Nanometals-Containing Polymeric Membranes for Purification Processes
Abstract
1. Introduction
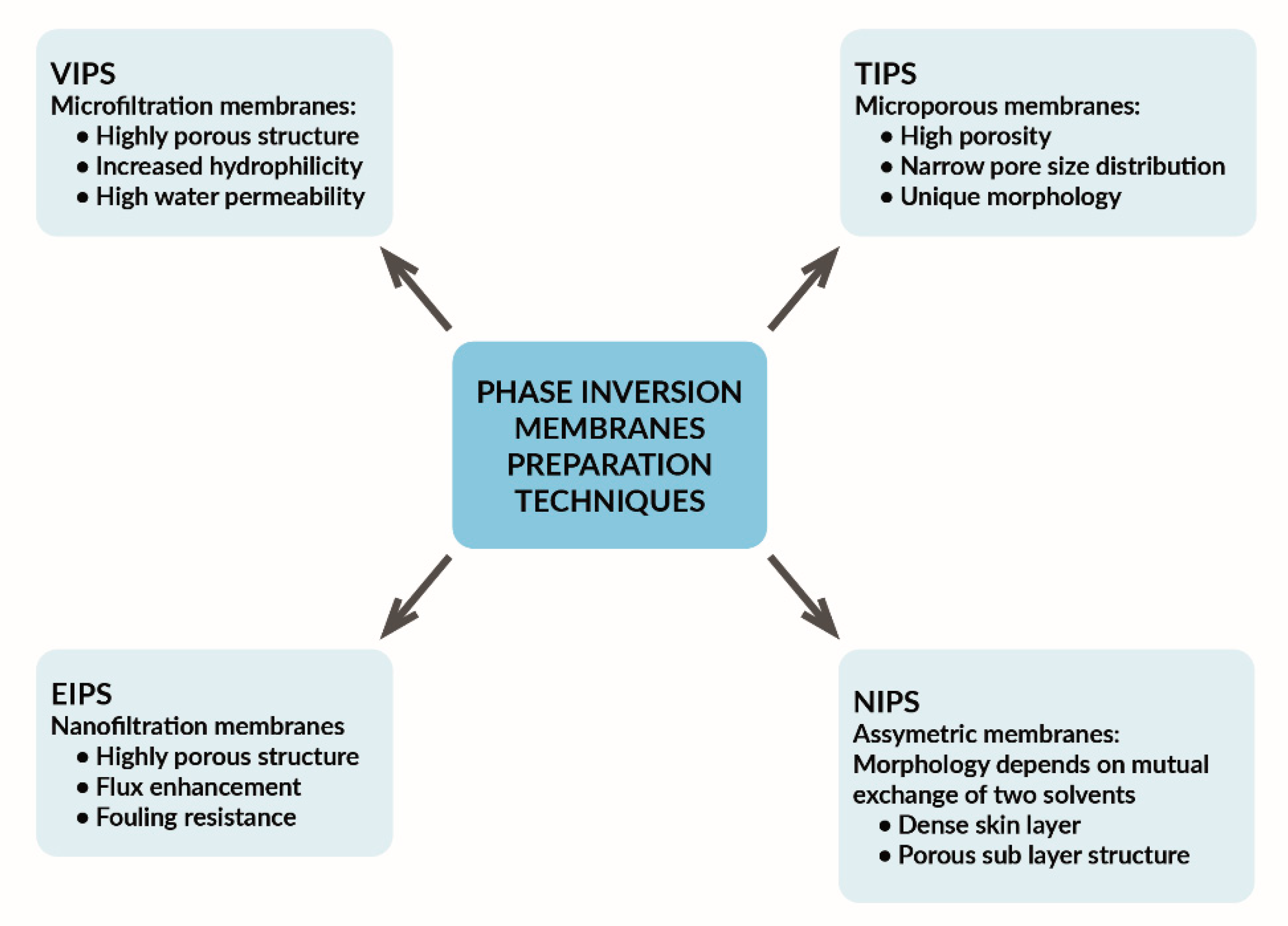
2. Membranes—Structure and Properties
- -
- -
- -
- Nanoparticles are dispersed in the solvent and stirred, and the polymer is dissolved in a solvent separately; the nanoparticle suspension is then added to the homogeneous polymeric solution [60].
3. Membranes Containing Nanoparticles of Silver
4. Membranes Containing Nanoparticles of Silica
5. Membranes Containing Nanoparticles of Aluminum
6. Membranes Containing Nanoparticles of Titanium
7. Membranes Containing Nanoparticles of Iron
8. Membranes Containing Nanoparticles of Other Nanoparticles of Metals
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALD | Atomic layer deposition |
| BSA | Bovine serum albumin |
| CA | Cellulose acetate |
| CPTES | 3-cyanopropyltriethoxysilane |
| CT | Carbon tetrachloride |
| Cu/TNTs | Titanate nanotubes modified with copper |
| EDTA | Ethylenediaminetetraacetic acid |
| EIPS | Evaporation-induced phase inversion |
| GO | Graphene oxide |
| HA | Humic acid |
| HAF | Humic acid flux |
| HAR | Humic acid rejection |
| HF | Hollow fiber |
| IP | Interfacial polymerization |
| ITF | Iron-tannin framework |
| MBR | Membrane bioreactor |
| MF | Microfiltration |
| MMMs | Mixed matrix membranes |
| MMNM | Nanocomposite mixed matrix membrane |
| MPD | m-phenylenediamine |
| NF | Nanofiltration |
| NIPS | Non-solvent-induced phase inversion |
| NOM | Natural organic matter |
| OM | Osmosis membranes |
| OSN | Organic solvent nanofiltration |
| PA | Polyamide |
| PAA | Poly(acrylic acid) |
| PA-CCTS | Polyamide-carboxylated chitosan composite |
| PAN | Polyacrylonitrile |
| PANI | Polyaniline |
| PBS | Phosphate-buffered saline |
| PCE | Tetrachloroethylene |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PEMEC | Proton exchange membrane fuel cells |
| PEO-PPO-PEO | Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) |
| PES | Polyethersulfone |
| PI | Polyimide |
| Polyamide 6,6 | Poly(hexamethylene adipamide) |
| POSS | Polysilsesquioxanes |
| PP | Polypropylene |
| PPMMs | Polypropylene macroporous membranes |
| PPSU | Polyphenylsulfone |
| PSF | Polysulfone |
| PTEE | Polytetrafluoroethylene |
| PVB | Polyvinylbutyral |
| PVC | Polyvinyl chloride |
| PVDF | Polyvinylidene fluoride |
| PVP | Polyvinylpyrrolidone |
| Pw | Water permeability |
| PWF | Pure water flux |
| RO | Reverse osmosis |
| ROS | Reactive oxygen species |
| RSM | Response surface methodology |
| SIS | Sequential infiltration synthesis |
| SMPR | Submerged membrane photoreactor |
| SPES | Sulfonated polyethersulfone |
| SRNF | Solvent-resistant nanofiltration |
| TA | Tannic acid |
| TCE | Trichloroethylene |
| TFN | Thin-layer nanocomposite |
| TFNC | Thin-film nanocomposite |
| TIPS | Thermally induced phase inversion |
| TMC | Trimesoylchloride |
| UF | Ultrafiltration |
| VIPS | Vapor-induced phase inversion |
| ZVI | Zero valent iron nanoparticles |
References
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, B.; Curcio, E.; Drioli, E. Process intensification in the textile industry: The role of membrane technology. J. Environ. Manag. 2004, 73, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.; Wahab, A. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 825910, 24. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Fíla, V.; Denis, P.C.; Ruby-Figueroa, R. Current Role of Membrane Technology: From the Treatment of Agro-Industrial by-Products up to the Valorization of Valuable Compounds. Waste Biomass Valorization 2018, 9, 513–529. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Lejon, L.; Vandecasteele, C. Reuse, treatment, and discharge of the concentrate of pressure-driven membrane processes. Environ. Sci. Technol. 2003, 37, 3733–3738. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Fane, A.G.; Tang, C.Y.; Wang, R. Membrane technology for water: Microfiltration, ultrafiltration, nanofiltration, and reverse osmosis. In Treatise on Water Science; Peter, W., Ed.; Elsevier: Oxford, UK, 2011; pp. 301–335. [Google Scholar]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- McBean, E.A. Evaluation of a bicycle-powered filtration system for removing ‘clumped’ coliform bacteria as a low-tech option for water treatment. Desalination 2009, 248, 138–143. [Google Scholar] [CrossRef]
- Archer, A.; Elmore, A.C. Use of ceramic pot filters for drinking water disinfection in Guatemal. In Proceedings of the World Environmental and Water Resources Congress 2010, Providence, RI, USA, 16–20 May 2010; pp. 545–558. [Google Scholar] [CrossRef]
- Boisson, S.; Kiyombo, M.; Sthreshley, L.; Tumba, S.; Makambo, J.; Clasen, T. Field Assessment of a Novel Household-Based Water Filtration Device: A Randomised, Placebo-Controlled Trial in the Democratic Republic of Congo. PLoS ONE 2010, 5, e12613. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Tagami, K.; Uchida, S. Can we remove iodine-131 from tap water in Japan by boiling?—Experimental testing in response to the Fukushima Daiichi Nuclear Power Plant accident. Chemosphere 2011, 84, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- HydroPack: The Simplest Way to Prepare Water for Emergencies. Available online: https://futuretechme.com/downloads/forward-osmosis/HydroPack.pdf (accessed on 1 December 2020).
