Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All inorganic halide perovskites nanosystem: Synthesis, structural features, optical properties and optoelectronic applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.B.; Zaiats, G.; Wappes, I.; Kamat, P.V. CsPbBr3 solar cells: Controlled film growth through layer-by-layer quantum dot deposition. Chem. Mater. 2017, 29, 9767–9774. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, X.; Song, J.; Xiao, L.; Zeng, H.; Sun, H. All-inorganic colloidal perovskite quantum dots: A new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.-S.; Li, H.-Z.; Ge, J.; Li, H.-D.; Yin, Y.-C.; Wang, K.-H.; Chen, C.; Yao, J.-S.; Zhang, Q.; Yao, H.-B. Room temperature precipitated dual phase CsPbBr3–CsPb2Br5 nanocrystals for stable perovskite light emitting diodes. Nanoscale 2018, 10, 19262–19271. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Li, J.; Chen, J.; Wu, Y.; Liu, K.; Song, J.; Zeng, H. Heterogeneous nucleation toward polar-solvent-free, fast, and one-pot synthesis of highly uniform perovskite quantum dots for wider color gamut display. Adv. Mater. Interfaces 2018, 5, 1800010. [Google Scholar] [CrossRef]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead halide perovskite nanocrystals in the research spotlight: Stability and defect tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Wang, L.-W. High defect tolerance in lead halide perovskite CsPbBr3. J. Phys. Chem. Lett. 2017, 8, 489–493. [Google Scholar] [CrossRef]
- Li, X.; Yu, D.; Cao, F.; Gu, Y.; Wei, Y.; Wu, Y.; Song, J.; Zeng, H. Healing all-inorganic perovskite films via recyclable dissolution–recyrstallization for compact and smooth carrier channels of optoelectronic devices with high stability. Adv. Funct. Mater. 2016, 26, 5903–5912. [Google Scholar] [CrossRef]
- Xia, H.; Wu, S.; Li, L.; Zhang, S. High binding ability ligand controlled formation of CsPbX3 (X = Cl/Br, Br, I) perovskite nanocrystals with high quantum yields and enhanced stability. RSC Adv. 2018, 8, 35973–35980. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Zhang, J.; Gao, Y.; Zheng, Y.; Wang, K.; Sun, X.W. All-inorganic perovskite nanocrystals for high-efficiency light emitting diodes: Dual-phase CsPbBr3-CsPb2Br5 composites. Adv. Funct. Mater. 2016, 26, 4595–4600. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Shi, T.; Liao, G.; Tang, Z. Controllable synthesis of all inorganic lead halide Perovskite nanocrystals with various appearances in multiligand reaction system. Nanomaterials 2019, 9, 1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooney, J.; Kambhampati, P. Get the basics right: Jacobian conversion of wavelength and energy scales for quantitative analysis of emission spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Lan, J.; Luo, L.; Wang, M.; Li, F.; Wu, X.; Wang, F. One pot gram-scale synthesis of CsPbBr3 nanocrystals and their application in green LED. J. Lumin. 2019, 210, 464–471. [Google Scholar] [CrossRef]
- Koolyk, M.; Amgar, D.; Aharon, S.; Etgar, L. Kinetics of cesium lead halide perovskite nanoparticle growth; focusing and de-focusing of size distribution. Nanoscale 2016, 8, 6403–6409. [Google Scholar] [CrossRef]
- Kim, S.H.; Man, M.T.; Lee, J.W.; Park, K.-D.; Lee, H.S. Influence of size and shape anisotropy on optical properties of CdSe quantum dots. Nanomaterials 2020, 10, 1589. [Google Scholar] [CrossRef]
- Kim, S.H.; Man, M.T.; Lee, H.S. Size and shell effects on CdSe quantum dots in binary ligand system. Appl. Sci. Converg. Technol. 2020, 29, 87–90. [Google Scholar] [CrossRef]
- Brennan, M.C.; Herr, J.E.; Nguyen-Beck, T.S.; Zinna, J.; Draguta, S.; Rouvimov, S.; Parkhill, J.; Kuno, M. Origin of the size-dependent stokes shift in CsPbBr3 perovskite nanocrystals. J. Am. Chem. Soc. 2017, 139, 12201–12208. [Google Scholar] [CrossRef] [PubMed]
- Campos-Gonzalez, E.; Rodriguez-Fragozo, P.; de la Cruz, G.G.; Santoyo-Salazar, J.; Zelaya-Angel, O. Synthesis of CdSe nanoparticles immersed in an organic matrix of amylopectin by means of rf sputtering. J. Cryst. Growth 2012, 338, 251–255. [Google Scholar] [CrossRef]
- Dutta, J.; Ajith, M.C.; Dutta, S.; Kadhane, U.R.; Kochupurackal, J.B.; Rai, B. An inherent instability study using ab initio computational methods and experimental validation of Pb(SCN)2 based perovskites for solar cell applications. Sci. Rep. 2020, 10, 15241. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Chukwuocha, E.O.; Onyeaju, M.C.; Harry, T.S.T. Theoretical studies on the effect of confinement on quantum dots using the Brus Equation. World J. Condens. Matter Phys. 2012, 2, 96–100. [Google Scholar] [CrossRef] [Green Version]
