Influence of Growth Defects on the Oxidation Resistance of Sputter-Deposited TiAlN Hard Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results
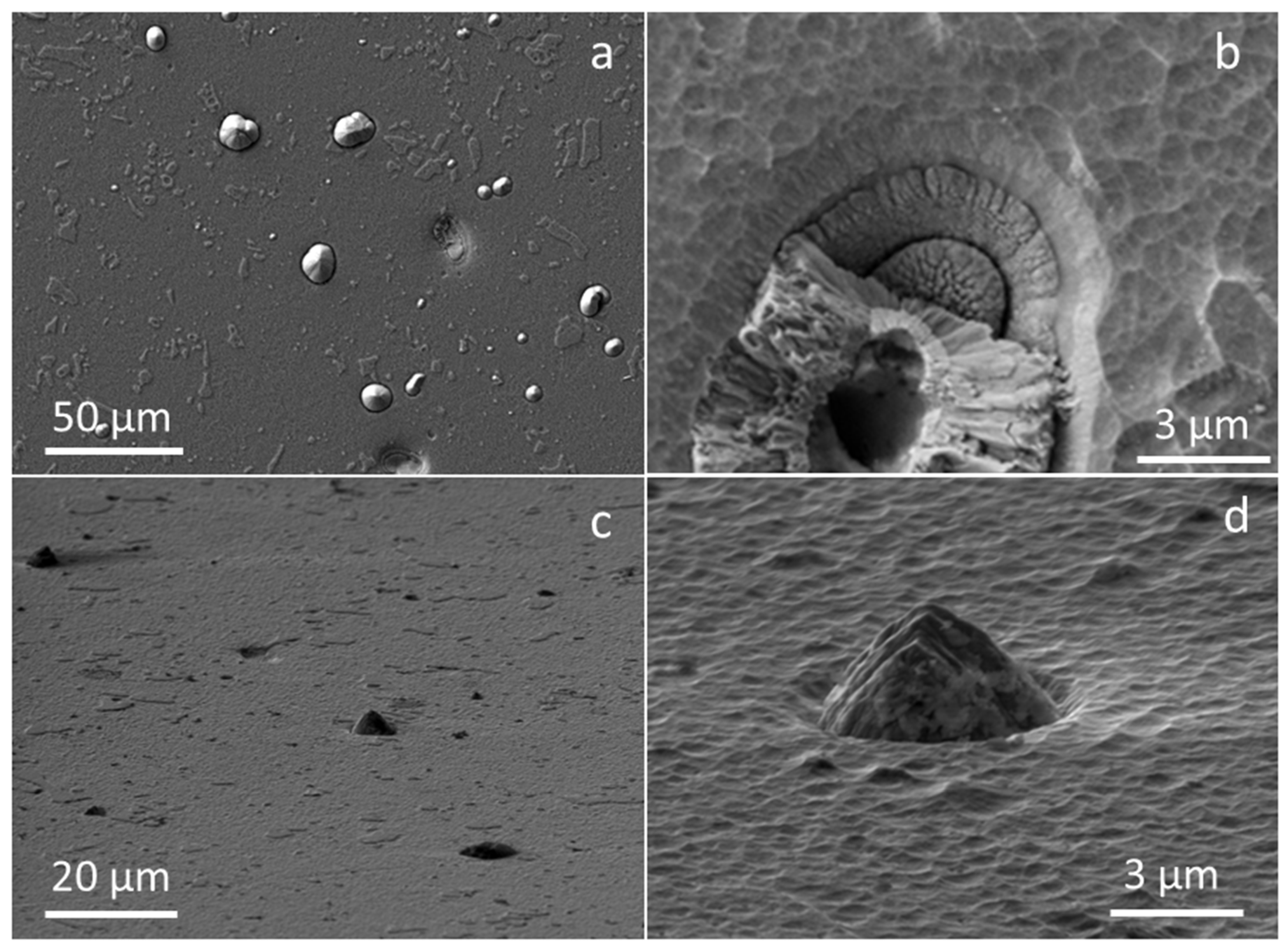
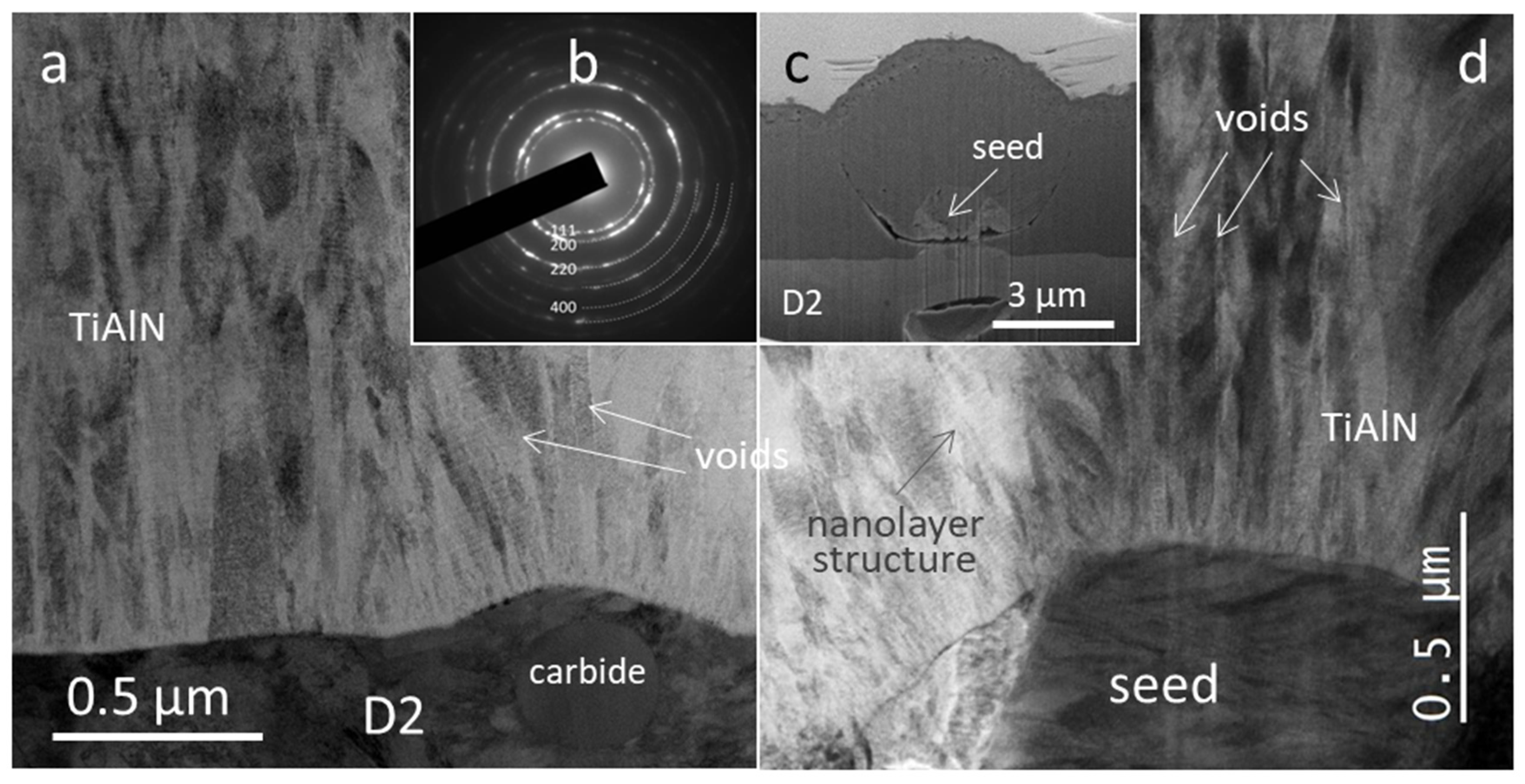
3.1. Morphology and Microstructure of As-Deposited TiAlN Hard Coating
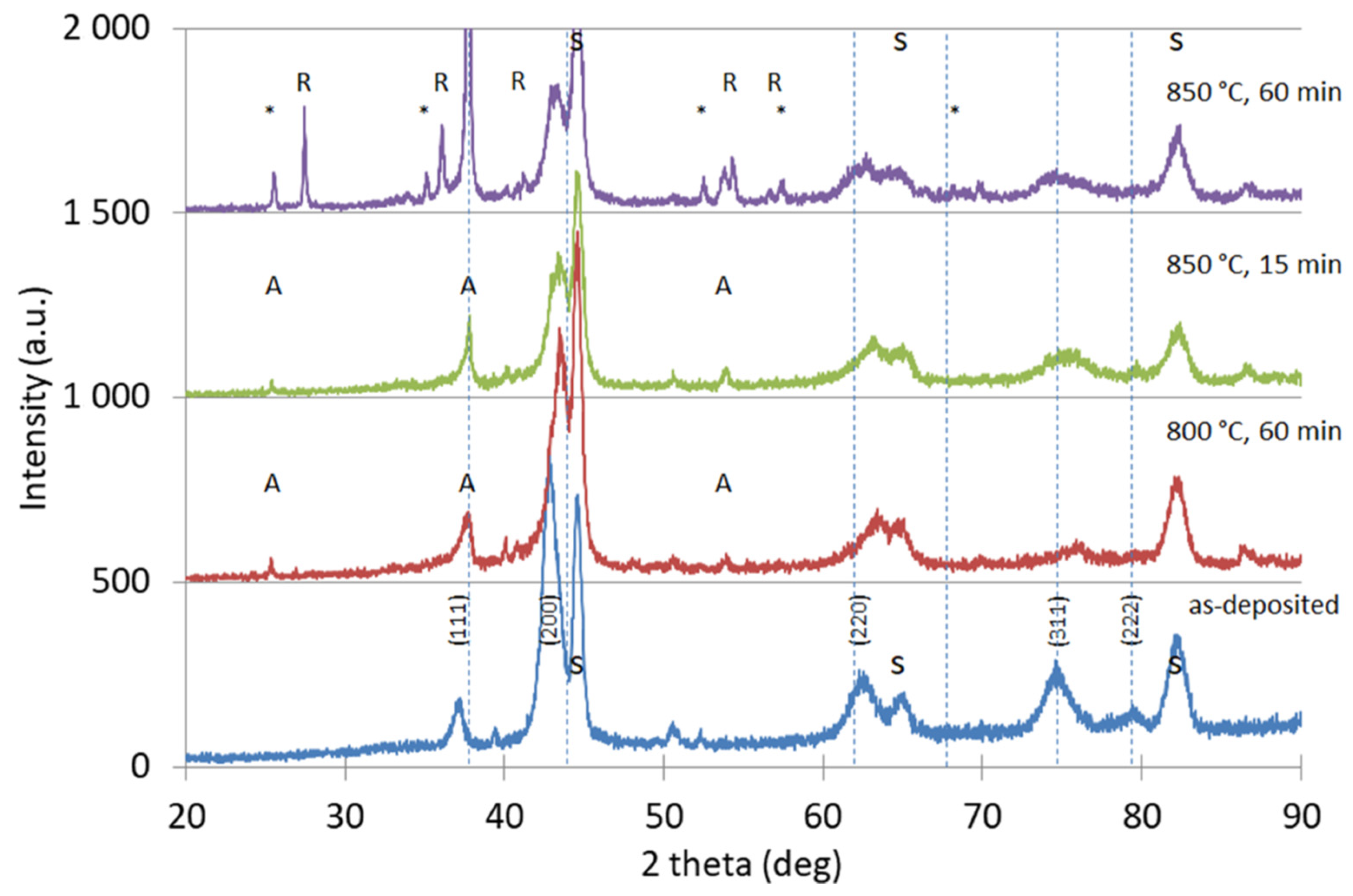
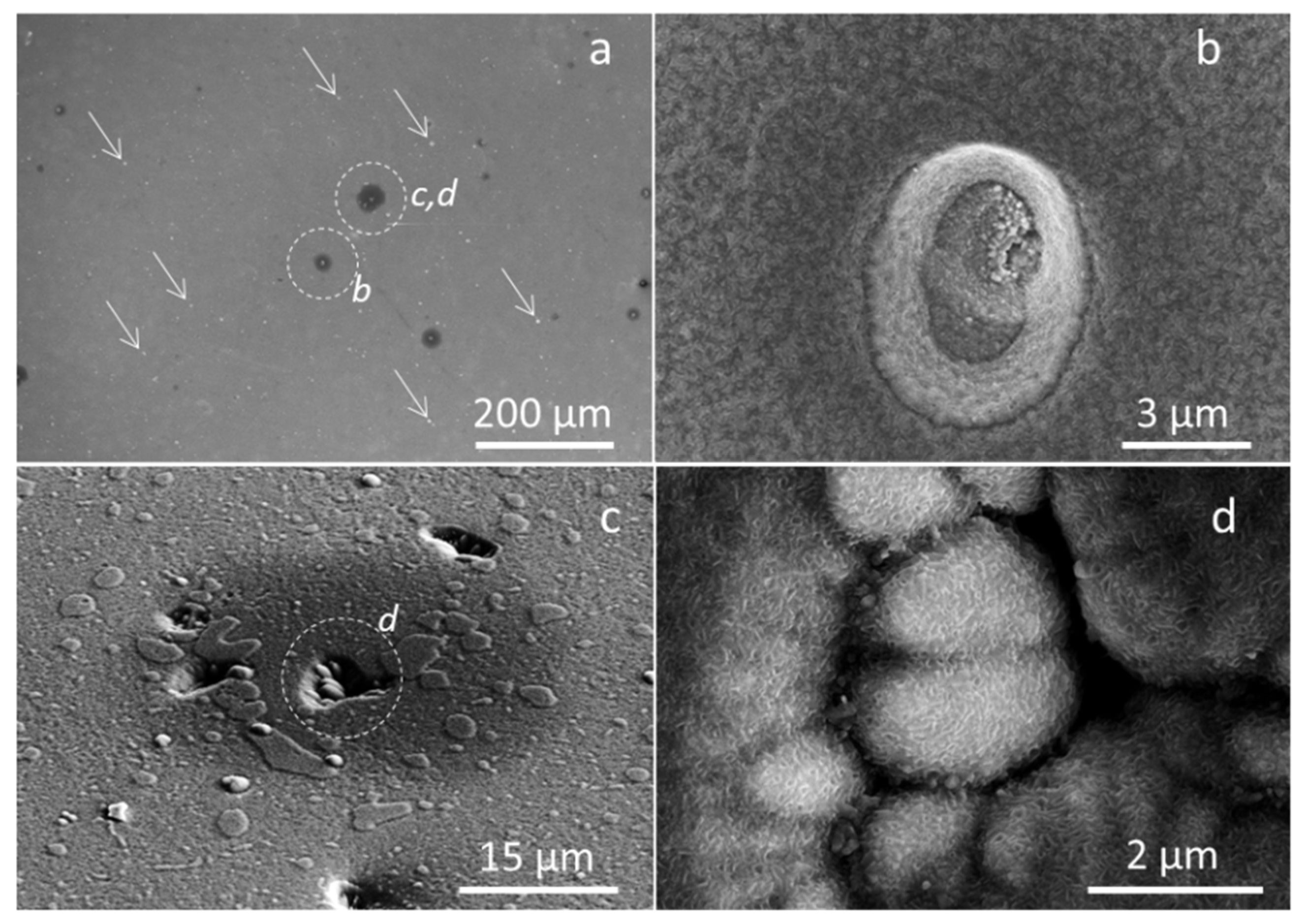
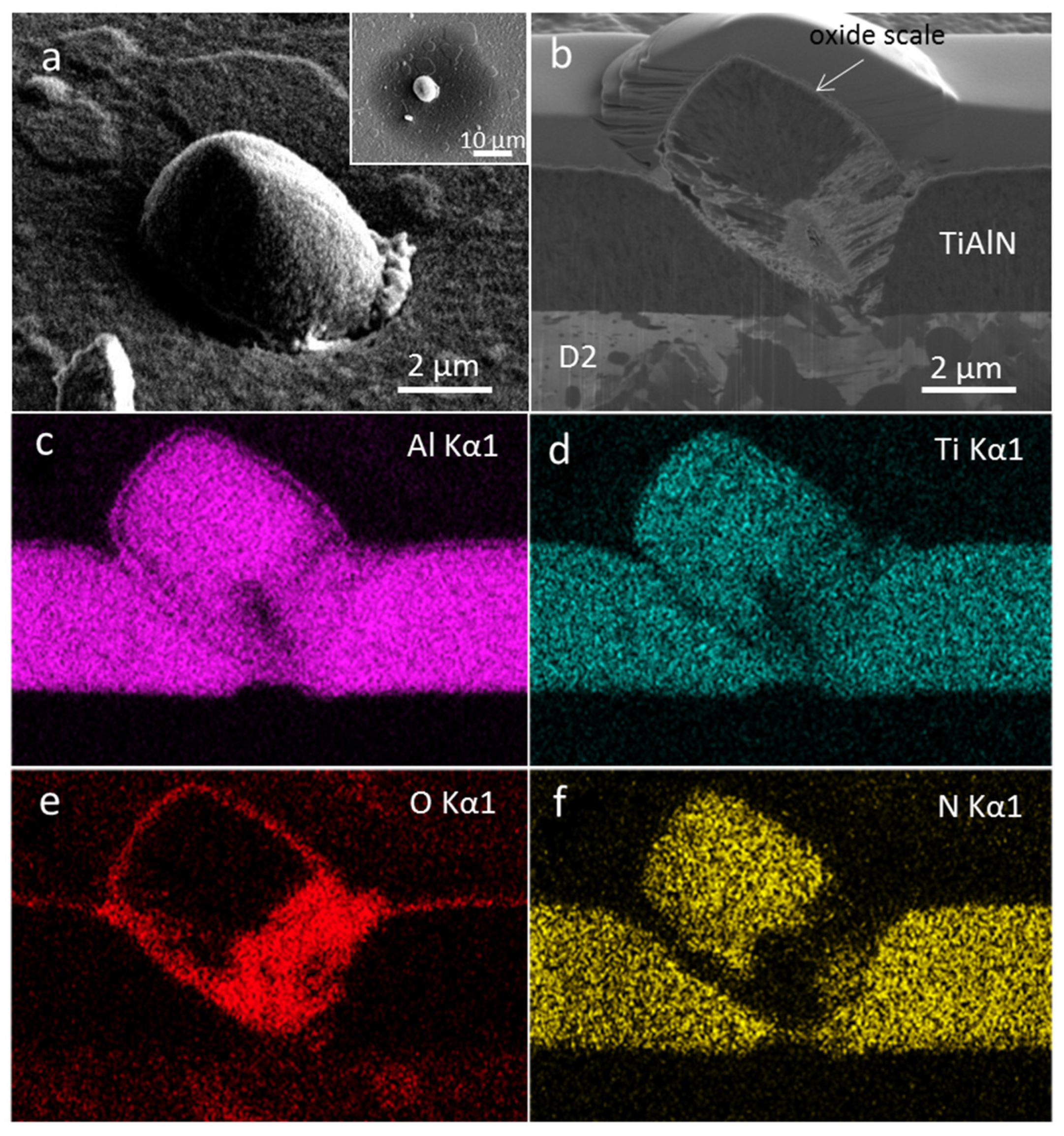
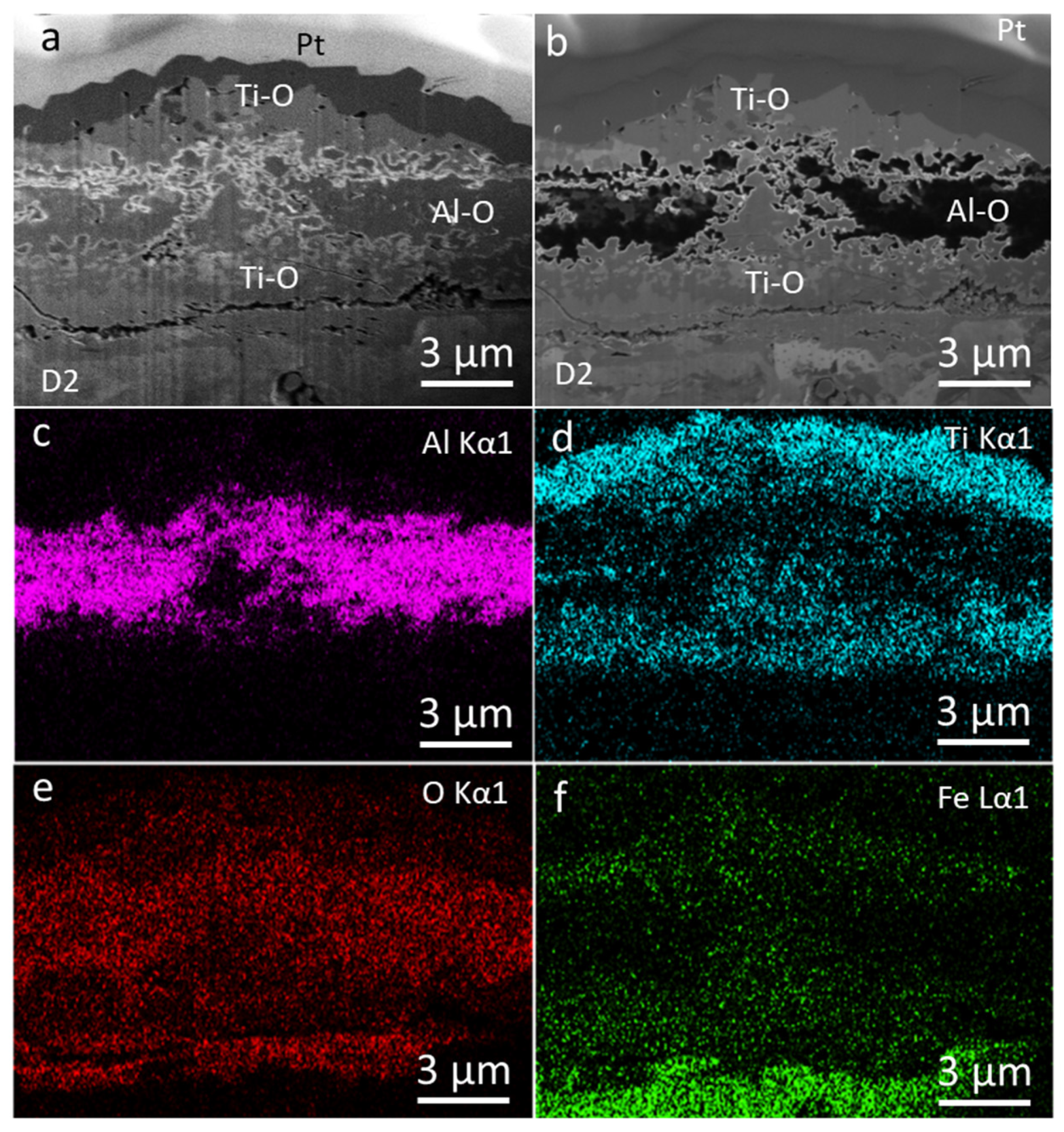
3.2. Coating Morphology and Microstructure after Oxidation at 800 °C
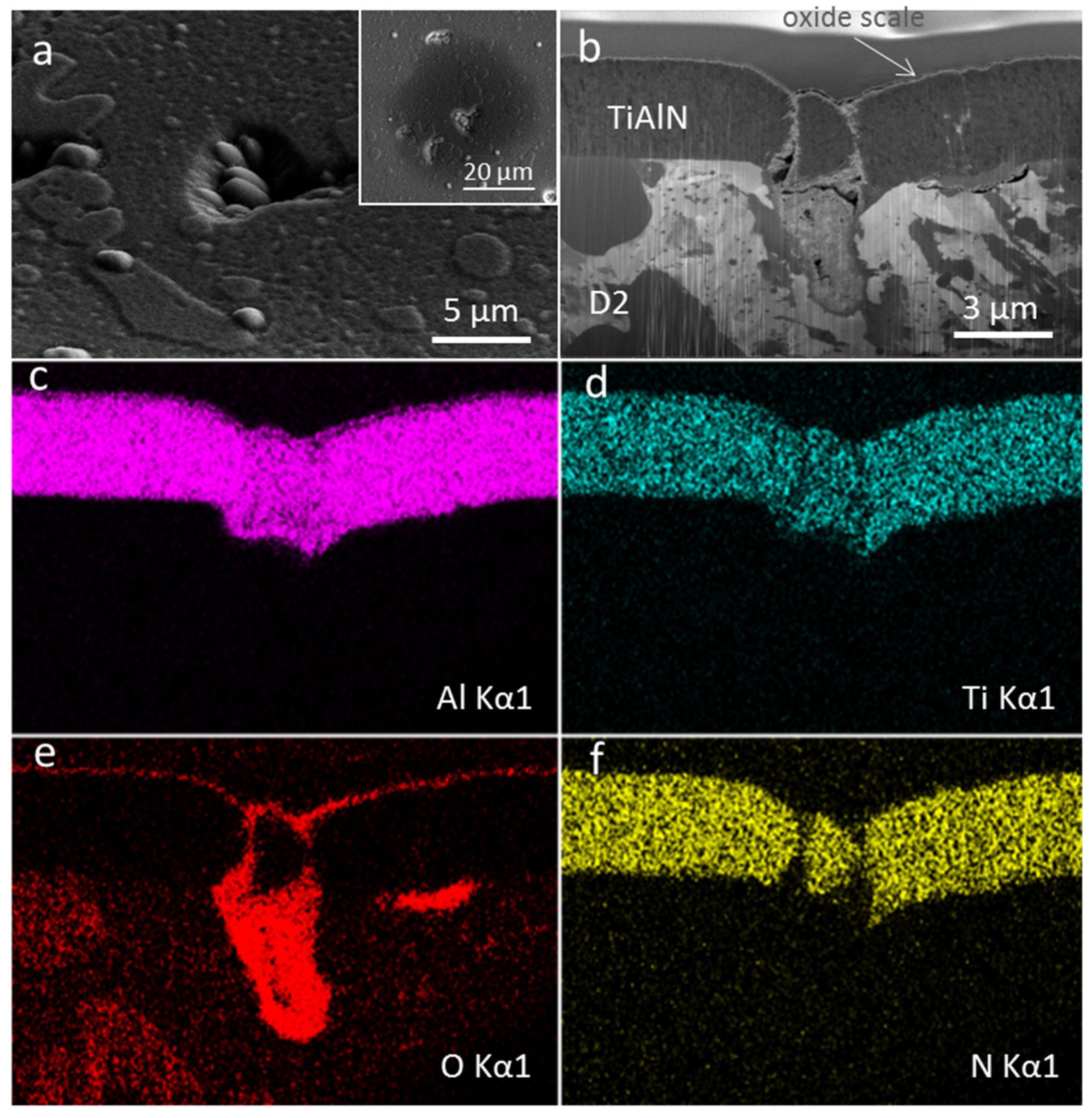
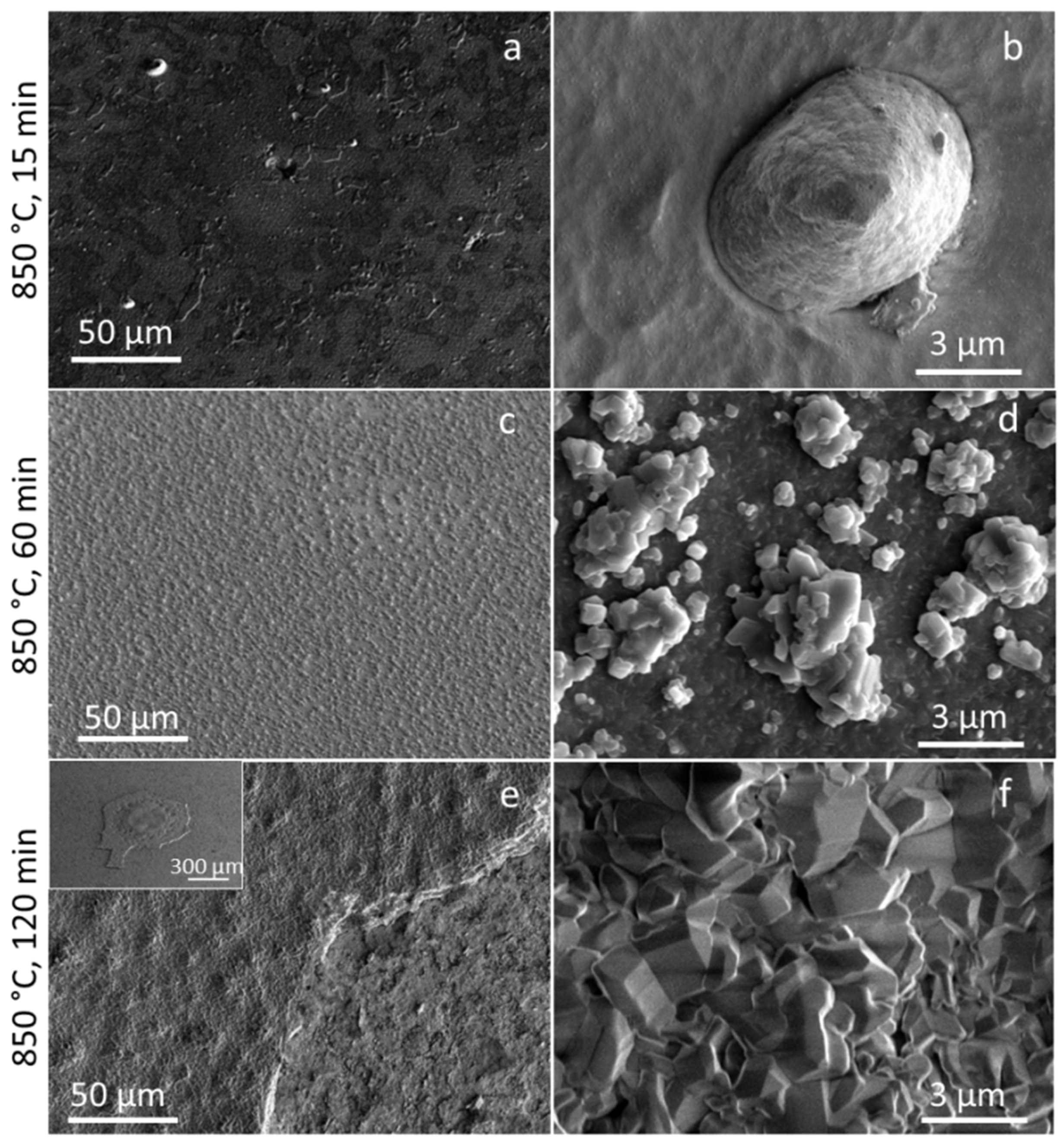
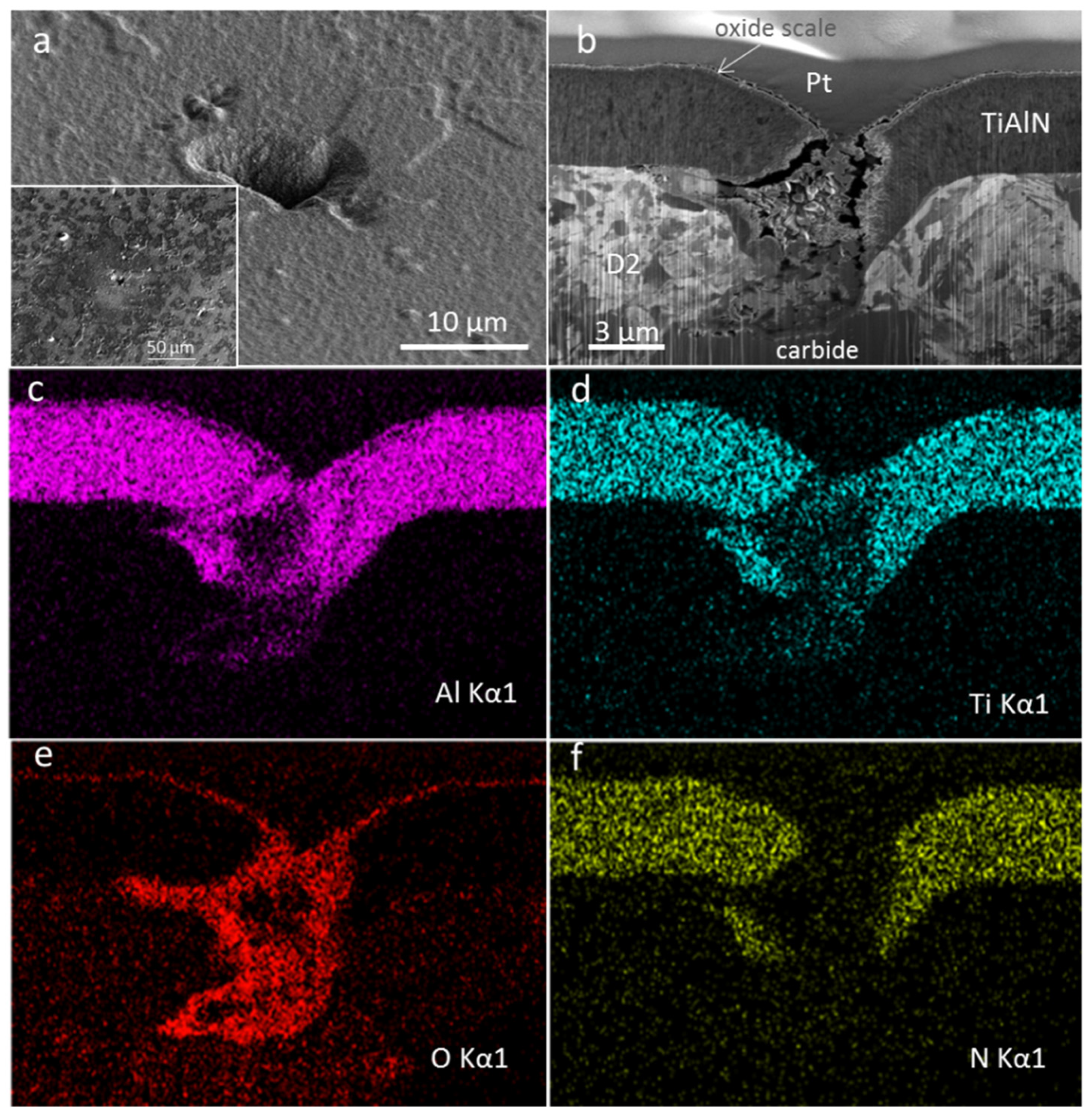
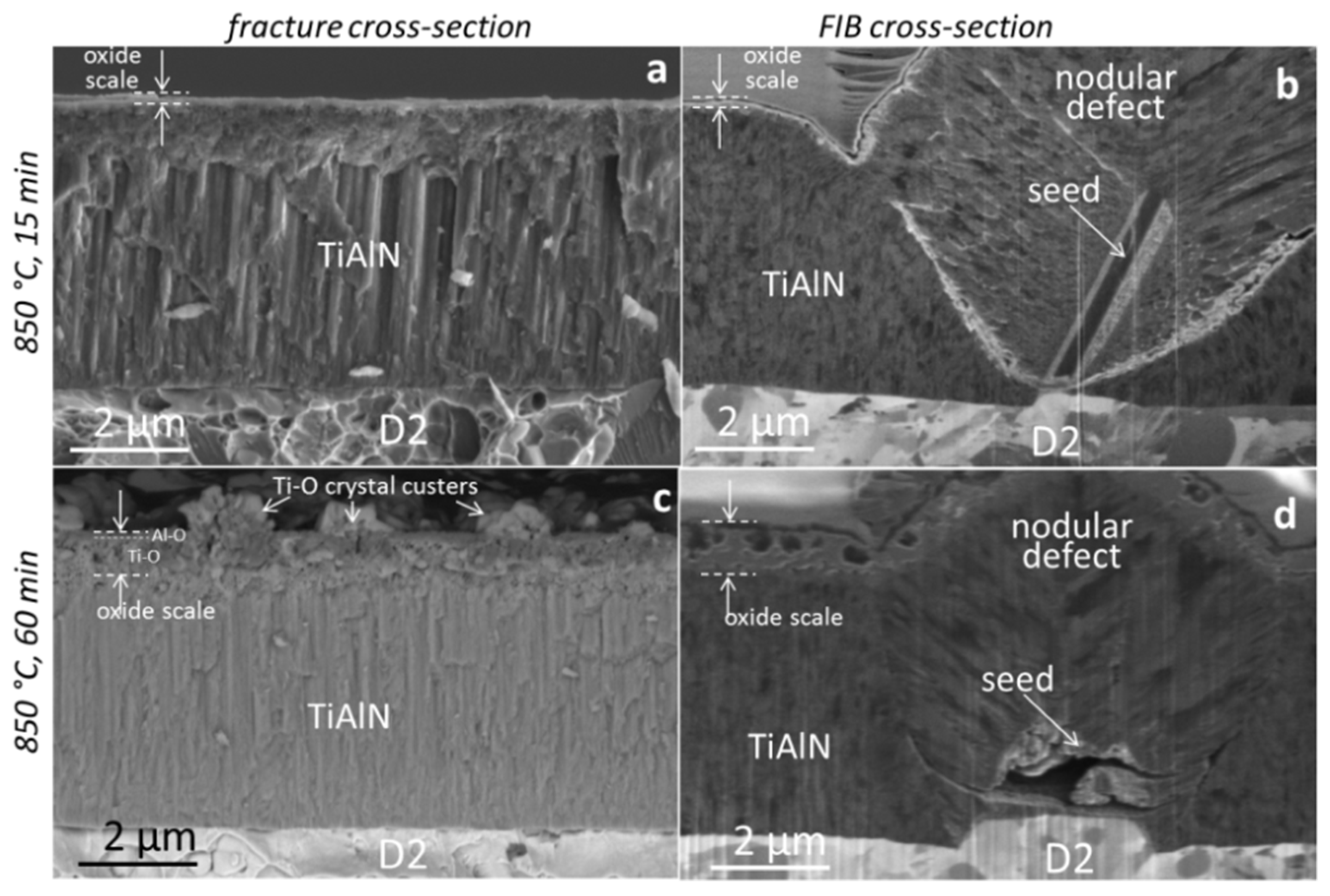
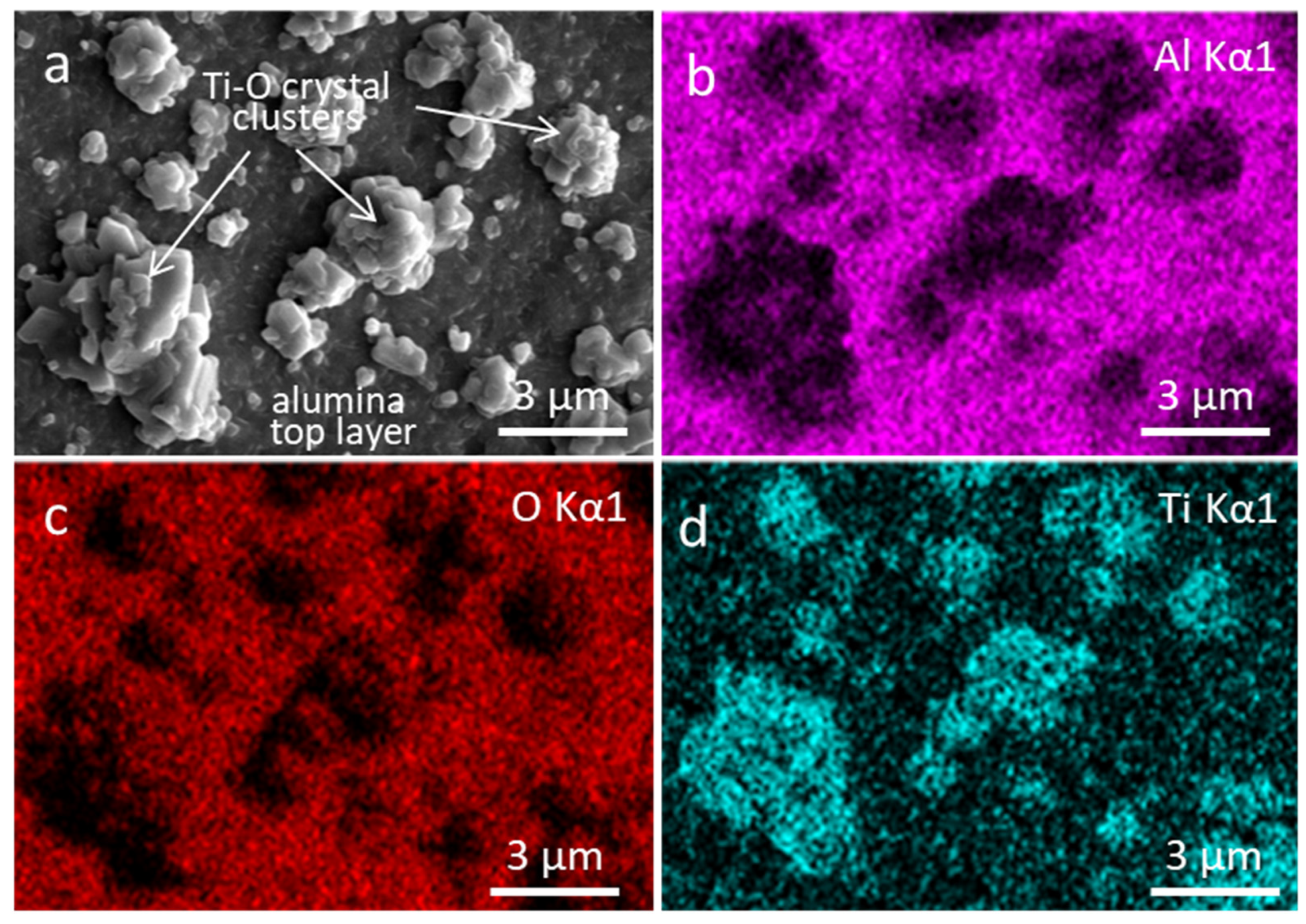
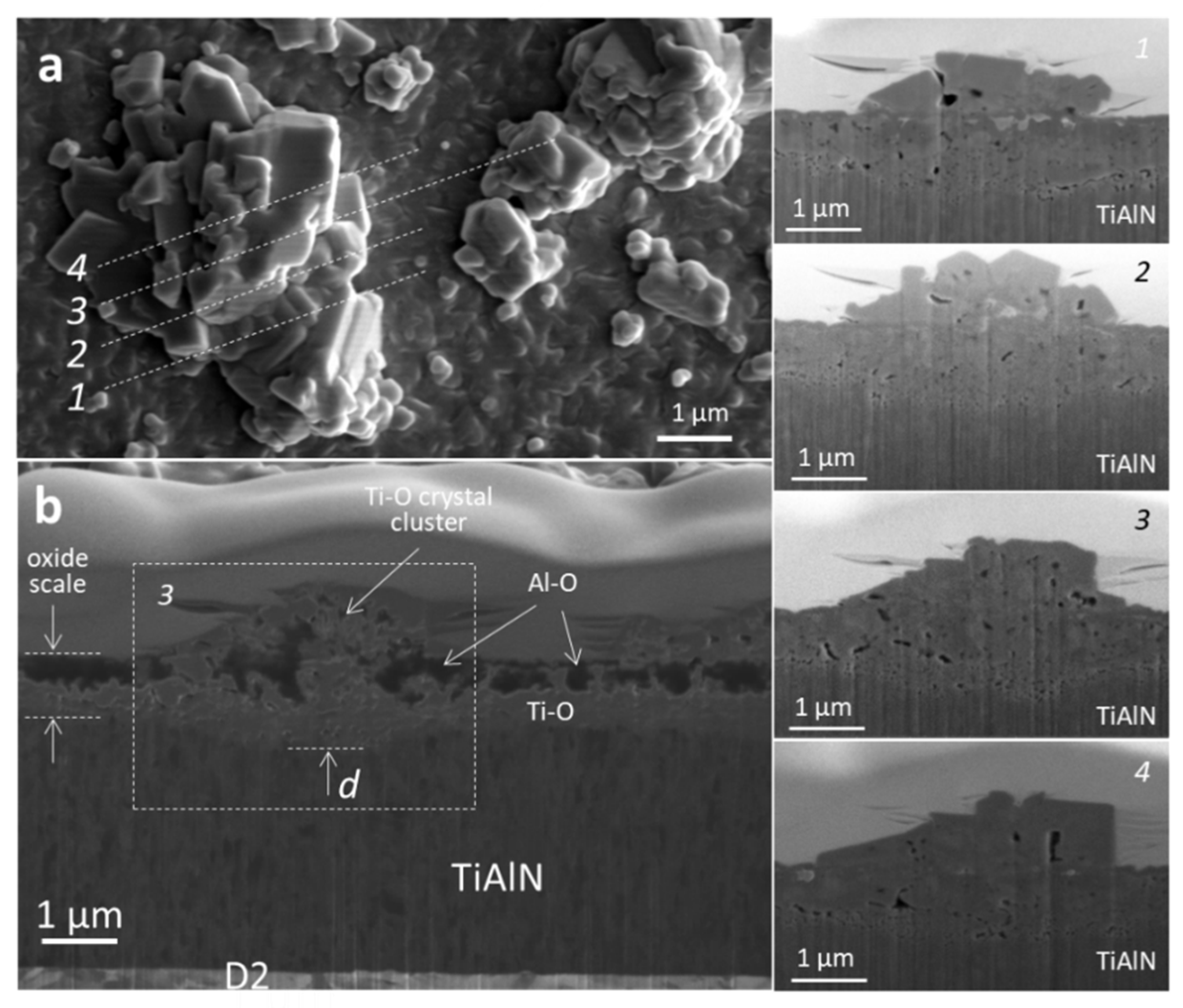
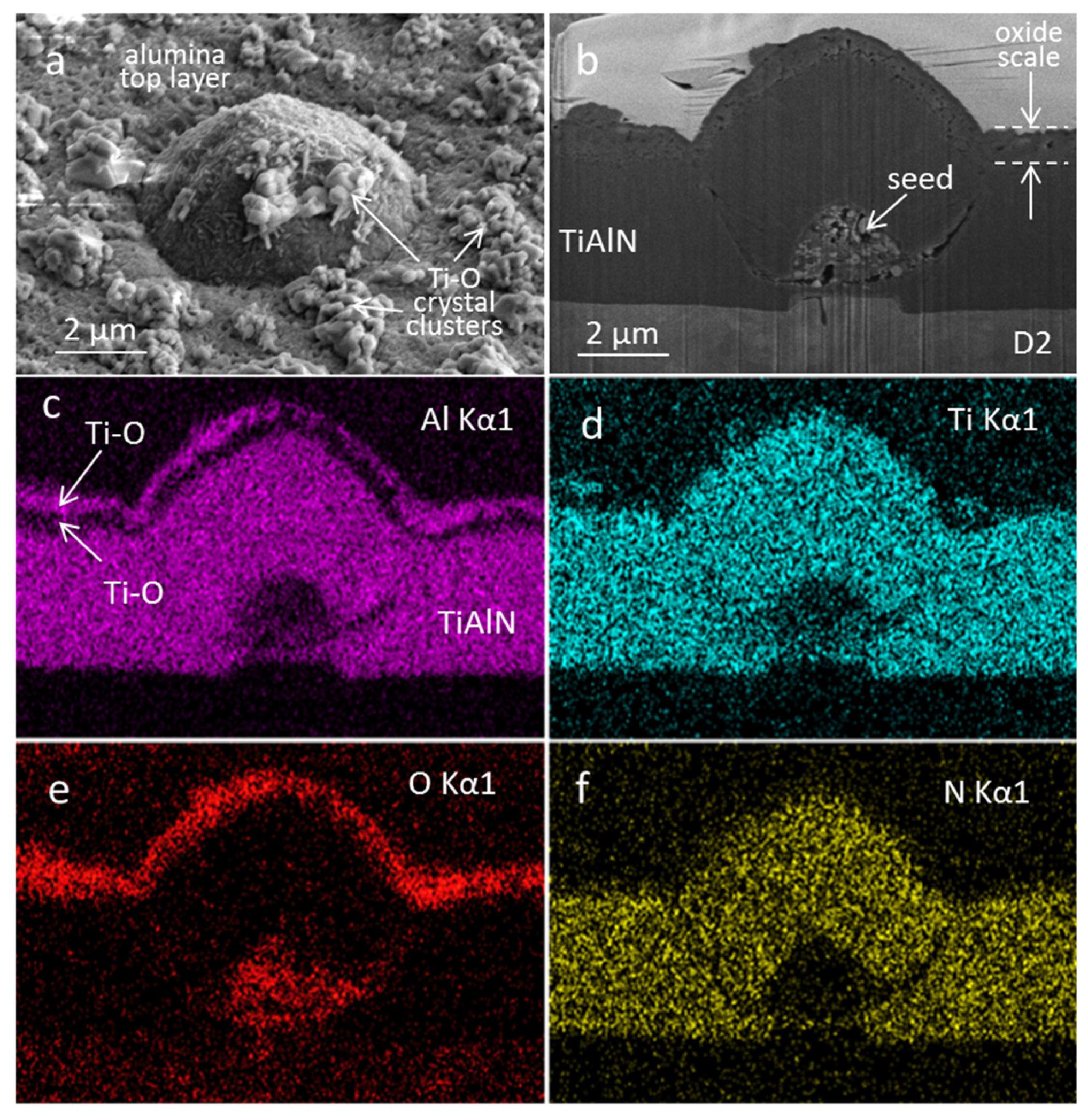
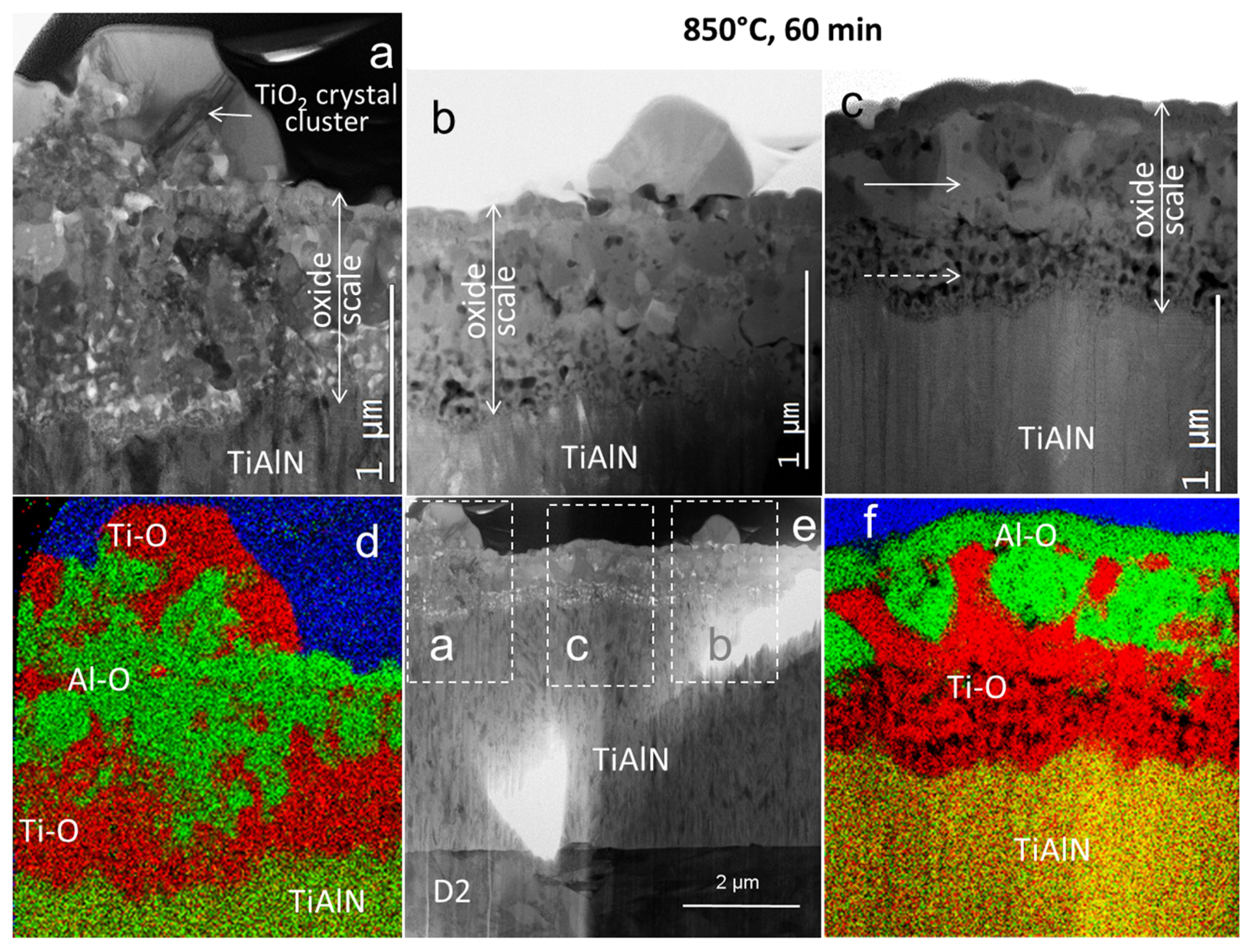
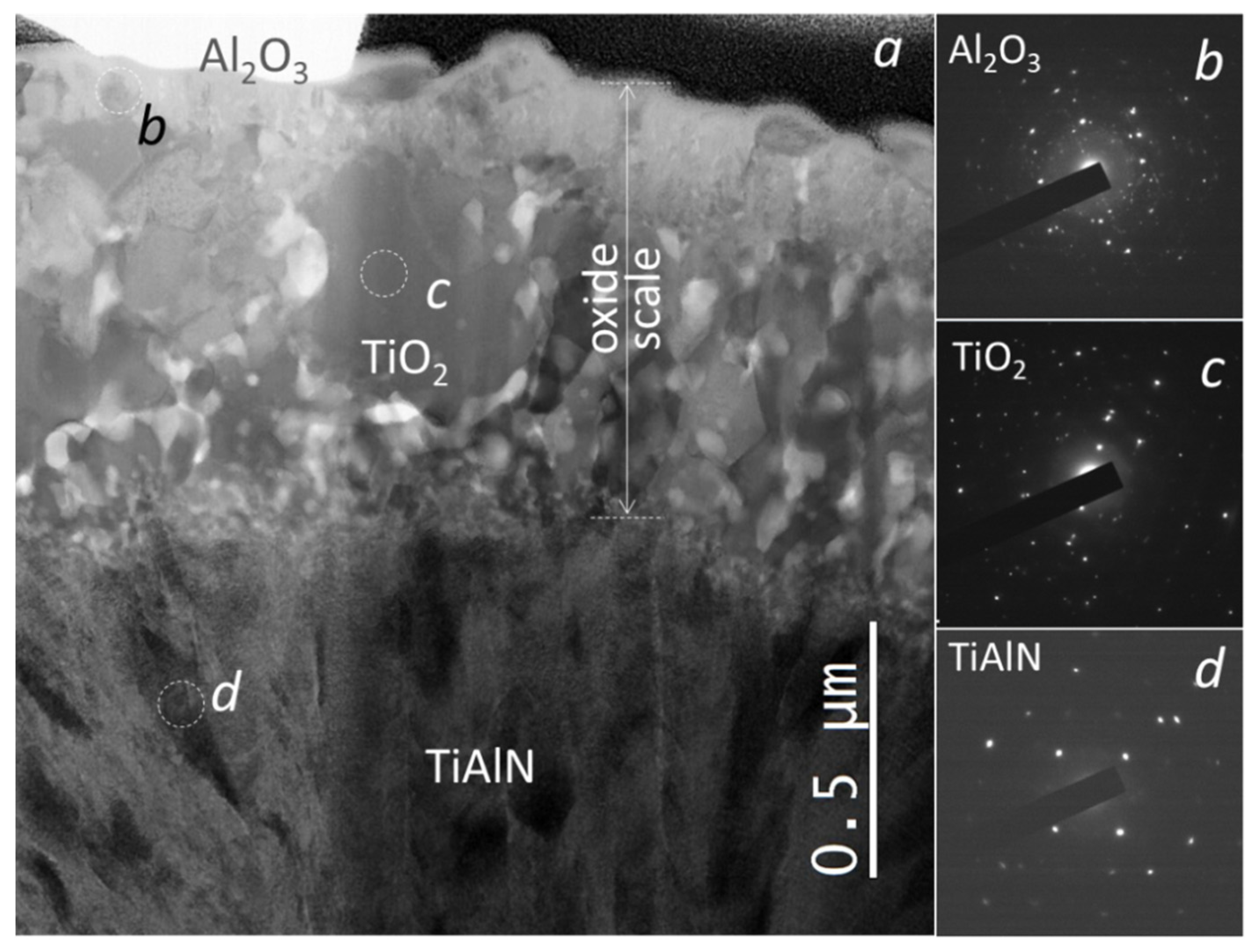
3.3. Coating Morphology and Microstructure after Oxidation at 850 °C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jindal, P.C.; Santhanam, A.T.; Schleinkofer, U.; Shuster, A.F. Performance of PVD TiN, TiCN, and TiAlN coated cemented carbide tools in turning. Int. J. Refract. Met. Hard Mater. 1999, 17, 163–170. [Google Scholar] [CrossRef]
- Chen, L.; Paulitsch, J.; Du, J.Y.; Mayrhofer, P.M. Thermal stability and oxidation resistance of Ti-Al-N coatings. Surf. Coat. Technol. 2012, 206–318, 2954–2960. [Google Scholar] [CrossRef]
