Depletion-Induced Chiral Chain Formation of Magnetic Spheres
Abstract
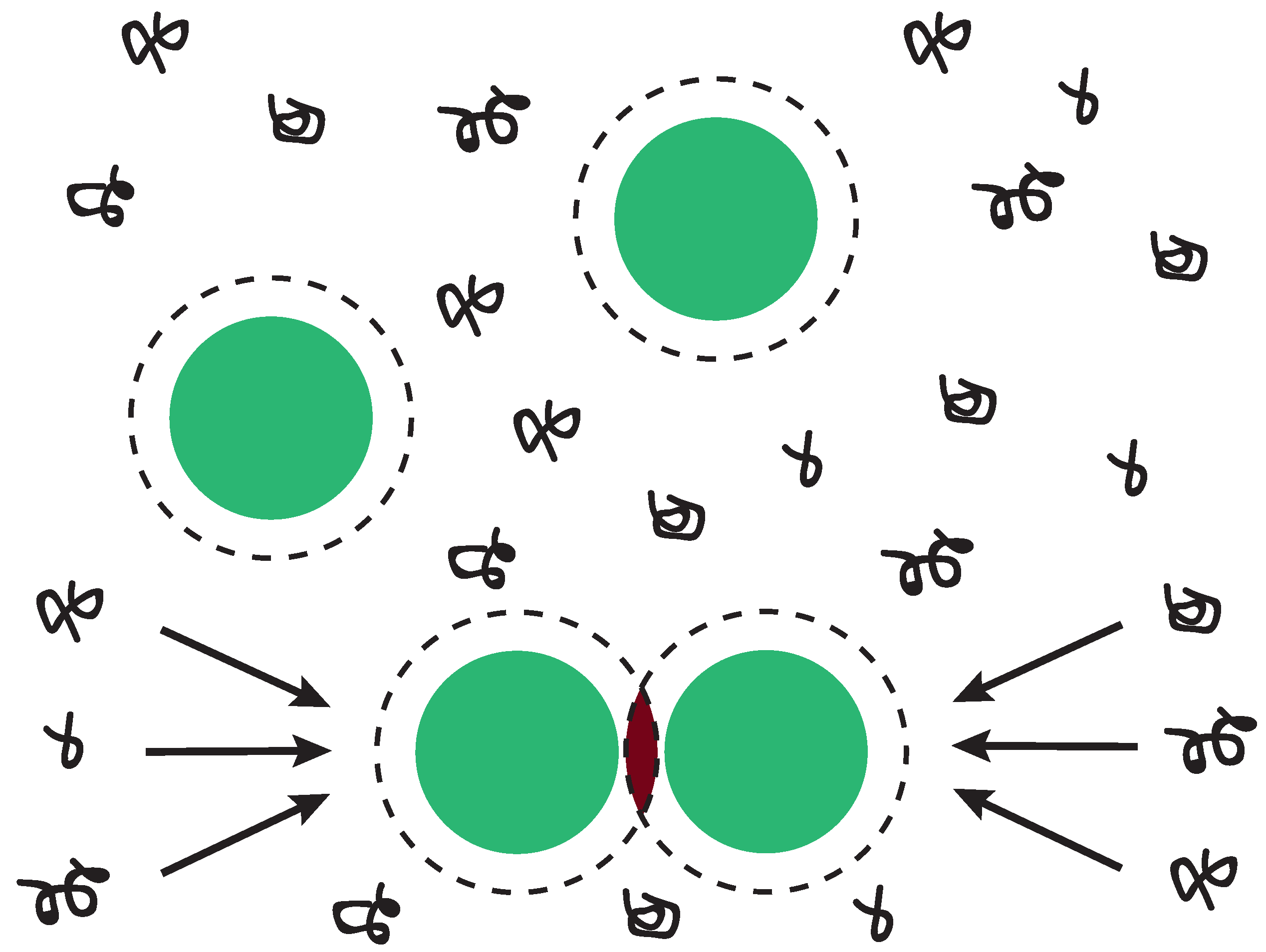
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Magnetic Field
2.3. Optical Microscopy
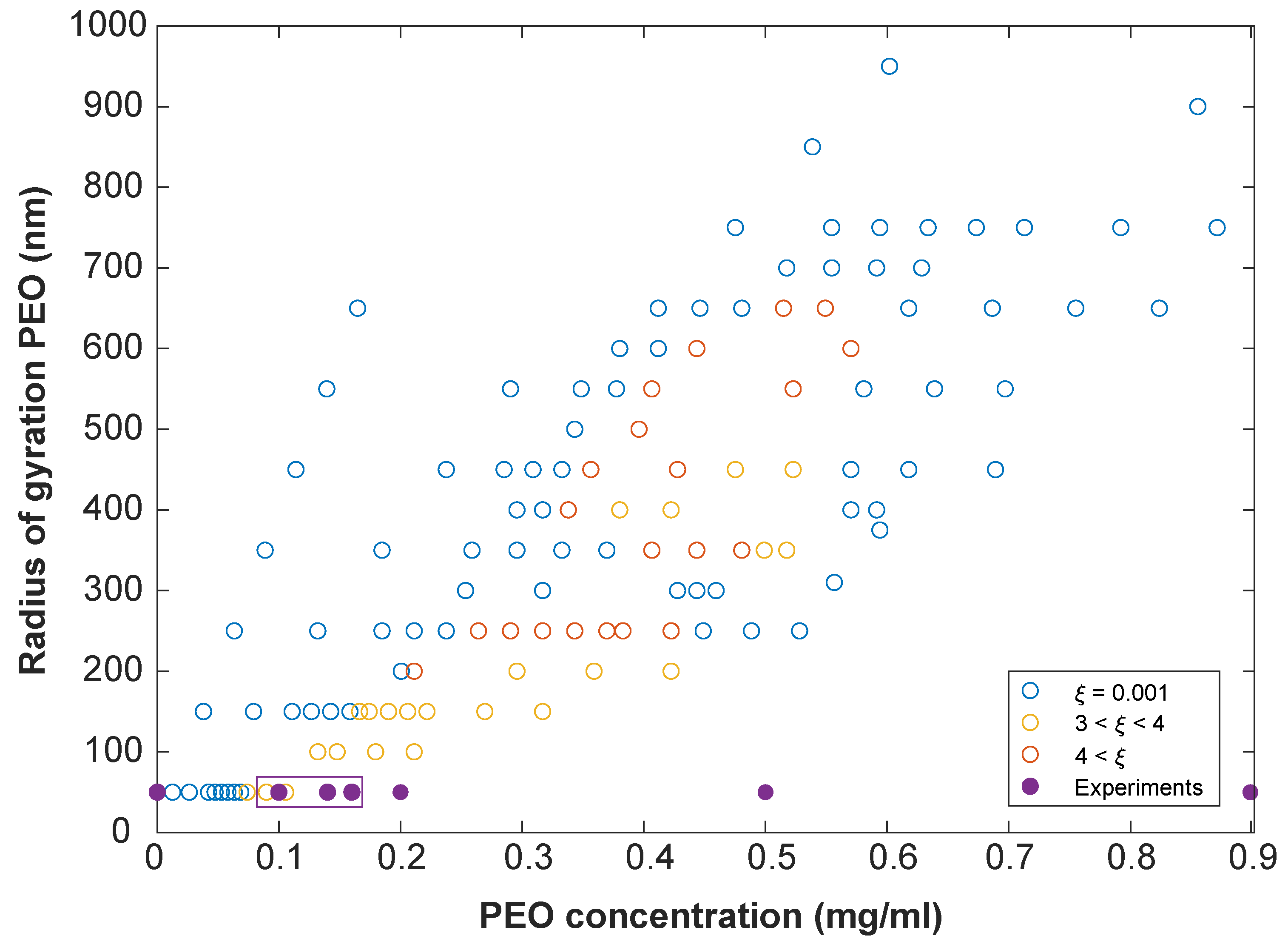
3. Results and Discussion
3.1. Model System
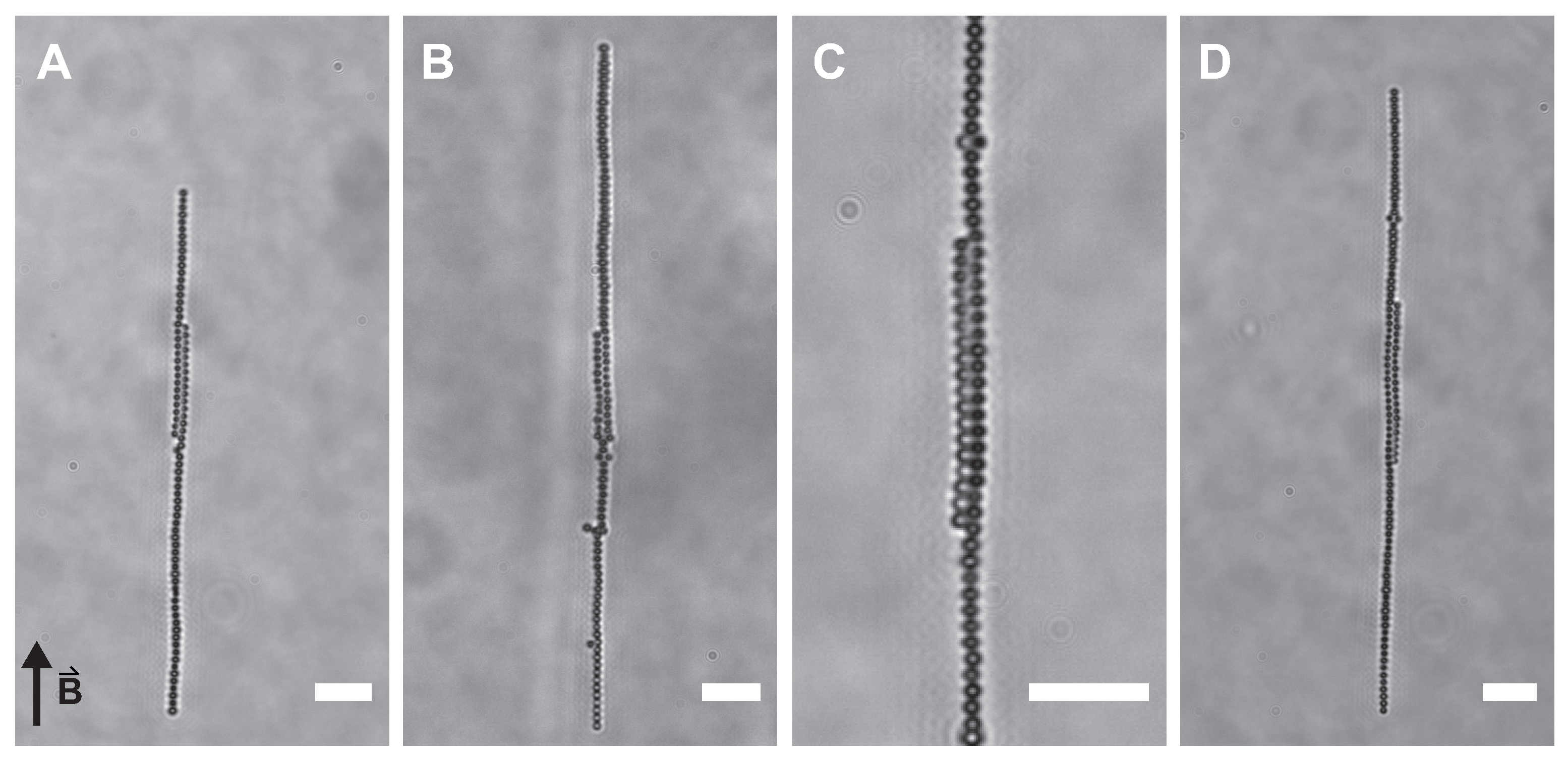
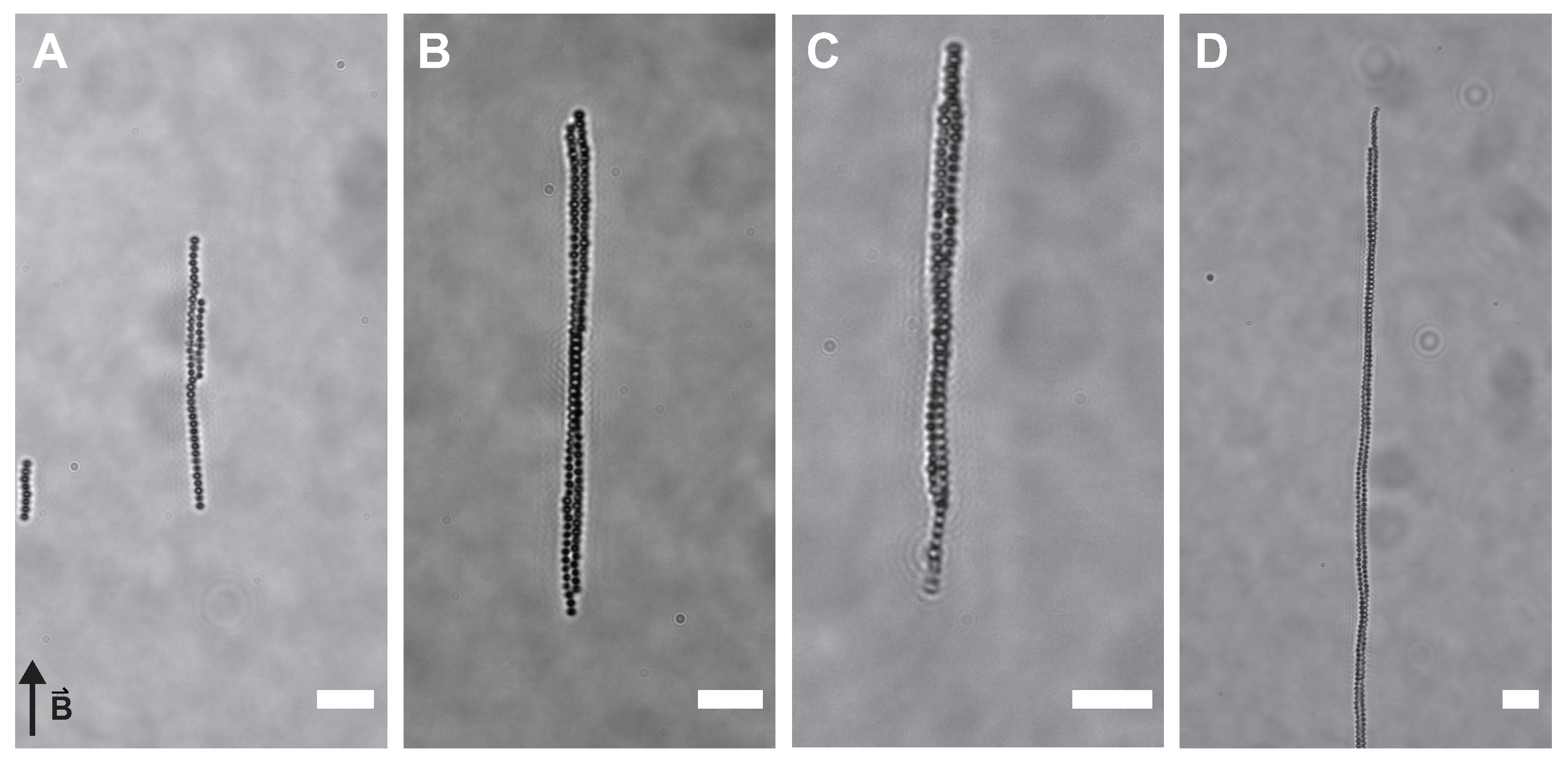
3.2. Observations
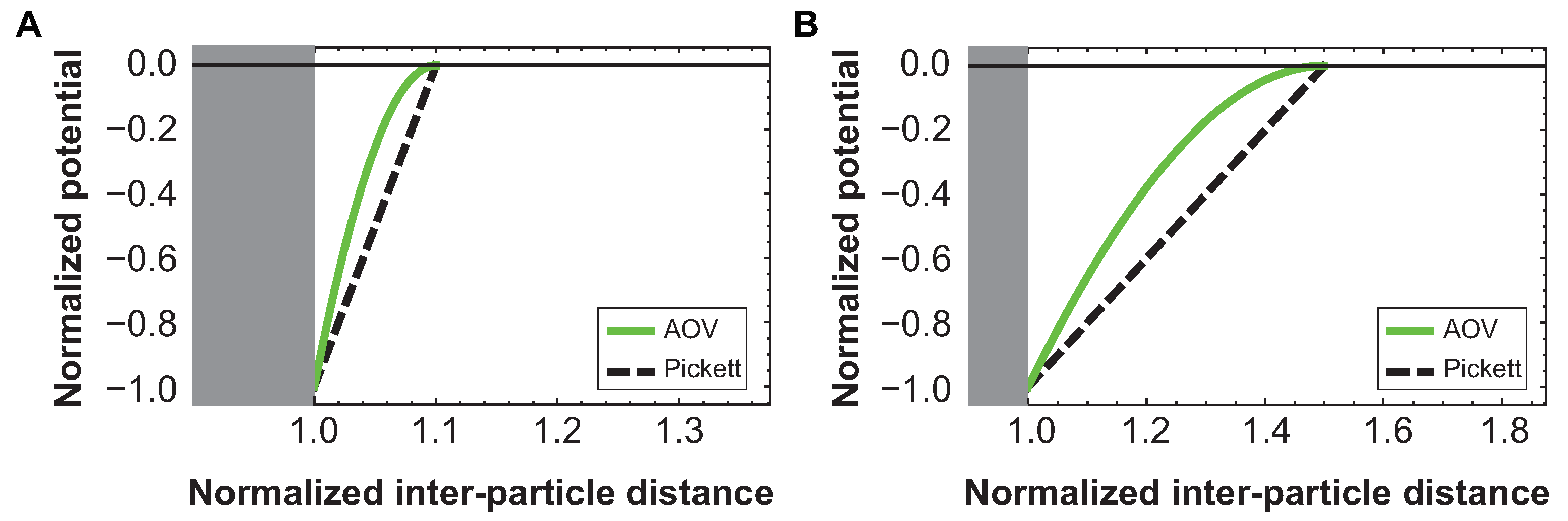
3.3. Comparison with Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Calculations
Appendix A.1. Debye Screening Length
Appendix A.2. Radius of Gyration
Appendix A.3. Depletion Potential
References
- Ma, W.; Xu, L.; de Moura, A.F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral Inorganic Nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.B.; Kamien, R.D.; Lubensky, T.C. Molecular