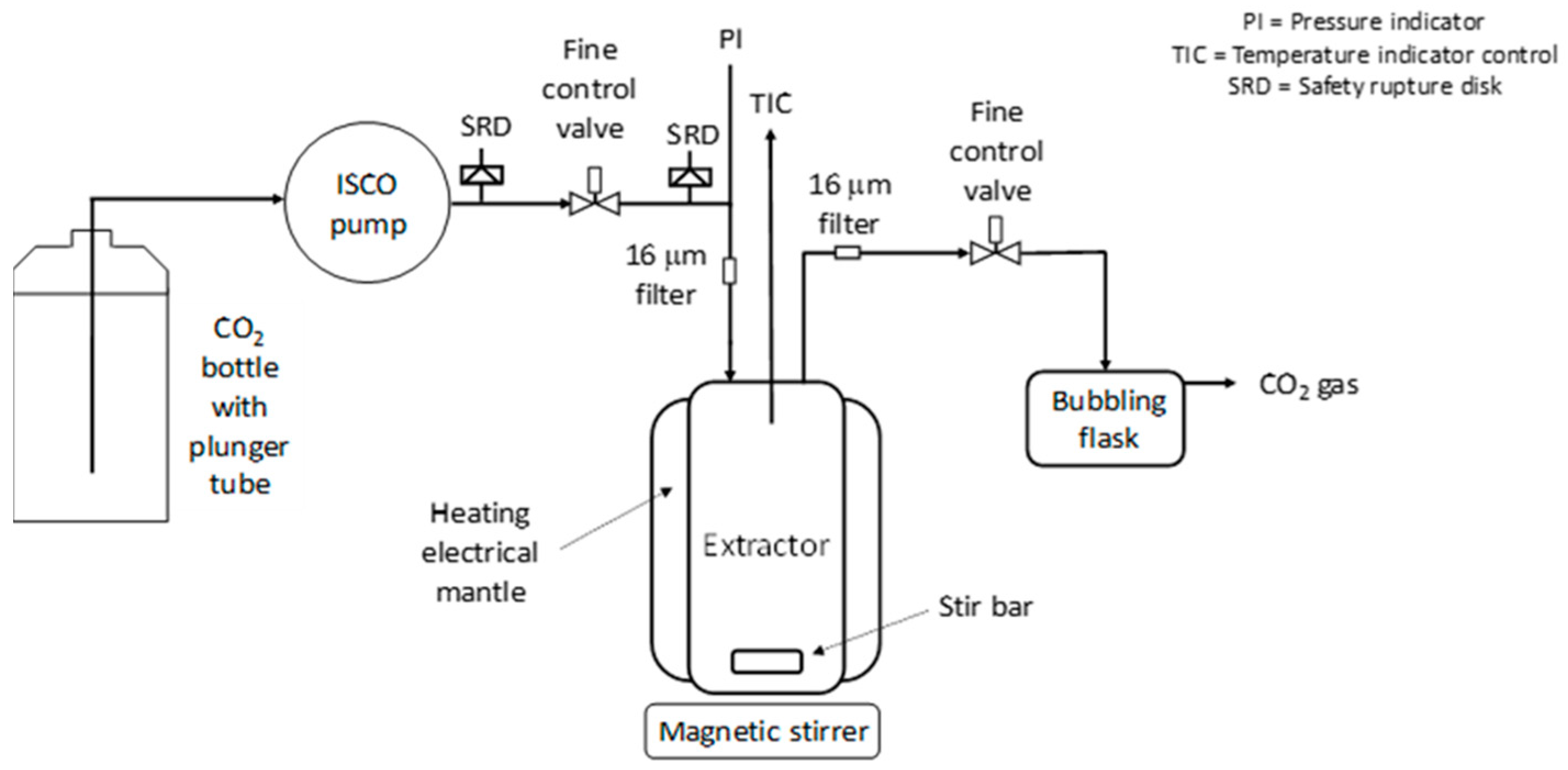
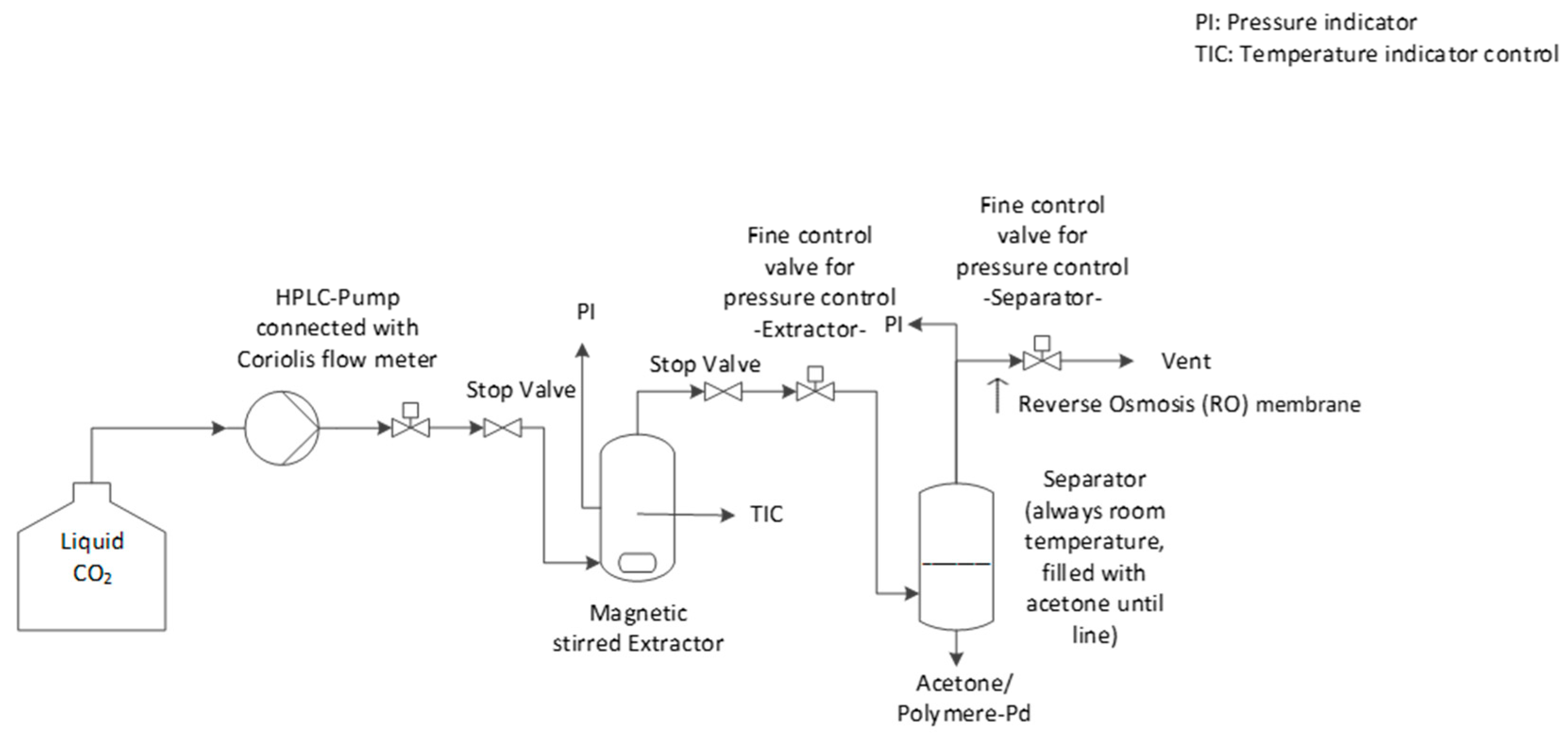
In this article, extraction screening experiments were performed in a small extraction cell (35 mL) to get a first impression of the extraction ability of the polymer/additive/Pd-catalyst system. Then, in a larger extractor (250 mL), promising extractions were investigated in more detail. In the extraction experiments, the extraction conversion, X
extraction, refers to the amount of Pd extracted from the catalyst support, and extraction yield, Y
extraction, refers to the amount of extracted Pd recovered. The amount of Pd extracted used to calculate X
extraction and Y
extraction was determined by inductively coupled plasma optical emission spectrometry (ICP-OES) in the different sample fractions, as detailed in the materials and methods section and in the
Supporting Information. The Pd-balance is the overall mass balance of palladium in the extraction system. The calculations for the Pd-balance are detailed in the materials and methods section.