- Horling, A.; Hultman, L.; Oden, M.; Sjolen, J.; Karlsson, L. Thermal stability of arc evaporated high aluminium-content TiAlN thin films. J. Vac. Sci. Technol. 2002, 20, 1815–1823. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Horling, A.; Karlsson, L.; Sjolen, J.; Larsson, T.; Mitterer, C.; Hultman, L. Self-organized nanostructures in the Ti–Al–N system. Appl. Phys. Lett. 2003, 83, 2049. [Google Scholar] [CrossRef]
- McIntyre, D.; Greene, J.E.; Håkansson, G.; Sundgren, J.E.; Münz, W.D. Oxidation of metastable single-phase polycristalline Ti0.5Al0.5N films: Kinetics and mechanisms. J. Appl. Phys. 1990, 67, 1542. [Google Scholar] [CrossRef]
- Ichimura, H.; Kawana, A. High-temperature oxidation of ion-plated TiN and TiAlN films. J. Mater. Res. 1993, 8, 1093–1100. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, L.; Ke, R.X.; Wn, Q.L.; Wang, Z.; Lu, Z.H. Thermal stability and oxidation behavior of AlTiN, AlCrN and AlCrSiWN coatings. Int. J. Refract. Met. Hard Mater. 2014, 43, 241–249. [Google Scholar] [CrossRef]
- Joshi, A.; Hu, H.S. Oxidation behavior of titanium-aluminium nitrides. Surf. Coat. Technol. 1995, 76−77, 499–507. [Google Scholar] [CrossRef]
- Lembke, M.I.; Lewis, D.B.; Münz, W.D. Localised oxidation defects in TiAlN/CrN superlattice structured hard coatings grown by cathodic arc/ unbalanced magnetron deposition on various substrate materials. Surf. Coat. Technol. 2000, 125, 263–268. [Google Scholar] [CrossRef]
