Large-Scale Laboratory Experiments on Mussel Dropper Lines in Ocean Surface Waves
Abstract
:1. Introduction
- To understand the adhesive forces of live mussels attached to farming rope;
- To quantify the drag and inertia coefficients of bivalve-encrusted dropper lines when exposed to waves in large, near full-scale experiments;
- To comparing the force response of live mussels with a surrogate specimen.
2. Materials and Methods
2.1. Testing Specimen
2.2. Surrogate Model of the Live Mussel Dropper Lines
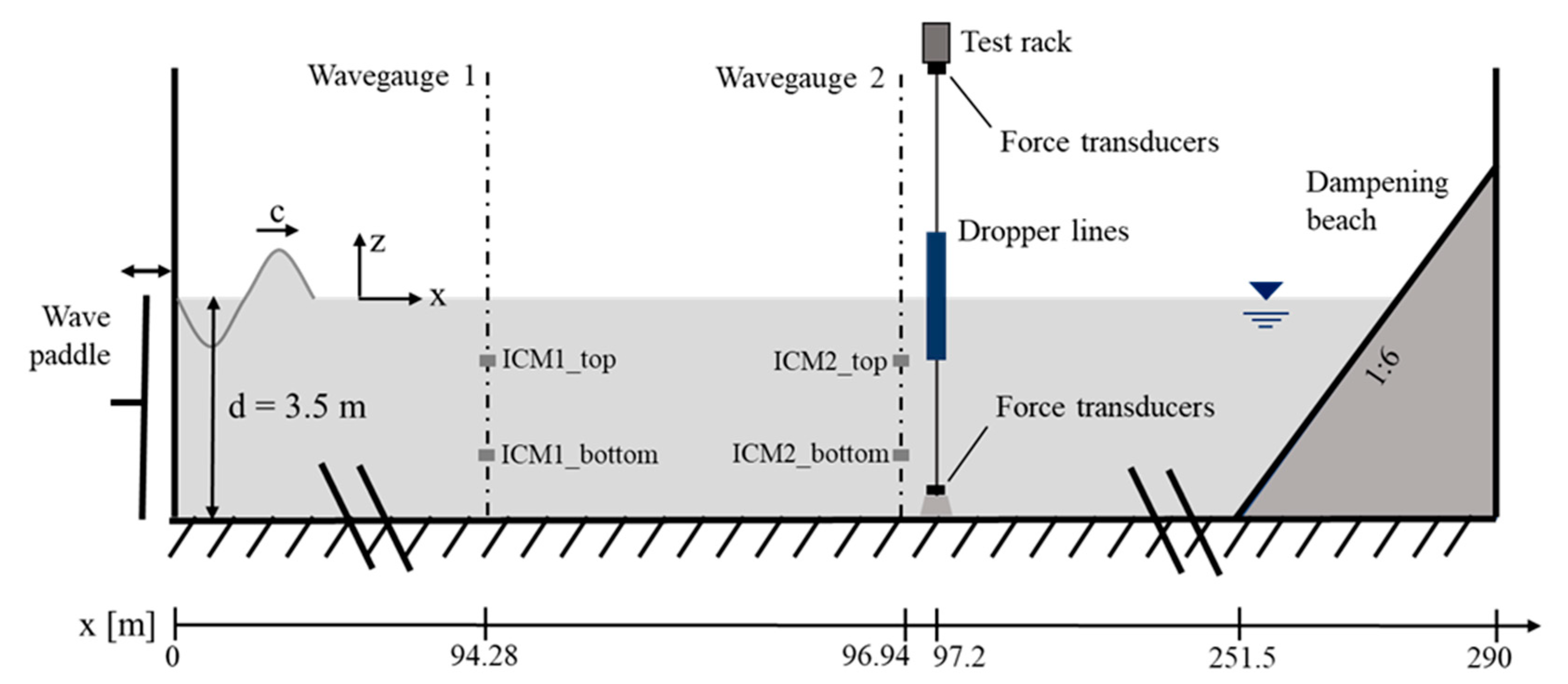
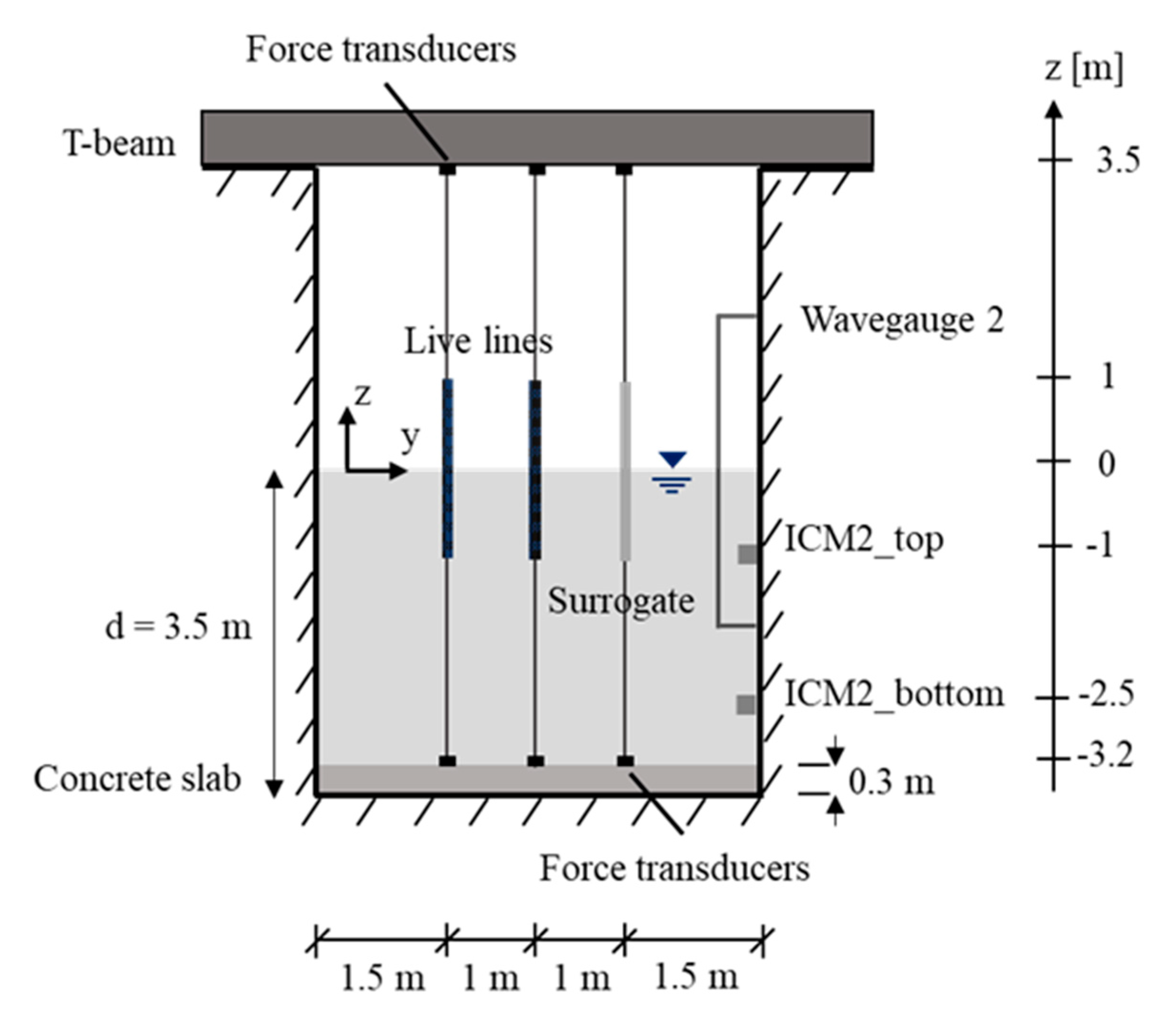
2.3. Experimental Setup
2.4. Instrumentation
2.5. Experimental Procedures
3. Results
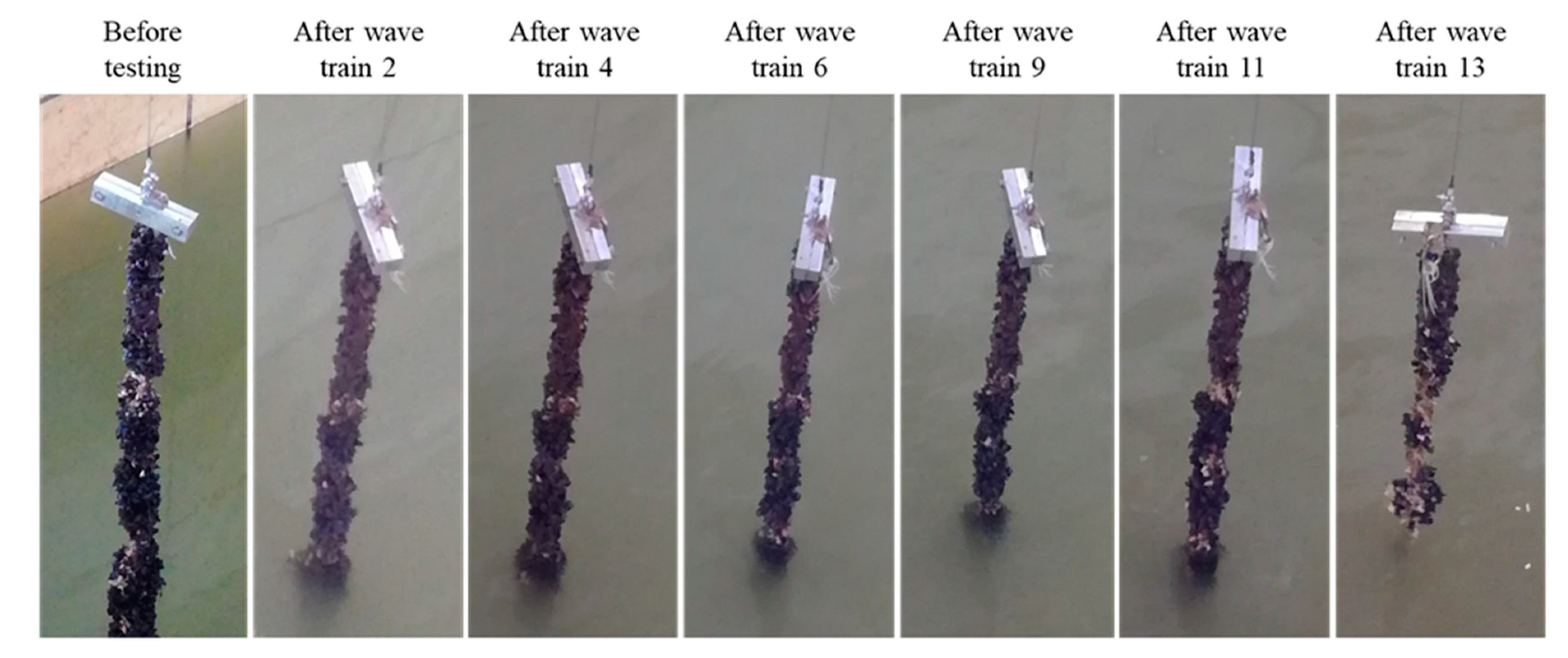
3.1. Dropper Line Testing
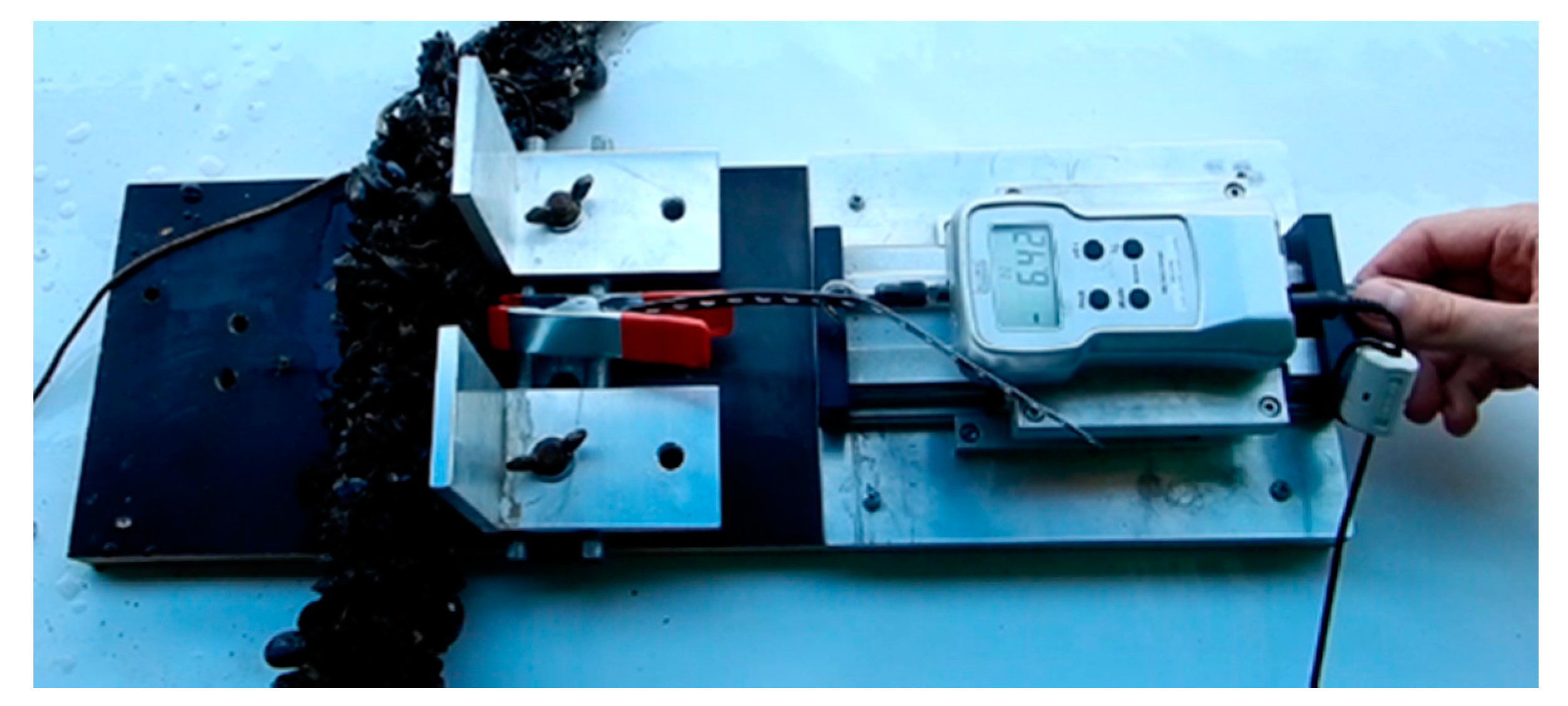
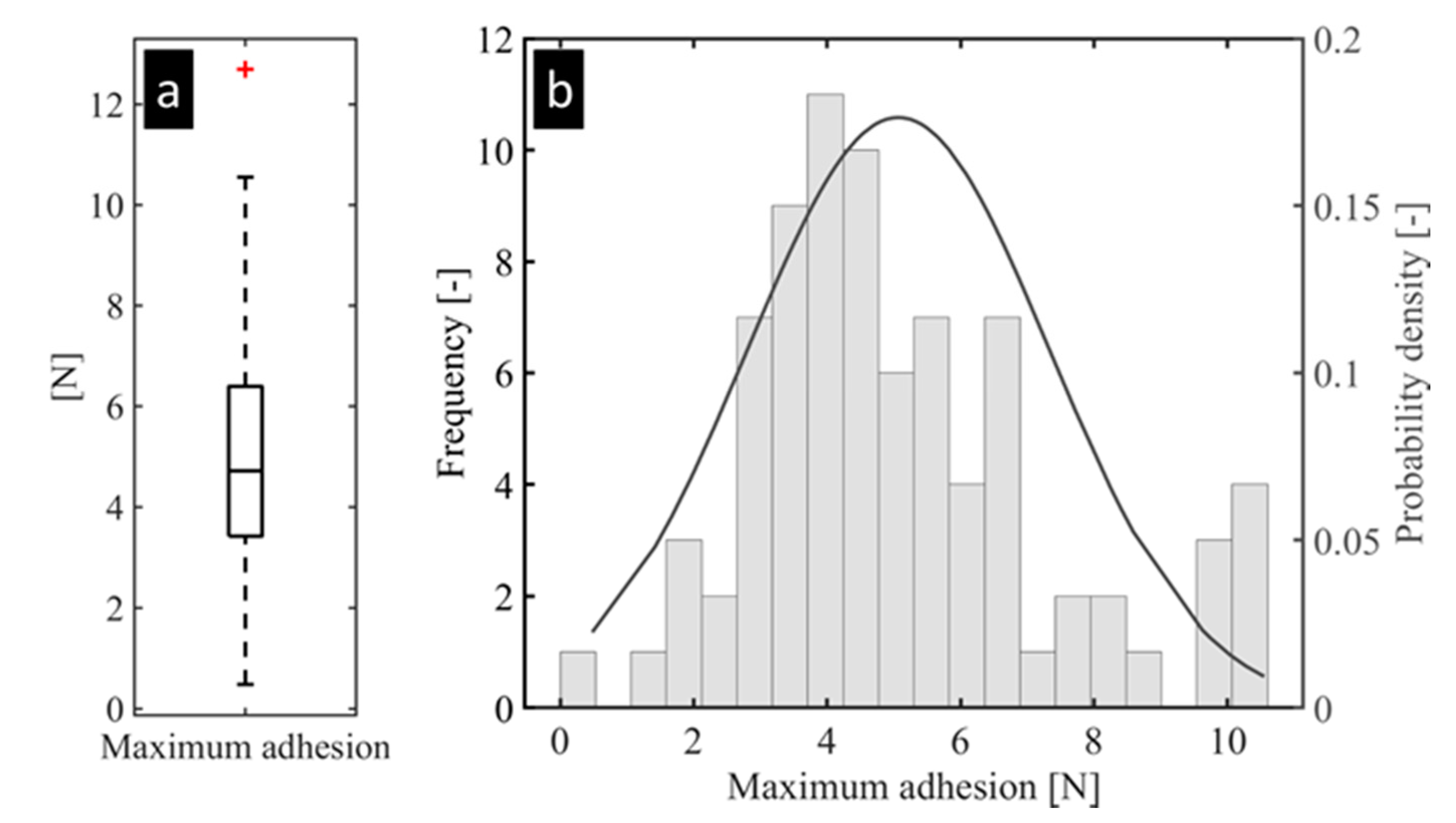
3.2. Individual Mussel Attachment Strength
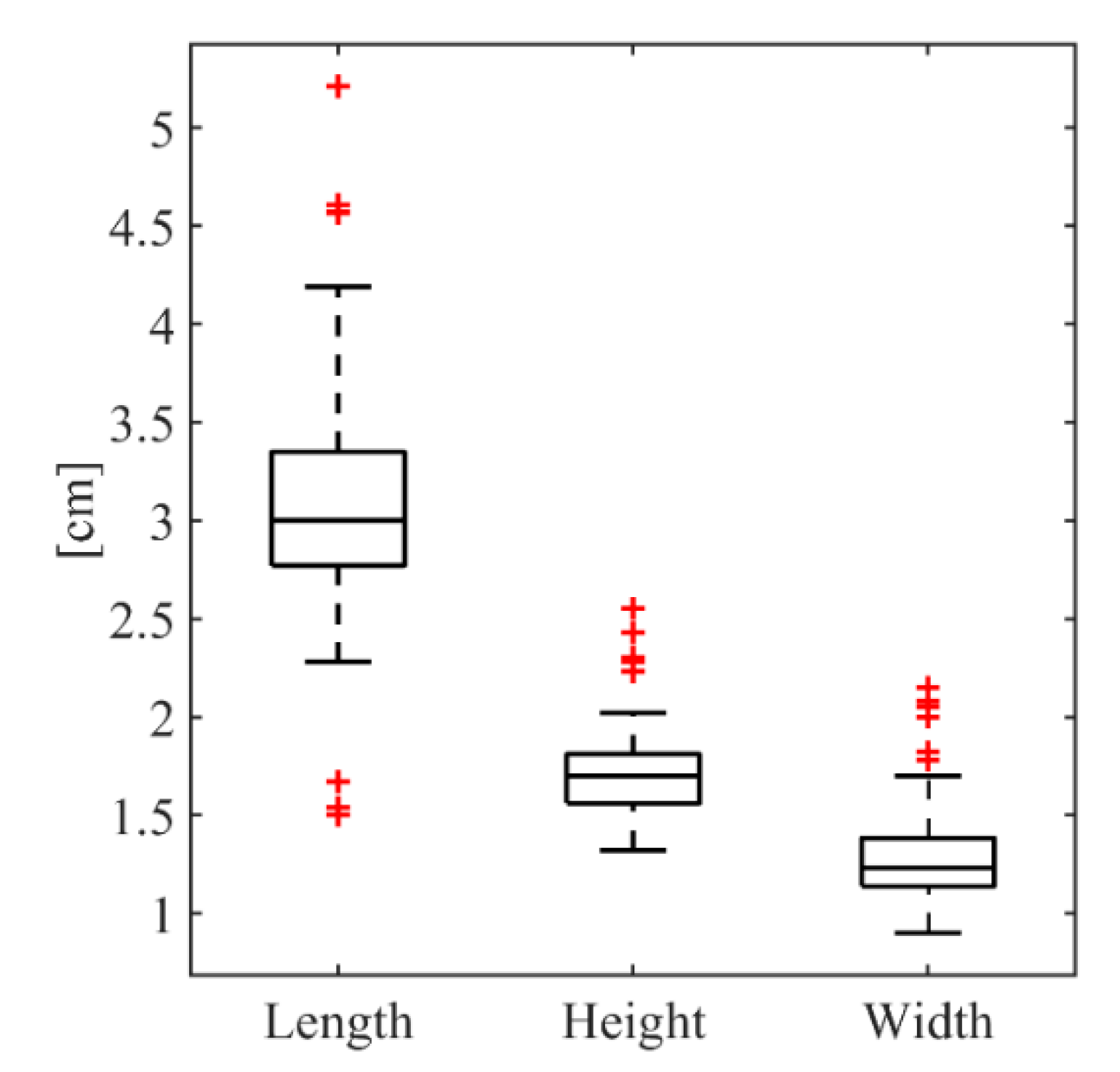
3.3. Properties of Mussel Individuals
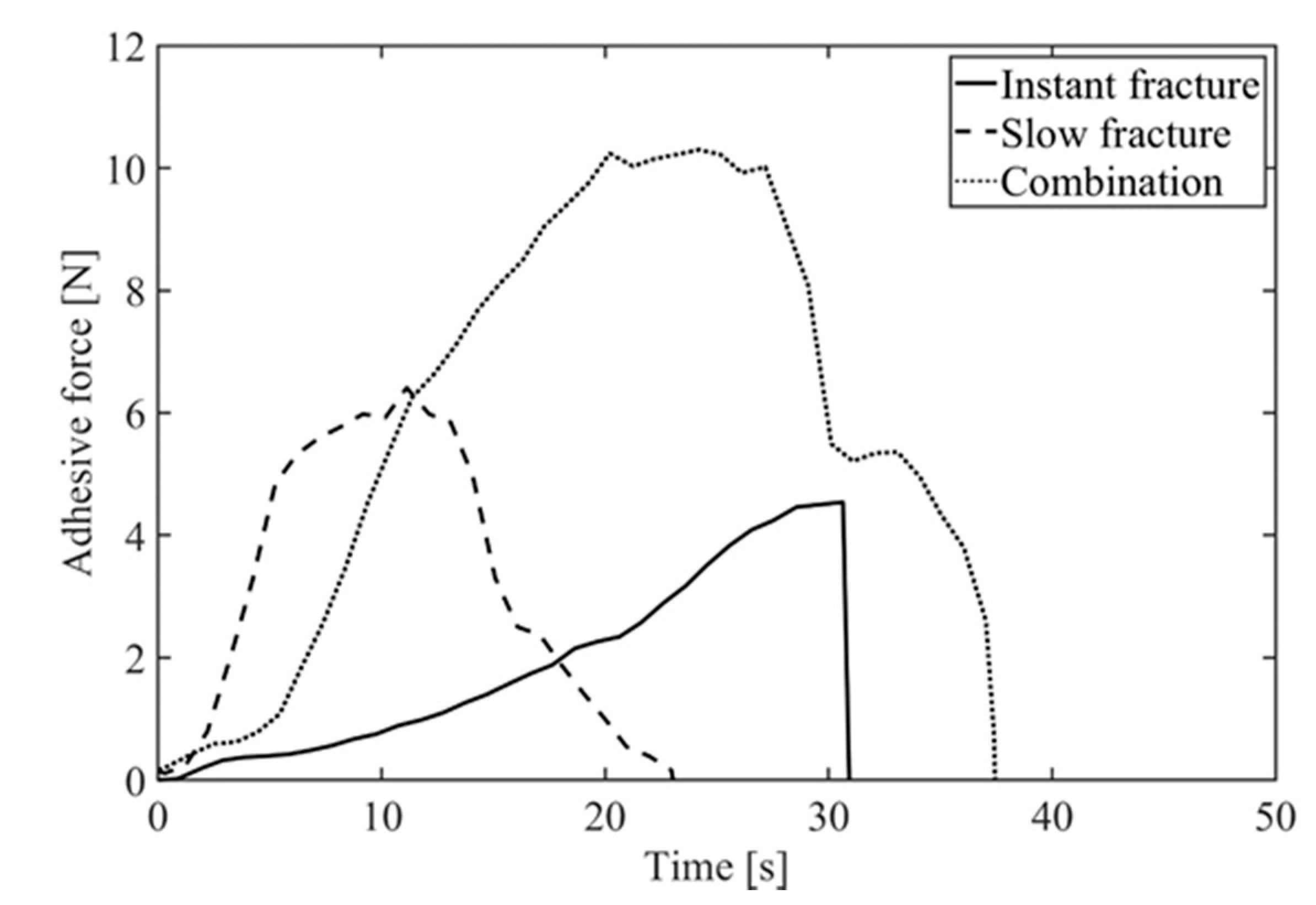
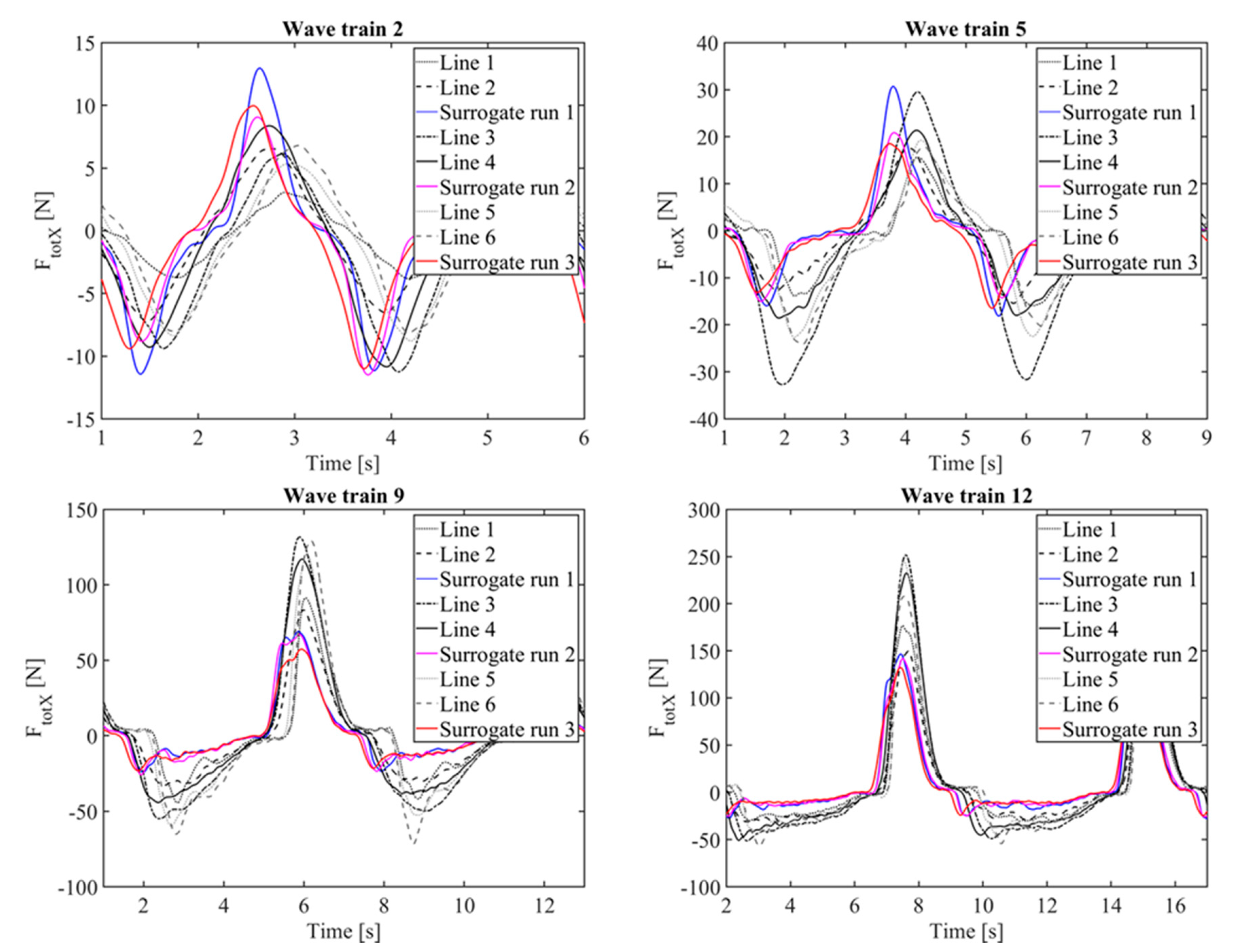
3.4. Force Time Histories
3.5. Force Coefficients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UN. World Population Prospects 2019: Highlights; UN: New York, NY, USA, 2019. [Google Scholar]
- UN. The 2030 Agenda for Sustainable Development; UN: New York, NY, USA, 2015. [Google Scholar]
- Buck, B.H.; Nevejam, N.; Wille, M.; Chambers, M.D.; Chopin, T. Aquaculture Perspectives of Multi-Use Sites in the Open Ocean; Buck, B.H., Langan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 23–69. [Google Scholar]
- Goseberg, N.; Chambers, M.D.; Heasman, K.; Fredricksson, D.; Frdheim, A.; Schlurmann, T. Aquaculture Perspective of Multi-Use Sites in the Open Ocean; The Untapped Potential for Marine Resources in the Anthropocene; Buck, B.H., Langan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–96. [Google Scholar]
- EFSA. Scientific Opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFSA J. 2014, 12, 3761. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; Volume 978. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture—Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Verdegem, M.C.J.; Bosma, R.H.; Verreth, J.A.V. Reducing water for animal production through aquaculture. Int. J. Water Resour. Dev. 2006, 22, 101–113. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: Vulnerability from climate change and population growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef] [Green Version]
- Döös, B.R. Population growth and loss of arable land. Global Environ. Chang. 2002, 12, 303–311. [Google Scholar] [CrossRef]