- Gálvez, J.B.; García-Rodríguez, L.; Martín-Mateosc, I. Seawater desalination by an innovative solar-powered membrane distillation system: The MEDESOL project. Desalination 2009, 246, 567–576. [Google Scholar] [CrossRef]
- Madaeni, S.S. The application of membrane technology for water disinfection. Water Res. 1999, 33, 301–308. [Google Scholar] [CrossRef]
- Ejraei, A.; Aroon, M.A.; Saravani, A.Z. Wastewater treatment using a hybrid system combining adsorption, photocatalytic degradation and membrane filtration processes. J. Water Process Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly(Vinylidene Fluoride). Polymers (Basel) 2019, 11, 1160. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Aburabie, J.; Villalobos, L.F.; Peinemann, K.V. Composite membrane formation by combination of reaction-induced and nonsolvent-induced phase separation. Macromol. Mater. Eng. 2017, 302, 1700131. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Figoli, A. The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation. Membranes 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Rajabzadeh, S.; Ma, W.; Zhou, Z.; Kakihana, Y.; Ohmukai, Y.; Miki, J.; Matsuyama, H. Preparation of PVDF/poly (tetrafluoroethylene-co-vinyl alcohol) blend membranes with antifouling propensities via nonsolvent induced phase separation method. J. Appl. Polym. Sci. 2016, 133, 43780–43789. [Google Scholar] [CrossRef]
- Hu, N.; Xiao, T.; Cai, X.; Ding, L.; Fu, Y.; Yang, X. Preparation and Characterization of Hydrophilically Modified PVDF Membranes by a Novel Nonsolvent Thermally Induced Phase Separation Method. Membranes (Basel) 2016, 6, 47. [Google Scholar] [CrossRef]
- Liang, H.Q.; Wu, Q.Y.; Wan, L.S.; Huang, X.J.; Xu, Z.K. Polar polymer membranes via thermally induced phase separation using a universal crystallizable diluent. J. Memb. Sci. 2013, 446, 482–491. [Google Scholar] [CrossRef]
- Othman, N.; Harruddin, N.; Idris, A.; Ooi, Z.Y.; Fatiha, N.; Sulaiman, R.N.R. Fabrication of polypropylene membrane via thermally induced phase separation as a support matrix of tridodecylamine supported liquid membrane for Red 3BS dye removal. Desalination Water Treat. 2016, 57, 12287–12301. [Google Scholar] [CrossRef]
- Kim, J.F. Recent Progress on Improving the Sustainability of Membrane Fabrication. J. Membr. Sci. Res. 2020, 6, 241–250. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.-P.; Bouyer, D.; Drioli, E.; Hassan Kiadeh, N.T. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2019, 597, 117601. [Google Scholar] [CrossRef]
- Hana, N.; Abu, H.; Tan, W.L. Natural Composite Membranes for Water Remediation: Toward a Sustainable Tomorrow. In Renewable Energy and Sustainable Technologies for Building and Environmental Applications; Ahmad, M.I., Ismail, M., Riffat, S., Eds.; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 25–49. [Google Scholar]
- Nackaerts, R. Are Membranes Implemented with Nanoparticles Able to Provide a Breakthrough in Water Purification? Master’s Dissertation, University of Johannesburg, Johannesburg, South Africa, 6 June 2014. [Google Scholar]
- Flemming, H.C. Reverse osmosis membrane biofouling. Exp. Fluid Sci. 1997, 14, 382–391. [Google Scholar] [CrossRef]
- Subramani, A.; Hoek, E.M.V. Direct observation of initial microbial deposition onto reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2008, 319, 111–125. [Google Scholar] [CrossRef]
- Boussu, K.; Belpaire, A.; Volodin, A.; van Haesendonck, C.; van der Meeren, P.; Vandecasteele, C.; van der Bruggen, B. Influence of membrane and colloid characteristics on fouling of nanofiltration membranes. J. Membr. Sci. 2007, 289, 220–230. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Kim, J.; van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Zheng, Y.X.; Zeng, H.M.; Walter, R.; Friedrich, K. Structure–property relationships of irradiation grafted nano-inorganic particle filled polypropylene composites. Polymer 2001, 42, 167–183. [Google Scholar] [CrossRef]
- Ahmadizadegan, H.; Esmaielzadeh, D.; Ranjbar, M.; Marzban, Z. Synthesis and characterization of polyester bionanocomposite membrane with ultrasonic irradiation process for gas permeation and antibacterial activity. Ultrason. Sonochem. 2018, 41, 538–550. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Zhang, Y.; Liu, J. Porous Graphene Nanosheets Functionalized Thin Film Nanocomposite Membrane Prepared by Interfacial Polymerization for CO2/N2 Separation. J. Membr. Sci. 2017, 543, 58–68. [Google Scholar] [CrossRef]
- Jiang, C.; Markutsya, S.; Pikus, Y.; Tsukruk, V.V. Freely suspended nanocomposite membranes as highly sensitive sensors. Nat. Mater. 2004, 3, 721–728. [Google Scholar] [CrossRef]
- Jalani, N.H.; Dunn, K.; Datta, R. Synthesis and characterization of Nafion®-MO2 (M = Zr, Si, Ti) nanocomposite membranes for higher temperature PEM fuel cells. Electrochim. Acta 2005, 51, 553–560. [Google Scholar] [CrossRef]