- Pfeiler, M.; Zechner, J.; Penoy, M.; Michotte, C.; Mitterer, C.; Kathrein, M. Improved oxidation resistance of TiAlN coatings by doping with Si or B. Surf. Coat. Technol. 2009, 203, 3104–3110. [Google Scholar] [CrossRef]
- Pfeiler, M.; Scheu, C.; Hutter, H.; Schnöller, J.; Michotte, C.; Mitterer, C.; Kathrein, M. On the effect of Ta on improved oxidation resistance of Ti-Al-Ta-N coatings. J. Vac. Sci. Technol. 2009, 27, 554. [Google Scholar] [CrossRef]
- Riedl, H.; Holec, D.; Rachbauer, R.; Polcik, P.; Hollerweger, R.; Paulitsch, J.; Mayrhofer, P.H. Phase stability, mechanical properties and thermal stability of Y alloyed Ti-Al-N coatings. Surf. Coat. Technol. 2013, 235, 174. [Google Scholar] [CrossRef]
- Asanuma, H.; Klimashin, F.F.; Polcik, P.; Kolozsvári, S.; Riedl, H.; Mayrhofer, P.H. Impact of lanthanum and boron on the growth, thermomechanical properties and oxidation resistance of Ti–Al–N thin films. Thin Solid Film. 2019, 688, 137239. [Google Scholar] [CrossRef]
- Hollerweger, R.; Riedl, H.; Paulitsch, J.; Arndt, M.; Rachbauer, R.; Polcik, P. Origin of high temperature oxidation resistance of Ti–Al–Ta–N coatings. Surf. Coat. Technol. 2014, 257, 78–86. [Google Scholar] [CrossRef]
- Wang, Q.M.; Garkas, W.; Renteria, A.F.; Leyens, C.; Kim, K.H. Oxidation Behaviour of Ti2AlN Films Composed Mainly of Nanolaminated MAX Phase. J. Nanosci. Nanotechnol. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Satoh, H. Phase formation and characterization of hard coatings in the Ti-Al-N system prepared by the cathodic arc ion plating method. Thin Solid Film. 1991, 195, 99–110. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, Z.C.; Nah, J.W.; Lee, J. High temperature oxidation of (Ti1-X AlX) N coatings made by plasma enhanced chemical vapor deposition. J. Vac. Sci. Technol. 1999, 17, 133. [Google Scholar] [CrossRef]
- Vaz, F.; Rebouta, L.; Andritschky, M.; da Silva, M.F.; Soares, J.C. Oxidation resistance of (Ti,Al,Si)N coatings in air. Surf. Coat. Technol. 1998, 98, 912–917. [Google Scholar] [CrossRef]
- Peng, J.; Su, D.; Wang, C. Combined effect of aluminium content and layer structure on the oxidation performance of Ti1-xAlxN based coatings. J. Mater. Sci. Technol. 2014, 30, 803–807. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Ehiasarian, A.P. Six strategies to produce application tailored nanoscale multilayer structured PVD coatings by conventional and High Power Impulse Magnetron Sputtering (HIPIMS). Thin Solid Film 2019, 688, 137409. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Xiong, X.; Chang, K.K.; Wu, M.J. Improved properties of Ti-Al-N coating by multilayer structure. Int. J. Refract. Met. Hard Mater. 2011, 29, 681–685. [Google Scholar] [CrossRef]
- Hollerweger, R.; Riedl, H.; Arndt, M.; Rachbauer, R.; Kolozsvari, S.; Primig, S.; Mayrhofer, P.H. Guidelines for increasing the oxidation resistance of Ti-Al-N based coatings. Thin Solid Film. 2019, 688, 137290. [Google Scholar] [CrossRef]
