Alternative Splicing Increases Sirtuin Gene Family Diversity and Modulates Their Subcellular Localization and Function
Abstract
1. Introduction
2. Results
2.1. Twenty-Three Sirtuin Isoforms in the Human Genome
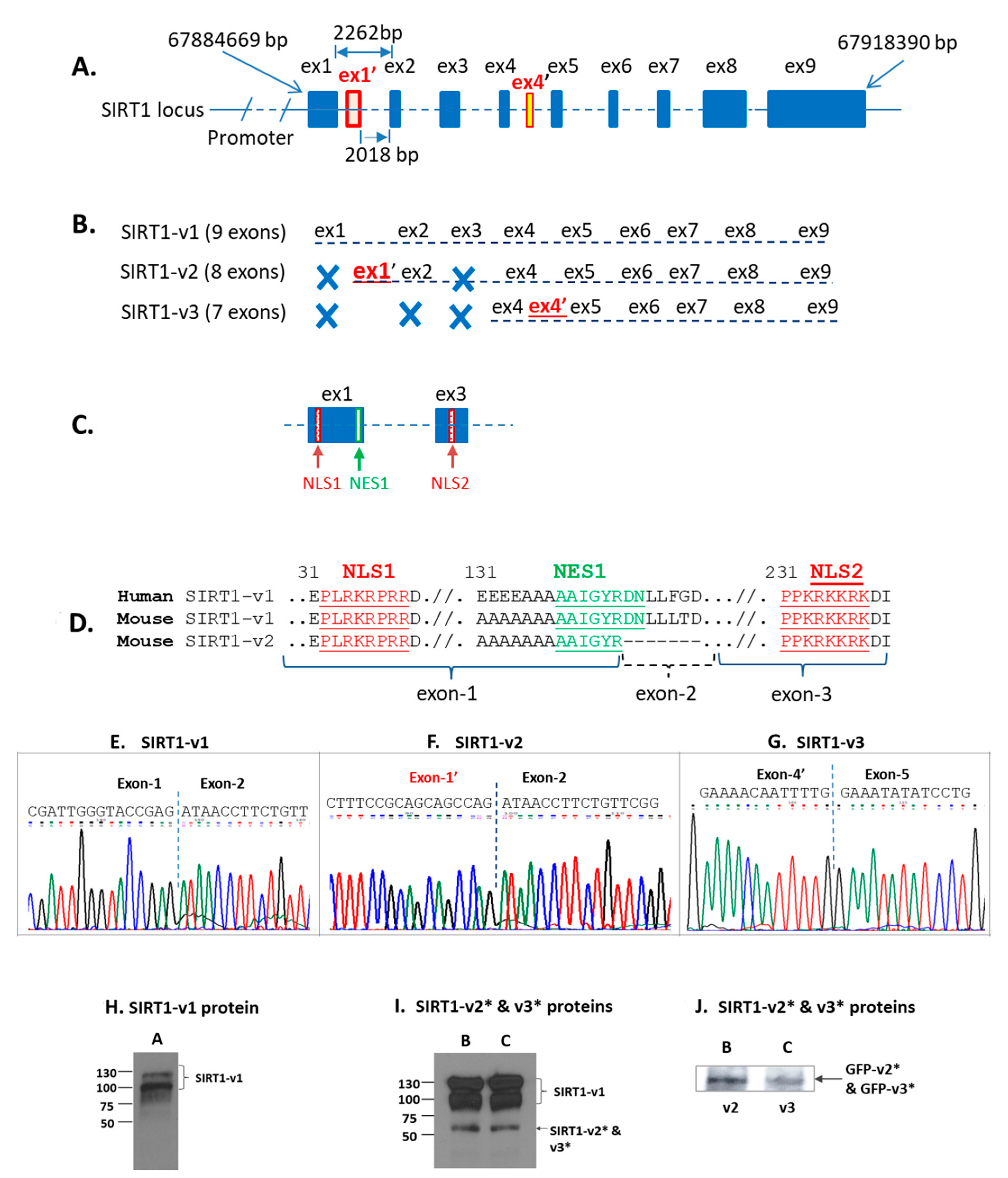
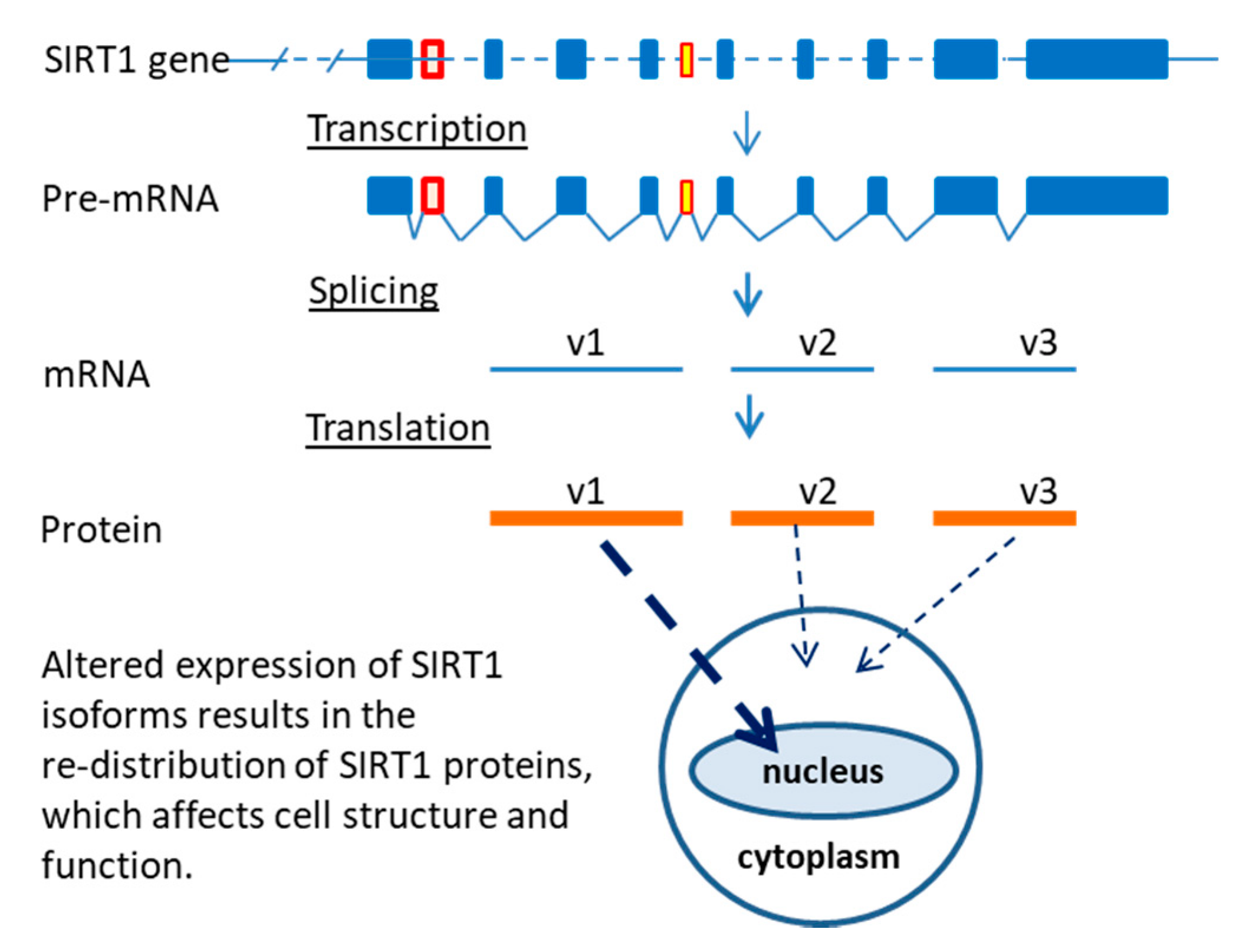
2.2. Sirtuin-1 (SIRT1) Gene Locus and Three Isoforms
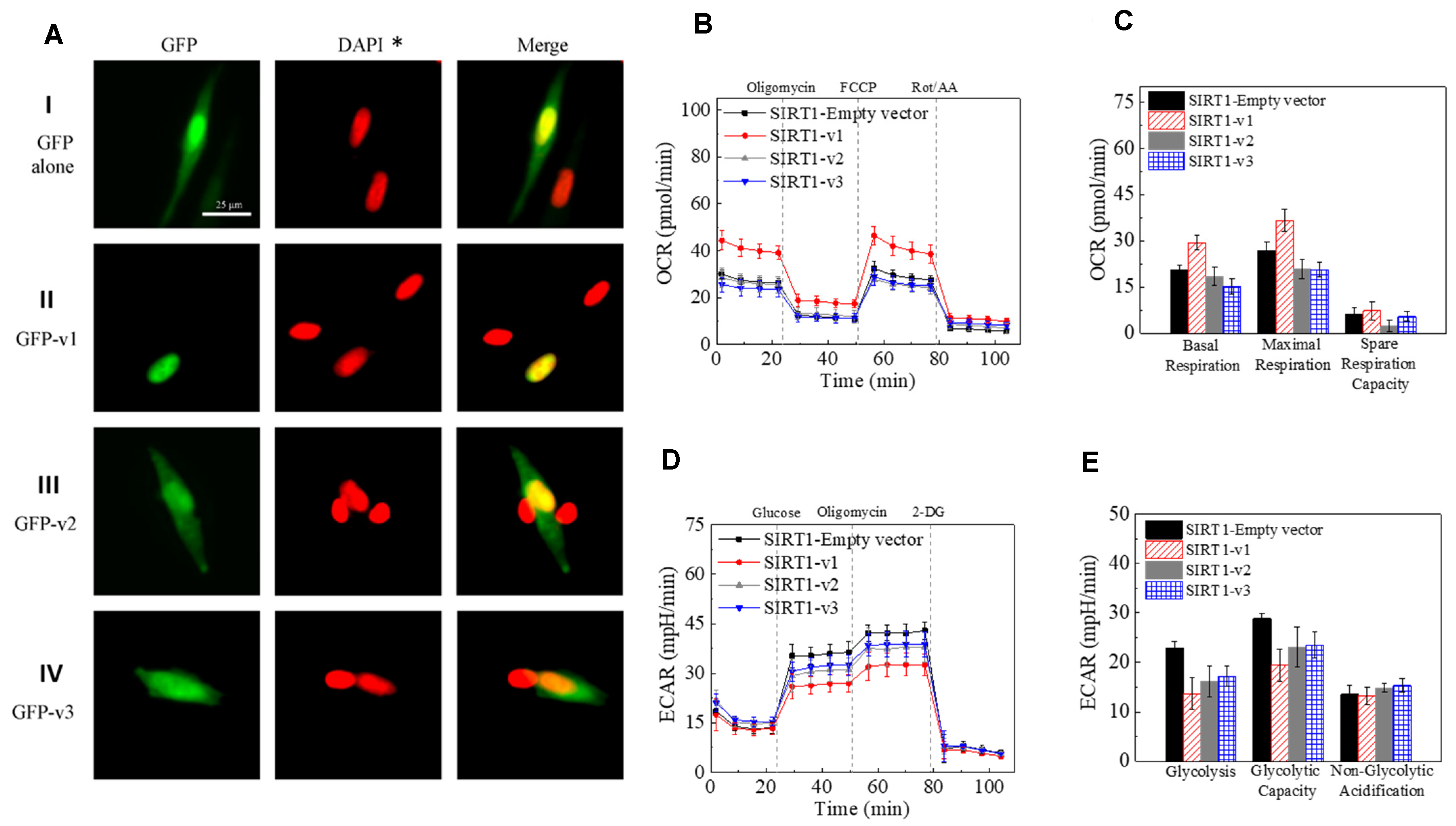
2.3. Differential Localization of Human SIRT1 Isoform Proteins in the Cells
2.4. The Effect of SIRT1 Isoforms Expression on Mitochondrial Function
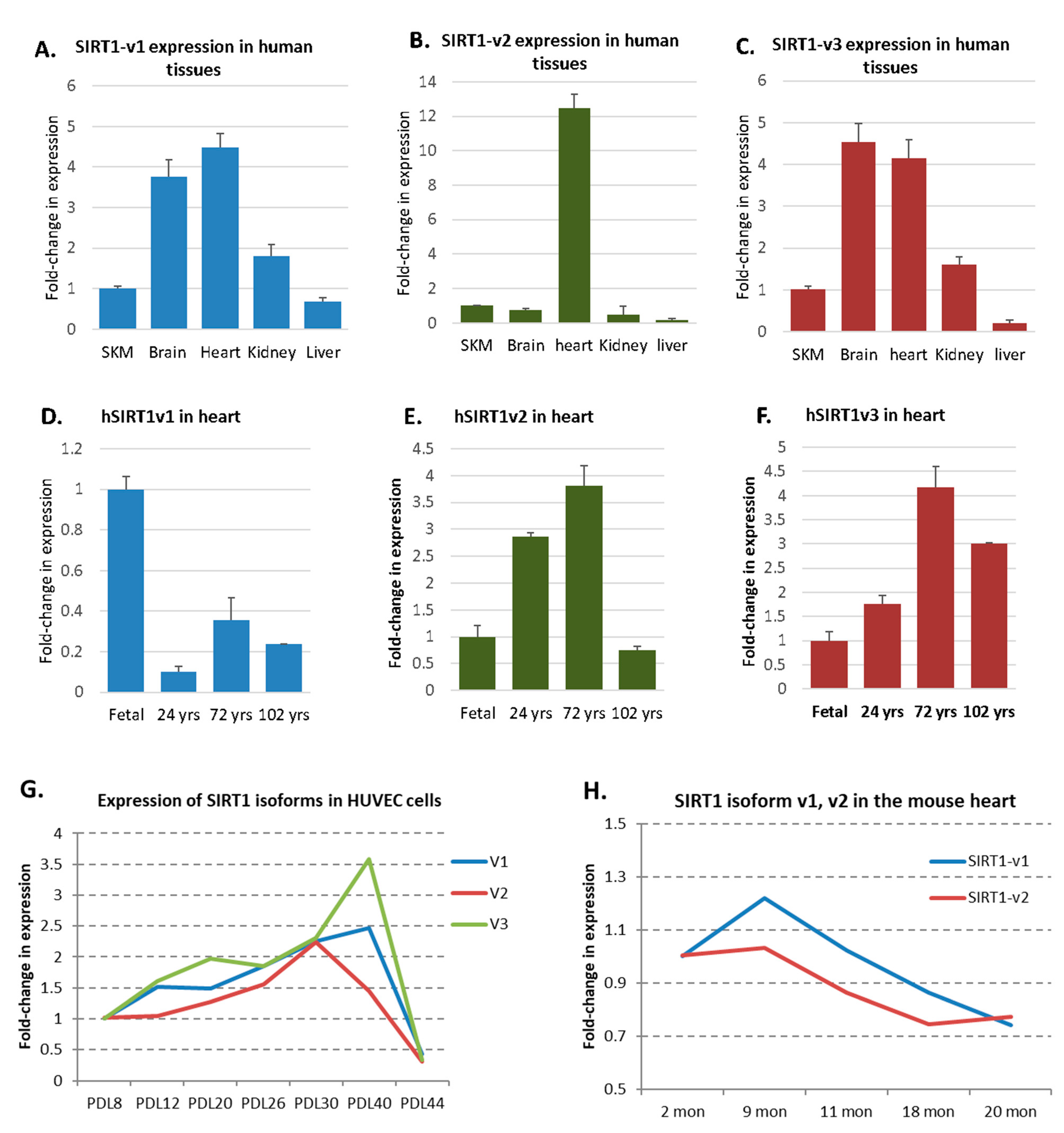
2.5. A Dynamic Expression Pattern of SIRT1 Isoforms in Human Tissues, HUVEC, and Mouse Hearts
3. Discussion
4. Materials and Methods
4.1. The Nomenclature of the Sirtuin Isoforms and Bioinformatics Analysis
4.2. Human and Mouse Tissues, mRNA and cDNA Samples
4.3. Cell Culture, Plasmid Constructs and Quantitative Reverse-Transcriptase PCR
4.4. Measurement of Mitochondrial Oxygen Consumption and Glycolytic Rate
4.5. Immunocytochemical Analysis
4.6. Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brachmann, C.B.; Sherman, J.M.; Devine, S.E.; Cameron, E.E.; Pillus, L.; Boeke, J.D. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995, 9, 2888–2902. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 2000, 273, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Koentges, C.; Pfeil, K.; Schnick, T.; Wiese, S.; Dahlbock, R.; Cimolai, M.C.; Meyer-Steenbuck, M.; Cenkerova, K.; Hoffmann, M.M.; Jaeger, C.; et al. SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res. Cardiol. 2015, 110, 36. [Google Scholar] [CrossRef] [PubMed]