chirality and chiral parameters. Rev. Mod. Phys. 1999, 71, 1745–1757. [Google Scholar] [CrossRef]
- Zafar, M.; Ragusa, A. Chirality at the Nanoparticle Surface: Functionalization and Applications. Appl. Sci. 2020, 10, 5357. [Google Scholar] [CrossRef]
- Nayani, K.; Kim, Y.K.; Abbott, N.L. Chiral interactions in liquid crystals. Nat. Mater. 2018, 17, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Gerbode, S.J.; Puzey, J.R.; McCormick, A.G.; Mahadevan, L. How the Cucumber Tendril Coils and Overwinds. Science 2012, 337, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Hensel, Z.; Dogic, Z.; Shribak, M.; Oldenbourg, R. Entropy-Driven Formation of a Chiral Liquid-Crystalline Phase of Helical Filaments. Phys. Rev. Lett. 2006, 96, 018305. [Google Scholar] [CrossRef] [PubMed]
- Wensink, H.H.; Morales-Anda, L. Chiral assembly of weakly curled hard rods: Effect of steric chirality and polarity. J. Chem. Phys. 2015, 143, 144907. [Google Scholar] [CrossRef]
- Collings, P.; Hird, M. Introduction to Liquid Crystals: Chemistry and Physics; Taylor & Francis: Abingdon, UK, 1997. [Google Scholar]
- Pickett, G.T.; Gross, M.; Okuyama, H. Spontaneous Chirality in Simple Systems. Phys. Rev. Lett. 2000, 85, 3652–3655. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Chan, H.K.; Weaire, D. Phyllotactic Description of Hard Sphere Packing in Cylindrical Channels. Phys. Rev. Lett. 2011, 106, 115704. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Chan, H.K.; Weaire, D.; Hutzler, S. Dense packings of spheres in cylinders: Simulations. Phys. Rev. E 2012, 85, 051305. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xia, Y. Self-assembly of spherical colloids into helical chains with well-controlled handedness. J. Am. Chem. Soc. 2003, 125, 2048–2049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; de Folter, J.W.J.; Huang, J.; Philipse, A.P.; Kegel, W.K.; Petukhov, A.V. Helical colloidal sphere structures through thermo-reversible co-assembly with molecular microtubes. Angew. Chem. Int. Ed. 2013, 52, 3364–3368. [Google Scholar] [CrossRef] [PubMed]
- Ouhajji, S.; van Ravensteijn, B.G.P.; Fernández-Rico, C.; Lacina, K.S.; Philipse, A.P.; Petukhov, A.V. Wet-Chemical Synthesis of Chiral Colloids. ACS Nano 2018, 12, 12089–12095. [Google Scholar] [CrossRef] [PubMed]
- Ouhajji, S. Chiral and Active Colloids. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2019. [Google Scholar]
- Lekkerkerker, H.N.W.; Tuinier, R. Colloids and the Depletion Interaction; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Asakura, S.; Oosawa, F. On Interaction between Two Bodies Immersed in a Solution of Macromolecules. J. Chem. Phys. 1954, 22, 1255–1256. [Google Scholar] [CrossRef]
- Devanand, K.; Selser, J.C. Asymptotic behavior and long-range interactions in aqueous solutions of poly(ethylene oxide). Macromolecules 1991, 24, 5943–5947. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heijnen, S.M.F.; van Vliet, P.; Kuipers, B.W.M.; Philipse, A.P.; Petukhov, A.V.; Ouhajji, S. Depletion-Induced Chiral Chain Formation of Magnetic Spheres. Materials 2021, 14, 507. https://doi.org/10.3390/ma14030507
Heijnen SMF, van Vliet P, Kuipers BWM, Philipse AP, Petukhov AV, Ouhajji S. Depletion-Induced Chiral Chain Formation of Magnetic Spheres. Materials. 2021; 14(3):507. https://doi.org/10.3390/ma14030507
Chicago/Turabian StyleHeijnen, Sandrine M. F., Patrick van Vliet, Bonny W. M. Kuipers, Albert P. Philipse, Andrei V. Petukhov, and Samia Ouhajji. 2021. "Depletion-Induced Chiral Chain Formation of Magnetic Spheres" Materials 14, no. 3: 507. https://doi.org/10.3390/ma14030507
APA StyleHeijnen, S. M. F., van Vliet, P., Kuipers, B. W. M., Philipse, A. P., Petukhov, A. V., & Ouhajji, S. (2021). Depletion-Induced Chiral Chain Formation of Magnetic Spheres. Materials, 14(3), 507. https://doi.org/10.3390/ma14030507