- Young, A. Is there really spare land? A critique of estimates of available cultivable land in developing countries. Environ. Dev. Sustain. 1999, 1, 3–18. [Google Scholar] [CrossRef]
- Pauly, D.; Alder, J.; Bennete, E.; Christensen, V.; Tyedmers, P.; Watson, R. The future for fisheries. Science 2003, 203, 1359–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 203, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Burke, M. Aquaculture and ocean resources: Raising tigers of the sea. Ann. Rev. Environ. Resour. 2005, 30, 185–218. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Holmer, M.; Olsen, Y.; Soto, D.; Marbà, N.; Guiu, J.; Black, K.; Karakssis, I. Will the oceans help feed humanity? BioScience 2009, 59, 967–976. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Hasan, M.R.; Subasinghe, R.P. Use of Fishery Resources as Feed Inputs for Aquaculture Development: Trend and Policy Implications; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Diana, J.S. Is lower intensity aquaculture a valuable means of producing food? An evaluation of its effects on near-shore and inland waters. Rev. Aquac. 2012, 4, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Olsen, Y.; Otterstad, O.; Duarte, C.M. Aquaculture in the Ecosystem; Holmer, M., Black, K., Duarte, C.M., Marbàn, N., Karakasis, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 293–3319. [Google Scholar]
- Pauly, D.; Trites, A.W.; Capuli, E.; Christensen, V. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 1998, 55, 467–481. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W.; Yang, Y.; Yang, C.; Yuan, Z.; Xiong, S.; Diana, J. Environmental impact of aquaculture and countermeasures to aquaculture pollution in China. Enviorn. Sci. Pollut. Res. 2007, 14, 452–462. [Google Scholar]
- Lloyd, B.D. Potential Effects of Mussel Farming on New Zealands’ Marine Mammals and Seabirds; Department of Conservation: Wellington, New Zealand, 2003. [Google Scholar]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallardi, D. Effects of bivalve aquaculture on the environment and their possible mitigation: A review. Fish. Aquac. J. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Primavera, J.H. Overcoming the impacts of aquaculture on the coastal zone. Ocean Coast. Manag. 2006, 49, 531–545. [Google Scholar] [CrossRef]
- Marra, J. When will we tame the oceans? Nature 2005, 436, 175–176. [Google Scholar] [CrossRef]
- Buck, B.H. Farming in a High Energy Environment: Potentials and Constraints of Sustainable Offshore Aquaculture in the German Bight (North Sea); Universtität Bremen: Bremen, Germany, 2007. [Google Scholar]
