Injectable Capsaicin for the Management of Pain Due to Osteoarthritis
Abstract
1. Introduction
2. Rationale for the Therapeutic Use of Capsaicin for Pain Management
3. Current Therapies for Osteoarthritis
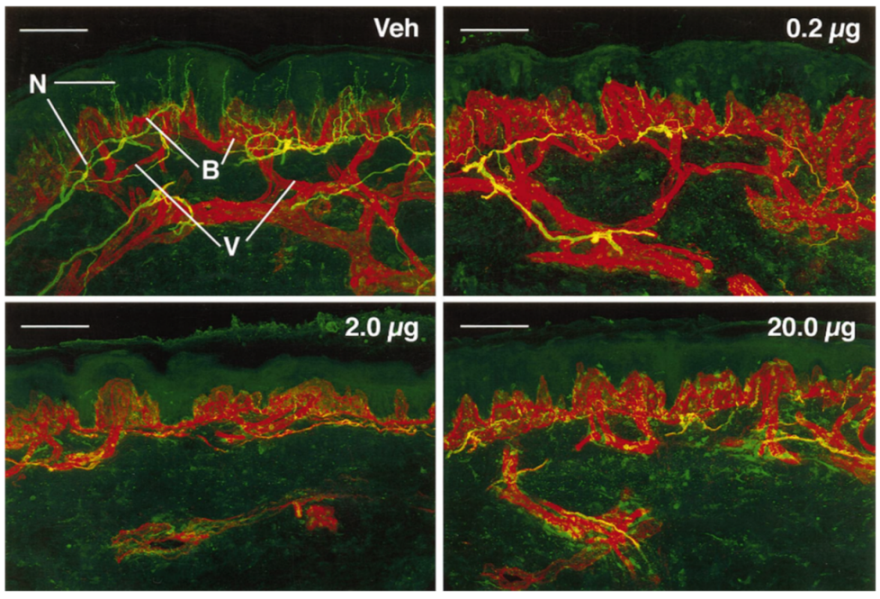
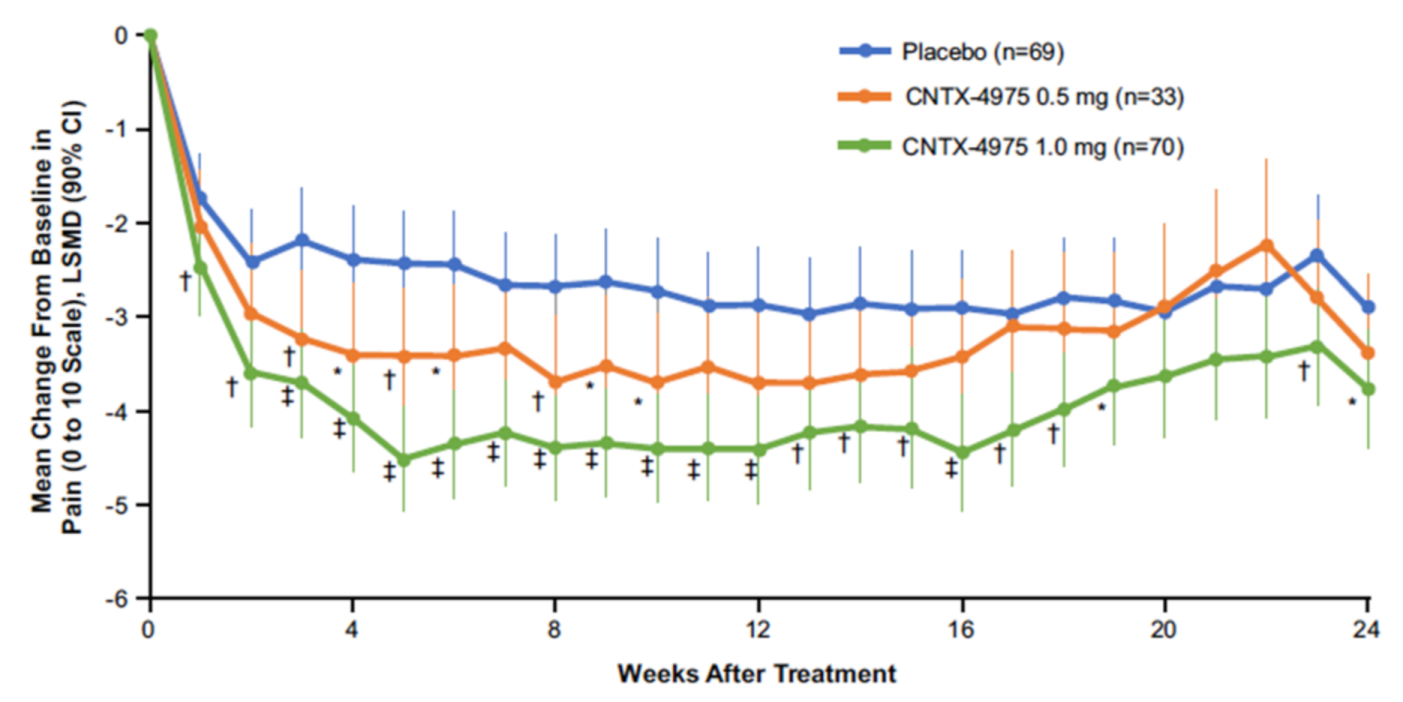
4. Capsaicin Injection for Osteoarthritis
5. Injection Versus Topical Delivery
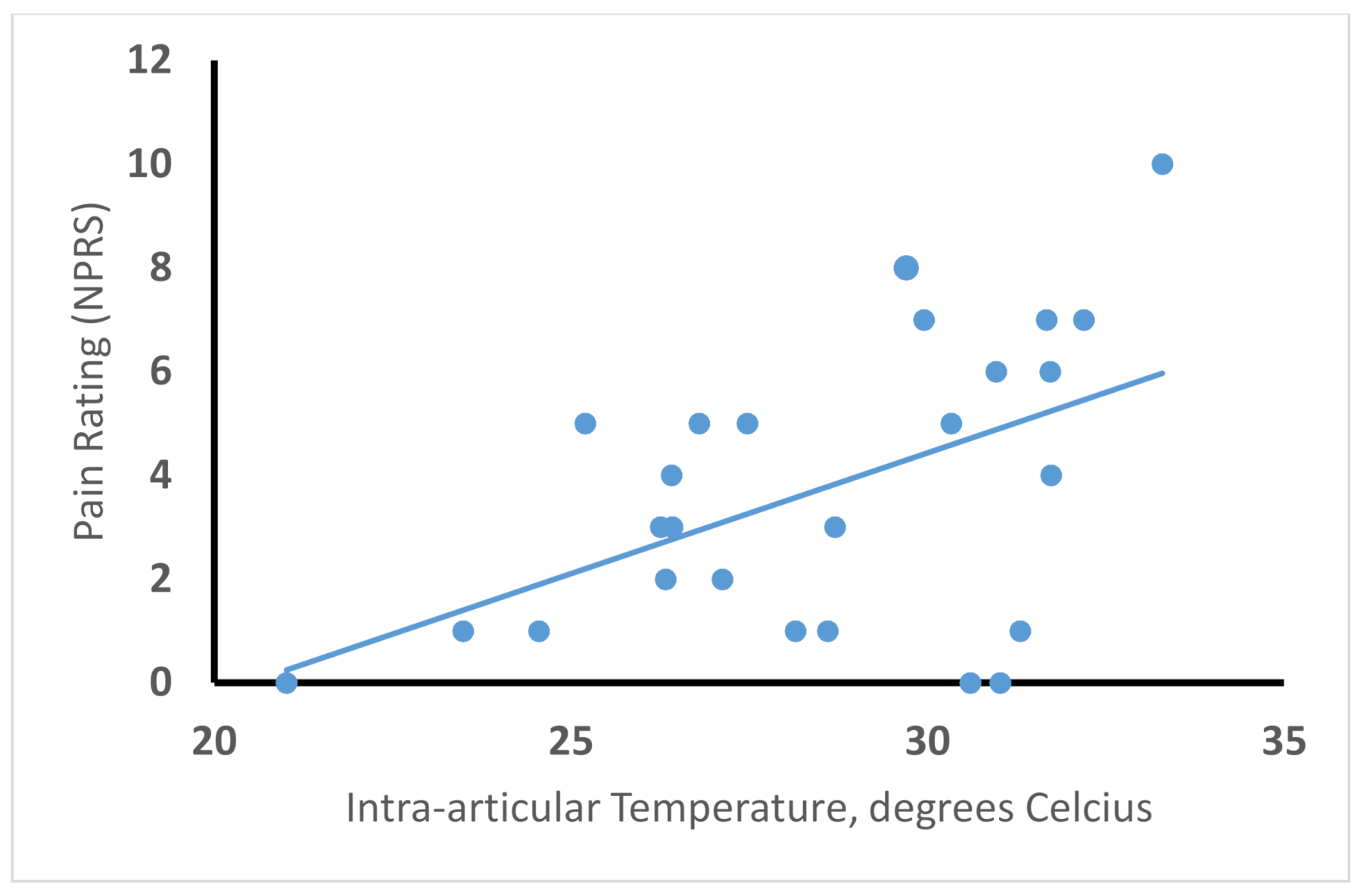
6. Cooling for Decreasing Procedural Pain
7. Experience with Bilateral Knee Injection
8. Expanded Opportunities
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 1, CD007393. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Malan, T.P.; Tuchman, M.M.; Mollen, M.D.; Tobias, J.K.; Vanhove, G.F. A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. J. Pain 2010, 11, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Van Nooten, F.; Treur, M.; Pantiri, K.; Stoker, M.; Charokopou, M. Capsaicin 8% Patch Versus Oral Neuropathic Pain Medications for the Treatment of Painful Diabetic Peripheral Neuropathy: A Systematic Literature Review and Network Meta-analysis. Clin. Ther. 2017, 39, 787–803.e18. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the mystery of capsaicin: A tool to understand and treat pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Campbell, J.N.; Chung, M.K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2020. [Google Scholar] [CrossRef]
- Chung, M.K.; Campbell, J.N. Use of Capsaicin to Treat Pain: Mechanistic and Therapeutic Considerations. Pharmaceuticals 2016, 9, 66. [Google Scholar] [CrossRef]
