Mealworm Oil (MWO) Enhances Wound Healing Potential through the Activation of Fibroblast and Endothelial Cells
Abstract
1. Introduction
2. Results
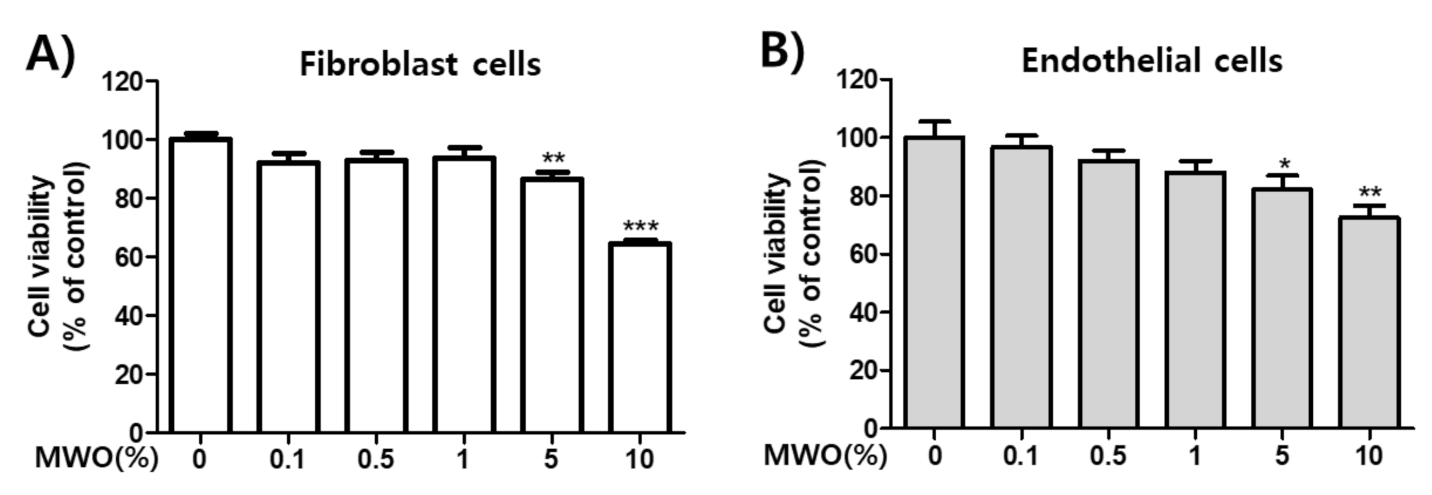
2.1. Effect of MWO on Cell Viability
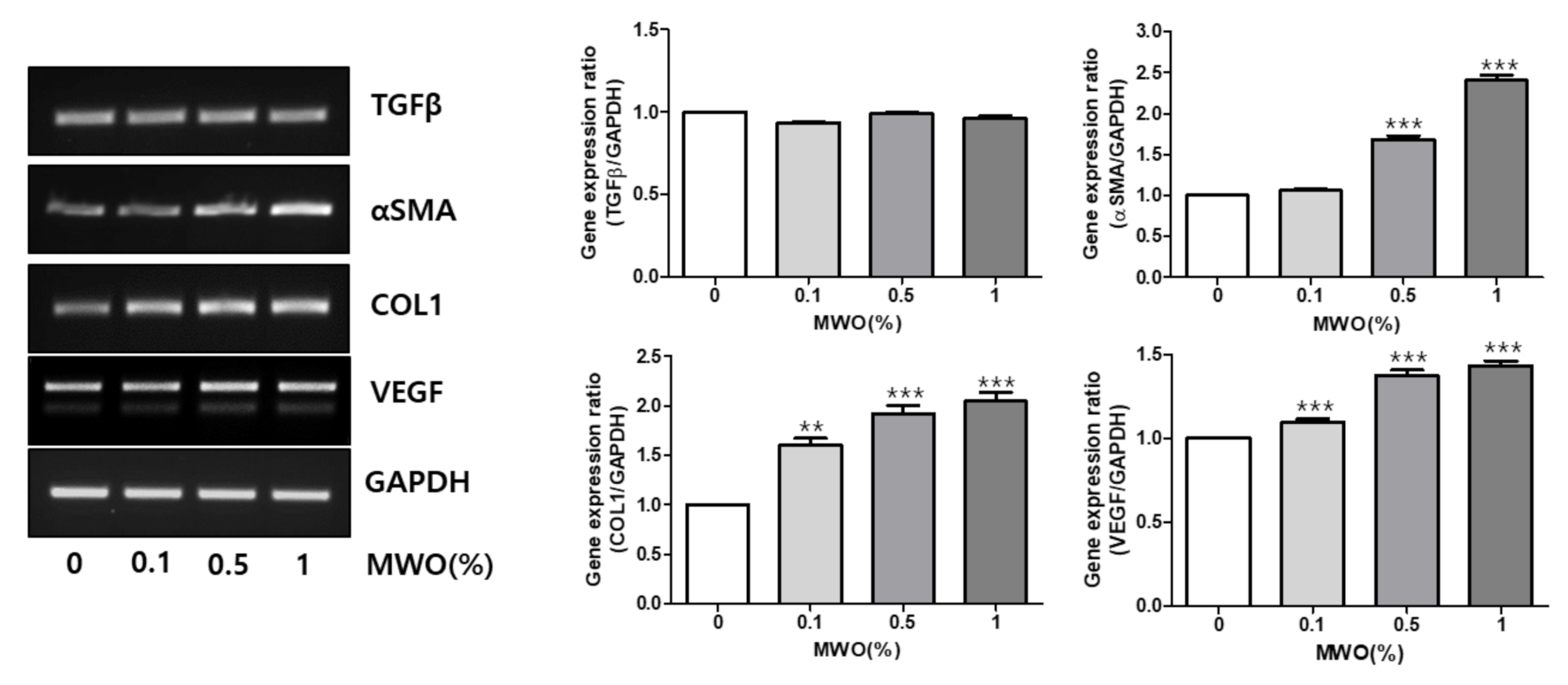
2.2. Regulation of mRNA Expressions of Genes Related to Wound Healing by MWO in Fibroblasts
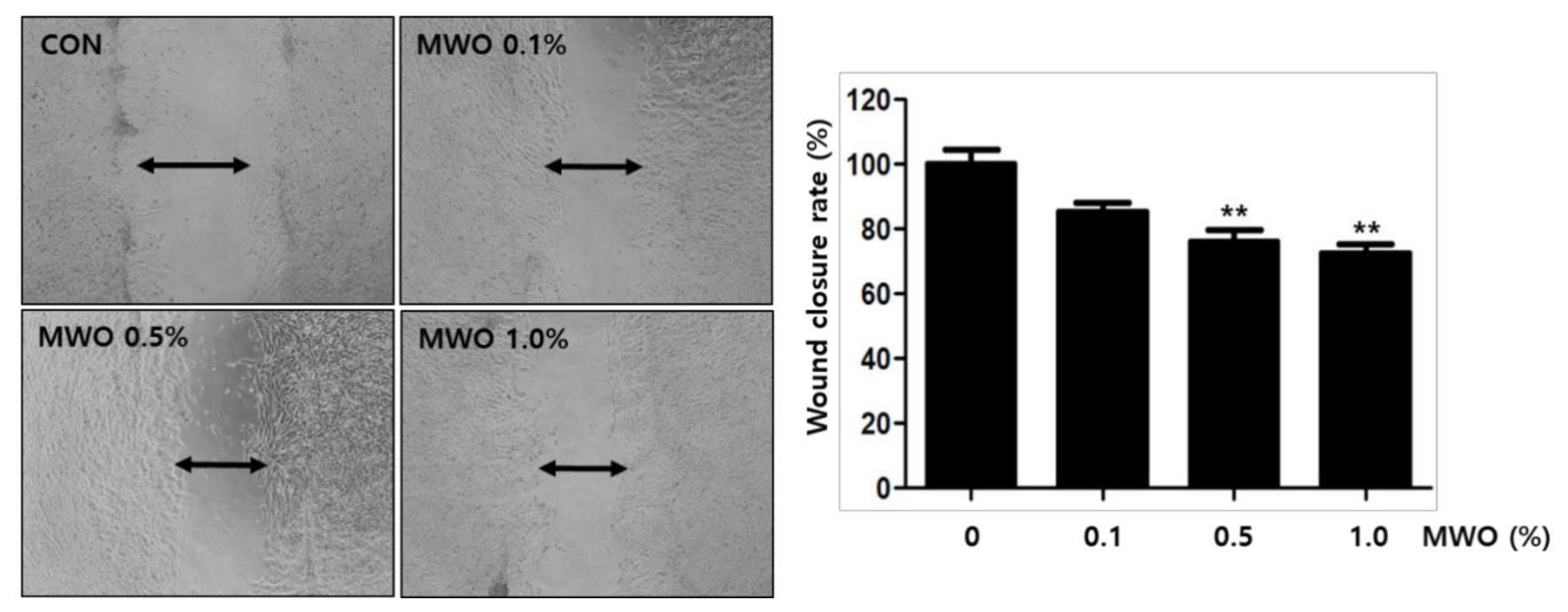
2.3. The Enhanced Migration of Fibroblasts by MWO
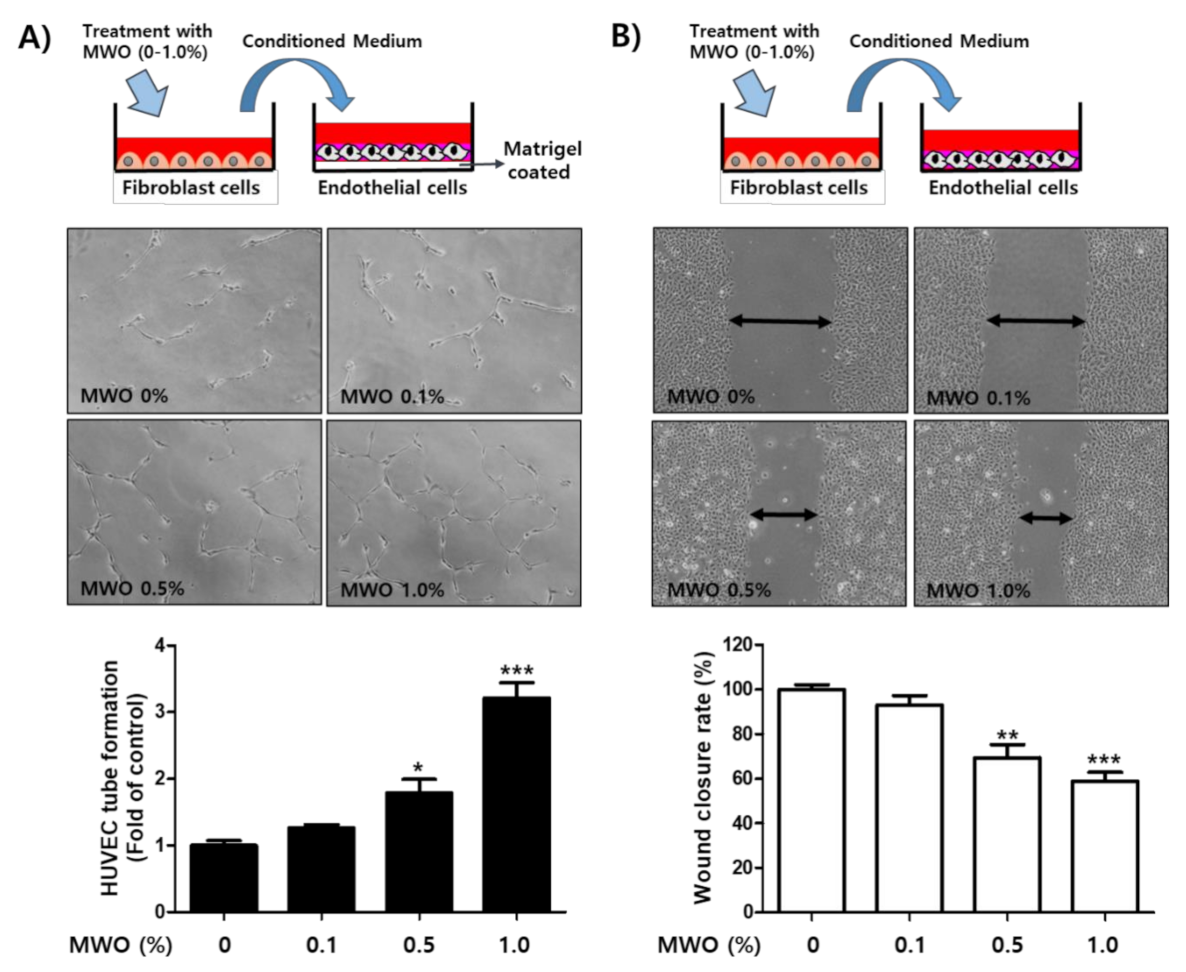
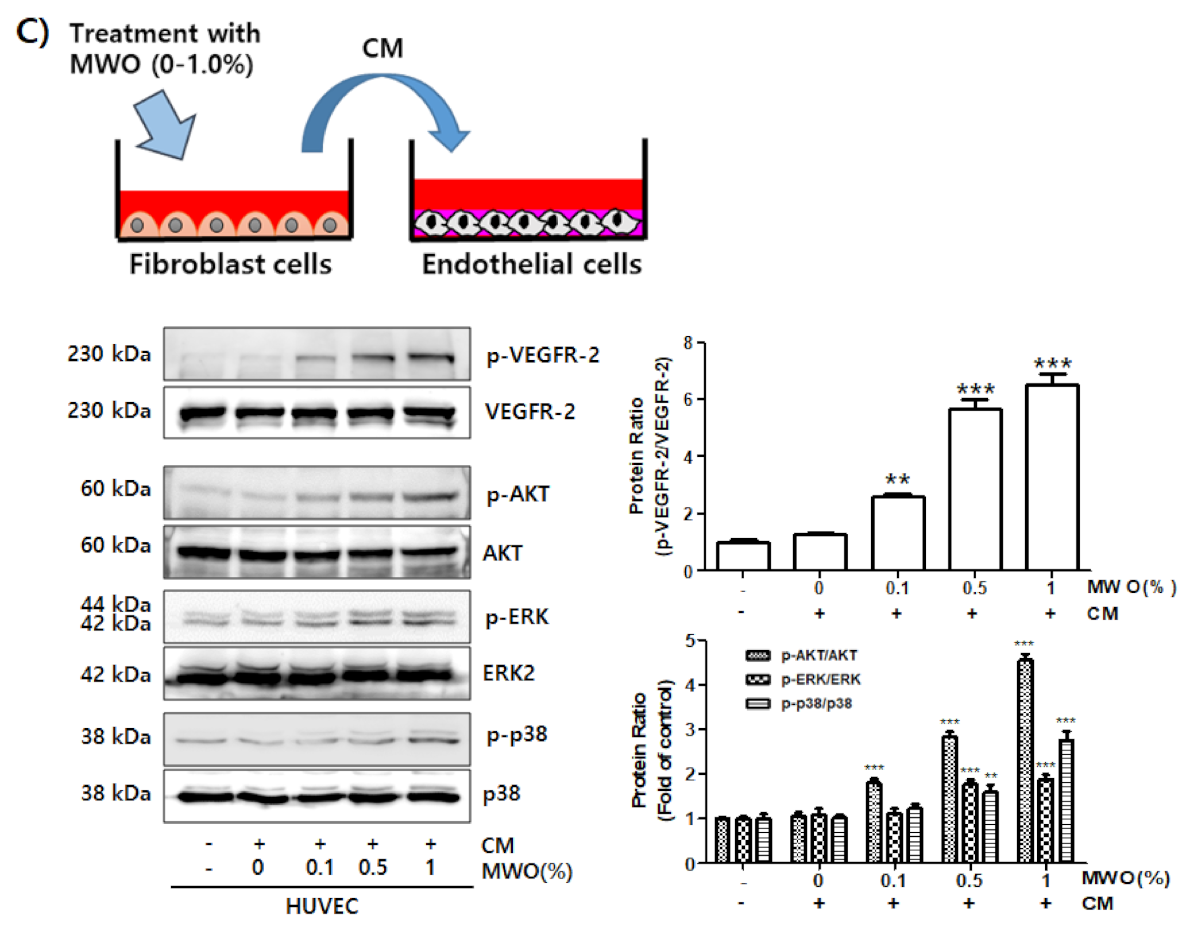
2.4. The Induction of Capillary-Like Tube Formation and Migration through VEGFR-2 Activation of Endothelial Cells by MWO-Stimulated Fibroblasts
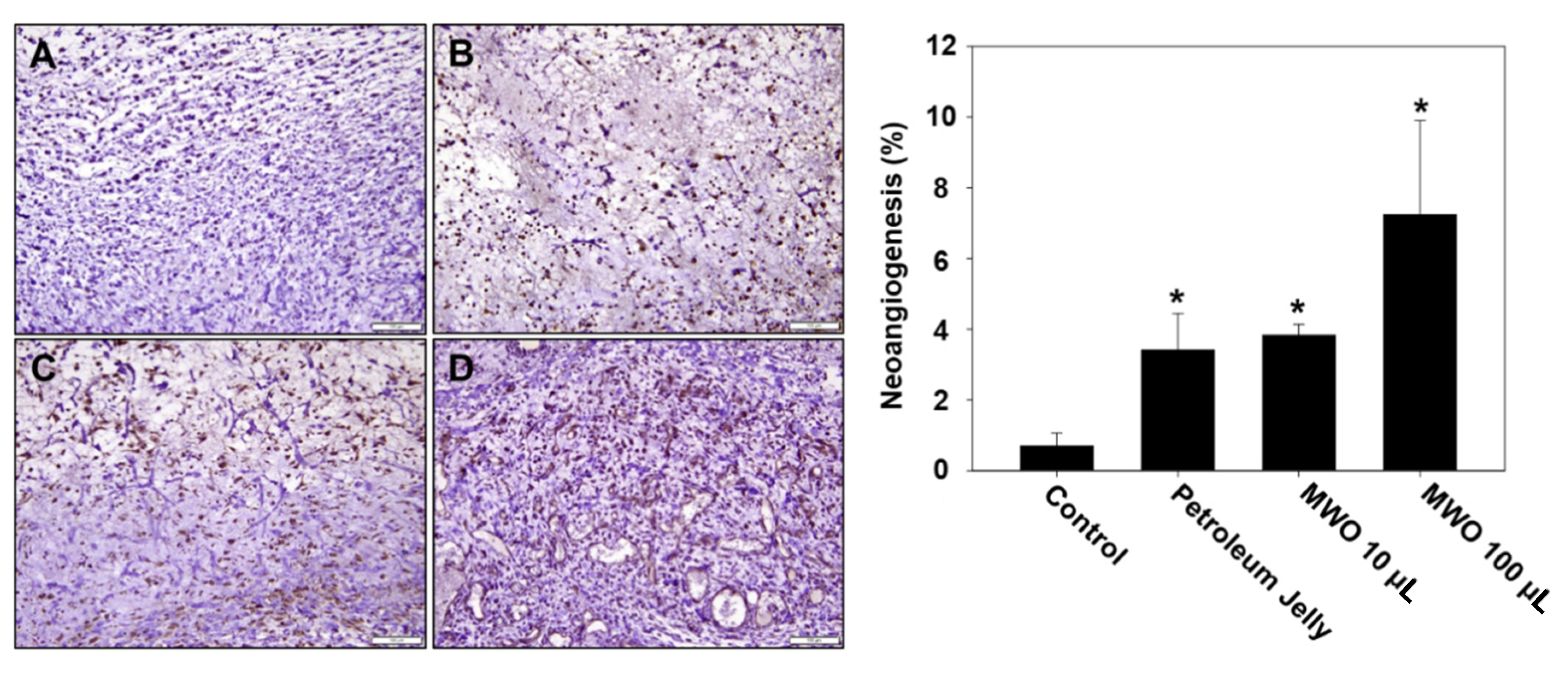
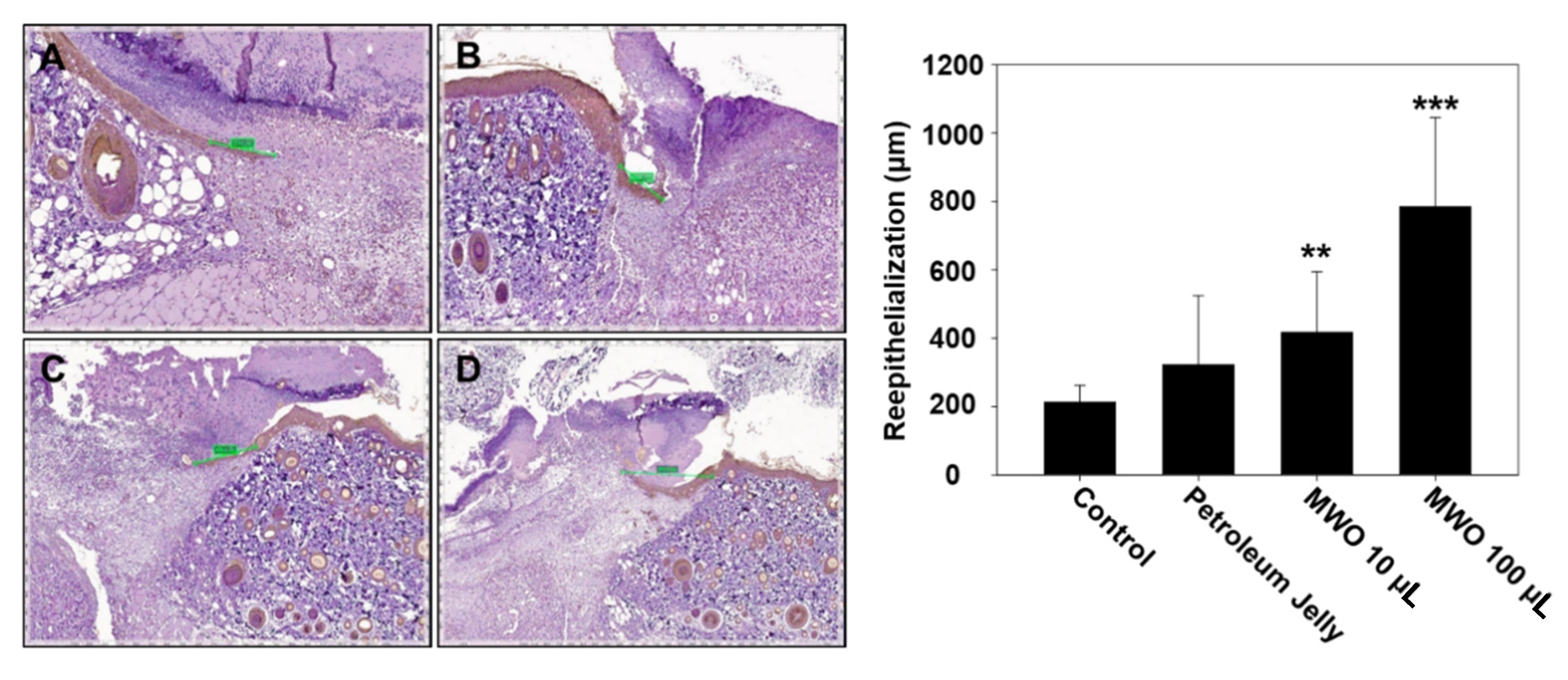
2.5. Effect of MWO on Skin Wound Healing in Rats
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. The Preparation of MWO