- Donohue, L.A.; Smith, I.J.; Münz, W.D.; Petrov, I.; Greene, J.E. Microstructure and oxidation-resistance of TiAlCrYN layers grown by combined steered-arc/unbalanced-magnetron-sputter deposition. Surf. Coat. Technol. 1997, 95–96, 226–231. [Google Scholar] [CrossRef]
- Vannemann, V.; Stock, H.R.; Kohlscheen, J.; Rambadt, S.; Erkens, G. Oxidation resistance of titanium-aluminium-silicon nitride coatings. Surf. Coat. Technol. 2003, 174–175, 408–415. [Google Scholar] [CrossRef]
- Moser, M.; Kiener, D.; Scheu, C.; Mayrhofer, P.H. Influence of Yttrium on the thermal stability of Ti-Al-N thin films. Materials 2010, 3, 1573–1592. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Lewis, D.; Luo, Q.; Münz, W.-D.; Mayrhofer, P.; Mitterer, C.; Zhou, Z.; Rainforth, W. TiAlN based nanoscale multilayer coatings designed to adapt their tribological properties at elevated temperaures. Thin Solid Film. 2005, 485, 160–168. [Google Scholar] [CrossRef]
- Wu, G.; Ma, S.; Xu, K.; Chu, P.K. Oxidation resistance of qintuple Ti-Al-Si-C-N coatings and associated mechanism. J. Vac. Sci. Technol. 2012, 30, 041508. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, M.; Ni, W.; Liu, Y. High temperature oxidation behavior of TiAlN coating and TiAlSiN coating. Vacuum 2012, 86, 1795–1799. [Google Scholar] [CrossRef]
- Polcar, T.; Cavaleiro, A. High temperature behavior of nanolayered CrAlTiN coating: Thermal stability, oxidation and tribological properties. Surf. Coat. Technol. 2014, 257, 70–77. [Google Scholar] [CrossRef]