- Boaretti, C.; Pasquini, L.; Sood, R.; Giancola, S.; Donnadio, A.; Roso, M.; Modesti, M.; Cavaliere, S. Mechanically stable nanofibrous sPEEK/Aquivion® composite membranes for fuel cell applications. J. Membr. Sci. 2017, 545, 66–74. [Google Scholar] [CrossRef]
- Chen, Z.; Holmberg, B.; Li, W.; Wang, X.; Deng, W.; Munoz, R.; Yan, Y. Nafion/zeolite nanocomposite membrane by in situ crystallization for a direct methanol fuel cell. Chem. Mater. 2006, 18, 5669–5675. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, H.P.; Zhang, P.; Li, G.C.; Wu, Y.P.; Zhou, X.D. Effects of the porous structure on conductivity of nanocomposite polymer electrolyte for lithium ion batteries. J. Membr. Sci. 2008, 322, 416–422. [Google Scholar] [CrossRef]
- Pandey, I.; Pandey, A.K.; Agrawal, P.C.; Das, N.R. Synthesis and characterization of dendritic polypyrrole silver nanocomposite and its application as a new urea biosensor. J. Appl. Polym. Sci. 2018, 135, 45705. [Google Scholar] [CrossRef]
- Yang, D.; Li, J.; Jiang, Z.; Lu, L.; Chen, X. Chitosan/TiO2 nanocomposite pervaporation membranes for ethanol dehydration. Chem. Eng. Sci. 2009, 64, 3130–3137. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef]
- Al Aani, S.; Wright, C.J.; Atieh, M.A.; Hilal, N. Engineering nanocomposite membranes: Addressing current challenges and future opportunities. Desalination 2017, 401, 1–15. [Google Scholar] [CrossRef]
- Schaep, J.; Vandecasteele, C.; Leysen, R.; Doyen, W. Salt retention of Zirfon® membranes. Sep. Purif. Technol. 1998, 14, 127–131. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Shen, J.; Liu, G.; Huang, K.; Li, Q.; Guan, K.; Li, Y.; Jin, W. UiO-66-polyether block amide mixed matrix membranes for CO2 separation. J. Membr. Sci. 2016, 513, 155–165. [Google Scholar] [CrossRef]
- Guo, X.; Huang, H.; Ban, Y.; Yang, Q.; Xiao, Y.; Li, Y.; Yang, W.; Zhong, C. Mixed matrix membranes incorporated with amine-functionalized titanium-based metal-organic framework for CO2/CH4 separation. J. Membr. Sci. 2015, 478, 130–139. [Google Scholar] [CrossRef]
- Kim, S.; Chen, L.; Johnson, J.K.; Marand, E. Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: Theory and experiment. J. Membr. Sci. 2007, 294, 147–158. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Guiver, M.D. Polysulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Fang, M.; Wu, C.; Yang, Z.; Wang, T.; Xia, Y.; Li, J. ZIF-8/PDMS mixed matrix membranes for propane/nitrogen mixture separation: Experimental result and permeation model validation. J. Membr. Sci. 2015, 474, 103–113. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Tabatabaei, S.H.; Carreau, P.J.; Ajji, A. Microporous membranes obtained from PP/HDPE multilayer films by stretching. J. Membr. Sci. 2009, 345, 148–159. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Teo, W.-E.; Ramakrishna, S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos. Sci. Technol. 2009, 69, 1804–1817. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhu, S.; Lu, H.; Meng, X. Shape evolution and thermal stability of Ag nanoparticles on spherical SiO2 substrates. J. Solid State Chem. 2008, 3, 587–592. [Google Scholar] [CrossRef]
- Huang, C.K.; Chen, C.Y.; Han, J.L.; Chen, C.C.; Jiang, M.D.; Hsu, J.S.; Chan, C.H.; Hsieh, K.H. Immobilization of silver nanoparticles on silica microspheres. J. Nanoparticle Res. 2010, 12, 199–207. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Sondi, J.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Jong-Min, L.; Dae-wook, K.; Young-Doo, J.; Seong-Geun, O. Preparation of silica–silver heterogeneous nanocomposite particles by one-pot preparation strategy using polyol process: Size-controlled immobilization of silver nanoparticles. Mater. Res. Bull. 2006, 41, 1407–1416. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Byeon, J.H.; Park, J.H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Ruperelia, J.P.; Chatterjje, K.A.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hu, D.; Cheng, E.W.C.; Vargas-Reus, M.A.; Reip, P.; Allaker, R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents 2009, 33, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid. Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Basri, H.; Ismail, A.F.; Aziz, K.; Nagai, M.; Matsuura, T.; Abdullah, M.S.; Ng, B.C. Silver-filled polyethersulfone membranes for antibacterial applications—Effect of PVP and TAP addition on silver dispersion. Desalination 2010, 261, 264–271. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, K. Polyethersulfone (PES)-silver composite UF membrane: Effect of silver loading and PVP molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- Jewrajka, S.K.; Haldar, S. Amphiphilic poly(acrylonitrile-co-acrylicacid)/silver nanocomposite additives for the preparation of antibiofouling membranes with improved properties. Polym. Compos. 2011, 32, 1851–1861. [Google Scholar] [CrossRef]
- Lind, M.L.; Jeong, B.H.; Subramani, A.; Huang, X.; Hoek, E.M.V. Effect of mobile cation on zeolite-polyamide thin film nanocomposite membranes. J. Mater. Res. 2009, 24, 1624–1631. [Google Scholar] [CrossRef]