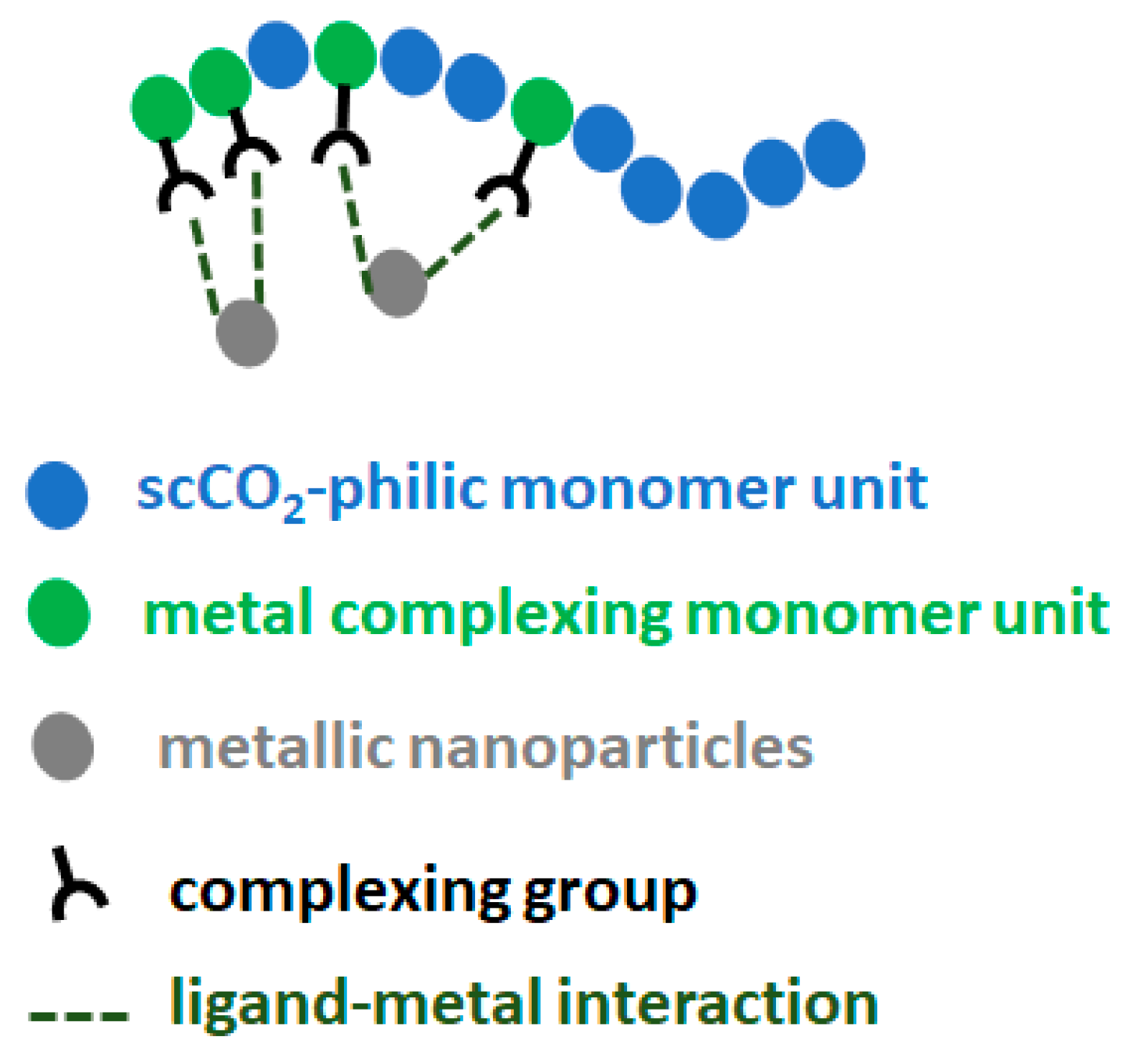
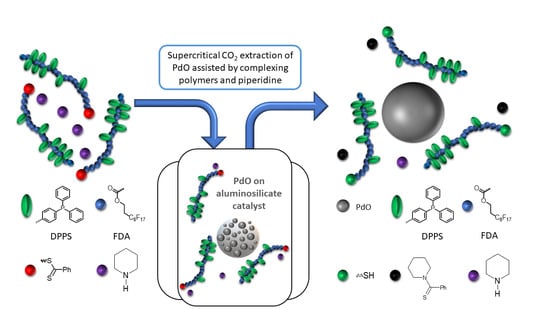
2.1. Design of scCO2-Soluble Polymers Capable of Complexing with Pd
In this study, fluorinated polymers containing FDA monomer units were chosen as assisting complexing polymers for their good solubility in scCO
2, while being aware of the harmful potential of this kind of fluorinated polymer. However, as the polymers are to be recycled later on (as will be taken into account in the life cycle analysis, LCA), there should be no exposure of the polymer to the environment. The first polymer was a homopolymer of FDA with a thiol complexing group, p(FDA)SH. The second polymer was a copolymer of FDA with DPPS, providing triphenylphosphine ligands for complexing with Pd (p(FDA-
co-DPPS)). The polymerization was carried out by a reversible addition-fragmentation chain transfer (RAFT) technique, using a chain transfer agent (CTA) (cf.