- Langan, R. Open Ocean Aquaculture—Moving Forward; Lee, C.S., O‘Bryen, P.J., Eds.; Oceanic Institute: Dana Point, CA, USA, 2007; pp. 57–60. [Google Scholar]
- Holm, P.; Buck, B.H.; Langan, R. Aquaculture Perspective of Multi-Use Sites in the Open Ocean; The Untapped Potential for Marine Resources in the, Anthropocene; Buck, B.H., Langan, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–20. [Google Scholar]
- Gosling, E. Marine Bivalve Molluscs, 2nd ed; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kaiser, M.J.; Synder, B.; Yu, Y. A review of the feasibility, costs, and benefits of platform-based open ocean aquaculture in the Gulf of Mexico. Ocean Coast. Manag. 2011, 54, 721–730. [Google Scholar] [CrossRef]
- Cheney, D.; Langan, R.; Heasman, K.; Friedman, B.; Davis, J. Shellfish culture in the open ocean: Lessons learned for offshore expansion. Mar. Technol. Soc. J. 2010, 44, 55–67. [Google Scholar] [CrossRef]
- Heasman, K.; Taylor, D.; Ericson, J.; Buck, B. Extending New Zealands’ marine shellfish aquaculture into exposed environments - Adapting to modern anthropogenic challenges. Front. Mar. Sci. 2020, 7, 751. [Google Scholar] [CrossRef]
- Hu, J.; Tang, X.; Lin, P.; Liu, P.L.F. Periodic water waves through suspended canopy. Coast. Eng. 2021, 163. [Google Scholar] [CrossRef]
- FAO. Cultured aquatic Species Information Programme: Mytilus Edulis (Linnaeus, 1758); Food and Agriculture Organization of the United Nations: Rome, Italy, 1996. [Google Scholar]
- Garen, P.; Robert, S.; Bougrier, S. Comparison of growth of mussel, Mytilus edulis, on longline, pole and bottom culture sites in the Pertuis Breton, France. Aquaculture 2004, 232, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Robinson, M.; Dewhurst, T. Dynamic of submersible mussel rafts in waves and current. China Ocean Eng. 2015, 29, 431–444. [Google Scholar] [CrossRef]
- Plew, D.R.; Stevens, C.L.; Spigel, R.H.; Hartstein, N.D. Hydrodynamic implications of large offshore mussel farms. IEEE J. Ocean. Eng. 2005, 30, 95–108. [Google Scholar] [CrossRef]
- Delaux, S.; Stevens, C.L.; Popinet, S. High-resolution computational fluid dynamics modelling of suspended shellfish structures. Environ. Fluid Mech. 2011, 11, 405–425. [Google Scholar] [CrossRef] [Green Version]
- O‘Donncha, F.; Hartnett, M.; Nash, S. Physical and numerical investigation of the hydrodynamic implications of aquaculture farms. Aqucultural Eng. 2013, 52, 14–26. [Google Scholar] [CrossRef]
- Gagnon, M.; Bergeron, P. Observations of the loading and motion of a submerged mussel longline at an open ocean site. Aquacult. Eng. 2017, 78, 114–129. [Google Scholar] [CrossRef]