- Mauter, M.S.; Wang, Y.; Okemgbo, K.C.; Osuji, C.O.; Giannelis, E.P.; Elimelech, M. Antifouling ultrafiltration membranes via post-fabrication grafting of biocidal nanomaterials. ACS Appl. Mater. Interfaces 2011, 3, 2861–2868. [Google Scholar] [CrossRef]
- Zielecka, M.; Bujnowska, E.; Kępska, B.; Wenda, M.; Piotrowska, M. Antimicrobial additives for architectural paints and impregnates. Prog. Org. Coat. 2011, 72, 193–201. [Google Scholar] [CrossRef]
- Zielecka, M.; Jeziórska, R.; Bujnowska, E.; Wenda, M.; Kępska, B.; Pytel, A.; Cyruchin, K.; Industrial Chemistry Research Institute. Method of Manufacturing the Silica Nanopowders with Biocidal Properties, Especially for Polymer Composites. U.S. Patent 9,371,586, 21 June 2016. [Google Scholar]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles showing improved biofouling resistance and virus removal. Water. Res. 2009, 43, 715–723. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, K.; DeGusseme, B.; Verstraete, W. Biogenic silver nanoparticles (bioAg0) decrease biofouling of bio-Ag0/PES nanocomposite membranes. Water Res. 2012, 46, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, J.; Ren, B.; Huang, Y.; Wang, D.; Liao, Z.; Lia, Q.; Wang, L.; Dionysiou, D.D. Polymeric ultrafiltration membrane with in situ formed nano-silver within the inner pores for simultaneous separation and catalysis. J. Membr. Sci. 2019, 579, 190–198. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes by Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Sawada, I.; Fachrul, R.; Ito, T.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J. Membr. Sci. 2012, 387–388, 1–6. [Google Scholar] [CrossRef]
- Mollahosseini, A.; Rahimpour, A.; Jahamshahi, M.; Peyravi, M.; Khavarpour, M. The effect of silver nanoparticle size on performance and antibacteriality of polysulfone ultrafiltration membrane. Desalination 2012, 306, 41–50. [Google Scholar] [CrossRef]
- Rehan, Z.A.; Gzara, L.; Khan, S.B.; Alamry, K.A.; El-Shahawi, M.S.; Albeirutty, M.H.; Figoli, A.; Drioli, A.; Asiri, A.M. Synthesis and Characterization of Silver Nanoparticles-Filled Polyethersulfone Membranes for Antibacterial and Anti-Biofouling Application. Recent Pat. Nanotechnol. 2016, 10. [Google Scholar] [CrossRef]
- Dolina, J.; Gončuková, Z.; Bobák, M.; Dvořák, L. Modification of a hollow-fibre polyethersulfone membrane using silver nanoparticles formed in situ for biofouling prevention. RSC Adv. 2018, 8, 14552–14560. [Google Scholar] [CrossRef]
- Mofradi, M.; Karimi, H.; Ghaedi, M. Hydrophilic polymeric membrane supported on silver nanoparticles surface decorated polyester textile: Toward enhancement of water flux and dye removal. Chin. J. Chem. Eng. 2020, 28, 901–912. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Ghosh, A.K.; Huang, X.; Liong, M.; Zink, J.I. Physical-chemical properties, separation performance, and fouling resistance of mixed-matrix ultrafiltration membranes. Desalination 2011, 283, 89–99. [Google Scholar] [CrossRef]
- Yamamoto, K.; Koge, S.; Gunji, T.; Kanezashi, M.; Tsuru, T.; Ohshita, J. Preparation of POSS-derived robust RO membranes for water desalination. Desalination 2017, 404, 322–327. [Google Scholar] [CrossRef]
- Singh, P.S.; Aswal, V.K. Characterization of physical structure of silica nanoparticles encapsulated in polymeric structure of polyamide films. J. Colloid Interface Sci. 2008, 326, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Jadav, G.L.; Singh, P.S. Synthesis of novel silica-polyamide nanocomposite membrane with enhanced properties. J. Membr. Sci. 2009, 328, 257–267. [Google Scholar] [CrossRef]
- Jadav, G.L.; Aswal, V.K.; Singh, P.S. SANS study to probe nanoparticle dispersion in nanocomposite membranes of aromatic polyamide and functionalized silica nanoparticles. J. Colloid Interface Sci. 2010, 351, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Kim, E.S.; Yang, J.; Deng, B. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM-41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012, 423–424, 238–246. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Optimizing polyamide thin film composite membrane covalently bonded with modified mesoporous silica nanoparticles. J. Membr. Sci. 2013, 428, 341–348. [Google Scholar] [CrossRef]
- Hu, D.; Xu, Z.L.; Wei, Y.M. A high performance silica-fluoropolyamide nanofiltration membrane prepared by interfacial polymerization. Sep. Purif. Technol. 2013, 110, 31–38. [Google Scholar] [CrossRef]
- Bandehali, S.; Parvizian, F.; Moghadassi, A.; Hosseini, S.M. High water permeable PEI nanofiltration membrane modified by L-cysteine functionalized POSS nanoparticles with promoted antifouling/separation performance. Sep. Purif. Technol. 2019, 237, 116361. [Google Scholar] [CrossRef]
- Roh, I.J.; Greenberg, A.R.; Khare, V.P. Synthesis and characterization of interfacially polymerized polyamide thin films. Desalination 2006, 191, 279–290. [Google Scholar] [CrossRef]
- Noor, M.Z.M.; Sollahunddin, N.A.; Irawan, S. Surface Modification of Aluminium Oxide (Al2O3) Nanoparticles (NPs) on Detection of Crude Oil Production. Proceedings 2018, 2, 1273. [Google Scholar] [CrossRef]
- TCTNanotech. Available online: https://www.tctnanotech.com/nanotech-ai2o3-nanoparticles-of-aluminium-oxide/ (accessed on 6 December 2020).
