Impact of Digestive Inflammatory Environment and Genipin Crosslinking on Immunomodulatory Capacity of Injectable Musculoskeletal Tissue Scaffold
Abstract
1. Introduction
2. Results
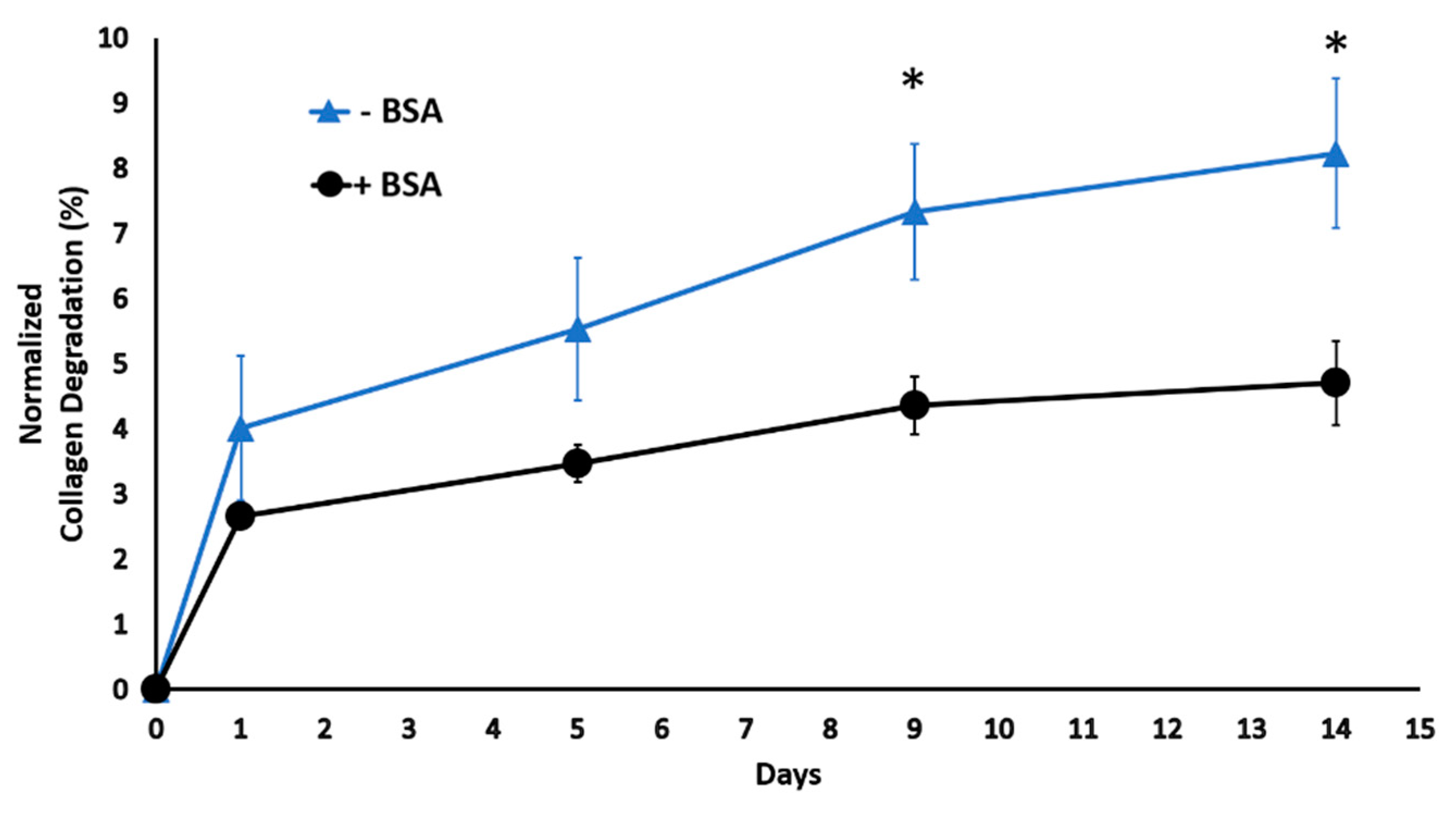
2.1. Bovine Serum Albumin Diminishes Scaffold Degradation in Digestive Inflammatory Environment
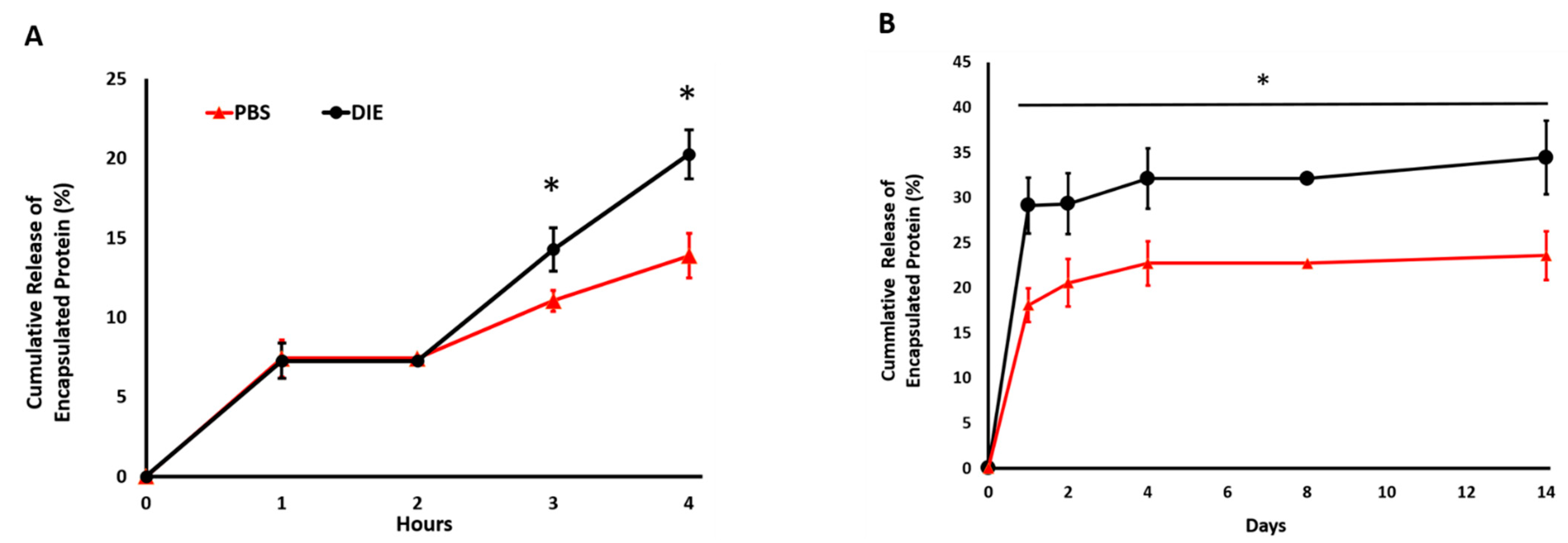
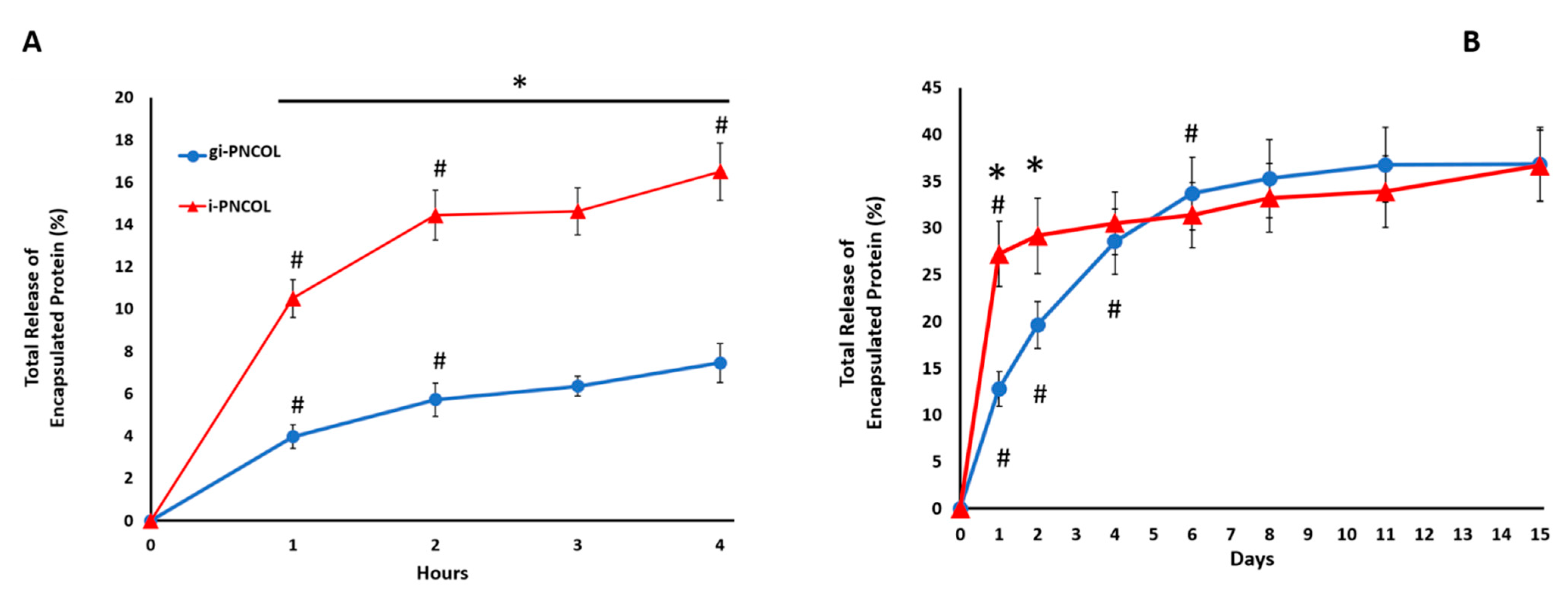
2.2. Digestive Inflammatory Environment (DIE) Attenuates Protein Retention within Scaffold
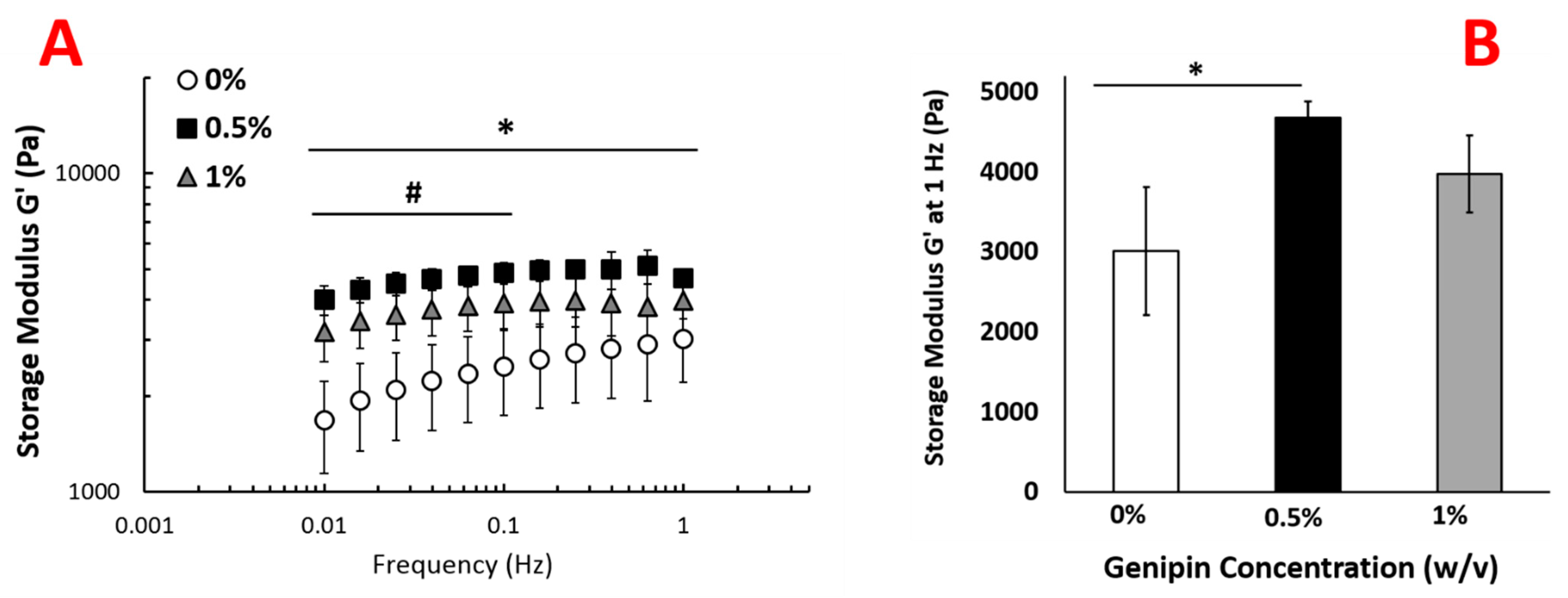
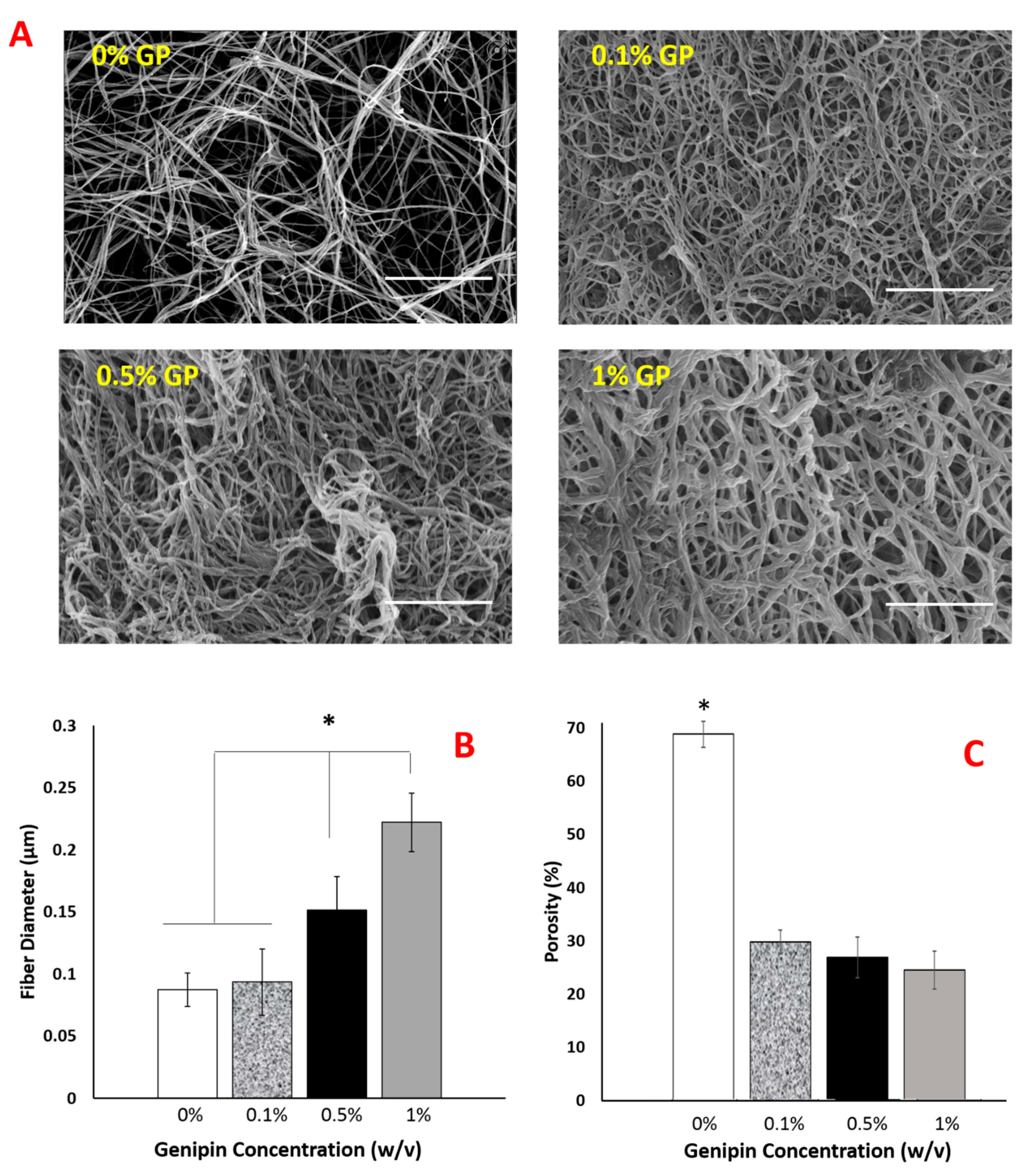
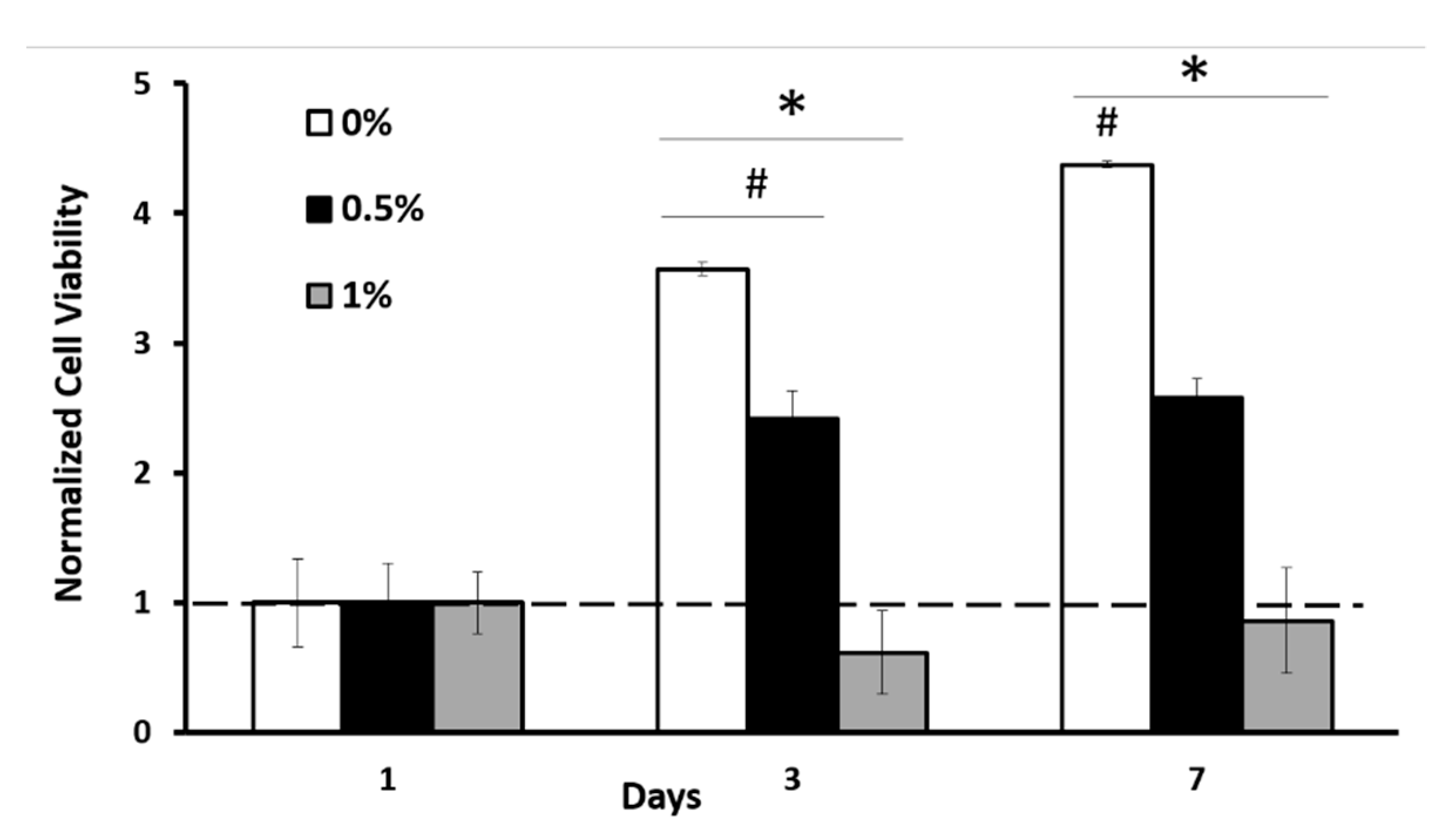
2.3. Genipin Crosslinking Improves Mechanical Strength but Diminishes the Encapsulated Cell Viability and Scaffold Porosity