- Moran, M.M.; Szallasi, A. Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. Br. J. Pharmacol. 2018, 175, 2185–2203. [Google Scholar] [CrossRef]
- Raja, S.N.; Ringkamp, M.; Guan, Y.; Campbell, J.N.; John, J. Bonica Award Lecture: Peripheral neuronal hyperexcitability: The “low-hanging” target for safe therapeutic strategies in neuropathic pain. Pain 2020, 161 (Suppl. 1), S14–S26. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Chesler, A.T.; Braz, J.M.; Shah, N.M.; Julius, D.; Basbaum, A.I. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 2011, 31, 10119–10127. [Google Scholar] [CrossRef]
- Cho, W.G.; Valtschanoff, J.G. Vanilloid receptor TRPV1-positive sensory afferents in the mouse ankle and knee joints. Brain Res. 2008, 1219, 59–65. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Guler, A.D.; Caterina, M.J. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat. Neurosci. 2008, 11, 555–564. [Google Scholar] [CrossRef] [PubMed]
- LaMotte, R.H.; Lundberg, L.E.; Torebjork, H.E. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J. Physiol. 1992, 448, 749–764. [Google Scholar] [CrossRef]
- Davis, K.D.; Meyer, R.A.; Turnquist, J.L.; Filloon, T.G.; Pappagallo, M.; Campbell, J.N. Cutaneous pretreatment with the capsaicin analog NE-21610 prevents the pain to a burn and subsequent hyperalgesia. Pain 1995, 62, 373–378. [Google Scholar] [CrossRef]