- Onyango, P.; Celic, I.; McCaffery, J.M.; Boeke, J.D.; Feinberg, A.P. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc. Natl. Acad. Sci. USA 2002, 99, 13653–13658. [Google Scholar] [CrossRef] [PubMed]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef]
- Sacconnay, L.; Carrupt, P.A.; Nurisso, A. Human sirtuins: Structures and flexibility. J. Struct. Biol. 2016, 196, 534–542. [Google Scholar] [CrossRef]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef]
- Schwerk, C.; Schulze-Osthoff, K. Regulation of apoptosis by alternative pre-mRNA splicing. Mol. Cell 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef]
- Tang, Y.; Horikawa, I.; Ajiro, M.; Robles, A.I.; Fujita, K.; Mondal, A.M.; Stauffer, J.K.; Zheng, Z.M.; Harris, C.C. Downregulation of splicing factor SRSF3 induces p53beta, an alternatively spliced isoform of p53 that promotes cellular senescence. Oncogene 2013, 32, 2792–2798. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Elowitz, M.B. Constitutive splicing and economies of scale in gene expression. Nat. Struct. Mol. Biol. 2019, 26, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. Tem 2014, 25, 138–145. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.; de Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res. 2013, 41, D344–D347. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Voelter-Mahlknecht, S.; Mahlknecht, U. Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int. J. Mol. Med. 2006, 17, 59–67. [Google Scholar] [CrossRef][Green Version]
