Laser Surface Texturing for Biomedical Applications: A Review
Abstract
:1. Introduction
2. Methods of Laser Surface Texturing
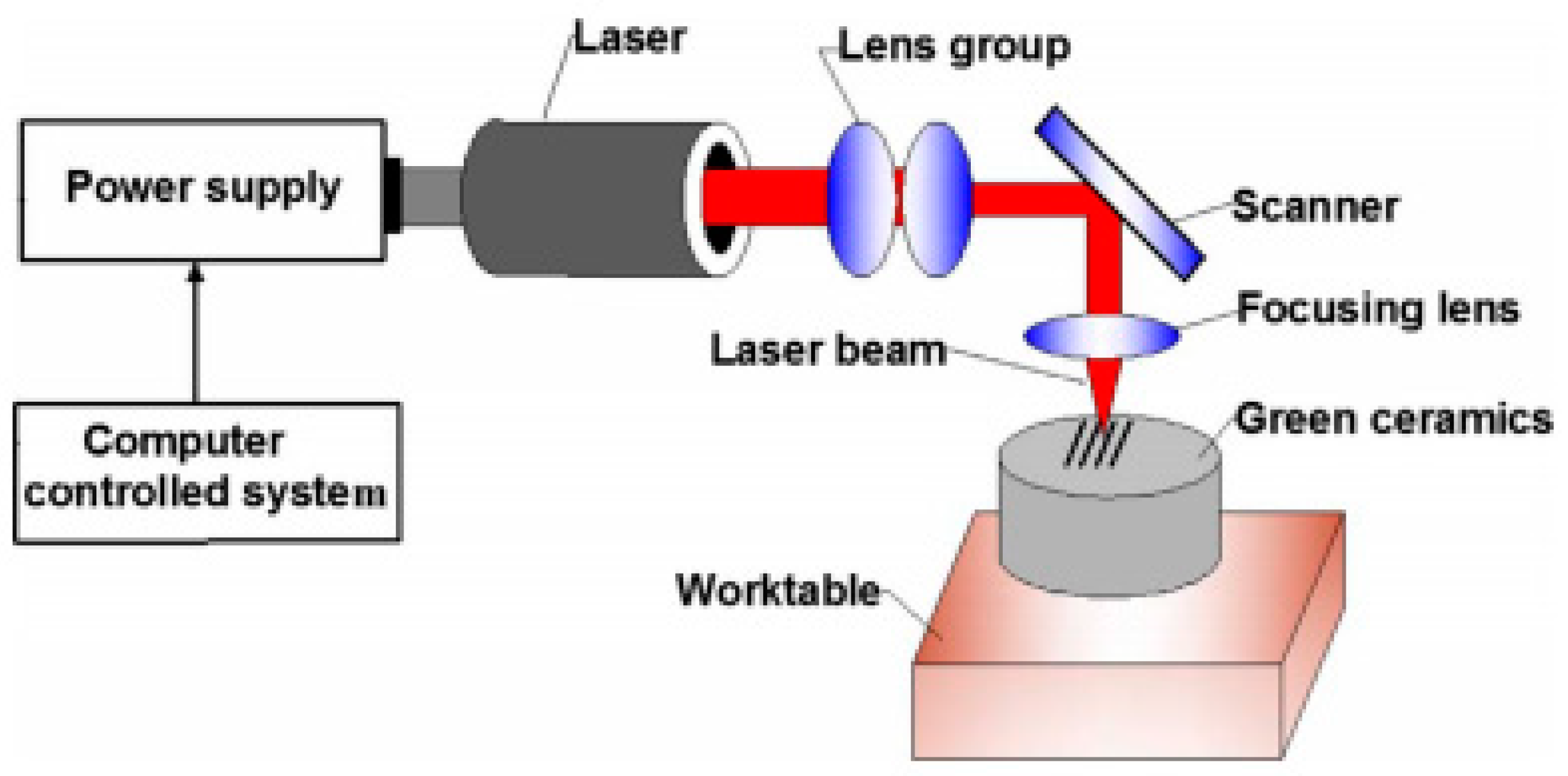
2.1. Texturing Using Laser Ablation
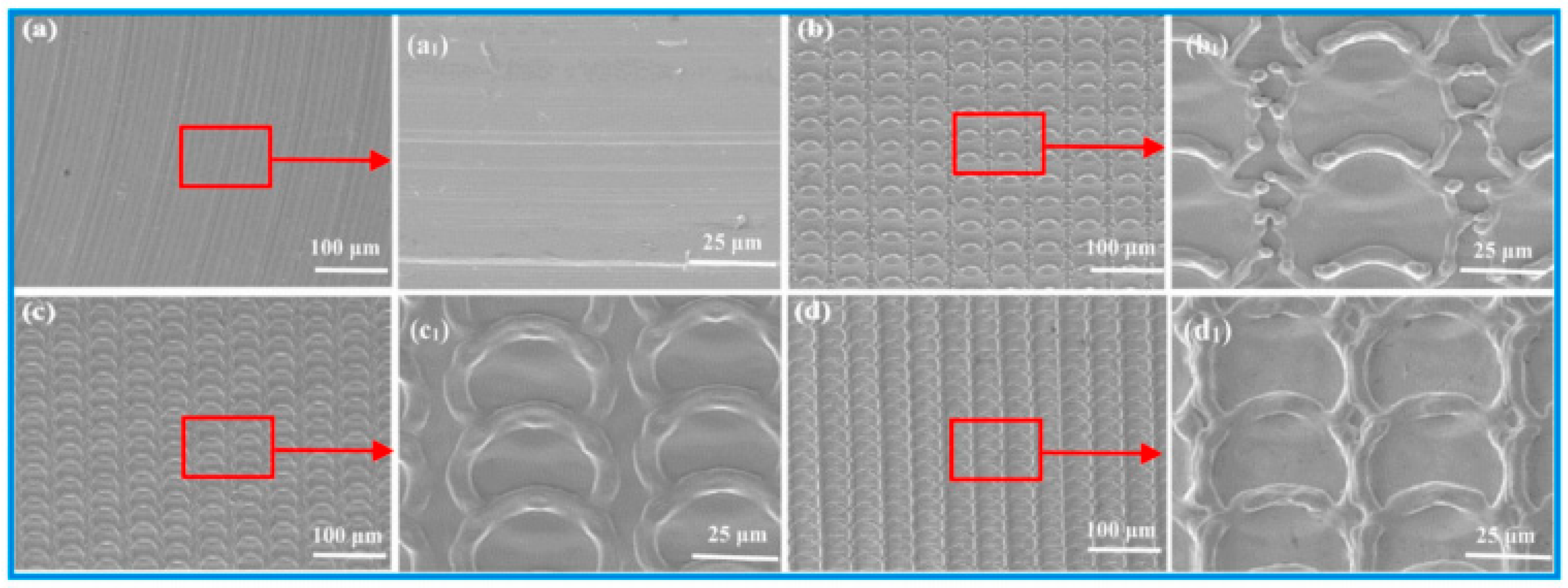
2.2. Texturing Using Laser Interference
3. Laser Surface Texturing for Biomedical Applications
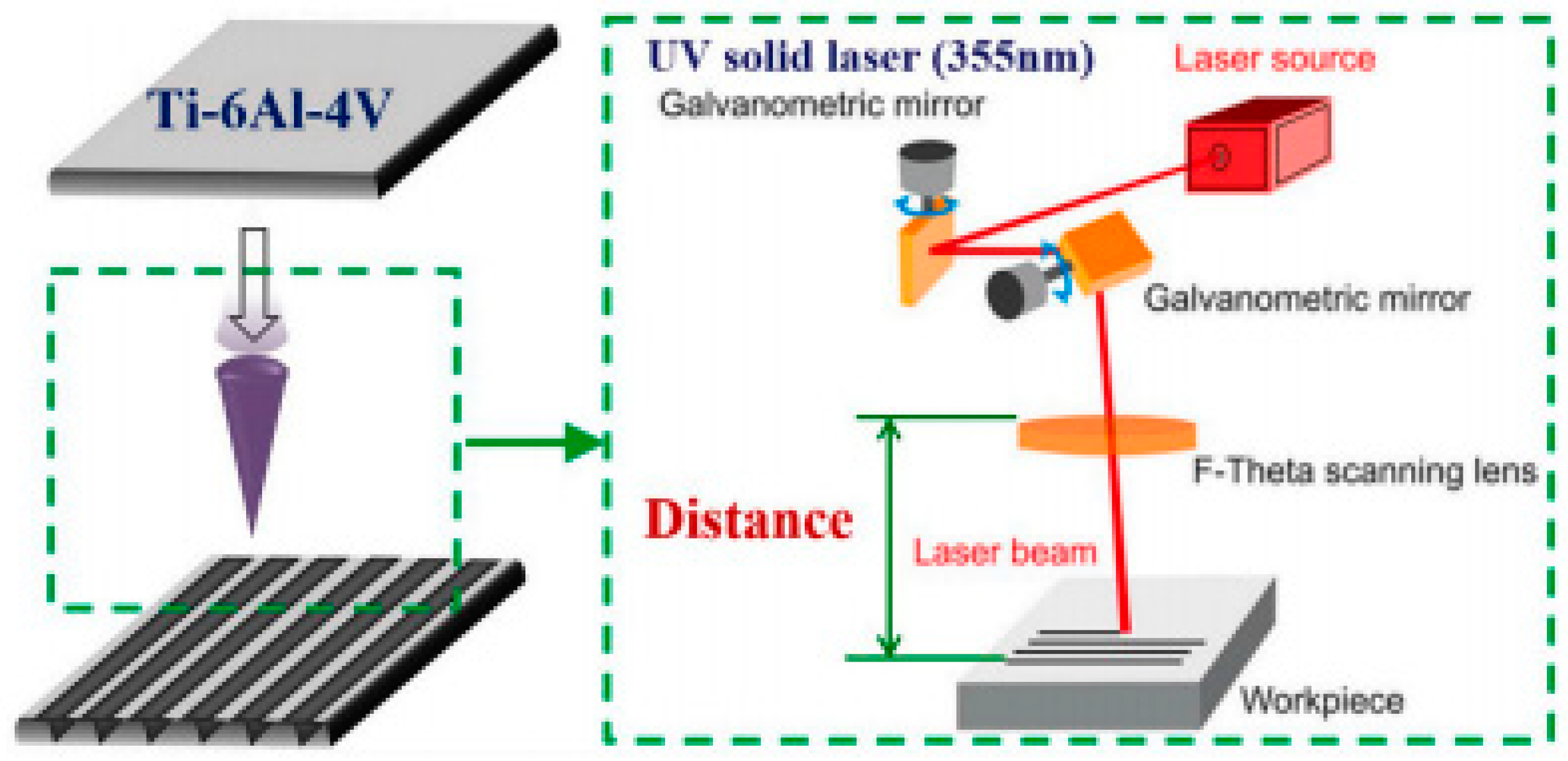
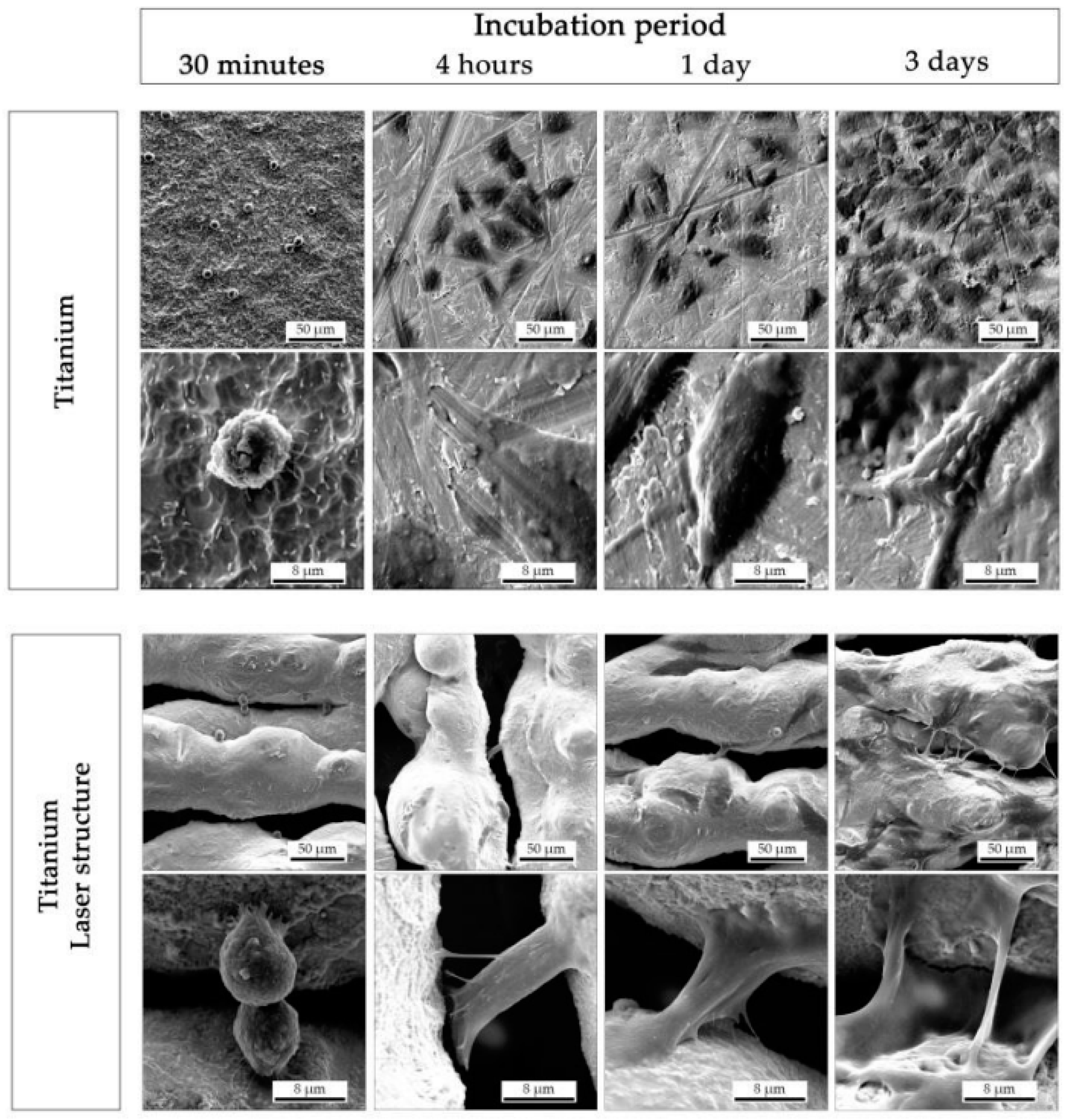
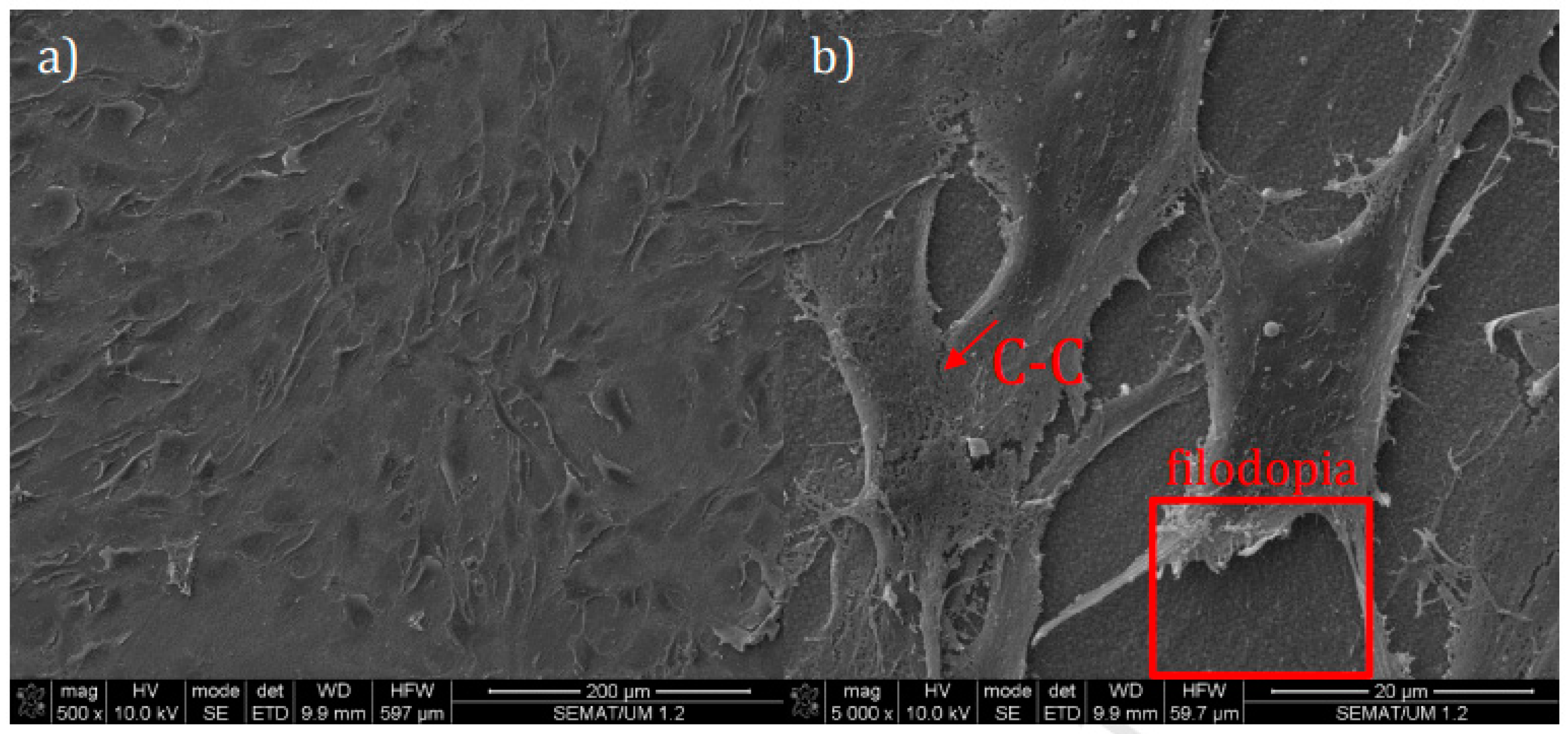
3.1. LST on Titanium and Its Alloys for Biomedical Applications
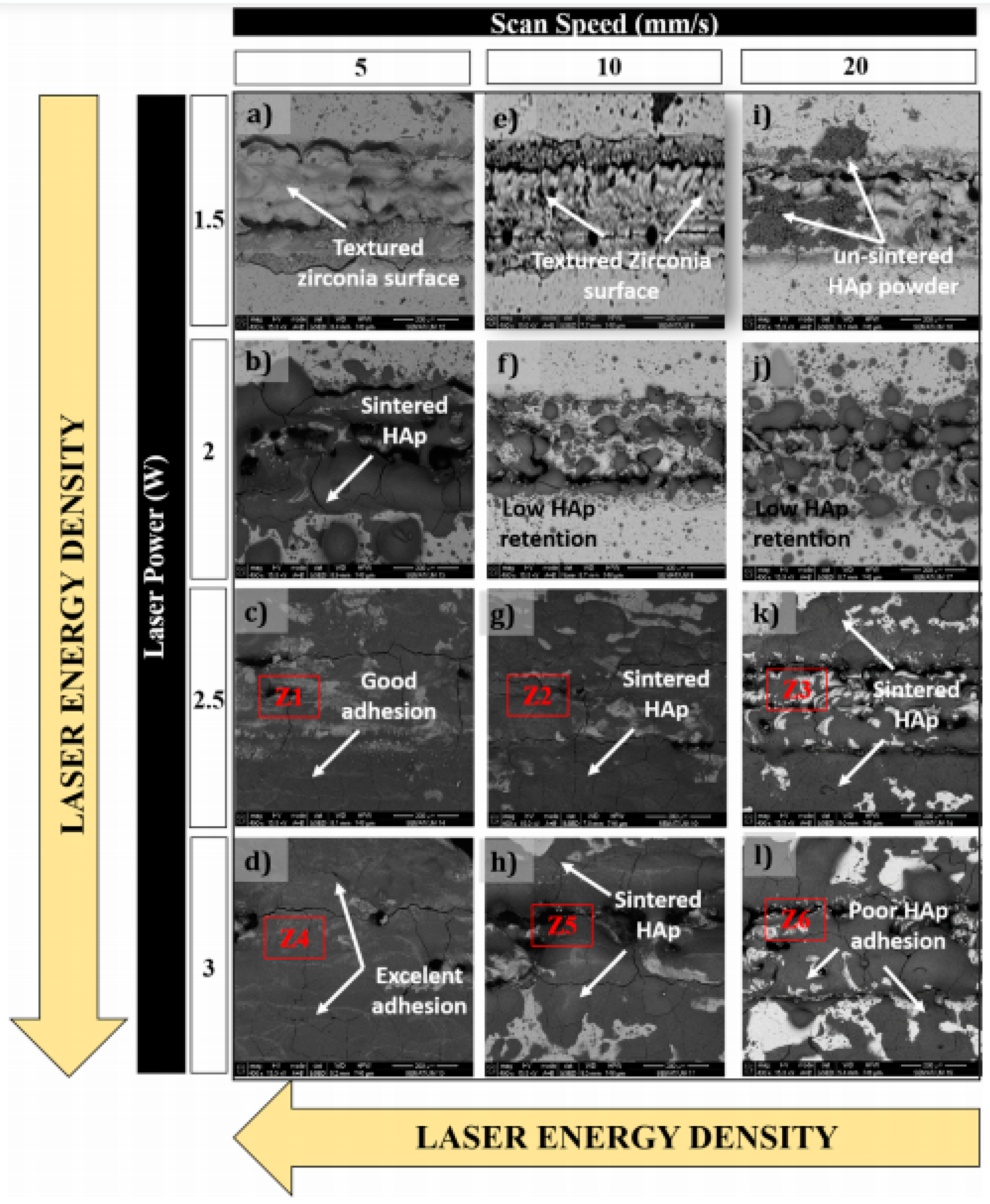
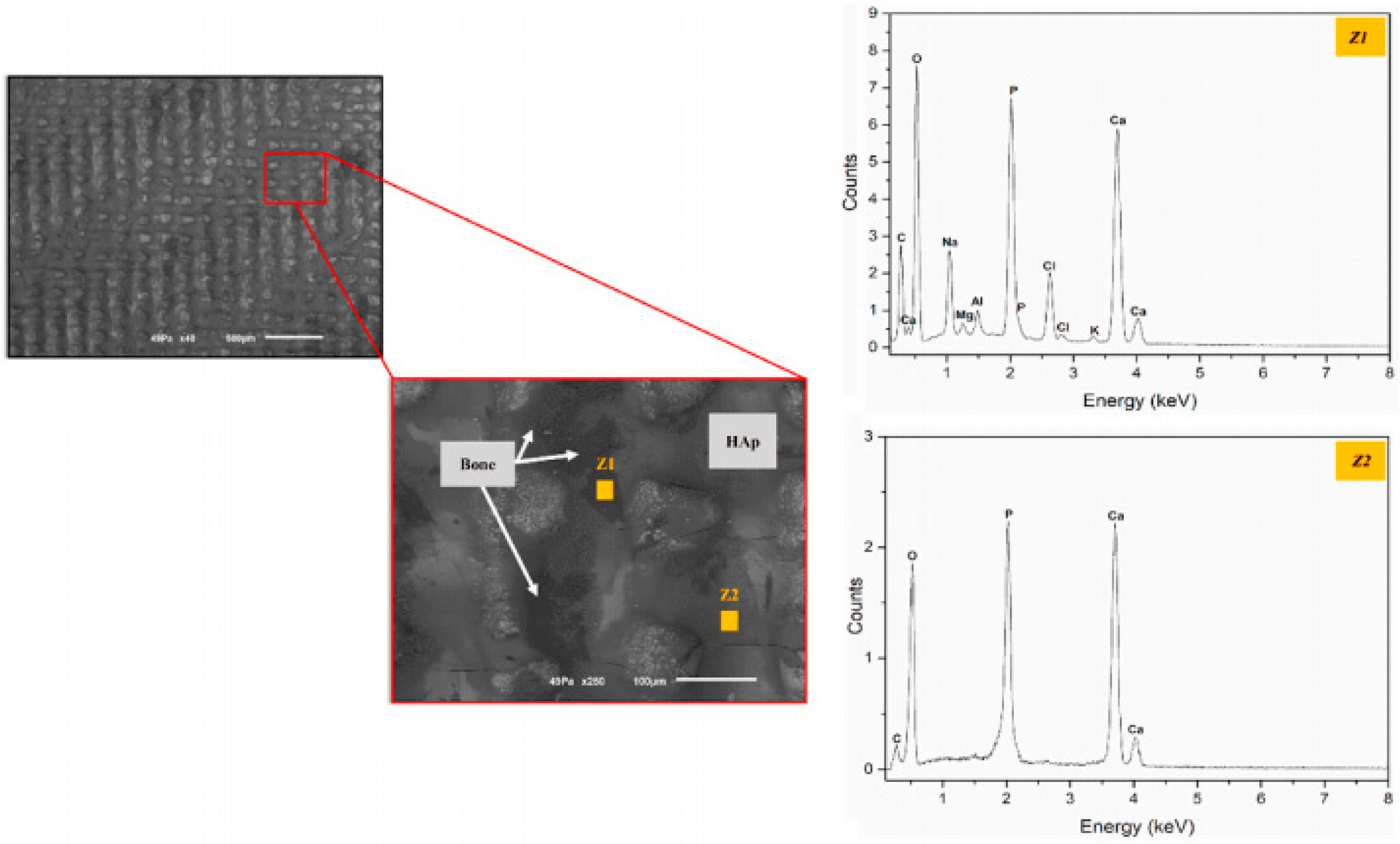
3.2. LST on Zirconia for Biomedical Application
3.3. LST on Polymer
3.4. LST on Other Material for Biomedical Application
4. Summary
- (i)
- Though there are many studies on titanium and its alloys and zirconia for biomedical applications, very limited work has been carried out considering the effect of laser parameters on the laser texturing process. The parametric analysis of several surface properties (like roughness, wettability, hardness, etc.) is essential for the overall improvement of the process and commercial application in the emerging area;
- (ii)
- For other material, more research needs to be carried out for exploring the physics in LST, and clinical trials must be carried out to confirm the feasibility for its application in the biomedical domain. Further, it was observed that laser surface texturing has become a potential method for surface modification of biomaterial and may be successfully utilized for biomedical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bhaduria, D.; Batala, A.; Dimova, S.S.; Zhangb, Z.; Dongb, H.; Fallqvistc, M.; M’Saoubid, R. On design and tribological behaviour of laser textured surfaces. Procedia CIRP 2017, 60, 20–25. [Google Scholar] [CrossRef]
- Hausmann, U.P.; Joerges, P.; Heinzl, J.; Talke, F.E. Nano-texturing of magnetic recording sliders via laser ablation. Microsyst. Technol. 2009, 15, 1747–1751. [Google Scholar] [CrossRef] [Green Version]
- Etsion, I.; Halperin, G. A laser surface textured hydrostatic mechanical seal. Tribol. Trans. 2002, 45, 430–434. [Google Scholar] [CrossRef]
- Mao, B.; Arpith Siddaiah, Y.L.; Pradeep, L.M. Laser surface texturing and related techniques for enhancing tribological performance of engineering materials: A review. J. Manuf. Process. 2020, 53, 153–173. [Google Scholar] [CrossRef]
- Arslan, A.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Mufti, R.A.; Mosarof, M.H.; Khuong, L.S.; Quazi, M.M. Surface texture manufacturing techniques and tribological effect of surface texturing on cutting tool performance: A review. Crit. Rev. Solid State Mater. Sci. 2016, 41, 447–481. [Google Scholar] [CrossRef]
- Joshi, B.; Tripathi, K.; Gyawali, G.; Lee, S.W. The effect of laser surface texturing on the tribological performance of different Sialon ceramic phases. Prog. Nat. Sci. Mater. Int. 2016, 26, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, X.; Guo, C.; Tao, J.; Tian, C.; Deng, Y.; Zhang, W. Micro surface texturing of alumina ceramic with nanosecond laser. Procedia Eng. 2017, 174, 370–376. [Google Scholar] [CrossRef]