SI-Chapter 1). The polymers serve as binding agents to bind to the Pd on the catalyst and carry the Pd through the scCO
2 medium, as the solubility of Pd in scCO
2 alone is negligible or null. Thus, polymers consisting of one type of scCO
2-soluble group (FDA) and one or more types of group capable of complexing with Pd (DPPS, -SH) were employed. This general design is shown in
Figure 1, with scCO
2-soluble groups in blue, and the Pd complexing groups in green.
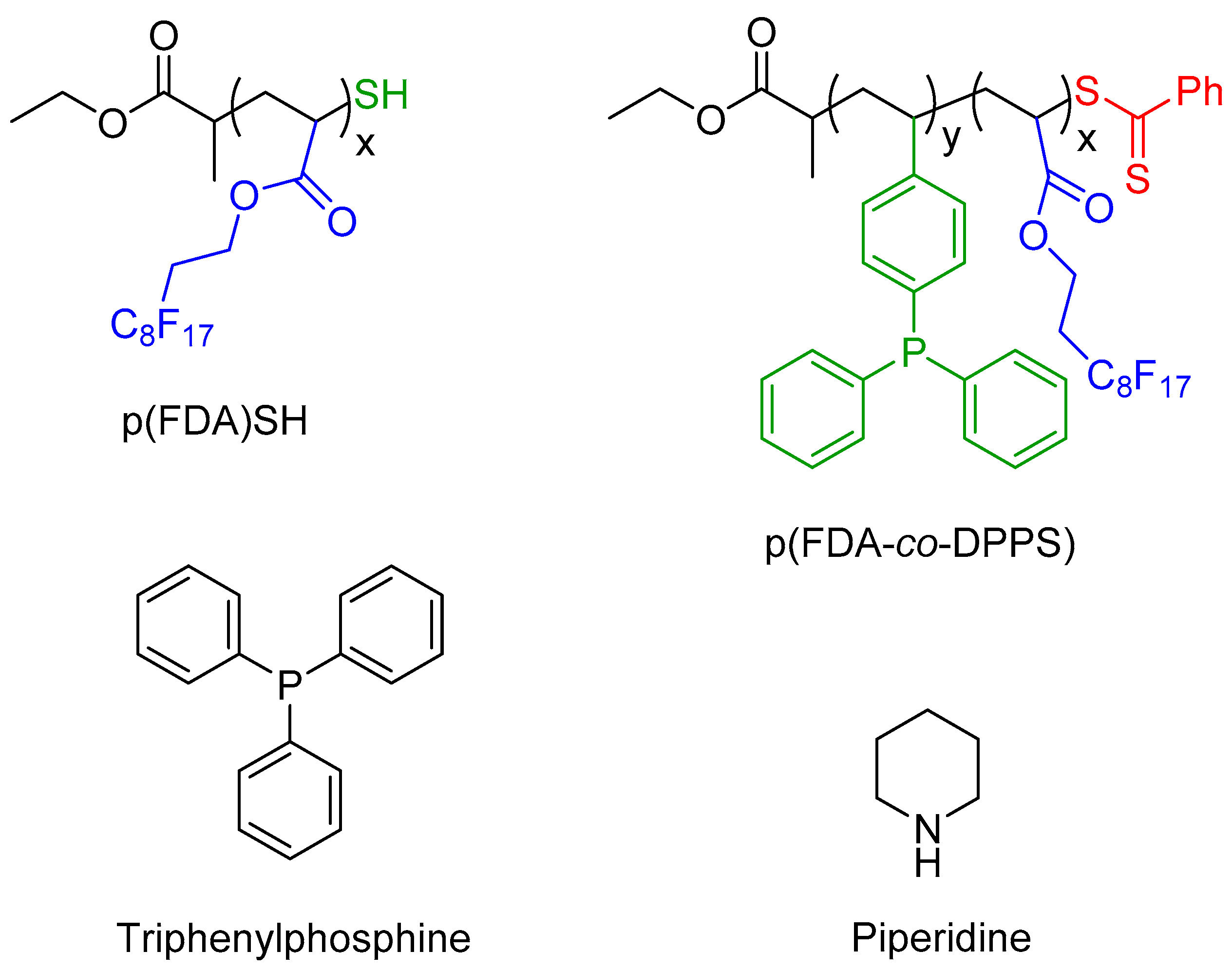
The two polymers used in this study are presented in
Figure 2, with scCO
2-soluble FDA groups in blue, metal complexing groups in green (thiol group for p(FDA)SH and triphenylphosphine groups for p(FDA-
co-DPPS)), and protected thiol groups in red. The p(FDA-
co-DPPS) copolymer bears two different metal complexing groups, given that the protected thiol group is activated by deprotection with an amine (aminolysis). This step was done in situ during the extraction using piperidine (
Figure 2), giving two groups capable of complexing with Pd (DPPS units and −SH end group).
2.2. Pd Extraction from Catalyst Cat D with Only scCO2
Before using the polymers in extraction, the extraction ability of scCO
2 alone was investigated. Control tests were performed in both experimental setups (60 min for screening experiments and 90 min for detailed investigations), with and without piperidine (pip), to determine the amount of precious metal extracted using only scCO
2. The extraction parameters are shown in
Table 1, and the extraction results in
Figure 3. Further information can be obtained from the
supporting information (cf. SI-Chapter 4.1).