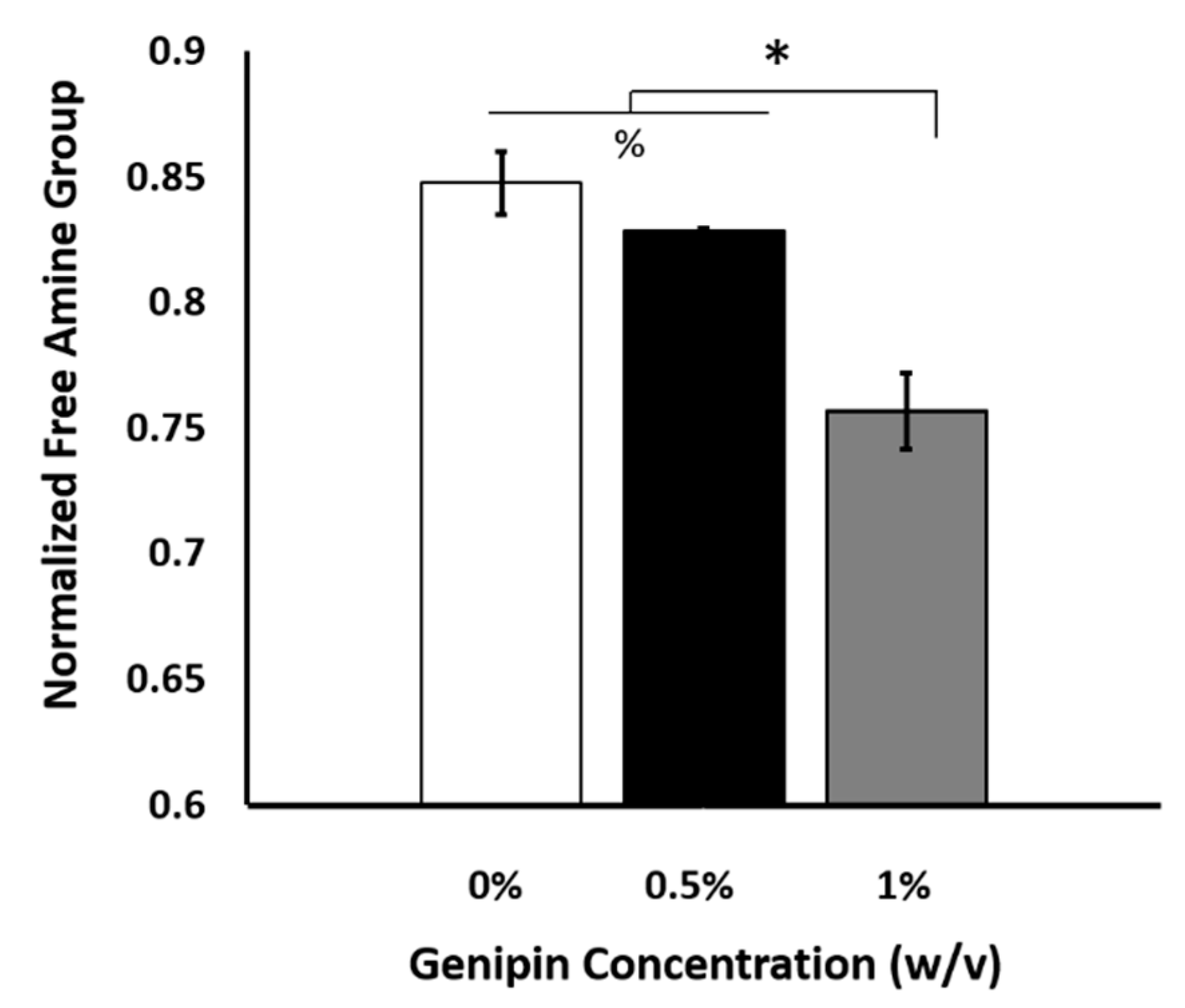
2.4. The Optimal Genipin Concentration for Kinetic Protein Release and Immunomodulation Studies
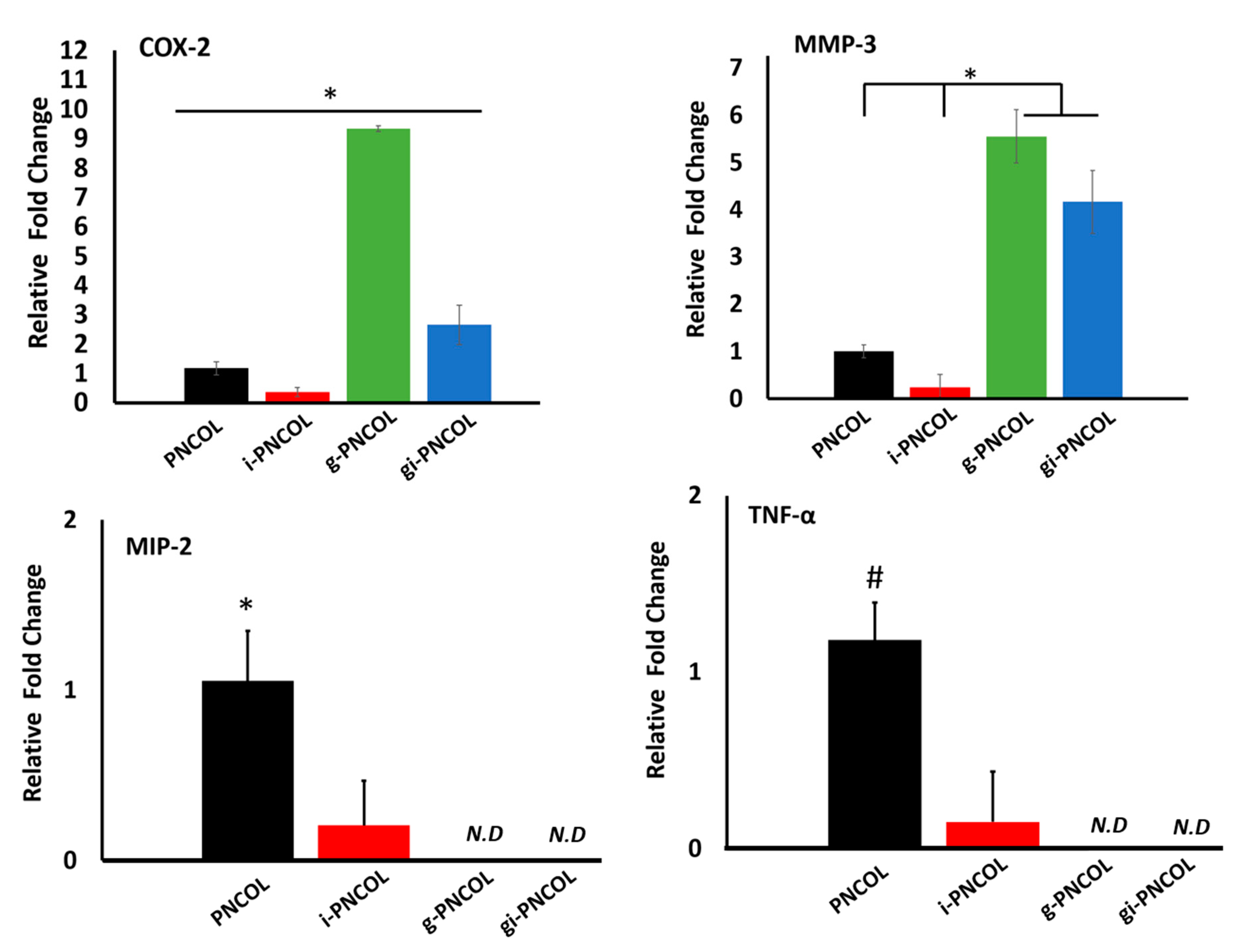
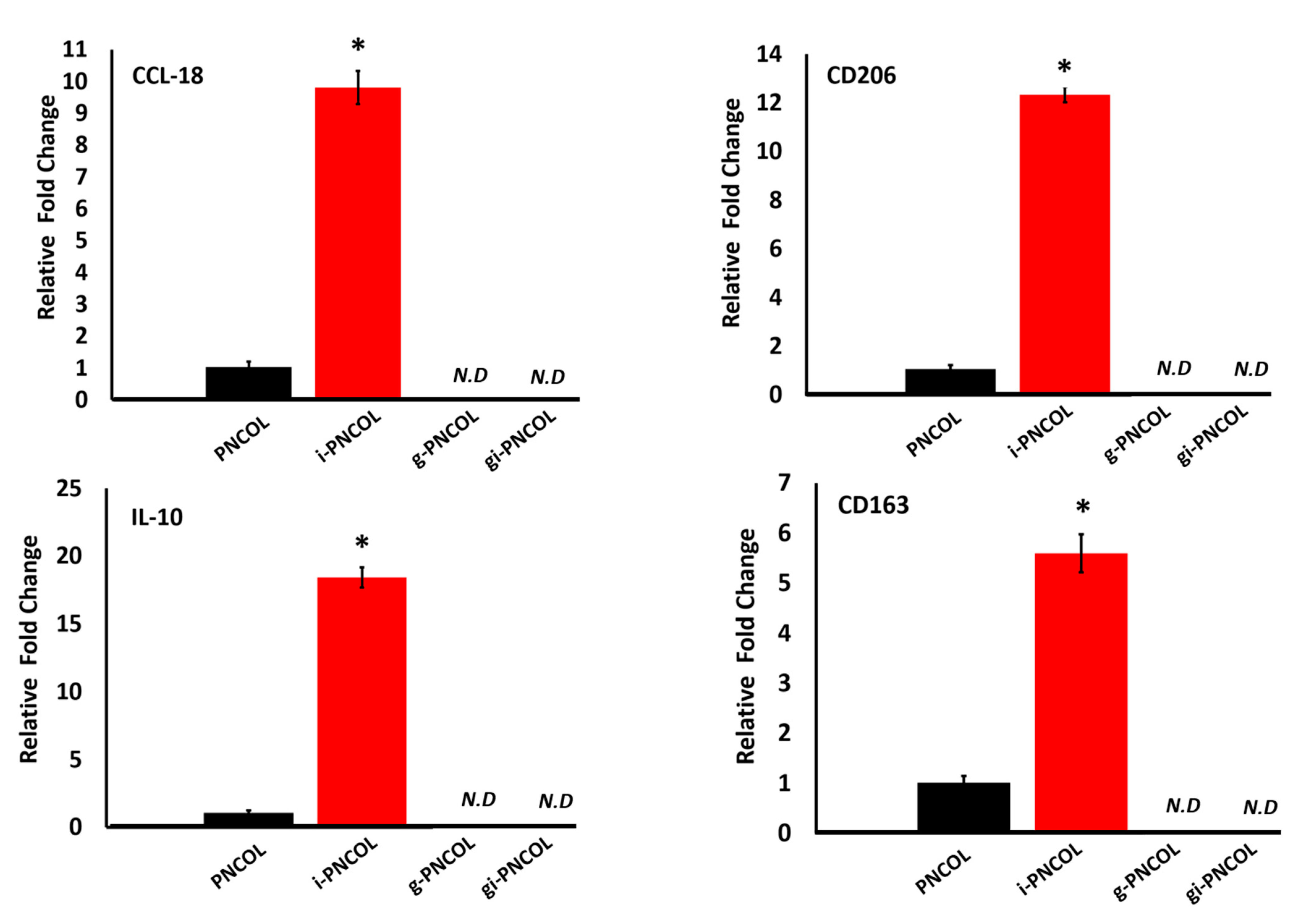
2.5. Interleukin-4 Release from Injectable Scaffold Alters Macrophage Polarization
3. Discussion
4. Method and Materials
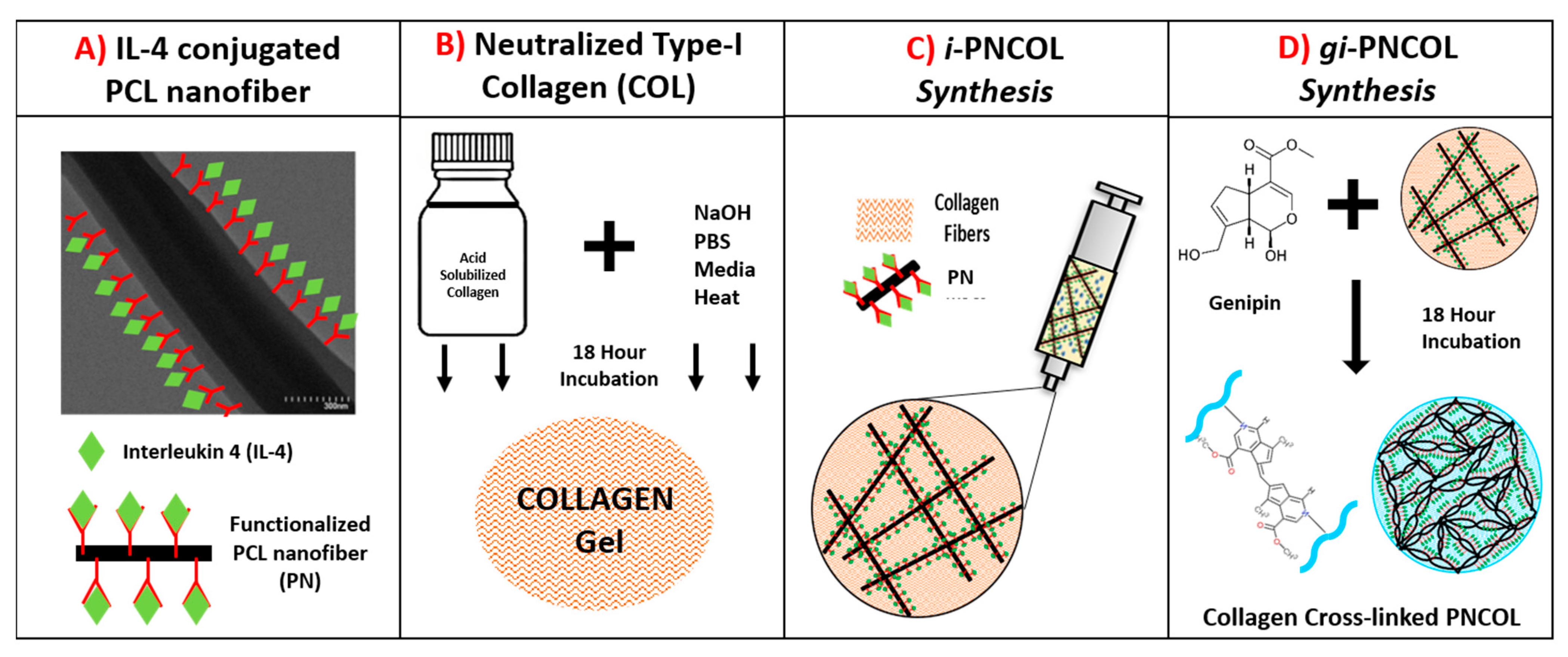
4.1. Synthesis of Injectable Immunomodulatory Scaffold
- PCL Nanofiber Fabrication and IL-4 Conjugation: Briefly, the polycaprolactone (PCL) nanofibers were fabricated by dissolving PCL (Mw = 45,000, Sigma-Aldrich, USA) at 16% (w/v) concentration