4.4. Cell Viability
4.5. Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
4.6. Tube Forming Assay
4.7. Wound Healing Assay
4.8. Western Blot Analysis
4.9. Animal Experimental Design
4.10. Histological Analysis
4.11. Immunohistochemistry
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol. Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Khemiri, I.; Essghaier Hedi, B.; Sadfi Zouaoui, N.; Ben Gdara, N.; Bitri, L. The Antimicrobial and Wound Healing Potential of Opuntia ficus indica L. inermis Extracted Oil from Tunisia. Evid. Based Complement. Alternat. Med. 2019, 2019, 9148782. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, G.W.; Park, K.H.; Mohamed, M.A.; Bang, M.H.; Baek, Y.S.; Son, Y.; Chung, D.K.; Baek, N.I.; Kim, J. The stimulatory effects of Stewartia koreana extract on the proliferation and migration of fibroblasts and the wound healing activity of the extract in mice. Int. J. Mol. Med. 2014, 34, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.J.; Chen, C.C.; Leu, Y.L.; Lin, M.W.; Chiu, M.M.; Wang, S.H. The effects of artocarpin on wound healing: In vitro and in vivo studies. Sci. Rep. 2017, 7, 15599. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.H.; Son, Y.J.; Kim, S.H.; Yun, E.Y.; Kang, H.J.; Hwang, I.K. Physicochemical properties and oxidative stabilities of mealworm (Tenebrio molitor) oils under different roasting conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; Choi, S.Y.; Hwang, I.K.; Nho, C.W.; Kim, S.H. Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients? Foods 2020, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.J.; Kang, D.G.; Jung, C.; Sohn, H.Y. Anti-Thrombotic, Anti-Oxidant and Haemolysis Activities of Six Edible Insect Species. Foods 