- Xing, Y.; Deng, J.; Feng, X.; Yu, S. Effect of laser surface texturing on Si3N4/TiC ceramic sliding against steel under dry friction. Mater. Design (1980–2015) 2013, 52, 34–45. [Google Scholar] [CrossRef]
- Šugár, P.; Šugárová, J.; Frnčík, M. Laser surface texturing of tool steel: Textured surfaces quality evaluation. Open Eng. 2016, 6, 90–97. [Google Scholar] [CrossRef]
- Tripathi, K.; Gyawali, G.; Joshi, B.; Amanov, A.; Wohn, S. Improved tribological behavior of grey cast Iron Under low and high viscous lubricants by laser surface texturing. Mater. Perform. Charact. 2017, 6, 24–41. [Google Scholar] [CrossRef]
- Riveiro, A.; Maçon, A.L.; del Val, J.; Comesaña, R.; Pou, J. Laser surface texturing of polymers for biomedical applications. Front. Phys. 2018, 6, 16. [Google Scholar] [CrossRef]
- Ezhilmaran, V.; Vasa, N.J.; Vijayaraghavan, L. Investigation on generation of laser assisted dimples on piston ring surface and influence of dimple parameters on friction. Surf. Coat. Technol. 2018, 335, 314–326. [Google Scholar] [CrossRef]
- Liang, L.; Yuan, J.; Li, X.; Yang, F.; Jiang, L. Wear behavior of the micro-grooved texture on WC-Ni3Al cermet prepared by laser surface texturing. Int. J. Refract. Met. Hard Mater. 2018, 72, 211–222. [Google Scholar] [CrossRef]
- White, N.; Eder, K.; Byrnes, J.; Cairney, J.M.; McCarroll, I.E. Laser ablation sample preparation for atom probe tomography and transmission electron microscopy. Ultramicroscopy 2020, 220, 113161. [Google Scholar] [CrossRef]
- Shum, P.W.; Zhou, Z.F.; Li, K.Y. To increase the hydrophobicity and wear resistance of diamond-like carbon coatings by surface texturing using laser ablation process. Thin Solid Film. 2013, 544, 472–476. [Google Scholar] [CrossRef]
- Eskandari, M.J.; Shafyei, A.; Karimzadeh, F. Investigation of wetting properties of gold nanolayer coated aluminum surfaces textured with continuous-wave fiber laser ablation. Thin Solid Film. 2020, 711, 138278. [Google Scholar] [CrossRef]
- Kong, M.C.; Miron, C.B.; Axinte, D.A.; Davies, S.; Kell, J. On the relationship between the dynamics of the power density and workpiece surface texture in pulsed laser ablation. CIRP Ann. 2012, 61, 203–206. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Khaled, M.; Abu-Dheir, N.; Al-Aqeeli, N.; Said, S.A.; Ahmed, A.O.; Varanasi, K.K.; Toumi, Y.K. Wetting and other physical characteristics of polycarbonate surface textured using laser ablation. Appl. Surf. Sci. 2014, 320, 21–29. [Google Scholar] [CrossRef]
- Shum, P.W.; Zhou, Z.F.; Li, K.Y. To increase the hydrophobicity, non-stickiness and wear resistance of DLC surface by surface texturing using a laser ablation process. Tribol. Int. 2014, 78, 1–6. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, K.; Huang, P.; Liu, L.; Wu, Z. Assessment machining of micro-channel textures on PCD by laser-induced plasma and ultra-short pulsed laser ablation. Opt. Laser Technol. 2020, 125, 106057. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Deng, J.; Meng, R.; Zou, X.; Wu, F. Fabrication of micro-scale textured grooves on green ZrO2 ceramics by pulsed laser ablation. Ceram. Int. 2017, 43, 6519–6531. [Google Scholar] [CrossRef]