- Xu, Y.X.; Riedl, H.; Holec, D.; Chen, L.; Du, Y.; Mayrhofer, P.H. Thermal stability and oxidation resistance of sputtered TiAlCrN hard coatings. Surf. Coat. Technol. 2017, 324, 48–56. [Google Scholar] [CrossRef]
- Sui, X.; Li, G.; Zhou, H.; Zhang, S.; Yu, Y.; Wang, Q.; Hao, J. Evolution behavior of oxide scales of TiAlCrN coatings at high temperature. Surf. Coat. Technol. 2019, 360, 133–139. [Google Scholar] [CrossRef]
- Glatz, S.A.; Hollerweger, R.; Polcik, P.; Rachbauer, R.; Paulitsch, J.; Mayrhofer, P.H. Thermal stability and mechanical properties of arc evaporated Ti–Al–Zr–N hard coatings. Surf. Coat. Technol. 2015, 266, 1–9. [Google Scholar] [CrossRef]
- Chen, L.; He, L.; Xu, Y.; Zhou, L.; Pei, F.; Du, Y. Influence of ZrN on oxidation resistance of Ti–Al–N coating. Surf. Coat. Technol. 2014, 244, 87–91. [Google Scholar] [CrossRef]
- Abadias, G.; Saladukhin, I.A.; Uglov, V.V.; Zlotski, S.V.; Eyidi, D. Thermal stability and oxidation behavior of quaternary TiZrAlN magnetron sputtered thin films: Influence of the pristine microstructure. Surf. Coat. Technol. 2013, 237, 187–195. [Google Scholar] [CrossRef]
- Koller, C.M.; Hollerweger, R.; Sabitzer, C.; Rachbauer, R.; Kolozsvári, S.; Paulitsch, J.; Mayrhofer, P.H. Thermal stability and oxidation resistance of arc evaporated TiAlN, TaAlN, TiAlTaN, and TiAlN/TaAlN coatings. Surf. Coat. Technol. 2014, 259, 599–607. [Google Scholar] [CrossRef]
- Rachbauer, R.; Holec, D.; Mayrhofer, P.M. Increased thermal stability of Ti-Al-N thin films by Ta alloying. Surf. Coat. Technol. 2012, 211, 98–103. [Google Scholar] [CrossRef]