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Goo, T.W.; Chung, M.Y.; Baek, M.; Hwang, J.S.; Kim, M.A.; Yun, E.Y. Tenebrio molitor Larvae Inhibit Adipogenesis through AMPK and MAPKs Signaling in 3T3-L1 Adipocytes and Obesity in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, Y.S.; Jang, S.Y.; Jun, S.Y.; Lim, J.H.; Kim, I.K.; Kim, H.M.; Park, J.S. Clinical application of invalid foods using mealworms and evaluation of nutrition status and immune function: A study protocol for a randomized, double blind, placebo-controlled trial. BMC Nutr. 2019, 5, 44. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. General principles of wound healing. Surg. Clin. N. Am. 1997, 77, 509–528. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.; Dallon, J.; Sherratt, J.; Maini, P. Fibroblast migration and collagen deposition during dermal wound healing: Mathematical modelling and clinical implications. Philos. T. R. Soc. A 2006, 364, 1385–1405. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.H.C. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches to maximize healing trajectories—In brief. Curr. Prob. Surg. 2001, 38, 65–140. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.D.; Clark, R.A.F. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Coppe, J.P.; Kauser, K.; Campisi, J.; Beausejour, C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef]
- Detsch, R.; Stoor, P.; Grunewald, A.; Roether, J.A.; Lindfors, N.C.; Boccaccini, A.R. Increase in VEGF secretion from human fibroblast cells by bioactive glass S53P4 to stimulate angiogenesis in bone. J. Biomed. Mater. Res. A 2014, 102, 4055–4061. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Wang, M.Z.; Yuan, T.J.; Xu, X.H.; Dad, H.A.; Yu, C.L.; Hou, J.; Peng, L.H. The crude ethanol extract of Periplaneta americana