- Harries, L.W.; Hernandez, D.; Henley, W.; Wood, A.R.; Holly, A.C.; Bradley-Smith, R.M.; Yaghootkar, H.; Dutta, A.; Murray, A.; Frayling, T.M.; et al. Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell 2011, 10, 868–878. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Grochova, D.; McKenna, T.; Borate, B.; Trivedi, N.S.; Erdos, M.R.; Eriksson, M. Global genome splicing analysis reveals an increased number of alternatively spliced genes with aging. Aging Cell 2016, 15, 267–278. [Google Scholar] [CrossRef]
- Zhang, X.; Azhar, G.; Huang, C.; Cui, C.; Zhong, Y.; Huck, S.; Wei, J.Y. Alternative splicing and nonsense-mediated mRNA decay regulate gene expression of serum response factor. Gene 2007, 400, 131–139. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Naftelberg, S.; Schor, I.E.; Ast, G.; Kornblihtt, A.R. Regulation of alternative splicing through coupling with transcription and chromatin structure. Annu. Rev. Biochem. 2015, 84, 165–198. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.D.; Jackson, B.; Marmorstein, R. Structural basis for sirtuin function: What we know and what we don’t. Biochim. Et Biophys. Acta 2010, 1804, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Marmorstein, R. Structural basis for sirtuin activity and inhibition. J. Biol. Chem. 2012, 287, 42428–42435. [Google Scholar] [CrossRef]
- Moniot, S.; Weyand, M.; Steegborn, C. Structures, substrates, and regulators of Mammalian sirtuins—Opportunities and challenges for drug development. Front. Pharmacol. 2012, 3, 16. [Google Scholar] [CrossRef]