- Simone, D.A.; Nolano, M.; Johnson, T.; Wendelschafer-Crabb, G.; Kennedy, W.R. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: Correlation with sensory function. J. Neurosci. 1998, 18, 8947–8959. [Google Scholar] [CrossRef]
- Seif, S.; Hansen, S. Measuring the stratum corneum reservoir: Desorption kinetics from keratin. J. Pharm. Sci. 2012, 101, 3718–3728. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Asgar, J.; Joseph, J.; Ro, J.Y.; Wei, F.; Campbell, J.N.; Chung, M.K. Ca(2+) and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J. Biol. Chem. 2017, 292, 8291–8303. [Google Scholar] [CrossRef]
- Nielsen, T.A.; Eriksen, M.A.; Gazerani, P.; Andersen, H.H. Psychophysical and vasomotor evidence for interdependency of TRPA1 and TRPV1-evoked nociceptive responses in human skin: An experimental study. Pain 2018, 159, 1989–2001. [Google Scholar] [CrossRef]
- Wang, S.; Bian, C.; Yang, J.; Arora, V.; Gao, Y.; Wei, F.; Chung, M.K. Ablation of TRPV1+ Afferent Terminals by Capsaicin Mediates Long-Lasting Analgesia for Trigeminal Neuropathic Pain. eNeuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.C.; Mishra, S.K.; Maric, D.; Kaszas, K.; Gonnella, G.L.; Clokie, S.J.; Kominsky, H.D.; Gross, J.R.; Keller, J.M.; Mannes, A.J.; et al. Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J. Pain 2014, 15, 1338–1359. [Google Scholar] [CrossRef] [PubMed]
- Chem Matters: Muy Caliente. Available online: https://www.acs.org/content/acs/en/education/resources/highschool/chemmatters/past-issues/archive-2013-2014/peppers.html (accessed on 28 December 2020).