- Yan, L.; Li, Y.S.; Xiang, C.B.; Xianda, S. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Richards, H.; Baker, P.; Iwuoha, E. Metal Nanoparticle Modified Polysulfone Membranes for Use in Wastewater Treatment: A Critical Review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 183–193. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Preparation, characterization and performance of Al2O3/PES membrane for wastewater filtration. J. Membr. Sci. 2009, 341, 67–75. [Google Scholar] [CrossRef]
- Liu, F.; Abed, M.R.M.; Li, K. Preparation and characterization of poly(vinylidene fluoride) (PVDF) based ultrafiltration membranes using nano γ-Al2O3. J. Membr. Sci. 2011, 366, 97–103. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B. Preparation of poly(vinylidene fluoride)(pvdf) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 2005, 46, 7701–7706. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; Sotto, A.; del Rosario, G.; Martınez, A.; Molina, S.; Teli, S.B.; de Abajo, J. Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes. J. Membr. Sci. 2013, 428, 131–141. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Mehrnia, M.R.; Rahmani, S.; Mojtahedi, Y.M. Fabrication of alumina/polysulfone nanocomposite membranes with biofouling mitigation approach in membrane bioreactors. J. Ind. Eng. Chem. 2015, 22, 357–367. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep. Purif. Technol. 2011, 89, 245–251. [Google Scholar] [CrossRef]
- Lin, Y.; Loh, C.H.; Shi, L.; Fan, Y.; Wang, R. Preparation of high-performance Al2O3/PES composite hollow fiber UF membranes via facile in-situ vapor induced hydrolyzation. J. Membr. Sci. 2017, 539, 65–75. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rana, D.; Lan, C.Q.; Matsuura, T. Effects of inorganic nano-additives on properties and performance of polymeric membranes in water treatment. Sep. Purif. Rev. 2015, 45, 141–167. [Google Scholar] [CrossRef]
- Li, F.; Li, L.; Liao, X.; Wang, Y. Precise pore size tuning and surface modifications of polymeric membranes using the atomic layer deposition technique. J. Membr. Sci. 2011, 385–386, 1–9. [Google Scholar] [CrossRef]
- Waldman, R.Z.; Mandia, D.J.; Yanguas-Gil, A.; Martinson, A.B.F.; Elam, J.W.; Darling, S.B. The chemical physics of sequential infiltration synthesis—A thermodynamic and kinetic perspective. J. Chem. Phys. 2019, 151, 190901. [Google Scholar] [CrossRef] [PubMed]
- Waldman, R.Z.; Choudhury, D.; Mandia, D.J.; Elam, J.W.; Nealey, P.F.; Martinson, A.B.F.; Darling, S.B. Sequential Infiltration Synthesis of Al2O3 in Polyethersulfone Membranes. JOM 2018, 71, 212–223. [Google Scholar] [CrossRef]
- Bergsman, D.S.; Getachew, B.A.; Cooper, C.B.; Grossman, J.C. Preserving nanoscale features in polymers during laser induced graphene formation using sequential infiltration synthesis. Nat. Commun. 2020, 11, 3636. [Google Scholar] [CrossRef]
- Sungur, Ş. Titanium Dioxide Nanoparticles. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O., Martínez, L., Kharisov, B., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Nguyen, A.; Zou, L.; Priest, C. Evaluating the antifouling effects of silver nanoparticles regenerated by TiO2 on forward osmosis membrane. J. Membr. Sci. 2014, 454, 264–271. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, Z.; Huang, J.; Wang, Y. PVDF membranes with simultaneously enhanced permeability and selectivity by breaking the tradeoff effect via atomic layer deposition of TiO2. J. Membr. Sci. 2013, 442, 57–64. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Peyravi, M.; Jahanshahi, M.; Rahimpour, A.; Javadi, A.; Hajavi, S. Novel thin film nanocomposite membranes incorporated with functionalized TiO2 nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 2014, 241, 155–166. [Google Scholar] [CrossRef]
- Wu, G.; Gan, S.; Cui, L.; Xu, Y. Preparation and characterization of PES/TiO2 composite membranes. Appl. Surf. Sci. 2008, 254, 7080–7086. [Google Scholar] [CrossRef]
- Luo, M.; Tang, W.; Zhao, J.; Pu, C. Hydrophilic modification of poly(ether sulfone) used TiO2 nanoparticles by a sol–gel process. J. Mater. Process. Technol. 2006, 172, 431–436. [Google Scholar] [CrossRef]
- Barahimi, V.; Taheri, R.A.; Mazaheri, A.; Moghimi, H. Fabrication of a novel antifouling TiO2/CPTES/metformin-PES nanocomposite membrane for removal of various organic pollutants and heavy metal ions from wastewater. Chem. Pap. 2020, 74, 3545–3556. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, H.; Wang, J.; Ding, R.; Du, Z.; Liu, J.; Cao, S. Mineralization-inspired preparation of composite membranes with polyethyleneimine–nanoparticle hybrid active layer for solvent resistant nanofiltration. J. Membr. Sci. 2014, 470, 70–79. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Yang, S.; Gu, J.-S.; Yu, H.-Y.; Zhou, J.; Li, S.-F.; Wu, X.-M.; Wang, L. Polypropylene membrane surface modification by RAFT grafting polymerization and TiO2 photocatalysts immobilization for phenol decomposition in a photocatalytic membrane reactor. Sep. Purif. Technol. 2011, 83, 157–165. [Google Scholar] [CrossRef]
- You, S.-J.; Semblante, G.U.; Lu, S.-C.; Damodar, R.A.; Wei, T.-C. Evaluation of the antifouling and photocatalytic properties of poly(vinylidene fluoride) plasma-grafted poly(acrylic acid) membrane with self-assembled TiO2. J. Hazard. Mater. 2012, 237–238, 10–19. [Google Scholar] [CrossRef]
- Kumar, M.; Gholamvand, Z.; Morrissey, A.; Nolan, K.; Ulbricht, M.; Lawler, J. Preparation and characterization of low fouling novel hybrid ultrafiltration membranes based on the blends of GO−TiO2 nanocomposite and polysulfone for humic acid removal. J. Membr. Sci. 2016, 506, 38–49. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.-Y.; Sohn, B.-H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Xue, X. Preparation of different shaped α-Fe2O3 nanoparticles with large particles of iron oxide red. CrystEngComm 2019, 21, 1097–1101. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J. Membr. Sci. 2012, 415–416, 250–259. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, A.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar] [CrossRef]
- Gholami, A.; Moghadassi, A.R.; Hosseini, S.M.; Shabani, S.; Gholami, F. Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J. Ind. Eng. Chem. 2014, 20, 1517–1522. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Van der Bruggen, B. Iron-tannin-framework complex modified PES ultrafiltration membranes with enhanced filtration performance and fouling resistance. J. Colloid Interface Sci. 2017, 505, 642–652. [Google Scholar] [CrossRef] [PubMed]
- AL-Hobaib, A.S.; AL-Sheetan, K.M.; El Mir, L. Effect of iron oxide nanoparticles on the performance of polyamide membrane for ground water purification. Mater. Sci. Semicond. Process. 2016, 42, 107–110. [Google Scholar] [CrossRef]
- Demirel, E.; Zhang, B.; Papakyriakou, M.; Xia, S.; Chen, Y. Fe2O3 nanocomposite PVC membrane with enhanced properties and separation performance. J. Membr. Sci. 2017, 529, 170–184. [Google Scholar] [CrossRef]