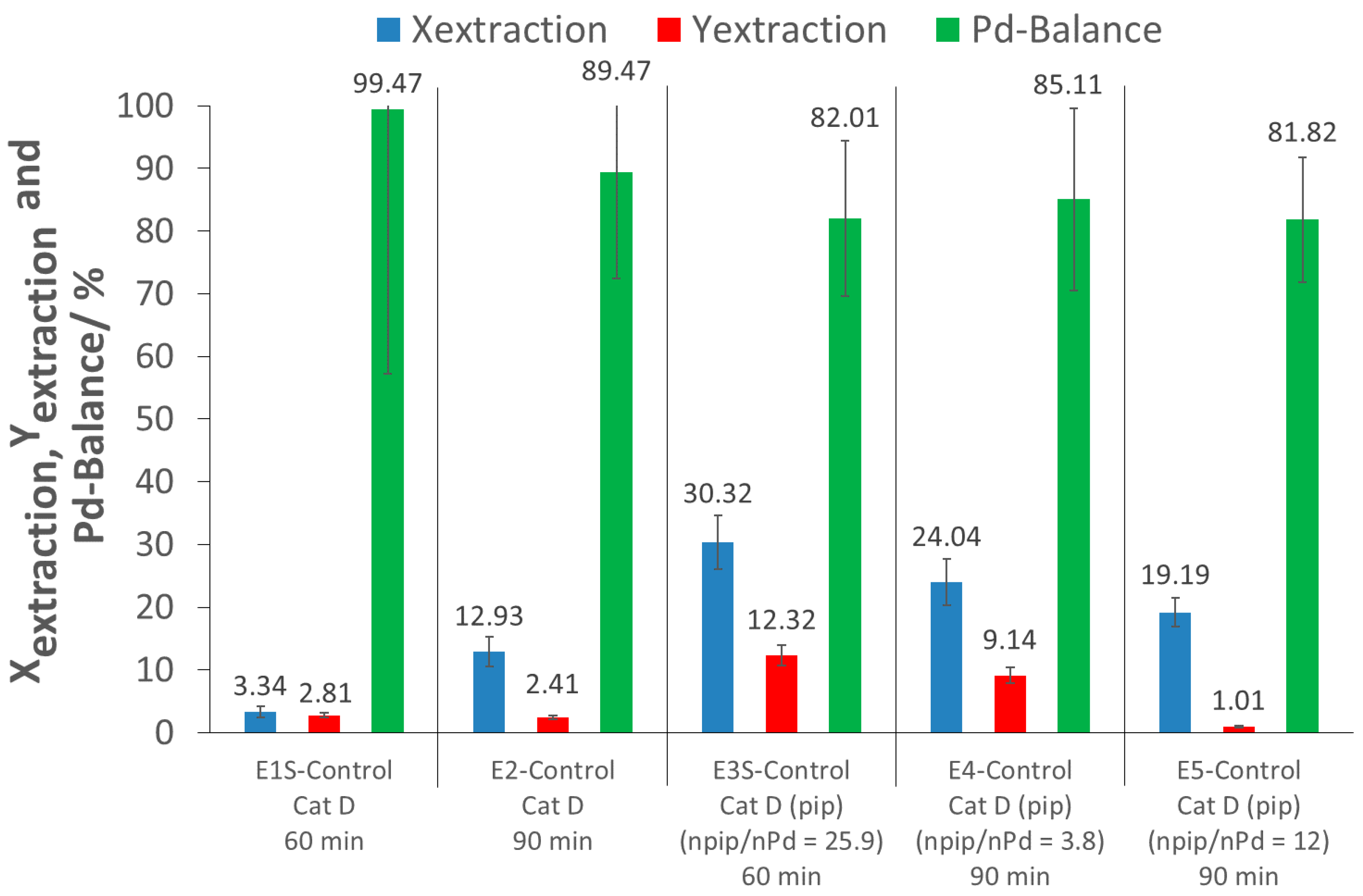
For the screening experiment, the extraction of Pd from the pristine catalyst after 60 min (E1S-Control) using only scCO2 resulted in a low extraction conversion (where extraction conversion, Xextraction, is the amount of Pd extracted from the catalyst support) and extraction yield (where extraction yield, Yextraction, is the amount of extracted Pd recovered), of 3% each. For the detailed investigation with a 90 min extraction time (E2-Control), an extraction conversion of about 13% was achieved with a low extraction yield of 2%. These low extraction conversions were expected, as there was no complexing agent for the precious metal. The extraction yield of E2-Control being lower than the extraction conversion was due to a loss of Pd during the Pd recovery process. As the Pd-balance was incomplete, some Pd was possibly lost to the atmosphere during CO2 depressurization and system flushing after extraction.
Piperidine was used as an in situ activation agent for the deprotection of the terminal dithioester group of the polymers to give a terminal –SH group. Since the –NH group of piperidine can potentially complex with Pd [
19], additional control experiments with scCO
2 and piperidine were performed at different piperidine/Pd ratios (E3S-Control, E4-Control, E5-Control) and extraction times. For the screening experiment, results showed that with 60 min of extraction time, the addition of piperidine, at a molar piperidine/Pd ratio of 25.9, increased the extraction conversion by up to 30% (E3S-Control). In the larger extractor for the detailed investigations, the addition of piperidine at a molar piperidine/Pd ratio of 3.8 and an extraction time of 90 min (E4-control) resulted in an extraction conversion of 24%, while with a piperidine/Pd ratio of 12 (E5-Control), a slightly lower extraction conversion of 19% was achieved. Thus, piperidine has a promoting effect, which is not fully understood at this point, and would deserve further attention. Nevertheless, the extraction conversions (<30%) were still too low for a scale-up and industrial application. Moreover, the extraction yields were even lower (less than 12%), due to loss of Pd during recovery.
2.3. Pd Extraction from Catalyst Cat D with PPh3
The previous control experiments showed that for a good extraction of Pd in scCO
2, an extracting agent (polymer), containing complexing groups, is necessary to bind to the metal. Taking into account the structure of p(FDA-
co-DPPS), it can be easily identified that the DPPS units mimic the triphenylphosphine ligand (PPh
3), which is extensively used to form Pd complexes [
19,
20]. Based on this assumption, the efficiency of the triphenylphosphine molecule as an extracting agent in supercritical CO
2 was tested (cf.