- Khetan, V.; Valle, N.; Duday, D.; Michotte, C.; Ogletree, M.P.D.; Choquet, P. Influence of temperature on oxidation mechanisms of fiber-textured AlTiTaN coatings. Appl. Mat. Interfaces 2014, 6, 4115–4125. [Google Scholar] [CrossRef]
- Lembke, M.I. Oxidation Behaviour of TiAlN Based Nanolayered Hard Coatings. Available online: http://shura.shu.ac.uk/19951/ (accessed on 22 January 2021).
- Fernandes, F.; Morgiel, J.; Polcar, T.; Cavaleiro, A. Oxidation and diffusion processes during annealing of TiSi(V)N films. Surf. Coat. Technol. 2015, 275, 120–126. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Kovač, J.; Gselman, P.; Bončina, T.; Paskvale, S.; Čekada, M.; Kek-Merl, D.; Panjan, M. Oxidation resistance of CrN/(Cr,V)N hard coatings deposited by DC magnetron sputtering. Thin Solid Film. 2015, 591, 323–329. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Kovač, J.; Čekada, M.; Panjan, M. Oxidation processes in vanadium-based single-layer and nanolayer hard coatings. Vacuum 2017, 138, 230–237. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, P.; Boncina, T.; Kek Merl, D. Influence of Growth Defects on the Corrosion Resistance of Sputter-Deposited TiAlN Hard Coatings. Coatings 2019, 9, 511. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, P. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Jehn, H.A. Improvement of the corrosion resistance of PVD hard coating–substrate system. Surf. Coat. Technol. 2000, 125, 212–217. [Google Scholar] [CrossRef]