- Stevens, C.L.; Plew, D.R.; Smith, M.J.; Frederiksson, D.W. Hydrodynamic forcing of long-line mussel farms: Observations. J. Waterw. Port Coast. Ocean Eng. 2007, 133, 192–199. [Google Scholar] [CrossRef]
- Raman-Nair, W.; Colbourne, B.; Gagnon, M.; Bergeron, P. Numerical model of a mussel longline system: Coupled dynamics. Ocean Eng. 2008, 35, 1372–1380. [Google Scholar] [CrossRef]
- Stevens, C.; Pley, D.; Hartein, N.; Frederiksson, D. The physics of open-water shellfish aquaculture. Aquacult. Eng. 2008, 38, 145–160. [Google Scholar] [CrossRef]
- Frederiksson, D.W.; Steppe, C.N.; Wallendorf, L.; Sweeney, S.; Kriebel, D. Biological and hydrodynamic design considerations for vertically oriented oyster grow out structures. Aquacult. Eng. 2010, 42, 57–69. [Google Scholar] [CrossRef]
- Plew, D.R. Depth-averaged drag coefficient for modeling flow through suspended canopies. J. Hydraul. Eng. 2011, 137, 234–247. [Google Scholar] [CrossRef]
- Gansel, L.C.; Endresen, P.C.; Steinhovden, K.B.; Dahle, S.W.; Svendsen, E.; Forbord, S.; Jensen, O. Drag on nets fouled with blue mussel (Mytilus Edulis) and sugar kelp (Saccharina Latissima) and parameterization of fouling. In Proceedings of the ASME 2017 36th International Conference on Ocean, Offshore and Arctic Engineering, Trondheim, Norway, 25–30 June 2017. [Google Scholar]
- Al-Yacouby, A.M.; Kurian, V.J.; Sebastian, A.A.; Liew, M.S.; Idichandy, V.G. Effects of marine growth on hydrodynamic coefficients of rigid tubular cylinders. Appl. Mech. Mater. J. 2014, 567, 247–252. [Google Scholar] [CrossRef]
- Henry, P.; Nedrebo, E.L.; Myrhaug, D. Visualisation of the effect of different types of marine growth on cylinders’ wake structure in low Re steady flows. Ocean Eng. 2016, 115, 182–188. [Google Scholar] [CrossRef]
- Loxton, J.; Macleod, A.K.; Nall, C.R.; McCollin, T.; Machado, I.; Simas, T.; Vance, T.; Kenny, C.; Want, A.; Miller, R. Setting an agenda for biofouling research for the marine renewable energy industry. Int. J. Mar. Energy 2017, 19, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Morison, J.R.; O‘Brien, M.P.; Johnson, J.W.; Schaaf, S.A. The force exerted by surface waves on piles. Pet. Trans. AIME 1950, 189. [Google Scholar] [CrossRef]
- Wolfram, J.; Naghipour, M. On the estimation of morison force coefficients and their predictive accuracy for very rough circular cylinders. Appl. Ocean Res. 1999, 21, 311–328. [Google Scholar] [CrossRef]
- Burrows, R.; Tickell, R.G.; Hames, D.; Najafian, G. Morison wave force coefficients for application to random seas. Appl. Ocean Res. 1997, 19, 183–199. [Google Scholar] [CrossRef]