- Zabila, Y.; Perzanowski, M.; Dobrowolska, A.; Kac, M.; Polit, A.; Marszalek, M. Direct laser interference patterning: Theory and application. Acta Phys. Pol. Ser. A Gen. Phys. 2009, 115, 591. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Hans, M.; Gachot, C.; Thome, A.; Bonk, S.; Mücklich, F. Direct laser interference patterning: Tailoring of contact area for frictional and antibacterial properties. Lubricants 2016, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Sanza, F.J.; Langheinrich, D.; Berger, J.; Hernandez, A.L.; Dani, S.; Casquel, R.; Lavin, A.; Otón, A.; Santamaría, B.; Laguna, M.; et al. Direct laser interference patterning (DLIP) technique applied to the development of optical biosensors based on biophotonic sensing cells (bicells). Int. Soc. Opt. Photonics 2015, 9351, 935114. [Google Scholar]
- El-Khoury, M.; Alamri, S.; Voisiat, B.; Kunze, T.; Lasagni, A.F. Fabrication of hierarchical surface textures using multi-pulse direct laser interference patterning with nanosecond pulses. Mater. Lett. 2020, 258, 126743. [Google Scholar] [CrossRef]
- Baumann, R.; Milles, S.; Leupolt, B.; Kleber, S.; Dahms, J.; Lasagni, A.F. Tailored wetting of copper using precise nanosecond direct laser interference patterning. Opt. Lasers Eng. 2020, 137, 106364. [Google Scholar] [CrossRef]
- Peter, A.; Onuseit, V.; Freitag, C.; Faas, S.; Graf, T. Flexible, compact and picosecond laser capable four-beam interference setup. In Proceedings of the Lasers in Manufacturing Conference, Munich, Germany, 18–22 June 2017; pp. 1–8. [Google Scholar]
- Peter, A.; Lutey, A.H.; Faas, S.; Romoli, L.; Onuseit, V.; Graf, T. Direct laser interference patterning of stainless steel by ultrashort pulses for antibacterial surfaces. Opt. Laser Technol. 2020, 123, 105954. [Google Scholar] [CrossRef]
- Choi, J.; Chung, M.H.; Dong, K.Y.; Park, E.M.; Ham, D.J.; Park, Y.; Song, I.S.; Pak, J.J.; Ju, B.K. Investigation on fabrication of nanoscale patterns using laser interference lithography. J. Nanosci. Nanotechnol. 2011, 11, 778–781. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Sabau, A.S.; Jones, J.F.; Hackett, A.C.; Daniel, C.; Warren, D. Aluminum surface texturing by means of laser interference metallurgy. In Light Metals; Springer: Cham, Switzerland, 2015; pp. 427–429. [Google Scholar]
- Furlan, V.; Biondi, M.; Demir, A.G.; Pariani, G.; Bianco, A.; Previtali, B. Direct laser texturing using two-beam interference patterning on biodegradable magnesium alloy. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, San Diego, CA, USA, 16–20 October 2016; p. 182. [Google Scholar]