- Hanukoglu, I. Proteopedia: Rossmann fold: A beta-alpha-beta fold at dinucleotide binding sites. Biochem. Mol. Biol. Educ. A Bimon. Publ. Int. Union Biochem. Mol. Biol. 2015, 43, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Finnin, M.S.; Donigian, J.R.; Pavletich, N.P. Structure of the histone deacetylase SIRT2. Nat. Struct. Biol. 2001, 8, 621–625. [Google Scholar] [CrossRef]
- Min, J.; Landry, J.; Sternglanz, R.; Xu, R.M. Crystal structure of a SIR2 homolog-NAD complex. Cell 2001, 105, 269–279. [Google Scholar] [CrossRef]
- Kim, E.J.; Kho, J.H.; Kang, M.R.; Um, S.J. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol. Cell 2007, 28, 277–290. [Google Scholar] [CrossRef]
- Kang, H.; Suh, J.Y.; Jung, Y.S.; Jung, J.W.; Kim, M.K.; Chung, J.H. Peptide switch is essential for Sirt1 deacetylase activity. Mol. Cell 2011, 44, 203–213. [Google Scholar] [CrossRef]
- Ghisays, F.; Brace, C.S.; Yackly, S.M.; Kwon, H.J.; Mills, K.F.; Kashentseva, E.; Dmitriev, I.P.; Curiel, D.T.; Imai, S.I.; Ellenberger, T. The N-Terminal Domain of SIRT1 Is a Positive Regulator of Endogenous SIRT1-Dependent Deacetylation and Transcriptional Outputs. Cell Rep. 2015, 10, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.N.; Finkel, T. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harbor Perspect. Biol. 2012, 4, a013102. [Google Scholar] [CrossRef]
- Lombard, D.B.; Tishkoff, D.X.; Bao, J. Mitochondrial sirtuins in the regulation of mitochondrial activity and metabolic adaptation. Handb. Exp. Pharmacol. 2011, 206, 163–188. [Google Scholar] [PubMed]