in an organic solvent mixture (3:1 mixture of chloroform/ methanol) and extruding PCL solution with a rate of 8 mL/h into an electric field created between the syringe tip (0 kV) and collecting plate (20 kV). The electrospun PCL fibers were further dried for 3 days and homogenized using a high-speed homogenizer (Ultra Turrax) to obtain tiny fragments of electrospun fibers. Our prior studies suggested that to prevent the denaturalization of biologics contact with PCL, its hydrophobicity needs to be lowered [56,91]. Thus, PCL nanofibers then were treated with plasma treatment (Harrick Plasma) for 3 min to reduce its hydrophobicity before IL-4 conjugation. IL-4 (Peprotech, Cranbury, NJ, USA) was used as an anti-inflammatory cytokine in this study. Bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA) and heparin (Sigma-Aldrich, St. Louis, MO, USA) were employed to protect IL-4 from denaturalization. IL-4 was first incubated with heparin and then BSA at a ratio of 1:40:2000, respectively, for 15 min at room temperature based on our established protocols [50,51]. Before mixing with the collagen, the IL-4 solution was incubated with functionalized PCL nanofibers for 20 min at room temperature to allow IL-4 to bind to the surface of PCL. Unless otherwise specified, 350 ng/mL of IL-4 was employed in the immunomodulatory scaffold, i-PNCOL.