- Aguilar-Morales, A.I.; Alamri, S.; Kunze, T.; Lasagni, A.F. Influence of processing parameters on surface texture homogeneity using Direct Laser Interference Patterning. Opt. Laser Technol. 2018, 107, 216–227. [Google Scholar] [CrossRef]
- Cardoso, J.T.; Aguilar-Morales, A.I.; Alamri, S.; Huerta-Murillo, D.; Cordovilla, F.; Lasagni, A.F.; Ocaña, J.L. Superhydrophobicity on hierarchical periodic surface structures fabricated via direct laser writing and direct laser interference patterning on an aluminium alloy. Opt. Lasers Eng. 2018, 111, 193–200. [Google Scholar] [CrossRef]
- Sola, D.; Lavieja, C.; Orera, A.; Clemente, M.J. Direct laser interference patterning of ophthalmic polydimethylsiloxane (PDMS) polymers. Opt. Lasers Eng. 2018, 106, 139–146. [Google Scholar] [CrossRef]
- Radmanesh, M.; Kiani, A. Effects of laser pulse numbers on surface biocompatibility of titanium for implant fabrication. J. Biomater. Nanobiotechnol. 2015, 6, 168. [Google Scholar] [CrossRef] [Green Version]
- Kurella, A.; Dahotre, N.B. Surface modification for bioimplants: The role of laser surface engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, S.; Zhang, W.; Jiang, X.; Hu, J. Picosecond laser texturing on titanium alloy for biomedical implants in cell proliferation and vascularization. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1494–1504. [Google Scholar] [CrossRef]
- Fiorucci, M.P.; López, A.J.; Ramil, A. Surface modification of Ti6Al4V by nanosecond laser ablation for biomedical applications. J. Phys. Conf. Ser. 2015, 605, 012022. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.S.; Moura, C.G.; Henriques, B.; Chevalier, J.; Silva, F.S.; Fredel, M.C. Influence of laser texturing on surface features, mechanical properties and low-temperature degradation behavior of 3Y-TZP. Ceram. Int. 2020, 46, 3502–3512. [Google Scholar] [CrossRef]
- Cunha, A.; Oliveira, V.; Serro, A.P.; Zouani, O.E.; Almeida, A.; Durrieu, M.C.; Vilar, R. Ultrafast laser texturing of Ti-6Al-4V surfaces for biomedical applications. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, Miami, FL, USA, 6–10 October 2013; pp. 910–918. [Google Scholar]
- Stango, S.A.; Karthick, D.; Swaroop, S.; Mudali, U.K.; Vijayalakshmi, U. Development of hydroxyapatite coatings on laser textured 316 LSS and Ti-6Al-4V and its electrochemical behavior in SBF solution for orthopedic applications. Ceram. Int. 2018, 44, 3149–3160. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, G.; Zhang, W.; Hu, J. Investigating the effect of picosecond laser texturing on microstructure and biofunctionalization of titanium alloy. J. Mater. Process. Technol. 2018, 255, 129–136. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- Schiff, N.; Grosgogeat, B.; Lissac, M.; Dalard, F. Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys. Biomaterials 2002, 23, 1995–2002. [Google Scholar] [CrossRef]