- Thanaraj, T.A.; Clark, F.; Muilu, J. Conservation of human alternative splice events in mouse. Nucleic Acids Res. 2003, 31, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Hannenhalli, S.; Choi, S.S. Conservation in first introns is positively associated with the number of exons within genes and the presence of regulatory epigenetic signals. BMC Genom. 2014, 15, 526. [Google Scholar] [CrossRef] [PubMed]
- Nurtdinov, R.N.; Neverov, A.D.; Favorov, A.V.; Mironov, A.A.; Gelfand, M.S. Conserved and species-specific alternative splicing in mammalian genomes. Bmc Evol. Biol. 2007, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Shah, Z.H.; Allison, S.J.; Ahmed, S.U.; Ford, J.; Warnock, L.J.; Li, H.; Serrano, M.; Milner, J. SIRT1 undergoes alternative splicing in a novel auto-regulatory loop with p53. PLoS ONE 2010, 5, e13502. [Google Scholar] [CrossRef]
- Rack, J.G.; VanLinden, M.R.; Lutter, T.; Aasland, R.; Ziegler, M. Constitutive nuclear localization of an alternatively spliced sirtuin-2 isoform. J. Mol. Biol. 2014, 426, 1677–1691. [Google Scholar] [CrossRef]
- Joly, A.L.; Andersson, J. Alternative splicing, FOXP3 and cardiovascular disease. Aging 2019, 11, 1905–1906. [Google Scholar] [CrossRef]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Carter, P.; Rost, B. NLSdb: Database of nuclear localization signals. Nucleic Acids Res. 2003, 31, 397–399. [Google Scholar] [CrossRef] [PubMed]