- Landmann, J.; Fröhling, L.; Gieschen, R.; Buck, B.H.; Heasman, K.; Scott, N.; Smeaton, M.; Goseberg, N.; Hildebrandt, A. Drag and inertia coefficients of live and surrogate shellfish dropper lines under steady and oscillatory flow. Ocean Eng. 2021. (Under Review). [Google Scholar]
- Landmann, J.; Ongsiek, T.; Goseberg, N.; Heasman, K.; Buck, B.H.; Pfaffenholz, J.-A.; Hildebrandt, A. Physical modelling of blue mussel dropper lines for the development of surrogates and hydrodynamic coefficients. J. Mar. Sci. Eng. 2019, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, J.G.; Yevich, P. Responses of an estuarine population of the blue mussel Mytilus edulis to heated water from a steam generating plant. J. Mar. Biol. 1976, 34, 177–189. [Google Scholar] [CrossRef]
- Harley, C.D.G. Tidal dynamics, topographic orientation, and temperature-mediated mass mortalities on rocky shores. Mar. Ecol. Prog. Ser. 2008, 371, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Hatcher, A.; Grant, J.; Schofield, B. Seasonal changes in the metabolism of cultured mussels (Mytilus edulis L.) from a Nova Scotian inlet: The effects of winter ice cover and nutritive stress. J. Exp. Mar. Biol. Ecol. 1997, 217, 63–78. [Google Scholar] [CrossRef]
- Mallet, A.L.; Carver, C.E.A.; Coffen, S.S.; Freeman, K.R. Winter growth of the blue mussel Mytilus edulis L.: Importance of stock and site. J. Exp. Mar. Biol. Ecol. 1987, 108, 217–228. [Google Scholar] [CrossRef]
- Pearce, J.B. Thermal addition and the benthos, Cape Cod Canal. Chesap. Sci. 1969, 10, 227–233. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Bøttiger, L.; Pleissner, D. Effect of salinity on growth of mussels, Mytilus edulis, with special reference to Great Belt (Denmark). Open J. Mar. Sci. 2012, 2, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Riisgård, H.U. Relationship between oxygen concentration, respiration and filtration rate in blue mussel Mytilus edulis. J. Oceanol. Limnol. 2018, 36, 395–404. [Google Scholar] [CrossRef]
- Field, I.A. Biology and economic value of the sea mussel Mytilus edulis. Bull. Bur. Fish. Wash. 1922, 38, 127–259. [Google Scholar]
- Waite, J.H. Results and Problems in Cell Differentiation: Biopolymers; Hennig, W., Nover, L., Scheer, U., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 27–54. [Google Scholar]
- Bell, E.C.; Gosline, J.M. Strategies for life in flow: Tenacity, morphometry and probability of dislodgement ot two mytilus species. Mar. Ecol. Prog. Ser. 1997, 159, 197–208. [Google Scholar] [CrossRef]