Table 2,
Figure 4 and
SI-Chapter 4.2). The extraction experiment E6S-PPh
3 was performed in the presence of PPh
3 alone for 60 min, without the use of other complexing or activating agents. This test showed the low efficiency of the low molecular weight additive, PPh
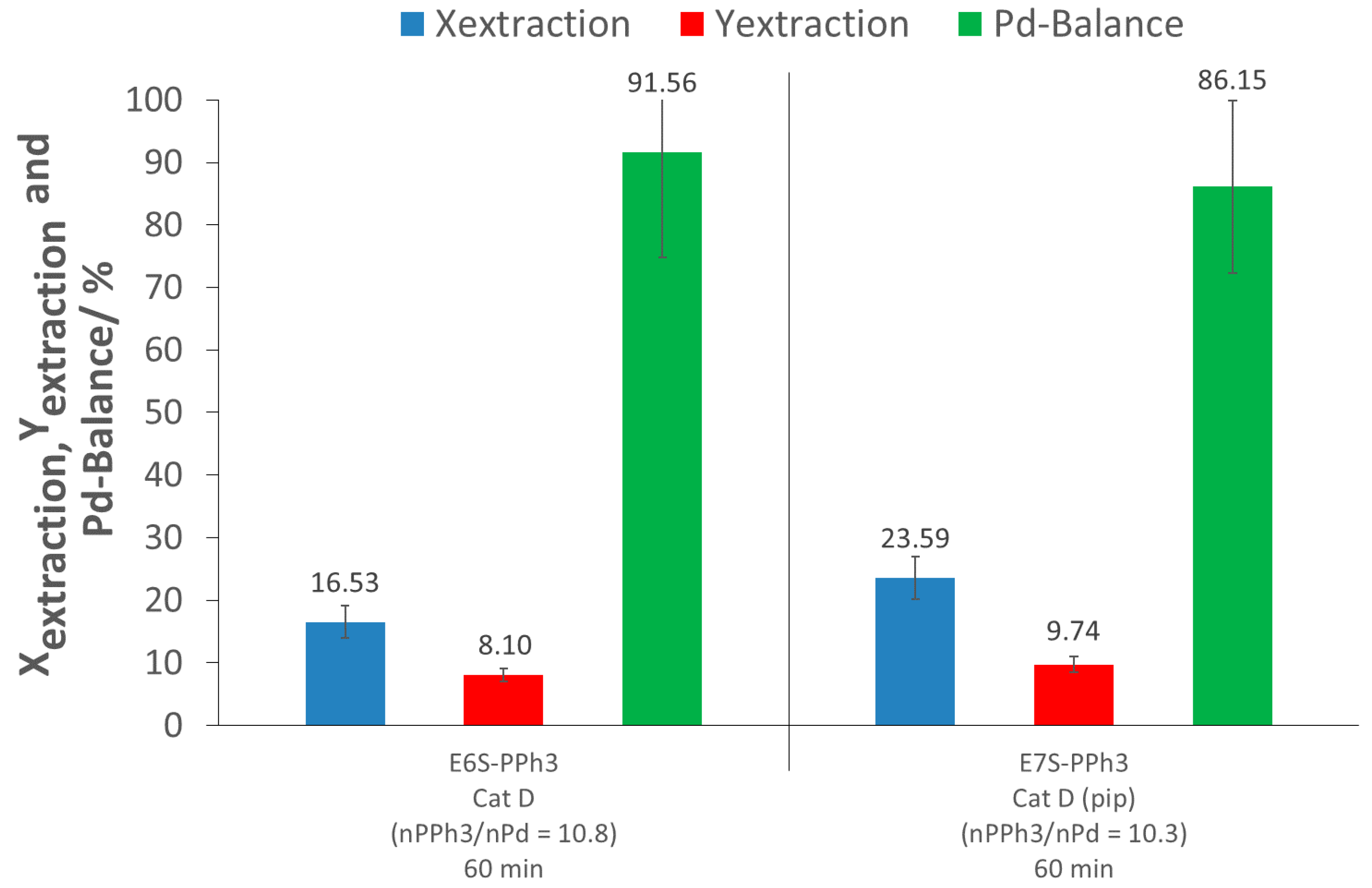
3, as an extracting agent, since the extraction conversion only reached 17%. In addition, the extraction yield was low (8%), due to the same reason as for the control tests (loss of Pd during depressurization and flushing). This result was likely due to the low solubility of PPh
3 in scCO
2 [
21], which would further decrease if Pd complexation occurred. Despite the low conversion, it was an improvement compared to the control test E1S-Control (in scCO
2 alone, 60 min). This indicates that the presence of the complexing agent in scCO
2 increases the extraction of Pd from the aluminosilicate support, simultaneously confirming the complexing ability of the PPh
3 group for Pd species.
As the p(FDA-co-DPPS) extraction experiments involve the in situ activation of the thiol group by piperidine, the addition of both piperidine and PPh3 in the scCO2 extraction was tested to see if there were any synergic extraction effects between the two complexing groups (E7S-PPh3). The results showed a small increase in the extraction conversion, of up to 24% (7% higher than E6S-PPh3), while the extraction yield remained nearly constant (10% instead of 8%). However, the amount of extracted palladium and recovered palladium using this combination (E7S-PPh3) was actually slightly lower than that measured when piperidine was used alone (E3S-Control) (X: 6% lower; Y: 2% lower), meaning no synergy, and even possibly an unfavorable interaction between the two systems on a molecular level.
2.4. Pd Extraction from Catalyst Cat D with Polymer p(FDA)SH
After the initial screening of the effect of only scCO
2, and that of molecular additives in Pd extraction, polymer-assisted extraction was investigated. The first polymer used for this investigation was p(FDA)SH (cf.
Figure 2;
Table 3;
Figure 5;
SI-Chapter 4.3). This fluorinated homopolymer is highly soluble in scCO
2, and contains a thiol end-group, which is a well-known ligand for transition metals, and was expected to be able to complex with Pd(II) species [
19,
22]. The thiol functionality (-SH) was activated by ex situ aminolysis of the protected polymer in order to have at least one potential complexing group, as the fluorinated units are not able to interact with Pd. The conditions used for the aminolysis of the polymer (N
2 atmosphere in the presence of PPh
3 as reducing agent) allowed limiting the disulfide formation during synthesis [
23,
24]. The thiol group of the resulting p(FDA)SH could potentially couple to give disulfide bonds, but as the polymer is solubilized in scCO
2 in a predominantly CO
2 environment (very little oxygen is present) during extraction, the formation of disulfide bonds is unlikely [
25]. During storage of the polymer, disulfide formation is possible due to exposure to oxygen [
26], but should be limited due to the semi-crystalline nature of the p(FDA) [
27], which drastically reduces the mobility of the polymer chains in the solid state [
28]. This polymer was used in a molar ratio of 10.3/1 with respect to the PdO present on the catalyst, meaning that a large, ten-fold excess of complexing units was used to perform the metal extraction. The polymer excess of 10.3 was chosen to ensure that a low extraction conversion would not be due to an insufficient number of complexing groups, but to a low polymer complexing ability.