- Neutralized Collagen Type-I Solution Preparation: A concentrated (9.6 mg/mL) and acidic (pH~3–4) collagen type-I (Corning, Corning, NY, USA) solution was diluted to 3 mg/mL and further neutralized with chilled 1N NaOH, 10× phosphate buffer saline (PBS), and deionized water according to the vendor’s protocol.
- Immunomodulatory scaffold, i-PNCOL, Synthesis, and Crosslinking: Then, the IL-4 conjugated-PCL nanofibers were admixed with neutralized collagen type-I solution at 3% (w/v) concentration to prepare injectable i-PNCOL. The PCL nanofiber concentration (3%) was chosen based on our prior studies [50]. Then, the i-PNCOL was transferred to the incubator (37 °C, 5% CO2) for further collagen polymerization for 1 h. Subsequently, either complete cell culture media, digestive inflammatory solution, or PBS were added to the scaffold for assessing the response of the scaffold to the different environments. Pure genipin (Purity > 98%, Challenge Bioproducts, Taiwan) with molecular weight (MW) = 226.2 g/mol was used as a crosslinking agent due to its collagen crosslinking capacity. Genipin crosslinking stock solutions were prepared by dissolving 1.1%( w/v) genipin powder (C11H14O5) in sterile PBS through mixing overnight at room temperature. Then, the genipin stock solution was filtered using a 0.21 μm pore-size filter to remove possible contaminants. Then, stock solution was diluted to various concentrations of 0.1%, 0.5%, and 1% (w/v). Then, the scaffolds were cultured with genipin solution in an incubator (37 °C, 5% CO2) for 18 h before further characterizations. On characterization day, the solution was aspirated, and the scaffolds were washed vigorously 3 times with sterile PBS to remove residual genipin solution. The genipin crosslinked scaffolds were referred to as gi-PNCOL throughout the study (Figure 10).
4.2. Degradation and Cytokine Retention Capacity of Scaffold in Digestive Inflammatory Environment
4.2.1. Measuring Scaffold Degradation in the Digestive Inflammatory Environment
4.2.2. Measuring Kinetic Release of Encapsulated Cytokine in Digestive Inflammatory Environment
4.3. Genipin-Mediated Cytotoxicity and Morphological Changes in Scaffold
4.3.1. Identifying Degree of Crosslinking Following Genipin
4.3.2. Measuring Cell Toxicity upon Genipin Crosslinking
4.3.3. Assessing Changes in Scaffold Morphology upon Genipin Crosslinking
4.3.4. Measuring Viscoelastic Properties of the Injectable Scaffold upon Genipin Crosslinking
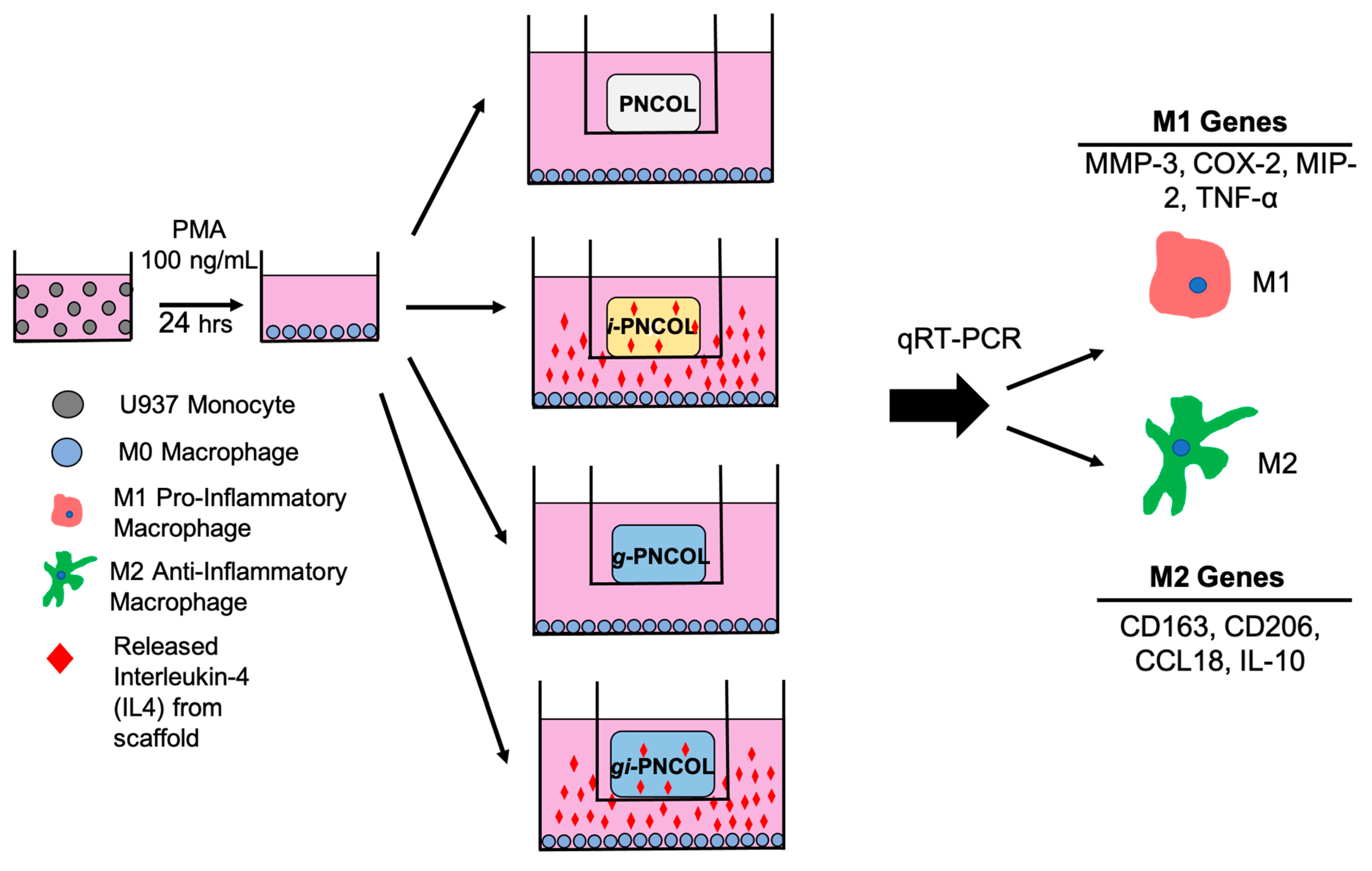
4.4. Assessing Immunomodulatory Capacity of Scaffold
4.4.1. Monocytic Cell Expansion and Differentiation to Macrophage
4.4.2. Response of Macrophage Polarization to Immunomodulatory Scaffolds
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| bFGF | Basic Fibroblast Growth Factor |
| BSA | Bovine Serum Albumin |
| C2C12 | mouse myoblast cell line |
| CCL-18 | Chemokine (C-C motif) ligand-18 |
| CD163 | Cluster of differentiation 163 |
| CD206 | Cluster of differentiation 206 |
| COX-2 | Cyclooxygenase-2 |
| DIE | Digestive Inflammatory Environment |
| DNA | Deoxyribonucleic Acid |
| dsDNA | Double Stranded Deoxyribonucleic Acid |
| ECM | Extracellular Matrix |
| EDC | 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Hydrochloride |
| FBS | Fetal Bovine Serum |
| G″ | Loss Modulus |
| G′ | Storage Modulus |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| gi-PNCOL | Genipin-Crosslinked PNCOL with IL-4 encapsulation |
| GP | Genipin |
| g-PNCOL | Genipin-Crosslinked PNCOL without IL-4 encapsulation |
| HEPES | Hydroxyethyl Piperazineethanesulfonic Acid |
| IL | Interleukin |
| IL-10 | Interleukin-10 |
| IL-4 | Interleukin-4 |
| i-PNCOL | Immunomodulatory Polycaprolactone Nanofibrous Collagen |
| LPS | lipid polysaccharide |
| M0 | naïve macrophage |
| M1 | pro-inflammatory macrophage |
| M2 | anti-inflammatory macrophage |
| MIP-2 | macrophage inflammatory protein-2 |
| MMP | Matrix Metalloproteinase |
| MMP-1 | Matrix Metalloproteinase-1 |
| mRNA | messenger Ribonucleic Acid |
| NHS | N-hydroxysuccinimide |
| NSAID | Non-steroidal anti-inflammatory drug |
| PBS | Phosphate Buffered Saline |
| PCL | Polycaprolactone |
| PCR | Polymerase chain reaction |
| PDGF | Platelet Derived Growth Factor |
| PMA | Phorbol 12-myristate 13-acetate |
| PNCOL | Polycaprolactone Nanofibrous Collagen |
| qRT-PCR | qualitative real-time polymerase chain reaction |
| RNA | Ribonucleic Acid |
| RPMI | Roswell Park Memorial Institute |
| SEM | Scanning Electron Microscope |
| TE | Tris-EDTA (Ethylenediamine Tetraacetic Acid) buffer solution |
| TNF-α | Tumor Necrosis Factor--α |
| Tris | tris(hydroxymethyl)aminomethane |
| U937 | human myeloid cell line |
References
- Hortensius, R.A.; Ebens, J.H.; Dewey, M.J.; Harley, B.A.C. Incorporation of the Amniotic Membrane as an Immunomodulatory Design Element in Collagen Scaffolds for Tendon Repair. ACS Biomater. Sci. Eng. 2018, 4, 4367–4377. [Google Scholar] [CrossRef] [PubMed]
- Snedeker, J.G.; Foolen, J. Tendon injury and repair—A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017, 63, 18–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon injuries: Basic science and new repair proposals. EFORT Open Rev. 2017, 2, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Fu, S.C. Anti-inflammatory management for tendon injuries—Friends or foes? Sports Med. Arthrosc. Rehabil. Technol. 2009, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Stride, M.; Scott, A. Tendons—Time to revisit inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Bosurgi, L.; Cao, Y.G.; Cabeza-Cabrerizo, M.; Tucci, A.; Hughes, L.D.; Kong, Y.; Weinstein, J.S.; Licona-Limon, P.; Schmid, E.T.; Pelorosso, F.; et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 2017, 356, 1072–1076. [Google Scholar] [CrossRef]
- Guo, Y.L.; Huang, H.; Zeng, D.X.; Zhao, J.P.; Fang, H.J.; Lavoie, J.P. Interleukin (IL)-4 induces production of cytokine-induced neutrophil chemoattractants (CINCs) and intercellular adhesion molecule (ICAM)-1 in lungs of asthmatic rats. J. Huazhong Univ. Sci. Technol. Med Sci. 2013, 33, 470–478. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314. [Google Scholar] [CrossRef]
- Gea-Sorlí, S.; Closa, D. BMC Immunology; BioMed Central: London, UK, 2009; Volume 10, ISSN 1471-2172. [Google Scholar]
- Hachim, D.; LoPresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef]
- Kumar, M.; Coburn, J.; Kaplan, D.L.; Mandal, B.B. Immuno-Informed 3D Silk Biomaterials for Tailoring Biological Responses. Acs Appl. Mater. Inter. 2016, 8, 29310–29322. [Google Scholar] [CrossRef]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Byun, J.H.; Cabrera, F.; Liu, X.; Ferrari, M.; Weiner, B.K.; Tasciotti, E. IL-4 Release from a Biomimetic Scaffold for the Temporally Controlled Modulation of Macrophage Response. Ann. Biomed. Eng. 2016, 44, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, H.; Zhang, N.; Hu, L.; Jing, W.; Pan, J. Interleukin-4-loaded hydrogel scaffold regulates macrophages polarization to promote bone mesenchymal stem cells osteogenic differentiation via TGF-beta1/Smad pathway for repair of bone defect. Cell Prolif. 2020, 53, e12907. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Taraballi, F.; Corradetti, B.; Minardi, S.; Powel, S.; Cabrera, F.; Van Eps, J.L.; Weiner, B.K.; Tasciotti, E. Biomimetic collagenous scaffold to tune inflammation by targeting macrophages. J. Tissue Eng. 2016, 7, 2041731415624667. [Google Scholar] [CrossRef] [PubMed]