- Torrey, J.D.; Killgore, J.P.; Bedford, N.M.; Greenlee, L.F. Oxidation behavior of zero-valent iron nanoparticles in mixed matrix water purification membranes. Environ. Sci. Water Res. Technol. 2015, 1, 146–152. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Sagadevan, V. Polyethylene glycol and iron oxide nanoparticles blended polyethersulfone ultrafiltration membrane for enhanced performance in dye removal studies. e-Polymers 2015, 15. [Google Scholar] [CrossRef]
- Raciny, I.; Zodrow, K.R.; Li, D.; Li, Q.; Alvarez, P.J.J. Addition of a magnetite layer onto a polysulfone water treatment membrane to enhance virus removal. Water Sci. Technol. 2011, 63, 2346–2352. [Google Scholar] [CrossRef][Green Version]
- Wu, L.; Ritchie, S.M.C. Enhanced dechlorination of trichloroethylene by membrane-supported Pd-coated iron nanoparticles. Environ. Prog. 2008, 27, 218–224. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Doong, R. Dechlorination of chlorinated hydrocarbons by bimetallic Ni/Fe immobilized on polyethylene glycol-grafted microfiltration membranes under anoxic conditions. Chemosphere 2012, 86, 392–399. [Google Scholar] [CrossRef]
- Armendariz Ontiveros, M.; Quintero, Y.; Llanquilef, A.; Morel, M.; Argentel Martínez, L.; García García, A.; Garcia, A. Anti-Biofouling and Desalination Properties of Thin Film Composite Reverse Osmosis Membranes Modified with Copper and Iron Nanoparticles. Materials 2019, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Akar, N.; Asar, B.; Dizge, N.; Koyuncu, I. Investigation of characterization and biofouling properties of PES membrane containing selenium and copper nanoparticles. J. Membr. Sci. 2013, 437, 216–226. [Google Scholar] [CrossRef]
- Kar, S.; Subramanian, M.; Ghosh, A.K.; Bindal, R.C.; Prabhakar, S.; Nuwad, J.; Pillai, C.G.S.; Chattopadhyay, S.; Tewari, P.K. Potential of nanoparticles for water purification: A case-study on anti-biofouling behaviour of metal based polymeric nanocomposite membrane. Desalination Water Treat. 2011, 27, 224–230. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Lu, X.; Nejati, S.; Jaramillo, H.; Elimelech, M. In situ surface functionalization of reverse osmosis membranes with biocidal copper nanoparticles. Desalination 2016, 388, 1–8. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Y.; Pan, G.; Xu, J.; Yan, H.; Liu, Y. In situ formation of copper nanoparticles in carboxylated chitosan layer: Preparation and characterization of surface modified TFC membrane with protein fouling resistance and long-lasting antibacterial properties. Sep. Purif. Technol. 2017, 176, 164–172. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, K.; Mo, Y.; Huang, X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J. Membr. Sci. 2012, 394–395, 184–192. [Google Scholar] [CrossRef]
- Jo, Y.J.; Choi, E.Y.; Choi, N.W.; Kim, C.K. Antibacterial and Hydrophilic Characteristics of Poly(ether sulfone) Composite Membranes Containing Zinc Oxide Nanoparticles Grafted with Hydrophilic Polymers. Ind. Eng. Chem. Res. 2016, 55, 7801–7809. [Google Scholar] [CrossRef]
- Chung, Y.T.; Ba-Abbad, M.M.; Mohammad, A.W.; Benamor, A. Functionalization of zinc oxide (ZnO) nanoparticles and its effects on polysulfone-ZnO membranes. Desalination Water Treat. 2015, 57, 7801–7811. [Google Scholar] [CrossRef]
- Pintilie, S.C.; Tiron, L.G.; Birsan, I.G.; Ganea, D.; Balta, S. Influence of ZnO Nanoparticle Size and Concentration on the Polysulfone Membrane Performance. Mater. Plast. 2017, 54, 257–261. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Effects of nano sized zinc oxide on the performance of PVDF microfiltration membranes. Desalination 2012, 302, 71–79. [Google Scholar] [CrossRef]
- Jia, H.; Wu, Z.; Liu, N. Effect of nano-ZnO with different particle size on the performance of PVDF composite membrane. Plast. Rubber Compos. 2016, 46, 1–7. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Z.; Liu, Y.; Li, R.; Xu, Y.; Jakaj, G.; Lin, H. Polymeric Membranes Incorporated With ZnO Nanoparticles for Membrane Fouling Mitigation: A Brief Review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Dipheko, T.D.; Matabola, K.P.; Kotlhao, K.; Moutloali, R.M.; Klink, M. Fabrication and Assessment of ZnO Modified Polyethersulfone Membranes for Fouling Reduction of Bovine Serum Albumin. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, W.; Shi, M.; Wang, Z.; Wang, J.; Wang, S. Improving permeability and antifouling performance of polyethersulfone ultrafiltration membrane by incorporation of ZnO-DMF dispersion containing nano-ZnO and polyvinylpyrrolidone. J. Membr. Sci. 2015, 478, 105–116. [Google Scholar] [CrossRef]
- Ghoul, J.E.L.; Ghiloufi, I.; Mir, L.E.L.; Arabia, S. Efficiency of polyamide thin-film nanocomposite membrane containing ZnO nanoparticles. J. Ovonic Res. 2017, 13, 83–90. [Google Scholar]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Ismail, S.; Seng, O.B. Optimization of PES/ZnO mixed matrix membrane preparation using response surface methodology for humic acid removal. Korean J. Chem. Eng. 2016, 33, 997–1007. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. Preparation and characterization of a novel photocatalytic self-cleaning PES nanofiltration membrane by embedding a visible-driven photocatalyst boron doped-TiO2SiO2/CoFe2O4 nanoparticles. Sep. Purif. Technol. 2019, 209, 764–775. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S. Self-cleaning properties of L-Histidine doped TiO2-CdS/PES nanocomposite membrane: Fabrication, characterization and performance. Sep. Purif. Technol. 2020, 240, 116591. [Google Scholar] [CrossRef]
- Yu, W.; Liu, Y.; Xu, Y.; Li, R.; Chen, J.; Liao, B.-Q.; Schen, L.; Lin, H. A conductive PVDF-Ni membrane with superior rejection, permeance and antifouling ability via electric assisted in-situ aeration for dye separation. J. Membrane Sci. 2019, 581, 401–412. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Performance of a novel ZrO2/PES membrane for wastewater filtration. J. Membr. Sci. 2010, 352, 222–230. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A. Preparation and characterization of novel porous PVDF-ZrO2 composite membranes. Desalination 2002, 146, 35–40. [Google Scholar] [CrossRef]
- Shalaby, M.S.; Abdallah, H.; Cenian, A.; Sołowski, G.; Sawczak, M.; Shaban, A.M.; Ramadan, R. Laser synthesized gold- nanoparticles, blend NF membrane for phosphate separation from wastewater. Sep. Purif. Technol. 2020, 247, 116994. [Google Scholar] [CrossRef]
- Dong, C.; He, G.; Li, H.; Zhao, R.; Han, Y.; Deng, Y. Antifouling enhancement of poly(vinylidene fluoride) microfiltration membrane by adding Mg(OH)2 nanoparticles. J. Membr. Sci. 2012, 387–388, 40–47. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Rana, D.; Doraiswamy, M. Separation of oil/water emulsions using nano MgO anchored hybrid ultrafiltration membranes for environmental abatement. Inc. J. Appl. Polym. Sci. 2016, 133, 42848. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Stanisławek, E.; Kacprzyńska-Gołacka, J.; Kaźmierczak, B.; Wieciński, P. The polyamide membranes modified by copper oxide using PVD techniques. J. Mach. Constr. Maint. 2018, 3, 49–55. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztech-c695358c-1090-42fb-8d64-ab88fc7537e8 (accessed on 6 December 2020).