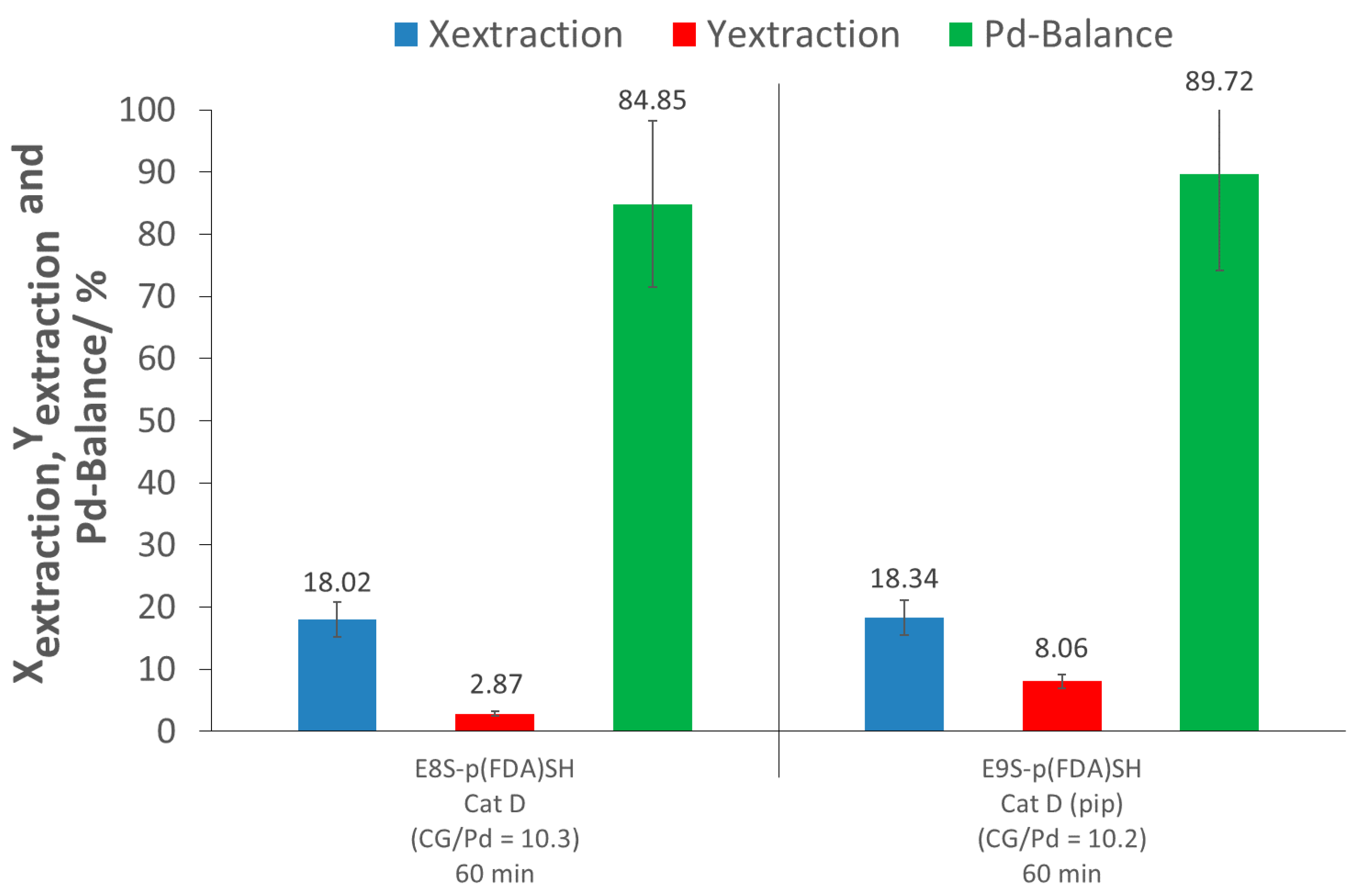
The extraction assisted by the thiol-terminated, fluorinated polymer (E8S-p(FDA)SH) showed limited extraction ability, achieving only 18% extraction conversion. The low efficiency can be explained by a strong interaction of the PdO with the support, as well as by the limited activity of organic ligands towards PdO. The extraction yield was also very low (3%), due to an important loss of Pd (Pd-balance of 85%). A second test was performed using the same polymer, but with the addition of piperidine to verify if this secondary amine is able to improve the solubility of the precious metal in the presence of the fluorinated polymer. The extraction experiment with the combination of p(FDA)SH and piperidine (E9S-p(FDA)SH) did not show any improvement in the extraction conversion (18%), showing an absence of synergic effect between p(FDA)SH and piperidine.
2.5. Pd Extraction from Catalyst Cat D with Polymer p(FDA-co-DPPS)
Extraction tests on the aluminosilicate-supported catalyst Cat D were performed with the polymer p(FDA-
co-DPPS) (cf.
Figure 2). Triphenylphosphine derivatives are well-known for their ability to form complexes with transition metals, especially with Pd [
19,
20], and DPPS was therefore targeted for use in the extraction experiments. All tests were performed at 25 MPa and 40 °C, with and without activation of the protected thiol group, with 60 min and 90 min of extraction time. The activation was done in situ by a simple addition of piperidine into the extractor, in excess relative to the polymer chains (2.5 to 5-fold molar excess of piperidine relative to the polymer chains depending on the extraction conditions). The extraction parameters and results are shown in
Table 4 and
Figure 6. Further information can be obtained from the
supporting information (cf. SI-Chapter 4.4).