- Gelain, F.; Unsworth, L.D.; Zhang, S. Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J. Control. Release 2010, 145, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Tchetverikov, I.; Lohmander, L.S.; Verzijl, N.; Huizinga, T.W.J.; TeKoppele, J.M.; Hanemaaijer, R.; DeGroot, J. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann. Rheum. Dis. 2005, 64, 694–698. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Sudbeck, B.D.; Pilcher, B.K.; Welgus, H.G.; Parks, W.C. Induction and Repression of Collagenase-1 by Keratinocytes is Controlled by Distinct Components of Different Extracellular Matrix Compartments. J. Biol. Chem. 1997, 272, 22103–22110. [Google Scholar] [CrossRef]
- Grier, W.K. Enhancement of Spatially-Controlled MSC Responses in a Multi-Compartment CG Scaffold for Tendon-Bone Junction Regeneration; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2017. [Google Scholar]
- Chen, X.; Yin, Z.; Chen, J.-L.; Liu, H.-H.; Shen, W.-L.; Fang, Z.; Zhu, T.; Ji, J.; Ouyang, H.-W.; Zou, X.-H. Scleraxis-Overexpressed Human Embryonic Stem Cell–Derived Mesenchymal Stem Cells for Tendon Tissue Engineering with Knitted Silk-Collagen Scaffold. Tissue Eng. Part A 2013, 20, 1583–1592. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. Structural and Biochemical Modification of a Collagen Scaffold to Selectively Enhance MSC Tenogenic, Chondrogenic, and Osteogenic Differentiation. Adv. Healthc. Mater. 2014, 3, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.J.; Dunn, A.J.; Marcinczyk, M.; Talovic, M.; Schwartz, M.; Scheidt, R.; Patel, A.D.; Hixon, K.R.; Elmashhady, H.; McBride-Gagyi, S.H.; et al. Biomimetic sponges for regeneration of skeletal muscle following trauma. J. Biomed. Mater. Res. Part A 2019, 107, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Kleinmann, H.K.; Rohrbach, D.H.; Terranova, V.P.; Varner, H.H.; Hewitt, A.T.; Grotendorst, G.R.; Wilkes, C.M.; Martin, G.R.; Seppä, H.; Schiffmann, E. Collagenous matrices as determinants of cell function. Immunochem. Extracell. Matrix 1982, 2, 151–174. [Google Scholar]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell. Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Howes, J.-M.; Bihan, D.; Slatter, D.A.; Hamaia, S.W.; Packman, L.C.; Knauper, V.; Visse, R.; Farndale, R.W. The recognition of collagen and triple-helical toolkit peptides by MMP-13: Sequence specificity for binding and cleavage. J. Biol. Chem. 2014, 289, 24091–24101. [Google Scholar] [CrossRef]
- Oliver, R.F.; Grant, R.A.; Cox, R.W.; Cooke, A. Effect of aldehyde cross-linking on human dermal collagen implants in the rat. Br. J. Exp. Pathol. 1980, 61, 544–549. [Google Scholar]
- Powell, H.M.; Boyce, S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef]
- Hanthamrongwit, M.; Reid, W.H.; Grant, M.H. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: The effect of cross-linking agents and diamines. Biomaterials 1996, 17, 775–780. [Google Scholar] [CrossRef]
- Yang, C. Enhanced physicochemical properties of collagen by using EDC/NHS-crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Romero, A.; Guerrero, A. Influence of collagen concentration and glutaraldehyde on collagen-based scaffold properties. J. Biomed. Mater. Res. Part A 2016, 104, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Caves, J.M.; Ravi, S.; Li, W.; Chaikof, E.L. Effects of crosslinking on the mechanical properties, drug release and cytocompatibility of protein polymers. Acta Biomater. 2014, 10, 26–33. [Google Scholar] [CrossRef]
- Huang-Lee, L.L.; Cheung, D.T.; Nimni, M.E. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J. Biomed. Mater. Res. 1990, 24, 1185–1201. [Google Scholar] [CrossRef]
- Saito, Y.; Nishio, K.; Yoshida, Y.; Niki, E. Cytotoxic effect of formaldehyde with free radicals via increment of cellular reactive oxygen species. Toxicology 2005, 210, 235–245. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Moshnikova, A.B.; Afanasyev, V.N.; Proussakova, O.V.; Chernyshov, S.; Gogvadze, V.; Beletsky, I.P. Cytotoxic activity of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide is underlain by DNA interchain cross-linking. Cell. Mol. Life Sci. 2006, 63, 229–234. [Google Scholar] [CrossRef]
- Meng, S.-X.; Peng, J.-H.; Feng, Q.; Cao, J.-M.; Hu, Y.-Y.J.A.; Medicine, I. The role of genipin and geniposide in liver diseases: A review. Altern. Integr. Med. 2013, 1–8. [Google Scholar] [CrossRef]
- Kim, B.R.; Jeong, Y.A.; Jo, M.J.; Park, S.H.; Na, Y.J.; Kim, J.L.; Jeong, S.; Yun, H.K.; Kang, S.; Lee, D.-H.J.M. Genipin enhances the therapeutic effects of oxaliplatin by upregulating BIM in colorectal cancer. J. Ethnopharmacol. 2019, 18, 751–761. [Google Scholar] [CrossRef]
- Koo, H.-J.; Lim, K.-H.; Jung, H.-J.; Park, E. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamazaki, M.; Chiba, K. Neuroprotective action of genipin on tunicamycin-induced cytotoxicity in neuro2a cells. Biol. Pharm. Bull. 2009, 32, 1220–1223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, R.; Stodulka, P.; Skopalova, K.; Saha, P. Dual Crosslinked Collagen/Chitosan Film for Potential Biomedical Applications. Polymer 2019, 11, 2094. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Wang, X.F.; Zhang, Z.; Wang, L.X.; Li, X.M.; Xu, Y.Y.; Ren, C.H.; Li, Q.; Turng, L.S. Programmed Release of Multimodal, Cross-Linked Vascular Endothelial Growth Factor and Heparin Layers on Electrospun Polycaprolactone Vascular Grafts. ACS Appl. Mater. Inter. 2019, 11, 32533–32542. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska-Lancucka, J.; Gilarska, A.; Bula, A.; Horak, W.; Latkiewicz, A.; Nowakowska, M. Genipin crosslinked bioactive collagen/chitosan/hyaluronic acid injectable hydrogels structurally amended via covalent attachment of surface-modified silica particles. Int. J. Biol. Macromol. 2019, 136, 1196–1208. [Google Scholar] [CrossRef]
- Uquillas, J.A.; Kishore, V.; Akkus, O. Genipin crosslinking elevates the strength of electrochemically aligned collagen to the level of tendons. J. Mech. Behav. Biomed. Mater. 2012, 15, 176–189. [Google Scholar] [CrossRef]
- Suchy, T.; Supova, M.; Sauerova, P.; Verdanova, M.; Sucharda, Z.; Ryglova, S.; Zaloudkova, M.; Sedlacek, R.; Kalbacova, M.H. The effects of different cross-linking conditions on collagen-based nanocomposite scaffolds-an in vitro evaluation using mesenchymal stem cells. Biomed. Mater. 2015, 10, 065008. [Google Scholar] [CrossRef]
- Elsaadany, M.; Winters, K.; Adams, S.; Stasuk, A.; Ayan, H.; Yildirim-Ayan, E. Equiaxial Strain Modulates Adipose-derived Stem Cell Differentiation within 3D Biphasic Scaffolds towards Annulus Fibrosus. Sci. Rep. 2017, 7, 12868. [Google Scholar] [CrossRef]
- Subramanian, G.; Bialorucki, C.; Yildirim-Ayan, E. Nanofibrous yet injectable polycaprolactone-collagen bone tissue scaffold with osteoprogenitor cells and controlled release of bone morphogenetic protein-2. Mater. Sci. Eng. C 2015, 51, 16–27. [Google Scholar] [CrossRef]