- la Cour, T.; Kiemer, L.; Molgaard, A.; Gupta, R.; Skriver, K.; Brunak, S. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng. Des. Sel. Peds 2004, 17, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Marquis, K.; Pei, J.; Fu, S.C.; Cagatay, T.; Grishin, N.V.; Chook, Y.M. LocNES: A computational tool for locating classical NESs in CRM1 cargo proteins. Bioinformatics 2015, 31, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Claros, M.G.; Vincens, P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 1996, 241, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Tsuji, J.; Fu, S.C.; Tomii, K.; Horton, P.; Imai, K. MitoFates: Improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol. Cell. Proteom. 2015, 14, 1113–1126. [Google Scholar] [CrossRef]
- Rogers, S.C.; Zhang, X.; Azhar, G.; Luo, S.; Wei, J.Y. Exposure to high or low glucose levels accelerates the appearance of markers of endothelial cell senescence and induces dysregulation of nitric oxide synthase. J. Gerontol. Ser. Abiological Sci. Med. Sci. 2013, 68, 1469–1481. [Google Scholar] [CrossRef]
- Zhang, X.; Azhar, G.; Wei, J.Y. The expression of microRNA and microRNA clusters in the aging heart. PLoS ONE 2012, 7, e34688. [Google Scholar] [CrossRef]
- Zhang, X.; Azhar, G.; Rogers, S.C.; Foster, S.R.; Luo, S.; Wei, J.Y. Overexpression of p49/STRAP alters cellular cytoskeletal structure and gross anatomy in mice. BMC Cell Biol. 2014, 15, 32. [Google Scholar] [CrossRef][Green Version]
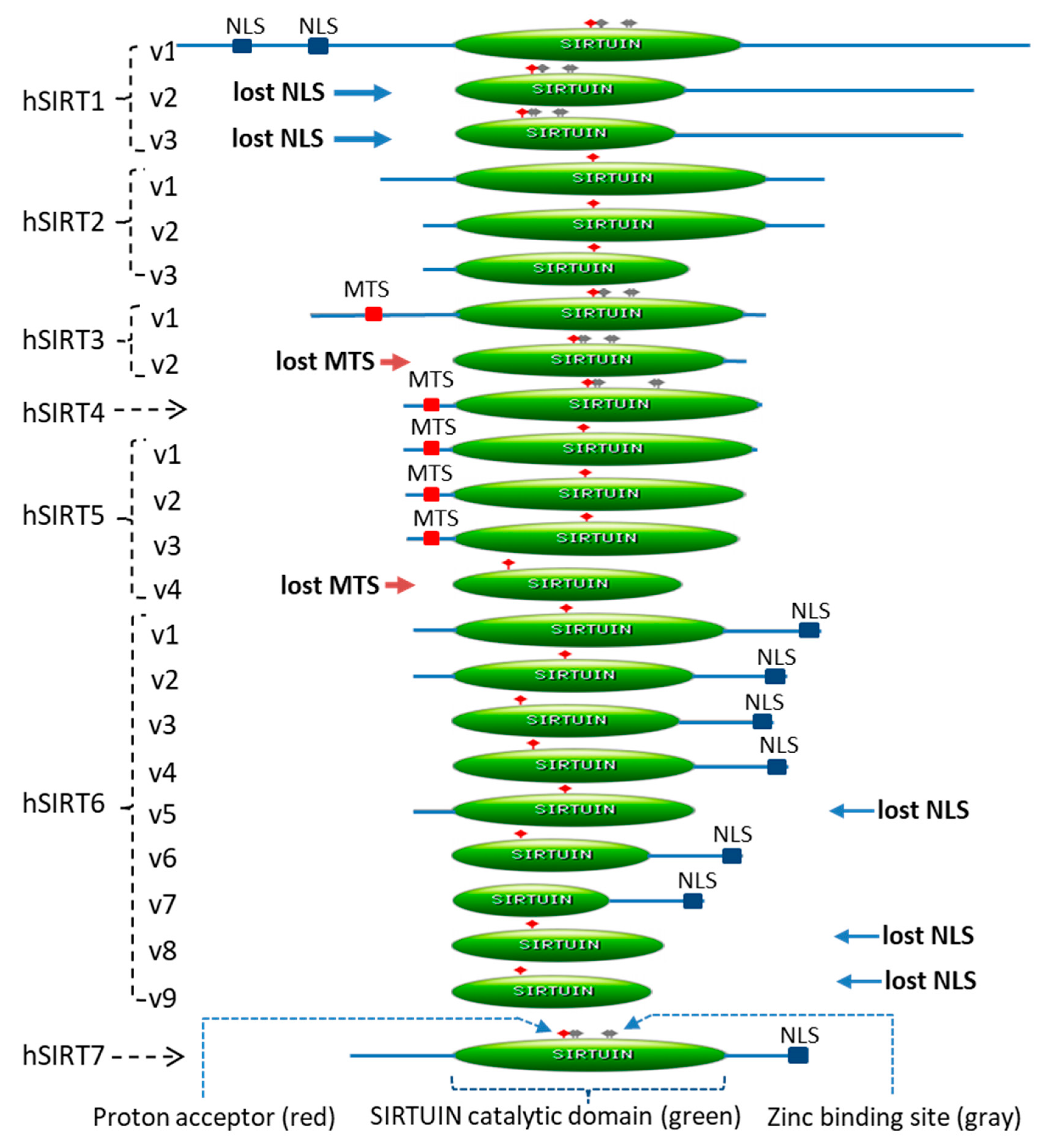
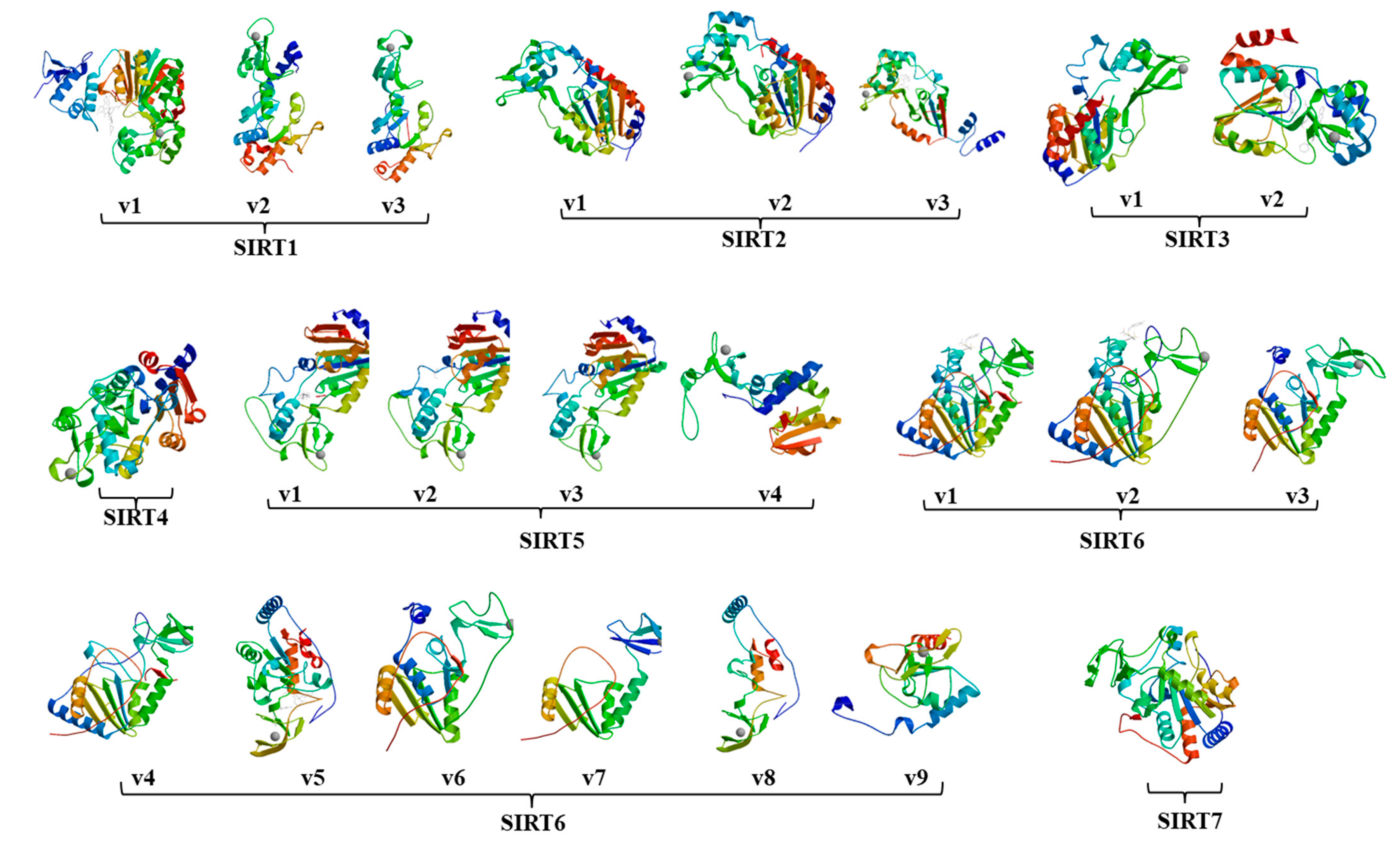