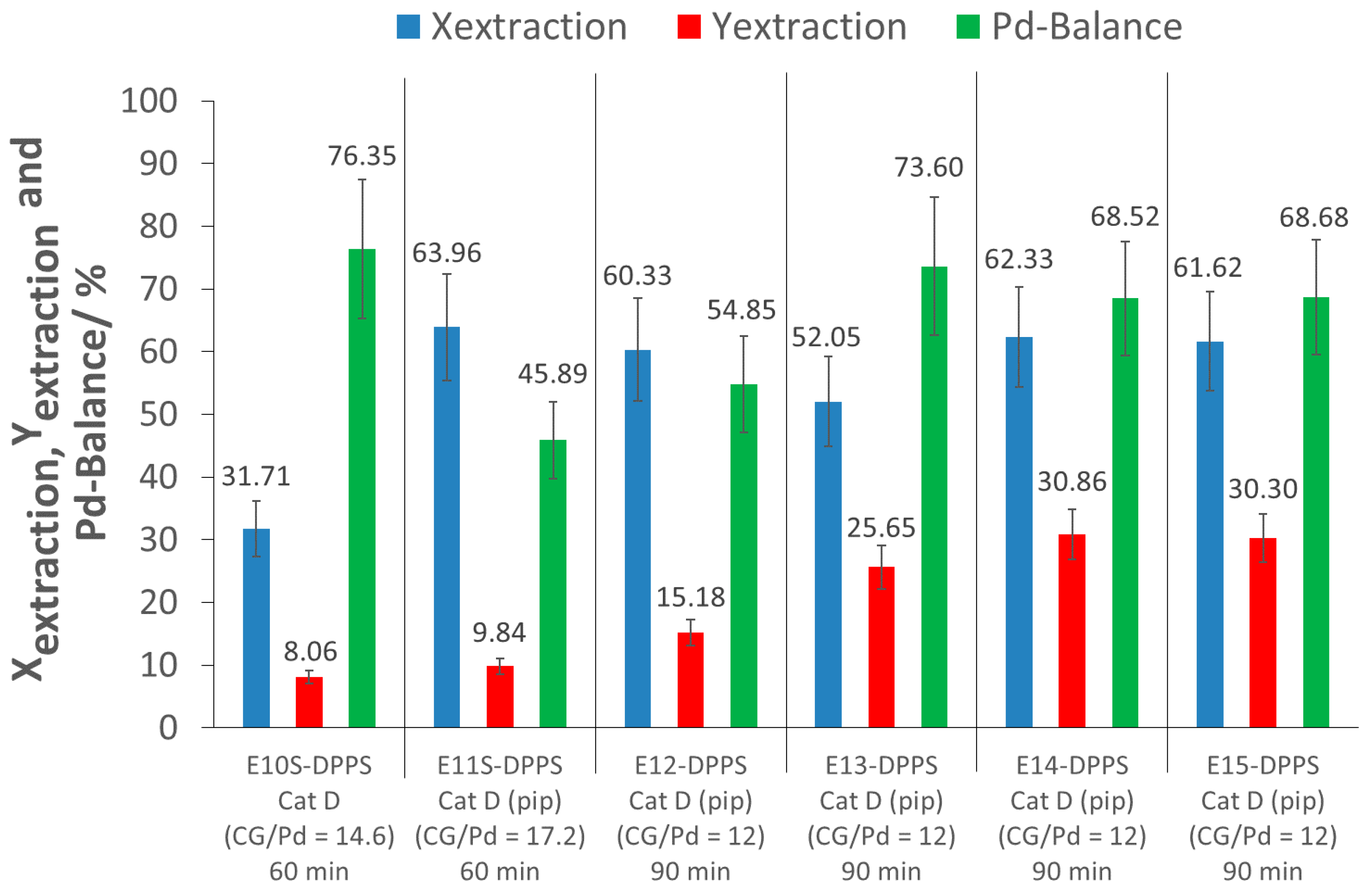
An initial screening extraction experiment with a 60 min extraction time was performed with p(FDA-
co-DPPS), with a complexing group (CG)/Pd ratio of 14 (large excess of complexing groups) (E10S-DPPS). This extraction gave an interesting result, achieving an extraction of 32% of the precious metal from the support, although the extraction yield was again low (8%), due to Pd loss during depressurization (Pd-balance = 76%). The next step was to try to improve the extraction conversion by activating the protected thiol group of the polymer (the dithiobenzoate end group) via aminolysis, as the thiol group is a well-known ligand for Pd complexation [
19,
22]. Although deprotection can be performed using several methods, piperidine, a secondary amine, was selected as the aminolyzing agent. This activation process was performed directly in situ in scCO
2, meaning that piperidine was added directly to the extractor at the beginning of the extraction experiments. This procedure is favored in order to avoid possible disulfide formation (oxidation of thiols). Furthermore, in situ activation was preferred to ex situ aminolysis in order to avoid a synthetic step, and thus optimizing the method for eventual scale-up applications. With the addition of piperidine, while keeping the other extraction parameters constant, the extraction process showed a remarkable improvement, allowing for the removal of 64% of the Pd from the support (E11S-DPPS). This is an increase of over 30% of extracted Pd, when compared to the same experiment without piperidine addition (E10S-DPPS). Nevertheless, the extraction yield was still low (10%) (E11S-DPPS). This promising extraction system was further investigated in more detail in the larger extractor. Four extractions were carried out (E12-DPPS–E15-DPPS) with a 90 min extraction time and a CG/Pd ratio of 12 (large excess of complexing groups), to see if the positive result of E11S-DPPS could also be confirmed in the larger extractor. Extraction conversions between 52 and 62% were achieved, confirming the good Pd complexing ability of p(FDA-
co-DPPS) in combination with piperidine. This indicates that a positive interaction between the polymer, piperidine, and the catalyst exists, which merits closer investigation. This interaction however, was not present when p(FDA)SH was used, which suggests an interaction specific to the DPPS groups. It is important to note that under the experimental conditions, disulfide formation by oxidative coupling of the thiol group is unlikely. Indeed, the DPPS groups may act as a reducing agent to limit disulfide formation, as triphenylphosphine is used for the reductive cleavage of disulfide bonds [
23,
24]. It has also been shown that polymer disulfide coupling decreases with increasing polymer molecular weight [
28]. Additionally, as the extractions were carried out in CO
2 atmosphere, there should not be enough oxygen present in the system to favor disulfide formation. However, the in situ formation of thiolactone cannot be completely discarded [
25,
28,
29].
In experiments E12-DPPS–E15-DPPS, two different batches of p(FDA-co-DPPS) polymers were investigated, differing in the number of FDA and DPPS monomer units per molecule. Reproducibility of the Pd extractions was confirmed across different polymer batches at a constant complexing group/Pd ratio.
The extraction yields of experiments E11S-DPPS and E12-DPPS–E15-DPPS were low (10 to 31%), as a large amount of Pd was lost during the depressurization and flushing of the cell (Pd-balance in the range of 46 to 74%). Nevertheless, in E13-DPPS to E15-DPPS, approximately 50% of the extracted Pd was recovered. To increase the Pd recovery (extraction yield), a cascade of separators could be used to ensure a slow stepwise depressurization of the CO2-Pd-polymer mixture to atmospheric pressure, minimizing this loss of Pd. During depressurization, the transition of CO2 from the supercritical domain to gas state occurs, separating the Pd-polymer mixture from CO2, as the polymer is insoluble in gaseous CO2.
To have a better understanding of the influence of the CG/Pd ratio, as well as the influence of the extraction time, extraction experiments with p(FDA-
co-DPPS) were performed at much higher (42) and lower (5) CG/Pd ratios, and at a much longer extraction time of 180 min. Extraction tests were also performed at 60 °C instead of 40 °C. The extraction parameters and results are shown in
Table 5,
Figure 7 and
Figure 8 (further information in
SI-Chapter 4.4).