- Pou, P.; Riveiro, A.; del Val, J.; Comesaña, R.; Penide, J.; Arias-González, F.; Soto, R.; Lusquiños, F.; Pou, J. Laser surface texturing of Titanium for bioengineering applications. Procedia Manuf. 2017, 13, 694–701. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. Jom 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Tiainen, L.; Abreu, P.; Buciumeanu, M.; Silva, F.; Gasik, M.; Guerrero, R.S.; Carvalho, O. Novel laser surface texturing for improved primary stability of titanium implants. J. Mech. Behav. Biomed. Mater. 2019, 98, 26–39. [Google Scholar] [CrossRef]
- Ramskogler, C.; Warchomicka, F.; Mostofi, S.; Weinberg, A.; Sommitsch, C. Innovative surface modification of Ti6Al4V alloy by electron beam technique for biomedical application. Mater. Sci. Eng. C 2017, 78, 105–113. [Google Scholar] [CrossRef]
- Shukla, P.; Waugh, D.G.; Lawrence, J.; Vilar, R. Laser surface structuring of ceramics, metals and polymers for biomedical applications: A review. In Laser Surface Modification of Biomaterials; Woodhead Publishing: Cambridge, UK, 2016; pp. 281–299. [Google Scholar]
- Kuczyńska, D.; Kwaśniak, P.; Marczak, J.; Bonarski, J.; Smolik, J.; Garbacz, H. Laser surface treatment and the resultant hierarchical topography of Ti grade 2 for biomedical application. Appl. Surf. Sci. 2016, 390, 560–569. [Google Scholar] [CrossRef]
- Behera, R.R.; Das, A.; Hasan, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Deposition of biphasic calcium phosphate film on laser surface textured Ti–6Al–4V and its effect on different biological properties for orthopedic applications. J. Alloy. Compd. 2020, 842, 155683. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Zhao, H.; Zhang, G.; Ren, T.; Zhang, Y. Enhanced anticorrosion and antiwear properties of Ti–6Al–4V alloys with laser texture and graphene oxide coatings. Tribol. Int. 2020, 152, 106475. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, W.; Zhang, G.; Li, Z.; Hu, H.; Wang, C.; Zeng, X.; Zhao, S.; Zhang, Y.; Ren, T. Friction stability and cellular behaviors on laser textured Ti–6Al–4V alloy implants with bioinspired micro-overlapping structures. J. Mech. Behav. Biomed. Mater. 2020, 109, 103823. [Google Scholar] [CrossRef]
- Uhlmann, E.; Schweitzer, L.; Kieburg, H.; Spielvogel, A.; Huth-Herms, K. The Effects of Laser Microtexturing of Biomedical Grade 5 Ti-6Al-4V Dental Implants (Abutment) on Biofilm Formation. Procedia CIRP 2018, 68, 184–189. [Google Scholar] [CrossRef]
- Paital, S.R.; Bunce, N.; Nandwana, P.; Honrao, C.; Nag, S.; He, W.; Banerjee, R.; Dahotre, N.B. Laser surface modification for synthesis of textured bioactive and biocompatible Ca–P coatings on Ti–6Al–4V. J. Mater. Sci. Mater. Med. 2011, 22, 1393–1406. [Google Scholar] [CrossRef]
- Zheng, Q.; Mao, L.; Shi, Y.; Fu, W.; Hu, Y. Biocompatibility of Ti-6Al-4V titanium alloy implants with laser microgrooved surfaces. Mater. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Mahmoodi, M.; Saeedinasab, H.; Tahriri, M. Evaluation of mechanical and electrochemical properties of laser surface modified Ti–6Al–4V for biomedical applications: In vitro study. Surf. Eng. 2008, 24, 209–218. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Tavakoli, J.; Mahmoodi, M. Analysis of bioadhesivity of osteoblast cells on titanium alloy surface modified by Nd: YAG laser. J. Adhes. 2007, 83, 151–172. [Google Scholar] [CrossRef]