- Subramanian, G.; Stasuk, A.; Elsaadany, M.; Yildirim-Ayan, E. Effect of Uniaxial Tensile Cyclic Loading Regimes on Matrix Organization and Tenogenic Differentiation of Adipose-Derived Stem Cells Encapsulated within 3D Collagen Scaffolds. Stem Cells Int. 2017, 2017, 6072406. [Google Scholar] [CrossRef]
- Bialorucki, C.; Subramanian, G.; Elsaadany, M.; Yildirim-Ayan, E. In situ osteoblast mineralization mediates post-injection mechanical properties of osteoconductive material. J. Mech. Behav. Biomed. Mater. 2014, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Elsaadany, M.; Harris, M.; Yildirim-Ayan, E. Design and Validation of Equiaxial Mechanical Strain Platform, EQUicycler, for 3D Tissue Engineered Constructs. Biomed. Res. Int. 2017, 2017, 3609703. [Google Scholar] [CrossRef] [PubMed]
- Elsaadany, M.; Yan, K.C.; Yildirim-Ayan, E. Predicting cell viability within tissue scaffolds under equiaxial strain: Multi-scale finite element model of collagen-cardiomyocytes constructs. Biomech. Model. Mechanobiol. 2017, 16, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Baylan, N.; Bhat, S.; Ditto, M.; Lawrence, J.G.; Lecka-Czernik, B.; Yildirim-Ayan, E. Polycaprolactone nanofiber interspersed collagen type-I scaffold for bone regeneration: A unique injectable osteogenic scaffold. Biomed. Mater. 2013, 8, 45011. [Google Scholar] [CrossRef] [PubMed]
- Finn, T.E.; Nunez, A.C.; Sunde, M.; Easterbrook-Smith, S.B. Serum albumin prevents protein aggregation and amyloid formation and retains chaperone-like activity in the presence of physiological ligands. J. Biol. Chem. 2012, 287, 21530–21540. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Carrilho, M.; Breschi, L.; Tjäderhane, L.; Nishitani, Y.; Carvalho, R.M.; Looney, S.; Tay, F.R.; et al. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent. Mater. 2010, 26, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Mandl, I.; Keller, S.; Manahan, J.J.B. Multiplicity of Clostridium histolyticum collagenases. Biochemistry 1964, 3, 1737–1741. [Google Scholar] [CrossRef]
- Tokoro, Y.; Eisen, A.Z.; Jeffrey, J.J. Characterization of a collagenase from rat skin. Biochim. Biophys. Acta Enzymol. 1972, 258, 289–302. [Google Scholar] [CrossRef]
- Eckhard, U.; Schönauer, E.; Brandstetter, H. Structural basis for activity regulation and substrate preference of clostridial collagenases G, H, and T. J. Biol. Chem. 2013, 288, 20184–20194. [Google Scholar] [CrossRef]
- Lipiäinen, T.; Peltoniemi, M.; Sarkhel, S.; Yrjönen, T.; Vuorela, H.; Urtti, A.; Juppo, A.J.J. Formulation and stability of cytokine therapeutics. J. Pharm. Sci. 2015, 104, 307–326. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Kurecic, M.; Mohan, T.; Virant, N.; Maver, U.; Stergar, J.; Gradisnik, L.; Kleinschek, K.S.; Hribernik, S. A green approach to obtain stable and hydrophilic cellulose-based electrospun nanofibrous substrates for sustained release of therapeutic molecules. RSC Adv. 2019, 9, 21288–21301. [Google Scholar] [CrossRef]
- Hasan, S.M.K.; Li, R.; Wang, Y.C.; Reddy, N.; Liu, W.S.; Qiu, Y.P.; Jiang, Q.R. Sustained Local Delivery of Diclofenac from Three-Dimensional Ultrafine Fibrous Protein Scaffolds with Ultrahigh Drug Loading Capacity. Nanomaterials 2019, 9, 918. [Google Scholar] [CrossRef]
- Edelman, E.R.; Nugent, M.A.; Karnovsky, M.J. Perivascular and intravenous administration of basic fibroblast growth factor: Vascular and solid organ deposition. Proc. Natl. Acad. Sci. USA 1993, 90, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Eppler, S.M.; Combs, D.L.; Henry, T.D.; Lopez, J.J.; Ellis, S.G.; Yi, J.-H.; Annex, B.H.; McCluskey, E.R.; Zioncheck, T.F. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin. Pharmacol. Ther. 2002, 72, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J. Cytokine delivery and tissue engineering. Yonsei Med. J. 2000, 41, 704–719. [Google Scholar] [CrossRef]
- Sato, T.; Widmer, M.; Finkelman, F.; Madani, H.; Jacobs, C.; Grabstein, K.; Maliszewski, C. Recombinant soluble murine IL-4 receptor can inhibit or enhance IgE responses in vivo. J. Immunol. 1993, 150, 2717–2723. [Google Scholar]
- Sung, H.W.; Chang, W.H.; Ma, C.Y.; Lee, M.H. Crosslinking of biological tissues using genipin and/or carbodiimide. J. Biomed. Mater. Res. Part A 2003, 64, 427–438. [Google Scholar] [CrossRef]
- Sung, H.W.; Liang, I.L.; Chen, C.N.; Huang, R.N.; Liang, H.F. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J. Biomed. Mater. Res. 2001, 55, 538–546. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.-F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Yoo, J.S.; Kim, Y.J.; Kim, S.H.; Choi, S.H. Study on genipin: A new alternative natural crosslinking agent for fixing heterograft tissue. Korean J. Thorac. Cardiovasc. Surg. 2011, 44, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chan-Park, M.B. Density quantification of collagen grafted on biodegradable polyester: Its application to esophageal smooth muscle cell. Anal. Biochem. 2007, 363, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ninh, C.; Iftikhar, A.; Cramer, M.; Bettinger, C. Diffusion-Reaction Models of Genipin Incorporation into Fibrin Networks. J. Mater. Chem. B 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.; Hafmans, T.; Veerkamp, J.; Van Kuppevelt, T.J.B. Development of tailor-made collagen–glycosaminoglycan matrices: EDC/NHS crosslinking, and ultrastructural aspects. Biomaterials 2000, 21, 581–593. [Google Scholar] [CrossRef]
- Lim, H.G.; Kim, S.H.; Choi, S.Y.; Kim, Y.J. Anticalcification effects of decellularization, solvent, and detoxification treatment for genipin and glutaraldehyde fixation of bovine pericardium. Eur. J. Cardio Thorac. 2012, 41, 383–390. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Tsao, S.-W.; Che, C.-M.; Yuen, M.-F.; Feng, Y. IRE1α inhibition by natural compound genipin on tumour associated macrophages reduces growth of hepatocellular carcinoma. Oncotarget 2016, 7, 43792–43804. [Google Scholar] [CrossRef]
- Rajanbabu, V.; Galam, L.; Fukumoto, J.; Enciso, J.; Tadikonda, P.; Lane, T.N.; Bandyopadhyay, S.; Parthasarathy, P.T.; Cho, Y.; Cho, S.H.; et al. Genipin suppresses NLRP3 inflammasome activation through uncoupling protein-2. Cell. Immunol. 2015, 297, 40–45. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yoon, S.-J.; Lee, S.-M.J.M.M. Genipin Attenuates Sepsis by Inhibiting Toll-Like Receptor Signaling. Mol. Med. 2012, 18, 455–465. [Google Scholar] [CrossRef]
- Lim, H.; Park, K.-R.; Lee, D.-U.; Kim, Y.-S.; Kim, H.-P.J.B. Effects of the constituents of gardenia fructus on prostaglandin and NO production. Therapeutics 2008, 16, 82–86. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, B.H.; Chang, I.M. Inhibitory Potencies of Several Iridoids on Cyclooxygenase-1, Cyclooxygnase-2 Enzymes Activities, Tumor Necrosis factor-alpha and Nitric Oxide Production In Vitro. Evid. Based Complementary Altern. Med. 2010, 7, 41–45. [Google Scholar] [CrossRef]