- Cavanaugh, D.J.; Lee, H.; Lo, L.; Shields, S.D.; Zylka, M.J.; Basbaum, A.I.; Anderson, D.J. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 9075–9080. [Google Scholar] [CrossRef]
- Mankowski, C.; Poole, C.D.; Ernault, E.; Thomas, R.; Berni, E.; Currie, C.J.; Treadwell, C.; Calvo, J.I.; Plastira, C.; Zafeiropoulou, E.; et al. Effectiveness of the capsaicin 8% patch in the management of peripheral neuropathic pain in European clinical practice: The ASCEND study. BMC Neurol. 2017, 17, 80. [Google Scholar] [CrossRef]
- Salio, C.; Aimar, P.; Malapert, P.; Moqrich, A.; Merighi, A. Neurochemical and Ultrastructural Characterization of Unmyelinated Non-peptidergic C-Nociceptors and C-Low Threshold Mechanoreceptors Projecting to Lamina II of the Mouse Spinal Cord. Cell Mol. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of Total Hip and Knee Replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Lundblad, H.; Kreicbergs, A.; Jansson, K.A. Prediction of persistent pain after total knee replacement for osteoarthritis. J. Bone Jt. Surg. Br. Vol. 2008, 90, 166–171. [Google Scholar] [CrossRef]
- Hawker, G.; Wright, J.; Coyte, P.; Paul, J.; Dittus, R.; Croxford, R.; Katz, B.; Bombardier, C.; Heck, D.; Freund, D. Health-related quality of life after knee replacement. J. Bone Jt. Surg. Am. 1998, 80, 163–173. [Google Scholar] [CrossRef]
- Baker, P.N.; Rushton, S.; Jameson, S.S.; Reed, M.; Gregg, P.; Deehan, D.J. Patient satisfaction with total knee replacement cannot be predicted from pre-operative variables alone: A cohort study from the National Joint Registry for England and Wales. Bone Jt. J. 2013, 95, 1359–1365. [Google Scholar] [CrossRef]
- Osani, M.C.; Vaysbrot, E.E.; Zhou, M.; McAlindon, T.E.; Bannuru, R.R. Duration of Symptom Relief and Early Trajectory of Adverse Events for Oral Nonsteroidal Antiinflammatory Drugs in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2020, 72, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Salsitz, E.A. Chronic Pain, Chronic Opioid Addiction: A Complex Nexus. J. Med. Toxicol. 2016, 12, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Dube, C.E.; Eaton, C.B.; Driban, J.B.; McAlindon, T.E.; Lapane, K.L. Longterm Effectiveness of Intraarticular Injections on Patient-reported Symptoms in Knee Osteoarthritis. J. Rheumatol. 2018, 45, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Barton, N.J.; McQueen, D.S.; Thomson, D.; Gauldie, S.D.; Wilson, A.W.; Salter, D.M.; Chessell, I.P. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp. Mol. Pathol. 2006, 81, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Helyes, Z.; Sandor, K.; Bite, A.; Pinter, E.; Nemeth, J.; Banvolgyi, A.; Bolcskei, K.; Elekes, K.; Szolcsanyi, J. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: In vivo study using gene-deficient mice. J. Pharmacol. Exp. Ther. 2005, 314, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Chapman, R.J.; Woodhams, S.; Sagar, D.R.; Turner, J.; Burston, J.J.; Bullock, C.; Paton, K.; Huang, J.; Wong, A.; et al. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann. Rheum. Dis. 2015, 74, 252–259. [Google Scholar] [CrossRef]
- Stevens, R.M.; Ervin, J.; Nezzer, J.; Nieves, Y.; Guedes, K.; Burges, R.; Hanson, P.D.; Campbell, J.N. Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated With Osteoarthritis of the Knee. Arthritis Rheumatol. 2019, 71, 1524–1533. [Google Scholar] [CrossRef]