- Coathup, M.J.; Blunn, G.W.; Mirhosseini, N.; Erskine, K.; Liu, Z.; Garrod, D.R.; Li, L. Controlled laser texturing of titanium results in reliable osteointegration. J. Orthop. Res. 2017, 35, 820. [Google Scholar] [CrossRef]
- Patil, D.; Aravindan, S.; Kaushal Wasson, M.; Rao, P.V. Fast fabrication of superhydrophobic titanium alloy as antibacterial surface using nanosecond laser texturing. J. Micro Nano-Manuf. 2018, 6, 011002. [Google Scholar] [CrossRef]
- Borcherding, K.; Marx, D.; Gätjen, L.; Specht, U.; Salz, D.; Thiel, K.; Wildemann, B.; Grunwald, I. Impact of Laser Structuring on Medical-Grade Titanium: Surface Characterization and In Vitro Evaluation of Osteoblast Attachment. Materials 2020, 13, 2000. [Google Scholar] [CrossRef]
- Kumari, R.; Scharnweber, T.; Pfleging, W.; Besser, H.; Majumdar, J.D. Laser Surface Textured Titanium Alloy (Ti-6Al-4V)-Part II-Studies on Bio-compatibity. Appl. Surf. Sci. 2015, 357, 750–758. [Google Scholar] [CrossRef]
- Cunha, A.; Elie, A.M.; Plawinski, L.; Serro, A.P.; do Rego, A.M.; Almeida, A.; Urdaci, M.C.; Durrieu, M.C.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Cunha, A.; Zouani, O.F.; Plawinski, L.; Botelho do Rego, A.M.; Almeida, A.; Vilar, R.; Durrieu, M.C. Human mesenchymal stem cell behavior on femtosecond laser-textured Ti-6Al-4V surfaces. Nanomedicine 2015, 10, 725–739. [Google Scholar] [CrossRef]
- Dumas, V.; Guignandon, A.; Vico, L.; Mauclair, C.; Zapata, X.; Linossier, M.T.; Bouleftour, W.; Granier, J.; Peyroche, S.; Dumas, J.C.; et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment. Biomed. Mater. 2015, 10, 055002. [Google Scholar] [CrossRef]
- Raimbault, O.; Benayoun, S.; Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.M.; Girard-Lauriault, P.L.; Valette, S.; Donnet, C. The effects of femtosecond laser-textured Ti-6Al-4V on wettability and cell response. Mater. Sci. Eng. C 2016, 69, 311–320. [Google Scholar] [CrossRef]
- Apratim, A.; Eachempati, P.; Salian, K.K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 147. [Google Scholar] [CrossRef] [Green Version]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.P.; Kiattavorncharoen, S.; Lauer, H.C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Moura, C.G.; Pereira, R.; Buciumeanu, M.; Carvalho, O.; Bartolomeu, F.; Nascimento, R.; Silva, F.S. Effect of laser surface texturing on primary stability and surface properties of zirconia implants. Ceram. Int. 2017, 43, 15227–15236. [Google Scholar] [CrossRef]
- Faria, D.; Henriques, B.; Souza, A.C.; Silva, F.S.; Carvalho, O. Laser-assisted production of HAp-coated zirconia structured surfaces for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 112, 104049. [Google Scholar] [CrossRef]
- Faria, D.; Madeira, S.; Buciumeanu, M.; Silva, F.S.; Carvalho, O. Novel laser textured surface designs for improved zirconia implants performance. Mater. Sci. Eng. C 2020, 108, 110390. [Google Scholar] [CrossRef]