- Brauch, J.E. 15—Underutilized Fruits and Vegetables as Potential Novel Pigment Sources. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 305–335. [Google Scholar]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; Discipio, R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Evers, S.; Roeder, D.; Parlow, A.F.; Risteli, J.; Risteli, L.; Lee, Y.C.; Feizi, T.; Langen, H.; Nussenzweig, M.C. Mannose Receptor-Mediated Regulation of Serum Glycoprotein Homeostasis. Science 2002, 295, 1898–1901. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Fabriek, B.O.; Dijkstra, C.D.; van den Berg, T.K. The macrophage scavenger receptor CD163. Immunobiology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Hu, J.M.; Liu, K.; Liu, J.H.; Jiang, X.L.; Wang, X.L.; Chen, Y.Z.; Li, S.G.; Zou, H.; Pang, L.J.; Liu, C.X.; et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 21526–21538. [Google Scholar] [CrossRef]
- Edelstein, C.L. Chapter Six—Biomarkers in Acute Kidney Injury. In Biomarkers of Kidney Disease, 2nd ed.; Edelstein, C.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 241–315. [Google Scholar]
- Eda, D.; Yildirim, D.P.; Guceri, S.; Sun, W. Enhanced Cellular Functions on Polycaprolactone Tissue Scaffolds by O2 Plasma Surface Modification. Plasma Process. Polym. 2011, 8, 256–267. [Google Scholar] [CrossRef]
- Riley, K.N.; Herman, I.M. Collagenase Promotes the Cellular Responses to Injury and Wound Healing In Vivo. J. Burn. Wounds 2005, 4, e8. [Google Scholar]
- Pardo, A.; Selman, M. MMP-1: The elder of the family. Int. J. Biochem. Cell Biol. 2005, 37, 283–288. [Google Scholar] [CrossRef]
- Rezwan, K.; Meier, L.P.; Gauckler, L.J. A prediction method for the isoelectric point of binary protein mixtures of bovine serum albumin and lysozyme adsorbed on colloidal titania and alumina particles. Langmuir 2005, 21, 3493–3497. [Google Scholar] [CrossRef]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- van de Weert, M.; van’t Hof, R.; van der Weerd, J.; Heeren, R.M.; Posthuma, G.; Hennink, W.E.; Crommelin, D.J. Lysozyme distribution and conformation in a biodegradable polymer matrix as determined by FTIR techniques. J. Control. Release 2000, 68, 31–40. [Google Scholar] [CrossRef]
- Park, T.G.; Lee, H.Y.; Nam, Y.S. A new preparation method for protein loaded poly (D, L-lactic-co-glycolic acid) microspheres and protein release mechanism study. J. Control. Release 1998, 55, 181–191. [Google Scholar] [CrossRef]
- Mekhail, M.; Wong, K.K.H.; Padavan, D.T.; Wu, Y.; O’Gorman, D.B.; Wan, W. Genipin-Cross-linked Electrospun Collagen Fibers. J. Biomater. Sci. Polymer Ed. 2011, 22, 2241–2259. [Google Scholar] [CrossRef]
- Oppong, F.; Li, Z.; Fakhrabadi, E.A.; Raorane, T.; Giri, P.M.; Liberatore, M.W.; Sarver, J.G.; Trabbic, C.J.; Hosier, C.E.; Erhardt, P.W.; et al. Investigating the Potential to Deliver and Maintain Plasma and Brain Levels of a Novel Practically Insoluble Methuosis Inducing Anticancer Agent 5-Methoxy MOMIPP Through an Injectable In Situ Forming Thermoresponsive Hydrogel Formulation. J. Pharm. Sci. 2020, 109, 2719–2728. [Google Scholar] [CrossRef]
- Senanayake, K.K.; Shokeen, N.; Fakhrabadi, E.A.; Liberatore, M.W.; Mukhopadhyay, A. Diffusion of nanoparticles within a semidilute polyelectrolyte solution. Soft Matter 2019, 15, 7616–7622. [Google Scholar] [CrossRef]
- Cho, D.-I.; Kim, M.R.; Jeong, H.-Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef]
- Hannon, D.B.; Thompson, J.T.; Khoo, C.; Juturu, V.; Vanden Heuvel, J.P. Effects of cranberry extracts on gene expression in THP-1 cells. Food Sci. Nutr. 2017, 5, 148–159. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, L.; Lizarraga, R.; Chen, Y. Human Airway Epithelial Cells Direct Significant Rhinovirus Replication in Monocytic Cells by Enhancing ICAM1 Expression. Am. J. Respir. Cell Mol. Biol. 2017, 57, 216–225. [Google Scholar] [CrossRef]
- KleinJan, A.; Dijkstra, M.D.; Boksa, S.S.; Severijnen, L.-A.W.F.M.; Mulder, P.G.H.; Fokkens, W.J. Increase in IL-8, IL-10, IL-13, and RANTES mRNA levels (in situ hybridization) in the nasal mucosa after nasal allergen provocation. J. Allergy Clin. Immunol. 1999, 103, 441–450. [Google Scholar] [CrossRef]
- Maeß, M.B.; Keller, A.-A.; Rennert, K.; Mosig, A.; Lorkowski, S. Optimization of the transfection of human THP-1 macrophages by application of Nunc UpCell technology. Anal. Biochem. 2015, 479, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Luo, Q.; Yao, F.; Qing, C.; Ye, J.; Deng, Y.; Li, J. Identification of differentially expressed long non-coding RNAs in polarized macrophages. Sci. Rep. 2016, 6, 19705. [Google Scholar] [CrossRef] [PubMed]
- Rennert, K.; Nitschke, M.; Wallert, M.; Keune, N.; Raasch, M.; Lorkowski, S.; Mosig, A.S. Thermo-responsive cell culture carrier: Effects on macrophage functionality and detachment efficiency. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.R.D.; Spiller, K.L.; Freytes, D.O.; Vunjak-Novakovic, G.; Kaplan, D.L. Controlled Release of Cytokines Using Silk-biomaterials for Macrophage Polarization. Biomaterials 2015, 73, 272–283. [Google Scholar] [CrossRef]
- Guo, L.; Xie, B.; Mao, Z. Autophagy in premature senescent cells is activated via AMPK pathway. Int. J. Mol. Sci. 2012, 13, 3563–3582. [Google Scholar] [CrossRef]
| Gene | Forward Primer | Reverse Primer | Ref |
|---|---|---|---|
| MMP-3 | 5′-CAAGGAGGCAGGCAAGACAGC-3′ | 5′-GCCACGCACAGCAACAGTAGG-3′ | [101] |
| COX-2 | 5′-CGGTGTTGAGCAGTTTTCTCC-3′ | 5′-AAGTGCGATTGTACCCGGAC-3′ | [102] |
| MIP-2 | 5′-CGCCCAAACCGAAGTCAT-3′ | 5′-GATTTGCCATTTTTCAGCATCTTT-3′ | [103] |
| TNF-α | 5′-AGAGGGAAGAGTTCCCCAGGGAC-3′ | 5′-TGAGTCGGTCACCCTTCTCCAG-3′ | [104] |
| CD163 | 5′-TCTGTTGGCCATTTTCGTCG-3′ | 5′-TGGTGGACTAAGTTCTCTCCTCTTGA-3′ | [105] |
| CCL18 | 5′-AAGAGCTCTGCTGCCTCGTCTA-3′ | 5′-CCCTCAGGCATTCAGCTTCA-3′ | [106] |
| IL-10 | 5′-CCTGTGAAAACAAGAGCAAGGC-3′ | 5′-TCACTCATGGCTTTGTAGATGCC-3′ | [107] |
| CD206 | 5′- CTACAAGGGATCGGGTTTATGGA-3′ | 5′- TTGGCATTGCCTAGTAGCGTA-3′ | [108] |
| GAPDH | 5′-AGAAGGCTGGGGCTGATTTG-3′ | 5′-AGGGCCCATCCACAGTCTTC-3′ | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shortridge, C.; Akbari Fakhrabadi, E.; Wuescher, L.M.; Worth, R.G.; Liberatore, M.W.; Yildirim-Ayan, E. Impact of Digestive Inflammatory Environment and Genipin Crosslinking on Immunomodulatory Capacity of Injectable Musculoskeletal Tissue Scaffold. Int. J. Mol. Sci. 2021, 22, 1134. https://doi.org/10.3390/ijms22031134
Shortridge C, Akbari Fakhrabadi E, Wuescher LM, Worth RG, Liberatore MW, Yildirim-Ayan E. Impact of Digestive Inflammatory Environment and Genipin Crosslinking on Immunomodulatory Capacity of Injectable Musculoskeletal Tissue Scaffold. International Journal of Molecular Sciences. 2021; 22(3):1134. https://doi.org/10.3390/ijms22031134
Chicago/Turabian StyleShortridge, Colin, Ehsan Akbari Fakhrabadi, Leah M. Wuescher, Randall G. Worth, Matthew W. Liberatore, and Eda Yildirim-Ayan. 2021. "Impact of Digestive Inflammatory Environment and Genipin Crosslinking on Immunomodulatory Capacity of Injectable Musculoskeletal Tissue Scaffold" International Journal of Molecular Sciences 22, no. 3: 1134. https://doi.org/10.3390/ijms22031134
APA StyleShortridge, C., Akbari Fakhrabadi, E., Wuescher, L. M., Worth, R. G., Liberatore, M. W., & Yildirim-Ayan, E. (2021). Impact of Digestive Inflammatory Environment and Genipin Crosslinking on Immunomodulatory Capacity of Injectable Musculoskeletal Tissue Scaffold. International Journal of Molecular Sciences, 22(3), 1134. https://doi.org/10.3390/ijms22031134