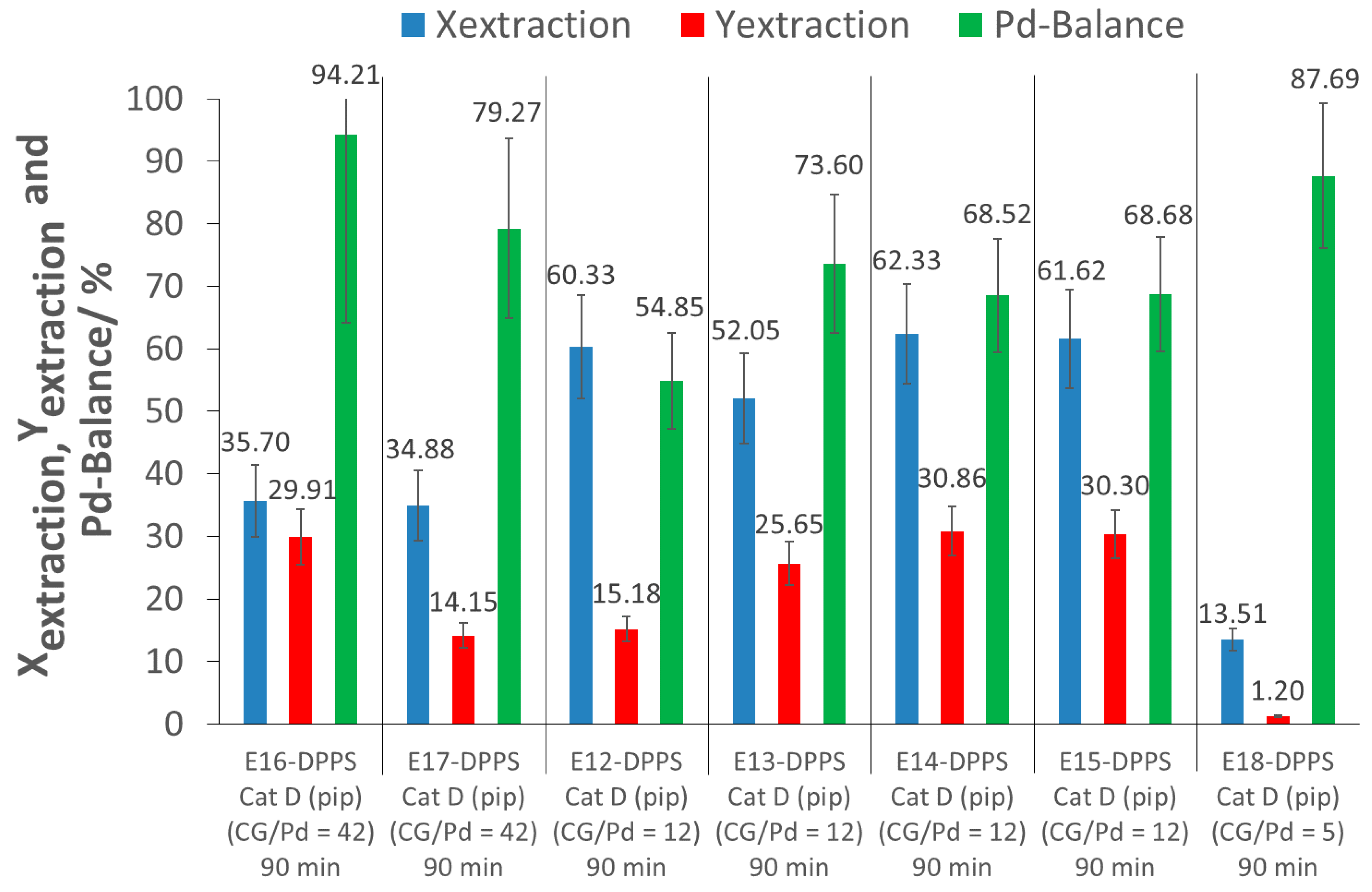
As can be observed in experiments E16-DPPS and E17-DPPS, a large increase of the CG/Pd ratio did not improve the extraction, but rather led to a poorer extraction performance as extraction conversions of only 35% were obtained, much lower than in the case of CG/Pd = 12 (approximately 60%). This might have been due to intermolecular polymer interaction as a result of the higher polymer concentration, making it more difficult for the polymers to interact with the catalyst, due to more competition between the polymers, and thus inhibiting complexation.
A decrease of the CG/Pd ratio to 5 (E18-DPPS) resulted in a lower extraction conversion as well, likely due to an insufficient amount of polymers for the extraction of the Pd nanoparticles.
The extraction yields obtained across extractions performed at different CG/Pd ratios were inconsistent, and varied between 1 and 31% (E12-DPPS to E18-DPPS). As indicated previously, a loss of Pd during the recovery process contributes to this variability. From the set of experiments E12-DPPS to E18-DPPS, a CG/Pd ratio of about 12 appeared to provide a good excess of complexing groups, yielding good extraction results.
As can be seen from
Figure 8, an increase in the extraction temperature to 60 °C resulted in a decrease in the extraction conversions and yields, regardless of the pressure used, 25 MPa (E22-DPPS) or, to increase the solubility of p(FDA-
co-DPPS), at 27 MPa (E23-DPPS). Further tests will have to be performed at other temperatures to verify this effect and explain this behavior.
When the extractions were extended to 180 min (doubled extraction time) at 40 °C, a decrease in extraction conversion and extraction yield was observed as well (E19-DPPS to E21-DPPS). The cause of the lower extraction efficiency with a longer extraction time cannot be explained at this point, and will require a more detailed investigation into the effects of the operating conditions and the extraction mechanism. From our present results, at an extraction temperature of 40 °C, it appears that an extraction time of 90 min is optimal for the larger extractor.
It can also be observed from
Figure 8 that the Pd-balances were high in the cases where extraction conversions were low. As mentioned previously, the loss of Pd during the depressurization step and flushing of the extractor led to low extraction yields. The fact that the Pd-balances were high when extraction conversions were low supports this explanation. If the Pd was not extracted from the catalyst, it remained on the support. However, Pd that was extracted by the polymer was able to flow through the sCO
2 medium, and could leave the extraction system during depressurization.
From this preliminary parameter study, it appears that the initially chosen conditions of 40 °C and 90 min of extraction time in the larger extractor gave the best results for the Pd extraction from an aluminosilicate-supported catalyst using p(FDA-co-DPPS) as complexing polymer, at a CG/Pd ratio of 12, with piperidine as in situ activator. An increase in the extraction temperature from 40 °C to 60 °C, a large extension of the extraction time from 90 to 180 min, or a significant increase or decrease of the CG/Pd ratio did not improve extraction conversion. Of course, a more complete parameter study using a wider range of operating conditions will be required to fully analyze the individual and combined effects of extraction time, temperature, and the CG/Pd ratio on extraction efficiencies.