- Carrington, E. The ecomechanics of mussel attachment: From molecules to ecosystems. Integr. Comp. Biol. 2002, 42, 846–852. [Google Scholar] [CrossRef] [Green Version]
- Brenner, M.; Buck, B.H. Attachment properties of blue mussel (Mytilus edulis L.) byssus threads on culture-based artificial collectors substrates. Aquacult. Eng. 2010, 42, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Bell, E.C.; Gosline, J.M. Mechanical design of mussel byssus: Material yield enhances attachment strength. J. Exp. Biol. 1996, 199, 1005–1017. [Google Scholar] [PubMed]
- Reimer, O.; Harms-Ringdahl, S. Predator-inducible changes in blue mussels from the predator-free Baltic Sea. Mar. Biol. 2001, 139, 959–965. [Google Scholar]
- Dolmer, P.; Swane, I. Attachment and orientation of Mytilus edulis L. in flowing water. Ophelia 1994, 40, 63–74. [Google Scholar] [CrossRef]
- Kautsky, N. Growth and size structure in a baltic Mytilus edulis population. Ma. Biol. 1982, 68, 117–133. [Google Scholar] [CrossRef]
- Seed, R. The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. Oecologia 1969, 3, 317–350. [Google Scholar] [CrossRef] [PubMed]
- Abbott, E.J.; Firestone, F.A. Specifying surface quality—A method based on accurate measurement and comparison. J. Mech. Eng. 1933, 55, 569–577. [Google Scholar]
- Plew, D.T.; Enright, M.P.; Nokes, R.I.; Dumas, J.K. Effect of mussel bio-pumping on the drag on and flow around a mussel crop rope. Aquacult. Eng. 2009, 40, 55–61. [Google Scholar] [CrossRef]
- Keulegan, G.H.; Carpenter, L.H. Forces on cylinders and plates in an oscillating fluid. J. Res. Natl. Bur. Stand. 1958, 60, 423–440. [Google Scholar] [CrossRef]
- Journeé, J.M.J.; Massie, W.W. Offshore Hydrodynamics; Delft University of Technology: Delft, The Netherlands, 2001. [Google Scholar]
- Allen, J.A.; Cook, M.; Jackson, D.J.; Preston, S.; Worth, E.M. Observations of the rate of production and mechanical properties of the byssus threads of Mytilus edulis L. J. Molluscan Stud. 1976, 42, 279–289. [Google Scholar]
- Harger, R. The effect of wave impact on some aspects of the biology of sea mussels. Veliger 1970, 12, 401–414. [Google Scholar]
- Chakrabarti, S.K. Wave force coefficients for rough vertical cylinders. J. Waterw. Port Coast. Ocean Eng. 1984, 110, 101–104. [Google Scholar] [CrossRef]
- Sarpkaya, T. Oscillating flow about smooth and rough cylinders. J. Offshore Mech. Arct. Eng. 1987, 112, 307–313. [Google Scholar] [CrossRef]
- Wolfram, J.; Theophanatos, A. The effects of marine fouling on the fluid loading of cylinders: Some experimental results. In Proceedings of the Proceedings of the Annual Offshore Technology Conference, Houston, TX, USA, 6–9 May 1985; pp. 517–526. [Google Scholar]