- Park, H.S.; Kappl, H.; Lee, K.H.; Lee, J.J.; Jehn, H.A.; Fenker, M. Structure modification of magnetron-sputtered CrN coatings by intermediate plasma etching steps. Surf. Coat. Technol. 2000, 133–134, 176–180. [Google Scholar] [CrossRef]
- Mattox, D.M. Atomistic Film Growth and Resulting Film Properties. Available online: http://www.htskorea.com/product/ambios/stress.pdf (accessed on 22 January 2021).
- Münz, W.D. Titanium aluminum nitride films: A new alternative to TiN coatings. J. Vac. Sci. Technol. A 1986, 4, 2717–2725. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panjan, P.; Drnovšek, A.; Dražić, G. Influence of Growth Defects on the Oxidation Resistance of Sputter-Deposited TiAlN Hard Coatings. Coatings 2021, 11, 123. https://doi.org/10.3390/coatings11020123
Panjan P, Drnovšek A, Dražić G. Influence of Growth Defects on the Oxidation Resistance of Sputter-Deposited TiAlN Hard Coatings. Coatings. 2021; 11(2):123. https://doi.org/10.3390/coatings11020123
Chicago/Turabian StylePanjan, Peter, Aljaž Drnovšek, and Goran Dražić. 2021. "Influence of Growth Defects on the Oxidation Resistance of Sputter-Deposited TiAlN Hard Coatings" Coatings 11, no. 2: 123. https://doi.org/10.3390/coatings11020123
APA StylePanjan, P., Drnovšek, A., & Dražić, G. (2021). Influence of Growth Defects on the Oxidation Resistance of Sputter-Deposited TiAlN Hard Coatings. Coatings, 11(2), 123. https://doi.org/10.3390/coatings11020123