- Bellamy, N.; Campbell, J.; Stevens, J.; Pilch, L.; Stewart, C.; Mahmood, Z. Validation study of a computerized version of the Western Ontario and McMaster Universities VA3.0 Osteoarthritis Index. J. Rheumatol. 1997, 24, 2413–2415. [Google Scholar]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Argoff, C.E. Topical analgesics in the management of acute and chronic pain. Mayo Clin. Proc. 2013, 88, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Seefried, L.; Blyth, M.; Maheshwari, R.; McDonnell, S.M.; Frappin, G.; Hagen, M.; Maybaum, N.; Moreira, S.; Pandit, H. Penetration of topical diclofenac into synovial tissue and fluid of osteoarthritic knees: A multicenter, randomized, placebo-controlled, pharmacokinetic study. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20943088. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J.W.; Somers, G.F. Percutaneous absorption. J. Pharm. Pharmacol. 1956, 8, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Hwang, S.W.; Kwak, J.; Lee, S.Y.; Kang, C.J.; Kim, W.B.; Kim, D.; Oh, U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999, 19, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab. Dispos. 2008, 36, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.S.; Sheth, R.N.; Bencherif, B.; Frost, J.J.; Campbell, J.N. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain 2002, 99, 207–216. [Google Scholar] [CrossRef]
- Simone, D.A.; Baumann, T.K.; LaMotte, R.H. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 1989, 38, 99–107. [Google Scholar] [CrossRef]
- Knolle, E.; Zadrazil, M.; Kovacs, G.G.; Medwed, S.; Scharbert, G.; Schemper, M. Comparison of cooling and EMLA to reduce the burning pain during capsaicin 8% patch application: A randomized, double-blind, placebo-controlled study. Pain 2013, 154, 2729–2736. [Google Scholar] [CrossRef]
- Stevens, R.; Guedes, K.; Mistry, N.; Lascelles, D.; Ball, D. AB0810 Intra-Articular CNTX-4975 for Painful Knee Osteoarthritis: Assessment of Cooling Methods for Reducing Procedural Pain. Ann. Rheum. Dis. 2019, 78, 1875–1876. [Google Scholar]
- Stevens, R.; Hanson, P.; Tiseo, P.J.; Guedes, K.; Campbell, J.; Connolly, J.; Ruggiero, S.; Corliss, M.; Smith, V.; Conaghan, P.G. OP0187 Determining Optimal Cooling and Administration Methods for CNTX-4975 Intra-articular Injection in Subjects With Moderate to Severe Osteoarthritis Knee Pain. Ann. Rheum. Dis. 2020, 79, 116. [Google Scholar] [CrossRef]
- Earp, B.; Cefalu, C.; Blazar, P. Thumb Metacarpophalangeal Joint Arthritis. J. Am. Acad. Orthop. Surg. 2019, 27, e1029–e1039. [Google Scholar] [CrossRef] [PubMed]
- Day, C.S.; Ramirez, M.A. Thumb metacarpophalangeal arthritis: Arthroplasty or fusion? Hand Clin. 2006, 22, 211–220. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, J.N.; Stevens, R.; Hanson, P.; Connolly, J.; Meske, D.S.; Chung, M.-K.; Lascelles, B.D.X. Injectable Capsaicin for the Management of Pain Due to Osteoarthritis. Molecules 2021, 26, 778. https://doi.org/10.3390/molecules26040778
Campbell JN, Stevens R, Hanson P, Connolly J, Meske DS, Chung M-K, Lascelles BDX. Injectable Capsaicin for the Management of Pain Due to Osteoarthritis. Molecules. 2021; 26(4):778. https://doi.org/10.3390/molecules26040778
Chicago/Turabian StyleCampbell, James N., Randall Stevens, Peter Hanson, James Connolly, Diana S. Meske, Man-Kyo Chung, and Benedict Duncan X. Lascelles. 2021. "Injectable Capsaicin for the Management of Pain Due to Osteoarthritis" Molecules 26, no. 4: 778. https://doi.org/10.3390/molecules26040778
APA StyleCampbell, J. N., Stevens, R., Hanson, P., Connolly, J., Meske, D. S., Chung, M.-K., & Lascelles, B. D. X. (2021). Injectable Capsaicin for the Management of Pain Due to Osteoarthritis. Molecules, 26(4), 778. https://doi.org/10.3390/molecules26040778






