | ||||
|---|---|---|---|---|---|
| 290 | 300 | 310 | 320 | 330 | |
| Co | 7.23 ± 0.01 | 7.27 ± 0.02 | 8 ± 0.013 | 8 ± 0.02 | 8.45 ± 0.02 |
| Cr | 4.33 ± 0.02 | 5.75 ± 0.01 | 5.82 ± 0.01 | 6.93 ± 0.01 | 10.69 ± 0.03 |
| W | 0 ± 0.01 | 0 ± 0.01 | 0 ± 0.01 | 0 ± 0.01 | 5 ± 0.02 |
| Mo | 7.07 ± 0.01 | 12.62 ± 0.01 | 13.52 ± 0.03 | 14.65 ± 0.02 | 18.34 ± 0.03 |
| Mn | 3.85 ± 0.02 | 5.12 ± 0.02 | 6.12 ± 0.01 | 6.66 ± 0.01 | 7.64 ± 0.02 |
| Si | 4.1 ± 0.02 | 5.33 ± 0.01 | 6.11 ± 0.01 | 7.54 ± 0.02 | 11.1 ± 0.03 |
| Ga | 5.98 ± 0.01 | 7.29 ± 0.02 | 8.24 ± 0.02 | 9.29 ± 0.02 | 12.47 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golgovici, F.; Prodana, M.; Ionascu, F.G.; Demetrescu, I. A Comparative Electrochemical and Morphological Investigation on the Behavior of NiCr and CoCr Dental Alloys at Various Temperatures. Metals 2021, 11, 256. https://doi.org/10.3390/met11020256
Golgovici F, Prodana M, Ionascu FG, Demetrescu I. A Comparative Electrochemical and Morphological Investigation on the Behavior of NiCr and CoCr Dental Alloys at Various Temperatures. Metals. 2021; 11(2):256. https://doi.org/10.3390/met11020256
Chicago/Turabian StyleGolgovici, Florentina, Mariana Prodana, Florentina Gina Ionascu, and Ioana Demetrescu. 2021. "A Comparative Electrochemical and Morphological Investigation on the Behavior of NiCr and CoCr Dental Alloys at Various Temperatures" Metals 11, no. 2: 256. https://doi.org/10.3390/met11020256
APA StyleGolgovici, F., Prodana, M., Ionascu, F. G., & Demetrescu, I. (2021). A Comparative Electrochemical and Morphological Investigation on the Behavior of NiCr and CoCr Dental Alloys at Various Temperatures. Metals, 11(2), 256. https://doi.org/10.3390/met11020256