- Chakrabarti, A.; Chen, Q.; Smith, H.D.; Liu, D. Large eddy simulation of unidirectional and wave flows through vegetation. J. Eng. Mech. 2016, 142, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Obasaju, E.D.; Bearmann, P.W.; Graham, J.M.R. A study of forces, circulation and vortex patterns around a circular cylinder in oscillating flow. J. Fluid Mech. 1988, 196, 467–494. [Google Scholar] [CrossRef]


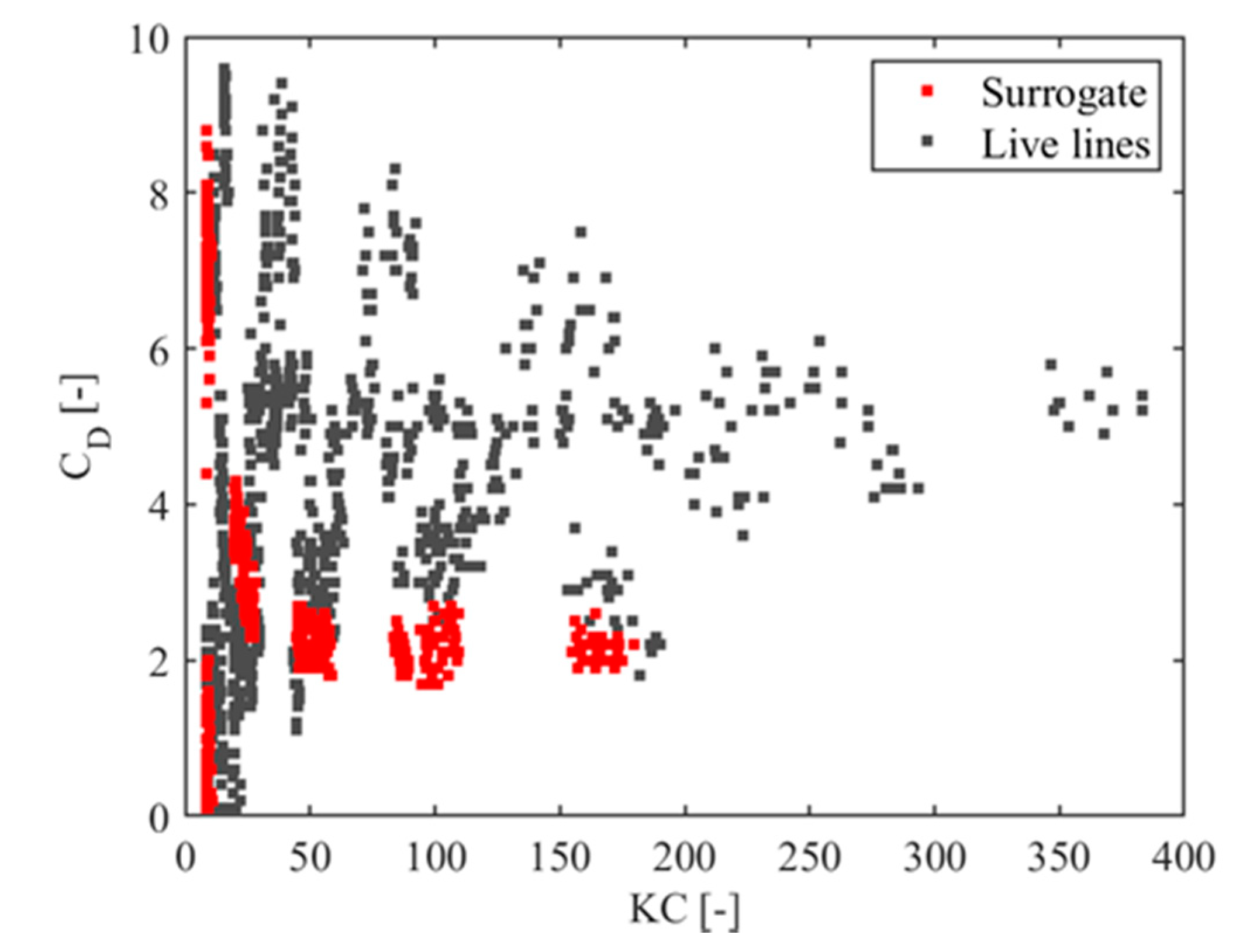
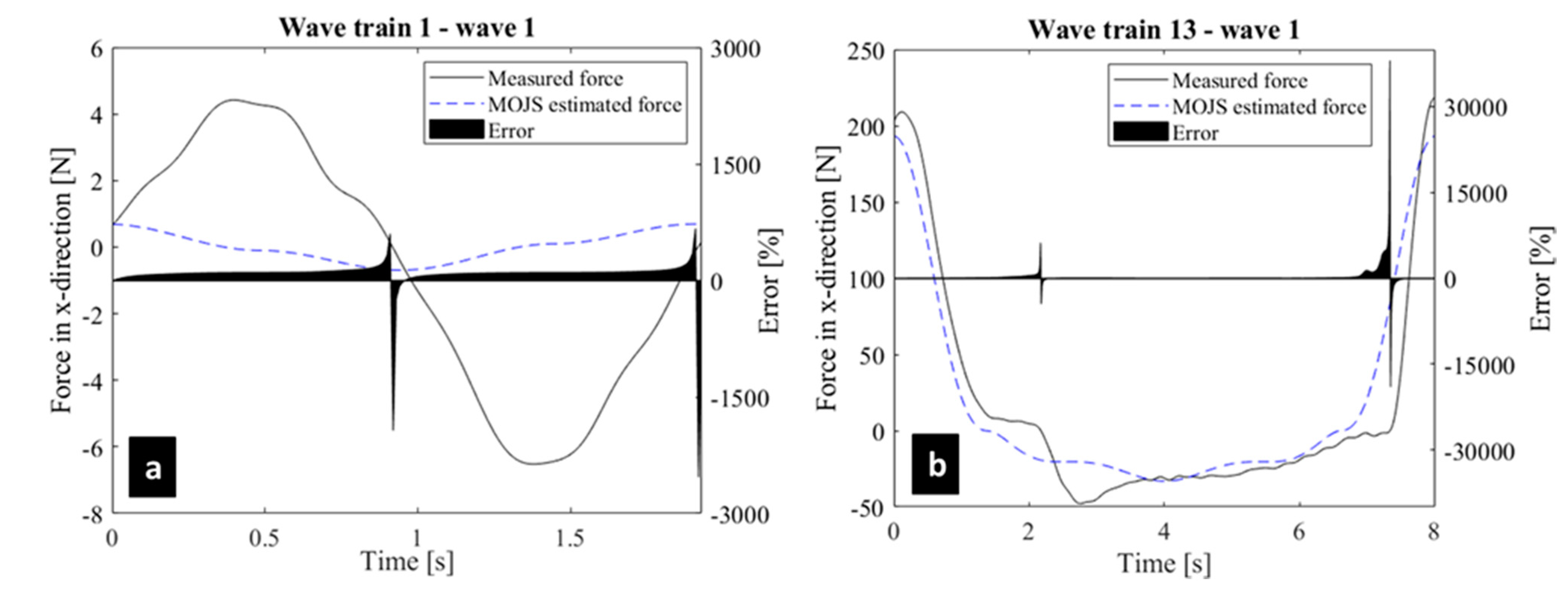
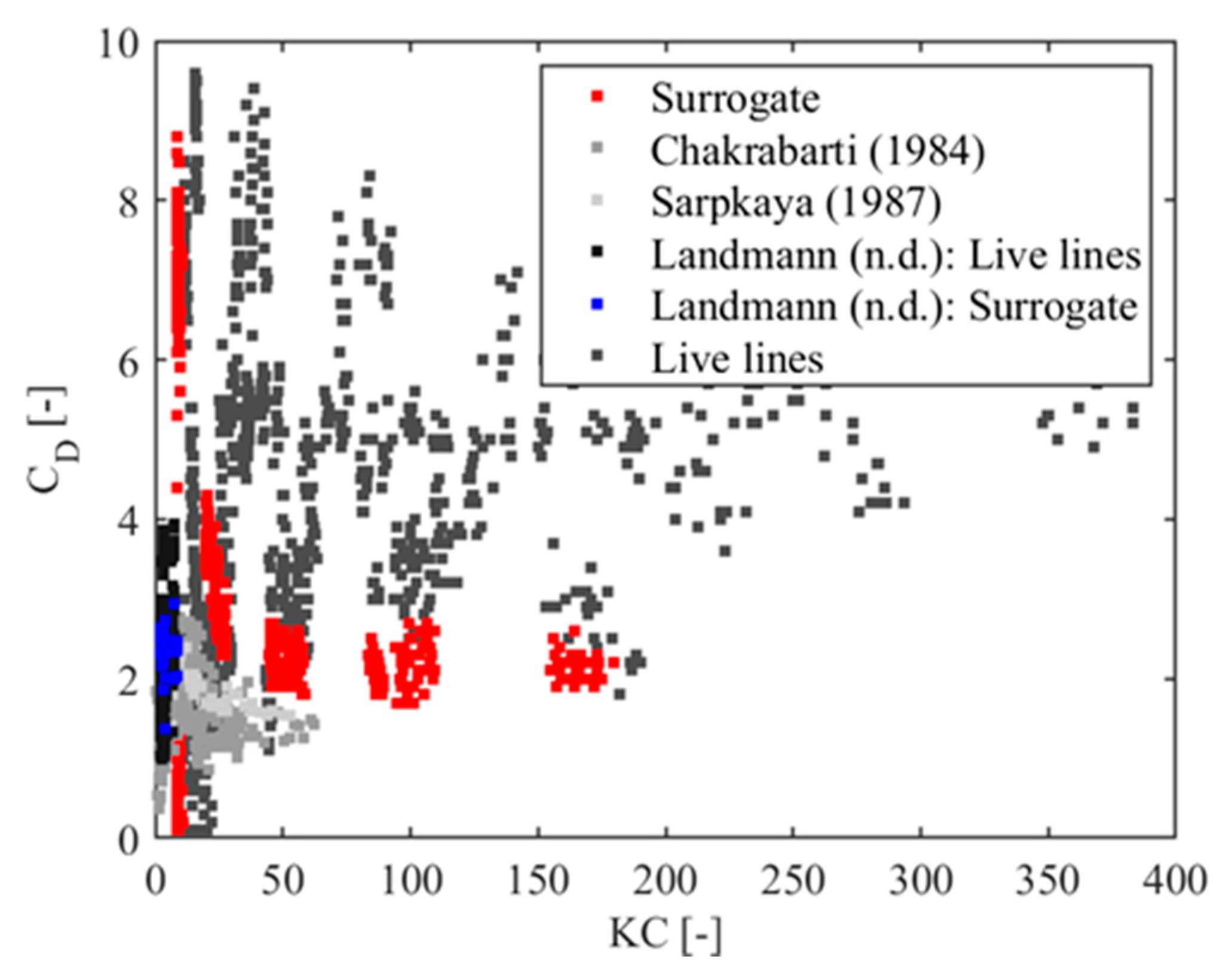
| Wave Train | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height H (m) | 0.2 | 0.2 | 0.4 | 0.4 | 0.4 | 0.6 | 0.6 | 0.6 | 0.8 | 0.8 | 0.8 | 1.0 | 1.0 |
| Period T (s) | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 6.5 | 7.0 | 7.5 | 8.0 |
| Length L (m) | 6.2 | 9.6 | 13.1 | 16.6 | 20.0 | 23.3 | 26.5 | 29.7 | 32.9 | 36.0 | 39.1 | 42.1 | 45.2 |
| Steepness H/L | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Wave Train | No. of Waves | Tests with Line 1, Line 2 and Surrogate | All Tests | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean H (m) | Std H (m) | Mean P (s) | Std P (s) | Mean H (m) | Std H (m) | Mean P (s) | Std P (s) | ||
| 1 | 28 | 0.19 | 0.01 | 2.00 | 0.03 | 0.19 | 0.01 | 2.00 | 0.04 |
| 2 | 28 | 0.18 | 0.00 | 2.50 | 0.04 | 0.19 | 0.01 | 2.50 | 0.04 |
| 3 | 23 | 0.39 | 0.01 | 2.99 | 0.06 | 0.39 | 0.01 | 3.00 | 0.06 |
| 4 | 18 | 0.42 | 0.01 | 3.49 | 0.06 | 0.42 | 0.01 | 3.50 | 0.05 |
| 5 | 15 | 0.43 | 0.01 | 3.98 | 0.05 | 0.43 | 0.01 | 4.00 | 0.06 |
| 6 | 13 | 0.64 | 0.01 | 4.51 | 0.06 | 0.64 | 0.01 | 4.50 | 0.06 |
| 7 | 11 | 0.63 | 0.01 | 4.98 | 0.05 | 0.64 | 0.01 | 5.01 | 0.05 |
| 8 | 10 | 0.64 | 0.00 | 5.49 | 0.06 | 0.64 | 0.01 | 5.50 | 0.05 |
| 9 | 8 | 0.83 | 0.00 | 5.98 | 0.05 | 0.84 | 0.01 | 5.99 | 0.05 |
| 10 | 8 | 0.85 | 0.01 | 6.53 | 0.06 | 0.85 | 0.01 | 6.53 | 0.07 |
| 11 | 7 | 0.84 | 0.01 | 7.02 | 0.05 | 0.84 | 0.02 | 7.02 | 0.06 |
| 12 | 6 | 1.09 | 0.01 | 7.51 | 0.02 | 1.10 | 0.01 | 7.52 | 0.04 |
| 13 | 6 | 1.06 | 0.01 | 8.03 | 0.08 | 1.07 | 0.02 | 8.02 | 0.06 |
| Line No. | Density (kg/m3) | Weight (kg/m) | Total Weight with Clamps (kg) | Ratio Weight Line to Weight Clamps | Mean Diameter (cm) | Std Diameter (cm) | Equivalent Diameter (cm) |
|---|---|---|---|---|---|---|---|
| 1 | 2593.55 | 1.82 | 6.53 | 0.70 | 8.09 | 1.79 | 2.99 |
| 2 | 1757.76 | 1.87 | 6.63 | 0.68 | 8.80 | 2.33 | 3.69 |
| 3 | 2841.60 | 3.86 | 10.79 | 0.33 | 10.31 | 2.80 | 4.16 |
| 4 | 1389.37 | 2.89 | 8.76 | 0.44 | 9.44 | 1.56 | 5.14 |
| 5 | 1304.05 | 4.63 | 12.41 | 0.28 | 9.66 | 1.87 | 6.72 |