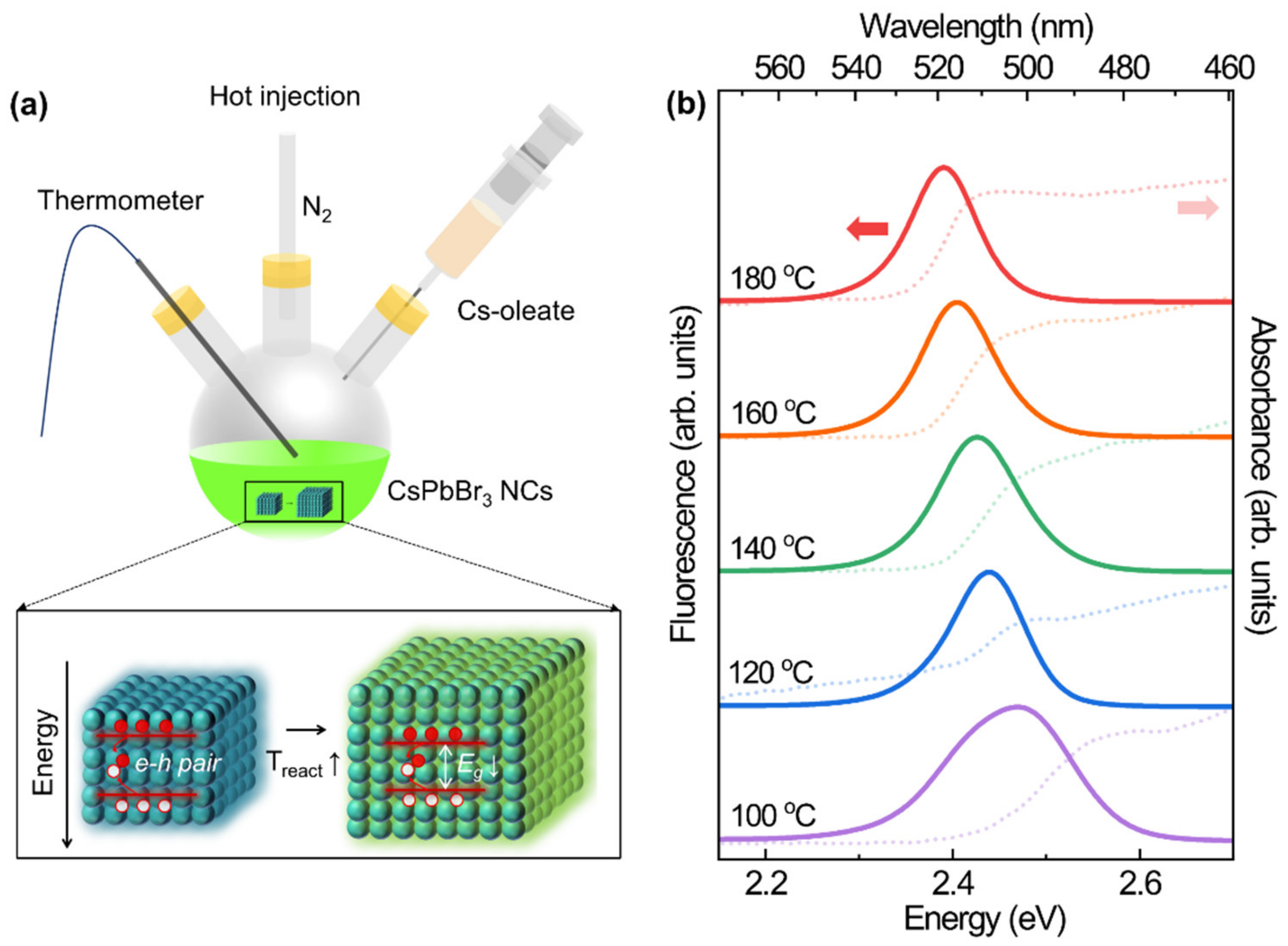
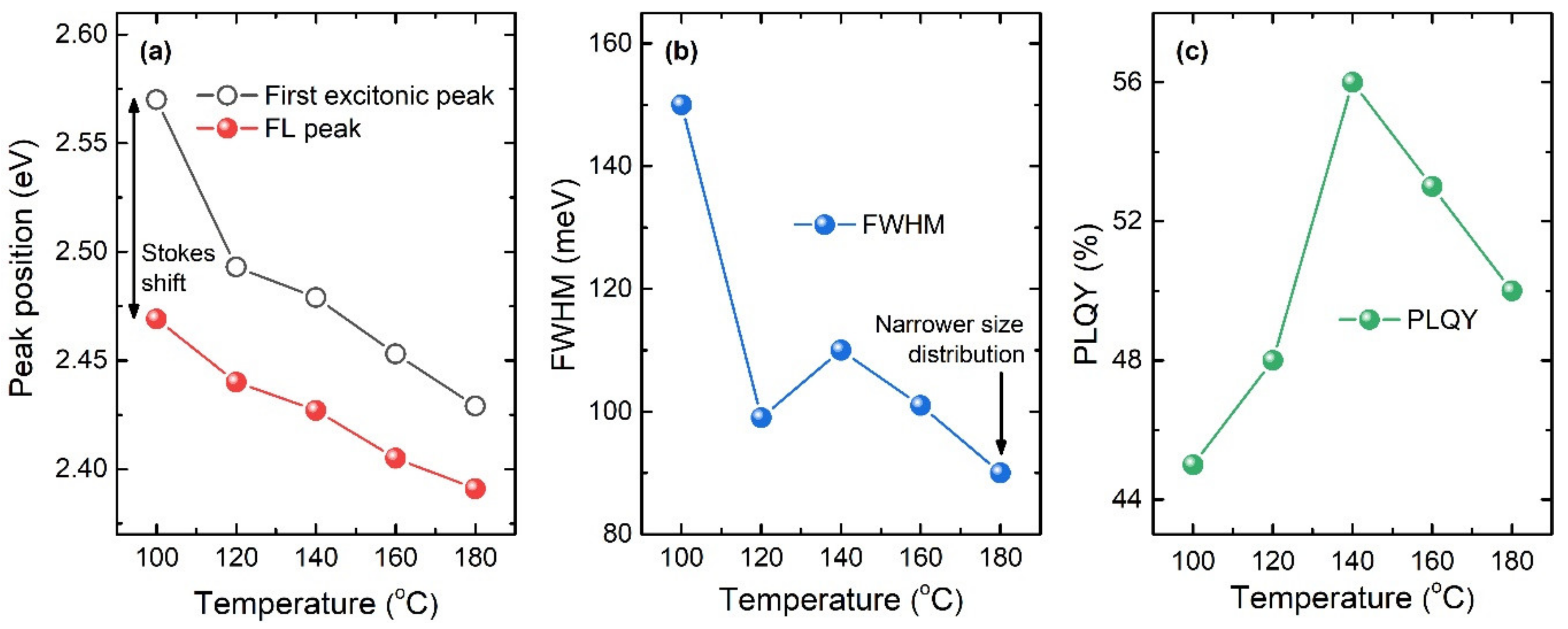
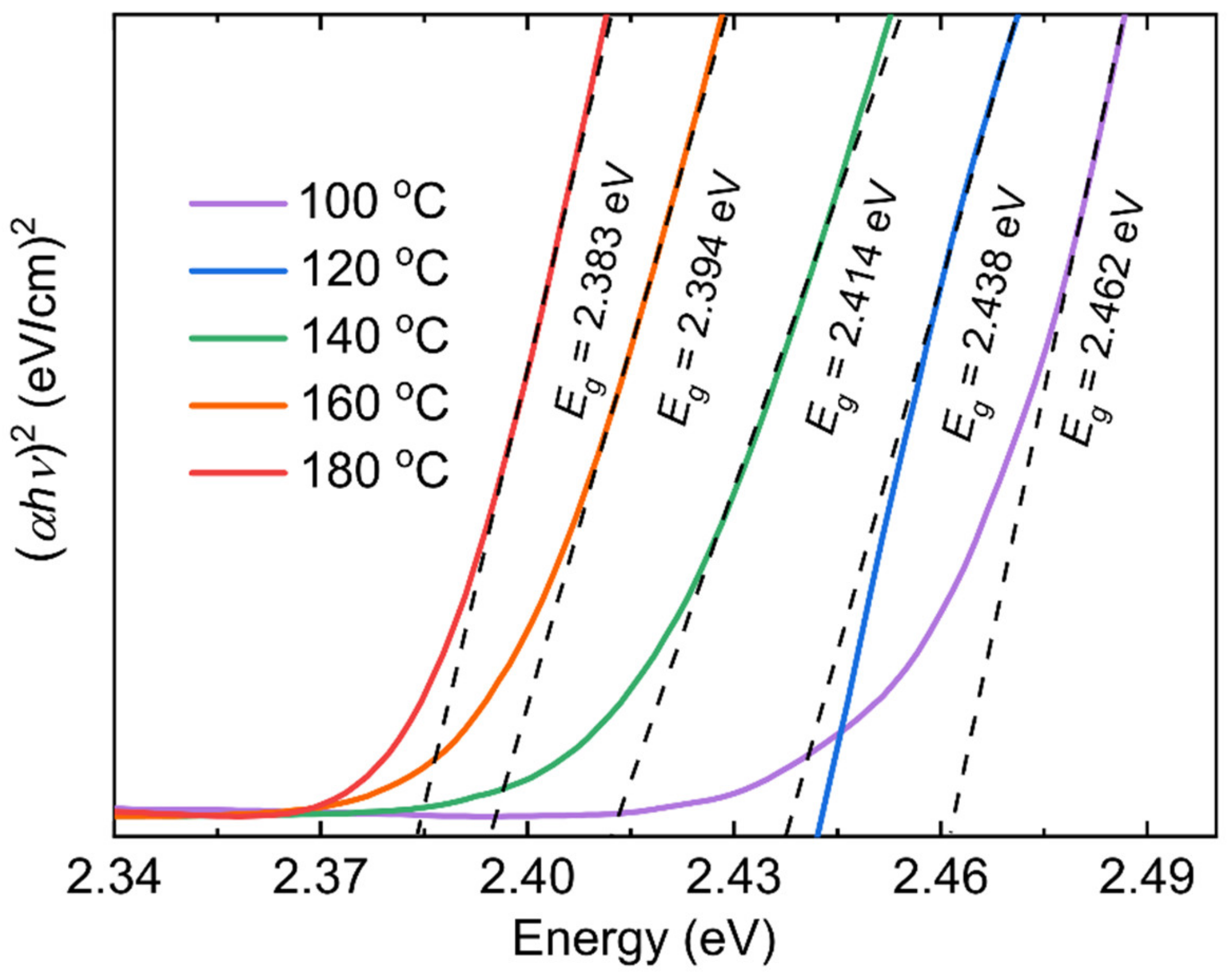
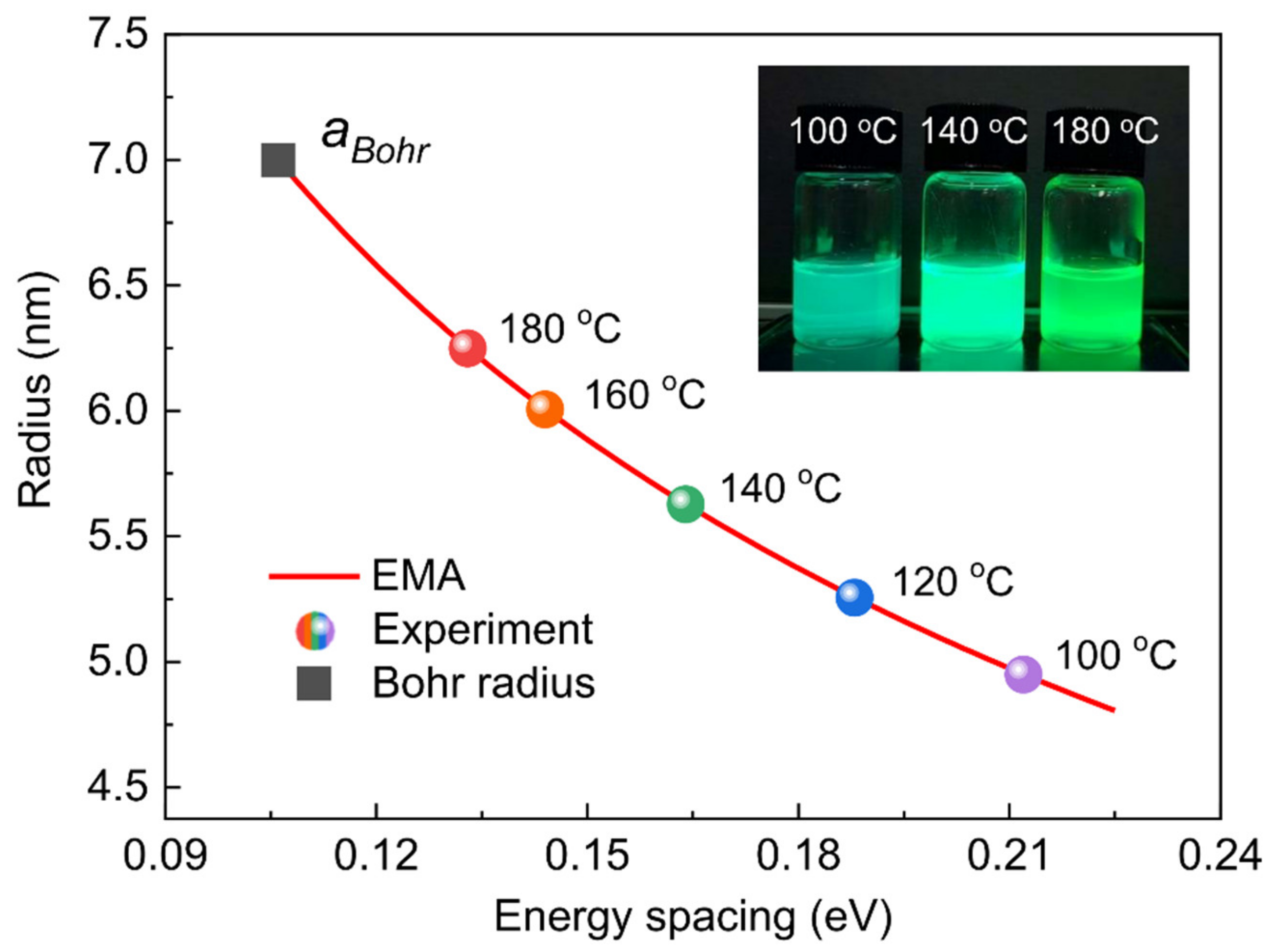
| Reaction Temperature (°C) | 100 | 120 | 140 | 160 | 180 |
|---|---|---|---|---|---|
| Energy Spacing (eV) | 0.21 | 0.19 | 0.16 | 0.14 | 0.13 |
| Diameter (nm) | 9.9 | 10.5 | 11.3 | 12.0 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Park, K.-D.; Lee, H.S. Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals. Energies 2021, 14, 275. https://doi.org/10.3390/en14020275
Kim SH, Park K-D, Lee HS. Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals. Energies. 2021; 14(2):275. https://doi.org/10.3390/en14020275
Chicago/Turabian StyleKim, Sung Hun, Kyoung-Duck Park, and Hong Seok Lee. 2021. "Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals" Energies 14, no. 2: 275. https://doi.org/10.3390/en14020275
APA StyleKim, S. H., Park, K.-D., & Lee, H. S. (2021). Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals. Energies, 14(2), 275. https://doi.org/10.3390/en14020275