- Szymański, K.; Darowna, D.; Sienkiewicz, P.; Jose, M.; Szymańska, K.; Zgrzebnicki, M.; Mozia, S. Novel polyethersulfone ultrafiltration membranes modified with Cu/titanate nanotubes. J. Water Process Eng. 2020, 33, 101098. [Google Scholar] [CrossRef]

| Process Type | Principle of Operation | Advantages/Disadvantages | Ref. |
|---|---|---|---|
| Pressure processes | |||
| Microfiltration | It is used mainly in ceramic filters or polymer membranes. | The main disadvantage is the low efficiency, and in order to increase it, a support is used, for example, a sand filter. The disadvantage of using ceramic membranes is the possibility of releasing arsenic from contaminated sintered materials. | [11,12] |
| Ultrafiltration | The use of a polymer ultrafiltration membrane enables good purification in one operation. | Microbiologically safe water is easily produced. | [13] |
| Nanofiltration | Nanomembranes remove polyvalent ions and organic compounds. | They cannot be used as the only solution for the desalination of sea water. | [14] |
| Reverse osmosis | It takes place in a solution with a higher concentration of the solute to a solution with a lower concentration, i.e., it leads to an increase in the concentration differences of both solutions. | Very good results are obtained in the removal of radionuclides, used to treat water after the 2011 Fukushima earthquake/very high energy consumption. | [15] |
| Osmotic processes | |||
| Spontaneous osmosis | It uses a two-layer filter with an inner layer containing a semipermeable osmotic layer, e.g., concentrated sugar solution. | Regeneration is achieved by exchanging the osmotic layer. It removes a wide range of contaminants without the use of pressure. | [16] |
| Thermal processes | |||
| Membrane distillation | It is a hybrid process: water evaporation, water vapor filtration through a membrane, and water vapor condensation. | The process can be carried out at a temperature of 50–90 °C and works well in regions with high sun exposure. | [17] |
| NP | Polymer | Particle Size/Loading, wt% | Fabrication Method | Application/Performance | Ref. |
|---|---|---|---|---|---|
| Ag-GO | Polyamide 6,6 | 0.8, 1.0 wt% | Graphene oxide was synthesized by using Hummers’ method/wet phase inversion method. | 135% flux increase, 40% less irreversible fouling, 46% increase in hydrophilicity, and excellent antibacterial properties against E. coli | [89] |
| Ag | PES | 10 nm | Acrylamide was grafted onto a PES membrane surface, and Ag nanoparticles were formed within the grafted layer. | positive effect on hydrophilicity, antifouling properties, and excellent antibacterial properties against E. coli (99.99%) | [90] |
| Ag | PSU | 30–70 nm/2–4 wt% | Phase inversion via immersion precipitation technique/4 wt% of PVP was used as a pore former in the casting solution. | UF/positive effect on hydrophilicity, antifouling properties, BSA rejection, and excellent antibacterial properties against E. coli | [91] |
| Ag | PES | <100 nm | NIPS process using PVP as an additive and N-Methyl-2-pyrrolidone as a solvent | superb antibacterial and anti-biofouling performances | [92] |
| Ag | PES | 25–50 nm | Own modification method consisting in soaking the PES hollow fiber membrane in a solution of silver ions, diffusion of ions into the polymer, and their reduction with ascorbic acid | positive effect on hydrophilicity and lower biofouling tendency (about 15% higher permeability) | [93] |
| Ag | PVDF | <100 nm | PEG as a hydrophilic agent, zeolitic-like framework-67 (ZIF-67), ethylenediamine as a cross-linking agent on n-Ag-decorated polyester textile support | good wettability, high pure water flux (PWF; 35.8 L/m2⋅h), flux recovery ratio (FRR; 90%), and dye removal efficiency (96.41%) | [94] |
| NP | Polymer | Particle Size/Loading, wt% | Fabrication Method | Application/Performance | Ref. |
|---|---|---|---|---|---|
| Silica (LUDOXs HS-40) | PA | ~13.2 nm/5–28% of PA | IP | Filtration of dioxane solution/positive effect on Pw; negative impact on solute rejection | [97] |
| Nanosilica | PA | 3 and 16 nm/0–0.4% (3 nm) and 0–0.5% (16 nm) in aqueous phase | IP | Reverse osmosis RO/positive effect on Pw and thermal stability; negative impact on NaCl rejection | [98] |
| Functionalized Silica | PA | 0.04–0.4% in aqueous phase | IP | RO; pervaporation PV/positive effect on thermal stability and Pw; negative impact on NaCl rejection | [99] |
| Mesoporous silica (MCM-41) and nonporous silica | PA | Both ~100 nm/0–0.1% in organic phase (0.05%) | IP | RO/positive effect on surface hydrophilicity and Pw; salt rejection no change porous; structures of filler contributed significantly | [100] |
| Modified mesoporous silica | PA | ~100 nm/0–0.07% in aqueous phase (0.03%) | IP | NF/under 87 psi, optimal water flux is 32.4 L/m2 h; Na2SO4 rejection (4 80%, 5 mmol/L) | [101] |
| Silica | Fluoropolyamide | 0–1.0% (w/v) in aqueous phase (0.1) | IP | NF/positive effect on Pw; Na2SO4 rejection under 87 psi; optimal water flux is 15.2 L/m2 h; Na2SO4 rejection (85.0%, 2000 mg/L) | [102] |
| L-cysteine modified-POSS nanoparticles | Polyetherimide | 33.77 nm/0.001–1 wt% | IP | NF/positive effect on salt rejection; the best separation performance for membrane with 1 wt% L-cysteine modified-POSS nanoparticles | [103] |
| NP | Polymer | Particle Size/Loading, wt% | Fabrication Method | Application/Performance | Ref. |
|---|---|---|---|---|---|
| γ-Al2O3 | PVDF | 20 nm/1–4 wt% | One-step conventional phase inversion technique | UF/solution of BSA/PBS (1 g/L, pH = 7.4)/positive effect on separation performances | [110] |
| Al2O3 | PVDF | 10 nm/2 wt% | Phase inversion technique | UF/oil–wastewater ultrafiltration/positive effect on surface hydrophilicity | [111] |
| Al2O3 | PES | 80 nm/0.4 wt% | Phase inversion technique | UF/BSA and humic acids as model organic foulants/more open and porous structure and antifouling property, and better long-term flux stability | [112] |
| γ-Al2O3 | PSF | 30 nm/0.02 and 0.03 wt% | Blending method and phase inversion technique | UF/bioreactors/prevents biofilm formation and reduces resistance by 75% | [113] |