| 6 | 1241.59 | 3.62 | 10.30 | 0.35 | 9.13 | 1.86 | 6.09 |
| 7 | 1797.95 | 2.13 | No wave test | No wave test | 8.36 | 1.33 | 3.99 |
| 8 | 363.58 | 0.85 | No wave tests | No wave tests | 7.79 | 1.63 | 5.47 |
| 9 | 922.39 | 1.19 | No wave tests | No wave tests | 7.52 | 1.67 | 4.05 |
| 10 | 509.21 | 1.02 | No wave tests | No wave tests | 8.04 | 1.52 | 5.04 |
| Sur. | 1220.00 | 4.25 | 8.5, no clamps | No clamps | 10.30 | - | 6.62 |
| Wave Train | Live Lines | Surrogate | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Waves | Fmax (N) | Mean Fmax (N) | Std Fmax (m) | No. of Waves | Fmax (N) | Mean Fmax (m) | Std Fmax (N) | |
| 1 | 168 | 7.39 | 4.70 | 1.33 | 84 | 17.28 | 9.23 | 3.39 |
| 2 | 168 | 9.44 | 5.86 | 1.79 | 84 | 12.97 | 10.00 | 0.98 |
| 3 | 138 | 29.79 | 17.83 | 4.58 | 69 | 34.72 | 23.89 | 3.87 |
| 4 | 108 | 34.09 | 18.56 | 5.12 | 54 | 28.07 | 22.04 | 3.42 |
| 5 | 90 | 36.75 | 18.61 | 4.82 | 45 | 30.70 | 20.29 | 3.84 |
| 6 | 78 | 83.11 | 52.42 | 11.76 | 39 | 56.63 | 42.33 | 7.17 |
| 7 | 66 | 80.26 | 56.43 | 12.66 | 33 | 53.32 | 40.40 | 7.71 |
| 8 | 60 | 83.82 | 60.66 | 11.51 | 30 | 47.56 | 36.57 | 7.06 |
| 9 | 48 | 136.68 | 104.73 | 17.52 | 24 | 70.12 | 58.19 | 6.58 |
| 10 | 48 | 135.13 | 103.83 | 19.36 | 24 | 75.61 | 63.12 | 7.52 |
| 11 | 43 | 162.79 | 124.04 | 24.47 | 31 | 94.25 | 77.25 | 7.63 |
| 12 | 36 | 245.47 | 207.51 | 34.24 | 18 | 147.68 | 133.15 | 7.91 |
| 13 | 36 | 244.23 | 190.11 | 32.85 | 18 | 146.83 | 132.65 | 8.62 |
| Wave Train | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Line 3 RMSE (%) | 85.7 | 70.7 | 35.7 | 20.6 | 11.6 | 13.7 | 16.2 | 14.4 | 12.4 | 8.1 | 18.3 | 18.5 | 13.0 | 26.1 |
| Surrogate RMSE (%) | 66.8 | 25.3 | 32.0 | 32.4 | 32.5 | 30.5 | 28.2 | 21.5 | 8.3 | 9.6 | 12.9 | 11.6 | 8.2 | 24.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gieschen, R.; Schwartpaul, C.; Landmann, J.; Fröhling, L.; Hildebrandt, A.; Goseberg, N. Large-Scale Laboratory Experiments on Mussel Dropper Lines in Ocean Surface Waves. J. Mar. Sci. Eng. 2021, 9, 29. https://doi.org/10.3390/jmse9010029
Gieschen R, Schwartpaul C, Landmann J, Fröhling L, Hildebrandt A, Goseberg N. Large-Scale Laboratory Experiments on Mussel Dropper Lines in Ocean Surface Waves. Journal of Marine Science and Engineering. 2021; 9(1):29. https://doi.org/10.3390/jmse9010029
Chicago/Turabian StyleGieschen, Rebekka, Christian Schwartpaul, Jannis Landmann, Lukas Fröhling, Arndt Hildebrandt, and Nils Goseberg. 2021. "Large-Scale Laboratory Experiments on Mussel Dropper Lines in Ocean Surface Waves" Journal of Marine Science and Engineering 9, no. 1: 29. https://doi.org/10.3390/jmse9010029