| Gene | Chromosome | Isoform | mRNA Accession# | Exon-Skipping | Protein Accession# | Amino Acid | Sirtuin Domain | ||
|---|---|---|---|---|---|---|---|---|---|
| from–to | Length | % (SIRT/Total) | |||||||
| SIRT1 | 10 | V1 | NM_012238 | NP_036370 | 747 | 244–498 | 254 | 34.0 | |
| V2 | NM_001142498 | ex1, ex3 | NP_001135970 | 452 | 1–203 | 202 | 44.7 | ||
| V3 | NM_001314049 | ex1, ex2, ex3 | NP_001300978 | 444 | 1–195 | 194 | 43.7 | ||
| SIRT2 | 19 | V1 | NM_012237 | NP_036369 | 389 | 65–340 | 275 | 70.7 | |
| V2 | NM_030593 | ex2 | NP_085096 | 352 | 28–303 | 275 | 78.1 | ||
| V3 | NM_001193286 | ex2, ex13, ex14 | NP_001180215 | 234 | 28–234 | 206 | 88.0 | ||
| SIRT3 | 11 | V1 | NM_012239 | NP_036371 | 399 | 126–382 | 256 | 64.2 | |
| V2 | NM_001017524 | ex2 | NP_001017524 | 257 | 1–240 | 239 | 93.0 | ||
| SIRT4 | 12 | V1 | NM_012240 | NP_036372 | 314 | 45–314 | 269 | 85.7 | |
| SIRT5 | 6 | V1 | NM_012241 | NP_036373 | 310 | 41–309 | 268 | 86.5 | |
| V2 | NM_031244 | ex1, ex10 | NP_112534 | 299 | 41–299 | 258 | 86.3 | ||
| V3 | NM_001193267 | ex1, ex7 | NP_001180196 | 292 | 41–291 | 250 | 85.6 | ||
| V4 | NM_001242827 | ex1, ex4 | NP_001229756 | 202 | 1–202 | 201 | 99.5 | ||
| SIRT6 | 19 | V1 | NM_016539 | NP_057623 | 355 | 35–274 | 239 | 67.3 | |
| V2 | NM_001193285 | ex6 | NP_001180214 | 328 | 35–247 | 212 | 64.6 | ||
| V3 | NM_001321058 | ex2 | NP_001307987 | 283 | 1–202 | 201 | 71.0 | ||
| V4 | NM_001321059 | ex3 | NP_001307988 | 294 | 1–213 | 212 | 72.1 | ||
| V5 | NM_001321060 | ex7 | NP_001307989 | 248 | 35–248 | 213 | 85.9 | ||