| Al2O3 | PA | 14 nm/1 wt% | In situ interfacial polymerization | OM/positive effect on permeate flow and hydrophilicity, maintaining salt rejection | [114] |
| Al2O3 | PES HF/PEO-PPO-PEO | 3.6, 7.4, and 10.9 wt% | In situ vapor-induced hydrolyzation | UF/antifouling properties to humic acid | [115] |
| NPs | Polymer | Particle Size/Loading, wt% | Fabrication Method | Application/Performance | Ref. |
|---|---|---|---|---|---|
| TiO2/SiO2 | PEI/PAN | 30, 45, 90, and 110 nm/0.2, 0.4, 0.6, and 0.8 wt% | mineralization, ultrasonic mixing, coating, casting, and drying | SRNF/organic solvents (n-heptane, toluene, butanone, ethyl acetate, isopropanol, and polyethylene glycol) | [129] |
| TiO2 | PVDF–PEG | 20–30 nm/0.25 to 2.0 wt% | the phase inversion process (PIP) method | UF/photocatalytic NOM degradation/good self-cleaning ability | [130] |
| TiO2 | PVDF | 16 nm/7.5 wt% | mixing, cooling, degassing with ultrasound, and spinning | UF/photocatalytic nonylphenol degradation | [131] |
| TiO2 | PPMMs | 50–100 nm/2.9–12.9 wt% | photoinduced reversible addition-fragmentation chain transfer grafting polymerization of acrylic acid | SMPR/phenol decomposition | [132] |
| TiO2 | PAA/PVDF | 0.5, 1.5, and 3.0%, m/v | plasma treatment at 100 W for 120 s followed by liquid grafting with 70% aqueous AA at 60 °C for 2 h | the highest pure water flux and the best protein antifouling property; photodegradation of strongly bound foulants; removal of 30–42% of 50 mg/L aqueous Reactive Black 5 (RB5) dye | [133] |
| GO-TiO2 | PSF/PVP | 0–5 wt% | in situ sol-gel | UF/humic acids removal | [134] |
| TiO2 | nonwoven fabric-reinforced PSF | 10 nm | interfacial polymerization of MPD in the aqueous phase (2 wt%) and TMC in the organic phase (0.1 wt%) | RO/photobactericidal effect on Escherichia coli | [135] |
| NPs | Polymer | Particle Size/Loading, wt% | Fabrication Method | Application/Performance | Ref. |
|---|---|---|---|---|---|
| γ-Fe2O3 | PSF | 10 nm/0.1–0.9 wt% | IP of thin film nanocomposite (TFNC) | RO/better hydrophilicity/high NaCl salt rejection of 98% | [142] |
| Fe2O3 | PVP | <50 nm/0–2 wt% (the most optimal: 1 wt%) | the phase inversion method | UF/positive effect on hardness, hydrophilicity, sodium alginate (SA) rejection rate (91.9%), and antifouling properties | [143] |
| ZVI | PSF/PVP | 100 nm (change due to the oxidation of the ZVI) | the phase inversion method | UF/oxidation of ZVI nanoparticles to FeO(OH), which are mechanically stable | [144] |
| FeO | PES | 20 nm/1–4 wt% | the phase inversion method | UF/positive effect on hydrophilicity, thermal stability, and dye rejection | [145] |
| Fe3O4 | PSF | <50 nm/3.9 wt% | membranes were coated by a simple filtration protocol | Low-pressure membrane systems/removal of a model virus (bacteriophage MS2) with efficiency exceeding 99.99% | [146] |
| Pd/Fe | MMMs from CA | 10 nm of Fe and 11–30 nm of Pd/Fe | magnetic stirring, dispersion, drying, and liquid ethanol bath | UF/de-chlorination of TCE | [147] |
| Fe/Ni | PVDF, Nylon-66, Millex GS, mixed cellulose ester membranes | 56 nm in nylon-66, 82 nm in PVDF, 43 nm in MCEM, and 36 nm in Millex G | immersion of membranes in coating solutions and heating | MF/degradation of chlorinated (PCE, TCE, CT, and dichloromethane) and non-chlorinated hydrocarbons (methane and ethane) | [148] |
| Fe/Fe2O3 | PA-PSF | <30 nm/0.25 wt% | interfacial polymerization process (PI) | RO/positive effect on roughness and bactericidal effect | [149] |
| NP | Polymer | Particle Size/Loading, wt% | Fabrication Method | Application/Performance | Ref. |
|---|---|---|---|---|---|
| ZrO2 | PES | <100 nm/0.01, 0.03, 0.05, 0.07, and 0.1 ZrO2/PES ratios (w/w) | Phase inversion technique | MBR/activated sludge filtration/positive effect on strength, permeability, and antifouling properties | [169] |
| ZrO2 | PVDF | <100 nm/20 wt% | Casting and immersion into a water bath of ternary suspensions | UF/positive effect on hydrophilicity and antifouling properties | [170] |
| Au | PES | 39 nm/0.1 wt% | Phase inversion technique | NF/for removing PO43−/positive effect on hydrophilicity and antifouling properties | [171] |
| Mg(OH)2 | PVDF | <100 nm/10 wt% | Phase inversion technique | positive effect on strength, permeability, antifouling properties, BSA rejection, and antibacterial properties against E. coli | [172] |
| MgO | PPSU | 55–105 nm/0.25 wt% | Phase inversion technique | improvement in membrane properties such as interlayer spacing, hydrophilicity, flux, rejection, porosity, pore size, and oleophobicity relative to oil/water emulsions | [173] |
| CuO | PA | <100 nm | Low-temperature metallic (metallic-gas) plasma and nonmetallic (gas) plasma of the physical vapor deposition process | high performance of the filtration materials with a strong antibacterial activity against gram-negative bacteria (E. coli) | [174] |
| Cu/TNT | PES | TNT: 50–200 nm, Cu: 2–3 nm (on the outer surface of the TNTs) | Wet phase inversion technique | positive effect on permeability, BSA rejection, and antibacterial properties against E. coli (lower in relation to S. epidermidis) | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabajczyk, A.; Zielecka, M.; Cygańczuk, K.; Pastuszka, Ł.; Jurecki, L. Nanometals-Containing Polymeric Membranes for Purification Processes. Materials 2021, 14, 513. https://doi.org/10.3390/ma14030513
Rabajczyk A, Zielecka M, Cygańczuk K, Pastuszka Ł, Jurecki L. Nanometals-Containing Polymeric Membranes for Purification Processes. Materials. 2021; 14(3):513. https://doi.org/10.3390/ma14030513
Chicago/Turabian StyleRabajczyk, Anna, Maria Zielecka, Krzysztof Cygańczuk, Łukasz Pastuszka, and Leszek Jurecki. 2021. "Nanometals-Containing Polymeric Membranes for Purification Processes" Materials 14, no. 3: 513. https://doi.org/10.3390/ma14030513
APA StyleRabajczyk, A., Zielecka, M., Cygańczuk, K., Pastuszka, Ł., & Jurecki, L. (2021). Nanometals-Containing Polymeric Membranes for Purification Processes. Materials, 14(3), 513. https://doi.org/10.3390/ma14030513