- Mesquita-Guimarães, J.; Detsch, R.; Souza, A.C.; Henriques, B.; Silva, F.S.; Boccaccini, A.R.; Carvalho, O. Cell adhesion evaluation of laser-sintered HAp and 45S5 bioactive glass coatings on micro-textured zirconia surfaces using MC3T3-E1 osteoblast-like cells. Mater. Sci. Eng. C 2020, 1091, 10492. [Google Scholar] [CrossRef]
- Madeira, S.; Barbosa, A.; Moura, C.G.; Buciumeanu, M.; Silva, F.S.; Carvalho, O. Aunps and Agμps-functionalized zirconia surfaces by hybrid laser technology for dental implants. Ceram. Int. 2020, 46, 7109–7121. [Google Scholar] [CrossRef]
- Daskalova, A.; Angelova, L.; Carvalho, A.; Trifonov, A.; Nathala, C.; Monteiro, F.; Buchvarov, I. Effect of surface modification by femtosecond laser on zirconia based ceramics for screening of cell-surface interaction. Appl. Surf. Sci. 2020, 26, 145914. [Google Scholar] [CrossRef]
- Carvalho, O.; Sousa, F.; Madeira, S.; Silva, F.S.; Miranda, G. HAp-functionalized zirconia surfaces via hybrid laser process for dental applications. Opt. Laser Technol. 2018, 106, 157–167. [Google Scholar] [CrossRef]
- Delgado-Ruíz, R.A.; Calvo-Guirado, J.L.; Moreno, P.; Guardia, J.; Gomez-Moreno, G.; Mate-Sánchez, J.E.; Ramirez-Fernández, P.; Chiva, F. Femtosecond laser microstructuring of zirconia dental implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Cangueiro, L.; Oliveira, V.; Vilar, R.; Fernandes, M.H.; Monteiro, F.J. Femtosecond laser microstructured Alumina toughened Zirconia: A new strategy to improve osteogenic differentiation of hMSCs. Appl. Surf. Sci. 2018, 435, 1237–1245. [Google Scholar] [CrossRef]
- Elena Sima, L.; Bonciu, A.; Baciu, M.; Anghel, I.; Dumitrescu, L.N.; Rusen, L.; Dinca, V. Bioinstructive Micro-Nanotextured Zirconia Ceramic Interfaces for Guiding and Stimulating an Osteogenic Response In Vitro. Nanomaterials 2020, 10, 2465. [Google Scholar] [CrossRef] [PubMed]
- Makropoulou, M.; Serafetinides, A.A.; Skordoulis, C.D. Ultra-violet and infra-red laser ablation studies of biocompatible polymers. Lasers Med Sci. 1995, 10, 201–206. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Del Val, J.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of ultra-high-molecular-weight polyethylene (UHMWPE) for biomedical applications. Appl. Surf. Sci. 2014, 302, 236–242. [Google Scholar] [CrossRef]
- Tomanik, M.; Kobielarz, M.; Filipiak, J.; Szymonowicz, M.; Rusak, A.; Mroczkowska, K.; Antończak, A.; Pezowicz, C. Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly (l-Lactide) Surface. Materials 2020, 13, 3786. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Del Val, J.; Comesaña, R.; Boutinguiza, M.; Quintero, F.; Lusquiños, F.; Pou, J. Texturing of polypropylene (PP) with nanosecond lasers. Appl. Surf. Sci. 2016, 374, 379–386. [Google Scholar] [CrossRef]
- Mirzadeh, H.; Katbab, A.A.; Burford, R.P. CO2-laser graft copolymerization of HEMA and NVP onto ethylene-propylene rubber (EPR) as biomaterial-(III). Radiat. Phys. Chem. 1995, 46, 859–862. [Google Scholar] [CrossRef]