| V6 | NM_001321061 | ex2, ex6 | NP_001307990 | 256 | 1–175 | 174 | 68.0 | ||
| V7 | NM_001321062 | ex2, 3x3 | NP_001307991 | 220 | 1–139 | 138 | 62.7 | ||
| V8 | NM_001321063 | ex3, ex7 | NP_001307992 | 187 | 1–187 | 186 | 99.5 | ||
| V9 | NM_001321064 | ex1, ex7 | NP_001307993 | 176 | 1–176 | 175 | 99.4 | ||
| SIRT7 | 17 | V1 | NM_016538 | NP_057622 | 400 | 90–331 | 241 | 60.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ameer, F.S.; Azhar, G.; Wei, J.Y. Alternative Splicing Increases Sirtuin Gene Family Diversity and Modulates Their Subcellular Localization and Function. Int. J. Mol. Sci. 2021, 22, 473. https://doi.org/10.3390/ijms22020473
Zhang X, Ameer FS, Azhar G, Wei JY. Alternative Splicing Increases Sirtuin Gene Family Diversity and Modulates Their Subcellular Localization and Function. International Journal of Molecular Sciences. 2021; 22(2):473. https://doi.org/10.3390/ijms22020473
Chicago/Turabian StyleZhang, Xiaomin, Fathima S. Ameer, Gohar Azhar, and Jeanne Y. Wei. 2021. "Alternative Splicing Increases Sirtuin Gene Family Diversity and Modulates Their Subcellular Localization and Function" International Journal of Molecular Sciences 22, no. 2: 473. https://doi.org/10.3390/ijms22020473
APA StyleZhang, X., Ameer, F. S., Azhar, G., & Wei, J. Y. (2021). Alternative Splicing Increases Sirtuin Gene Family Diversity and Modulates Their Subcellular Localization and Function. International Journal of Molecular Sciences, 22(2), 473. https://doi.org/10.3390/ijms22020473