L. stimulates wound healing in vitro & in vivo. Chin. Med. 2019, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Kim, S.J.; Choi, H.J.; Kim, K.J.; Kim, M.J.; Kim, S.H.; Lee, H.J.; Ko, J.H.; Lee, Y.C.; Suzuki, A.; et al. Ganglioside GM3 inhibits VEGF/VEGFR-2-mediated angiogenesis: Direct interaction of GM3 with VEGFR-2. Glycobiology 2009, 19, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Hensbergen, P.; Gibbs, S.; Kempenaar, J.; van der Schors, R.; Ponec, M. Fibroblasts facilitate re-epithelialization in wounded human skin equivalents. Lab. Investig. 2004, 84, 102–112. [Google Scholar] [CrossRef]
- Chung, T.W.; Kim, S.J.; Choi, H.J.; Kwak, C.H.; Song, K.H.; Suh, S.J.; Kim, K.J.; Ha, K.T.; Park, Y.G.; Chang, Y.C.; et al. CAPE suppresses VEGFR-2 activation, and tumor neovascularization and growth. J. Mol. Med. 2013, 91, 271–282. [Google Scholar] [CrossRef]

| Gene | Primer Sequences | PCR Condition | Size (bp) |
|---|---|---|---|
| mTGF-β1 | Forward: 5′-AGGAGACGGAATACAGGGCT-3′ Reverse: 5′-CCACGTAGTAGACGATGGGC-3′ | 60 °C 30 cycles | 482 |
| mα-SMA | Forward: 5′-CTGACAGAGGCACCACTGAA-3′ Reverse: 5′-CATCTCCAGAGTCCAGCACA-3′ | 55 °C 30 cycles | 160 |
| mCOL-1 | Forward: 5′-AAGAGGCGAGAGAGGTTTCC-3′ Reverse: 5′-AGAACCATCAGCACCTTTGG-3′ | 55 °C 25 cycles | 244 |
| mVEGF | Forward: 5′-CAGCACATAGGAGAGATGAGC-3′ Reverse: 5′-TCACCGCCTCGGCTTGTCACA-3′ | 60 °C cycles | 234, 306 |
| mGAPDH | Forward: 5′-AACTTTGGCATTGTGGAAGG-3′ Reverse: 5′-ACACATTGGGGGTAGGAACA-3′ | 60 °C 30 cycles | 223 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Kim, E.-Y.; Chung, K.J.; Lee, J.-H.; Choi, H.-J.; Chung, T.-W.; Kim, K.-J. Mealworm Oil (MWO) Enhances Wound Healing Potential through the Activation of Fibroblast and Endothelial Cells. Molecules 2021, 26, 779. https://doi.org/10.3390/molecules26040779
Kim J-H, Kim E-Y, Chung KJ, Lee J-H, Choi H-J, Chung T-W, Kim K-J. Mealworm Oil (MWO) Enhances Wound Healing Potential through the Activation of Fibroblast and Endothelial Cells. Molecules. 2021; 26(4):779. https://doi.org/10.3390/molecules26040779
Chicago/Turabian StyleKim, Joung-Hee, Eun-Yeong Kim, Kyu Jin Chung, Jung-Hee Lee, Hee-Jung Choi, Tae-Wook Chung, and Keuk-Jun Kim. 2021. "Mealworm Oil (MWO) Enhances Wound Healing Potential through the Activation of Fibroblast and Endothelial Cells" Molecules 26, no. 4: 779. https://doi.org/10.3390/molecules26040779
APA StyleKim, J.-H., Kim, E.-Y., Chung, K. J., Lee, J.-H., Choi, H.-J., Chung, T.-W., & Kim, K.-J. (2021). Mealworm Oil (MWO) Enhances Wound Healing Potential through the Activation of Fibroblast and Endothelial Cells. Molecules, 26(4), 779. https://doi.org/10.3390/molecules26040779