- Dinca, V.; Alloncle, P.; Delaporte, P.; Ion, V.; Rusen, L.; Filipescu, M.; Mustaciosu, C.; Luculescu, C.; Dinescu, M. Excimer laser texturing of natural composite polymer surfaces for studying cell-to-substrate specific response. Appl. Surf. Sci. 2015, 352, 82–90. [Google Scholar] [CrossRef]
- Koufaki, N.; Ranella, A.; Aifantis, K.E.; Barberoglou, M.; Psycharakis, S.; Fotakis, C.; Stratakis, E. Controlling cell adhesion via replication of laser micro/nano-textured surfaces on polymers. Biofabrication 2011, 3, 045004. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Yu, H.; Lim, K.P.; Ng, K.L.; Boey, Y.C.; Subbu, V.S.; Tan, L.P. Multiscale topological guidance for cell alignment via direct laser writing on biodegradable polymer. Tissue Eng. Part C Methods 2010, 16, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.; Lawrence, J. Laser surface processing of polymers for biomedical applications. In Laser-Assisted Fabrication of Materials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 275–318. [Google Scholar]
- Hu, G.; Guan, K.; Lu, L.; Zhang, J.; Lu, N.; Guan, Y. Engineered functional surfaces by laser microprocessing for biomedical applications. Engineering 2018, 4, 822–830. [Google Scholar] [CrossRef]
- Selvamani, V.; Zareei, A.; Elkashif, A.; Maruthamuthu, M.K.; Chittiboyina, S.; Delisi, D.; Li, Z.; Cai, L.; Pol, V.G.; Seleem, M.N.; et al. Hierarchical micro/mesoporous copper structure with enhanced antimicrobial property via laser surface texturing. Adv. Mater. Interfaces 2020, 7, 1901890. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, Q.; Wang, W.; Zhang, Y.; Dong, G. Response of MC3T3-E1 osteoblast cells to the microenvironment produced on Co–Cr–Mo alloy using laser surface texturing. J. Mater. Sci. 2014, 49, 2662–2671. [Google Scholar] [CrossRef]
- Purnama, A.; Furlan, V.; Dessi, D.; Demir, A.G.; Tolouei, R.; Paternoster, C.; Levesque, L.; Previtali, B.; Mantovani, D. Laser surface texturing of SS316L for enhanced adhesion of HUVECs. Surf. Eng. 2018, 1–10. [Google Scholar] [CrossRef]
- Krylach, I.V.; Kudryashov, S.I.; Olekhnovich, R.O.; Moskvin, M.K.; Uspenskaya, M.V. Tuning water wetting angle of a steel surface via nanosecond laser ablative nano/microtexturing for chemical and biomedical microfluidic applications. Laser Phys. Lett. 2019, 16, 105602. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivakoti, I.; Kibria, G.; Cep, R.; Pradhan, B.B.; Sharma, A. Laser Surface Texturing for Biomedical Applications: A Review. Coatings 2021, 11, 124. https://doi.org/10.3390/coatings11020124
Shivakoti I, Kibria G, Cep R, Pradhan BB, Sharma A. Laser Surface Texturing for Biomedical Applications: A Review. Coatings. 2021; 11(2):124. https://doi.org/10.3390/coatings11020124
Chicago/Turabian StyleShivakoti, Ishwer, Golam Kibria, Robert Cep, Bal Bahadur Pradhan, and Ashis Sharma. 2021. "Laser Surface Texturing for Biomedical Applications: A Review" Coatings 11, no. 2: 124. https://doi.org/10.3390/coatings11020124