Analytical Determination of the Optimal Feed Temperature for Hydrogen Peroxide Decomposition Process Occurring in Bioreactor with a Fixed-Bed of Commercial Catalase
Abstract
:1. Introduction
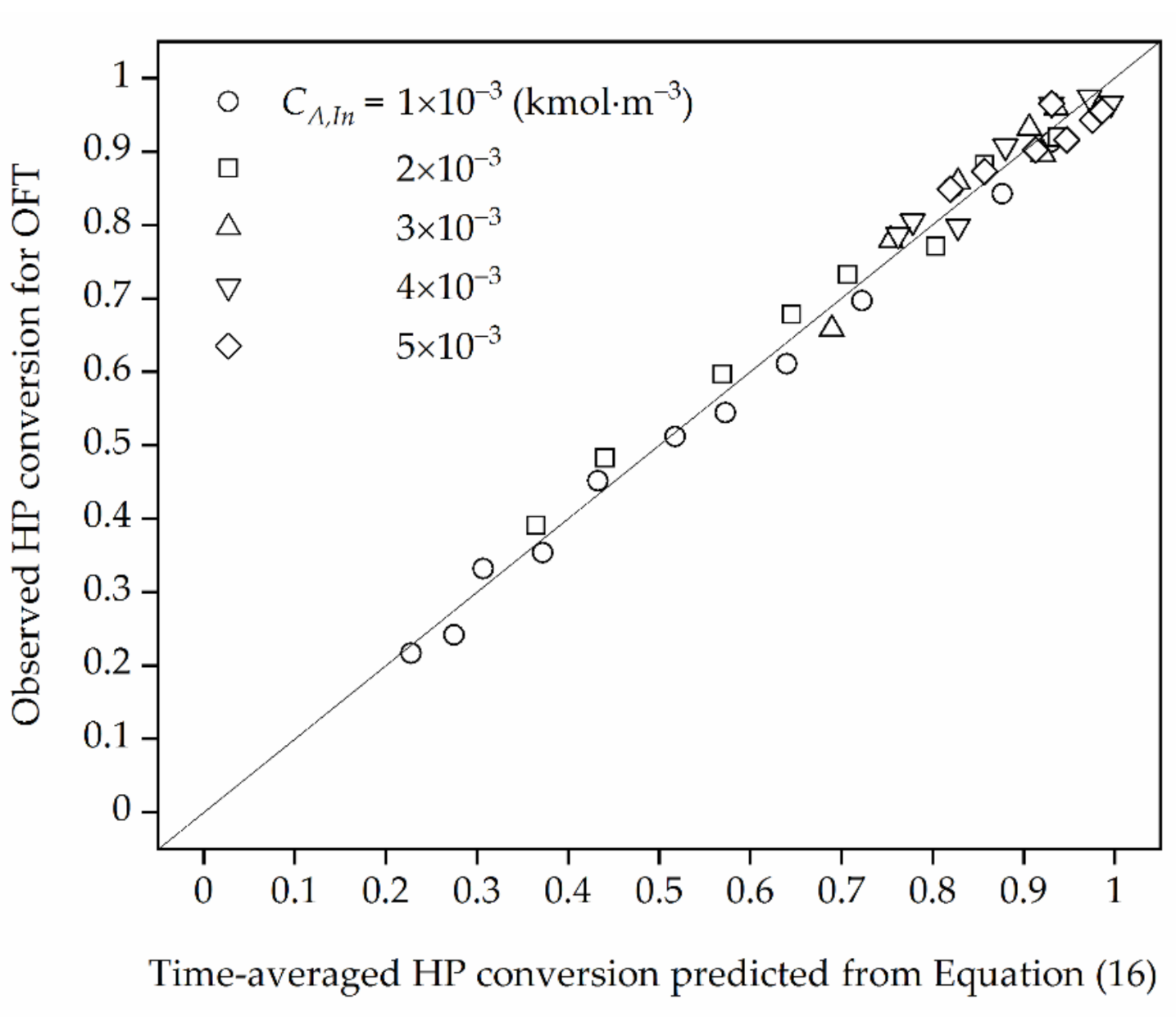
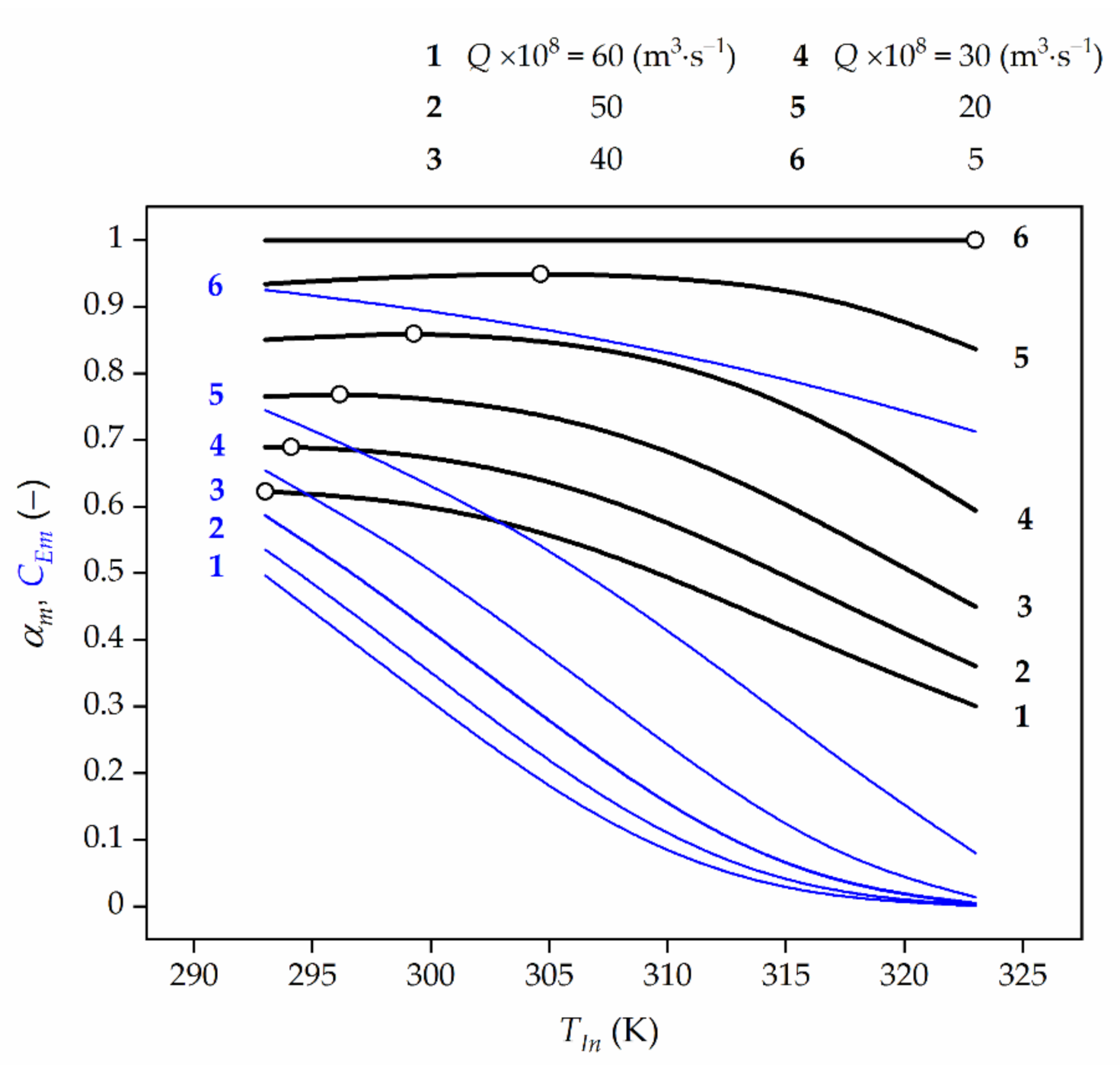
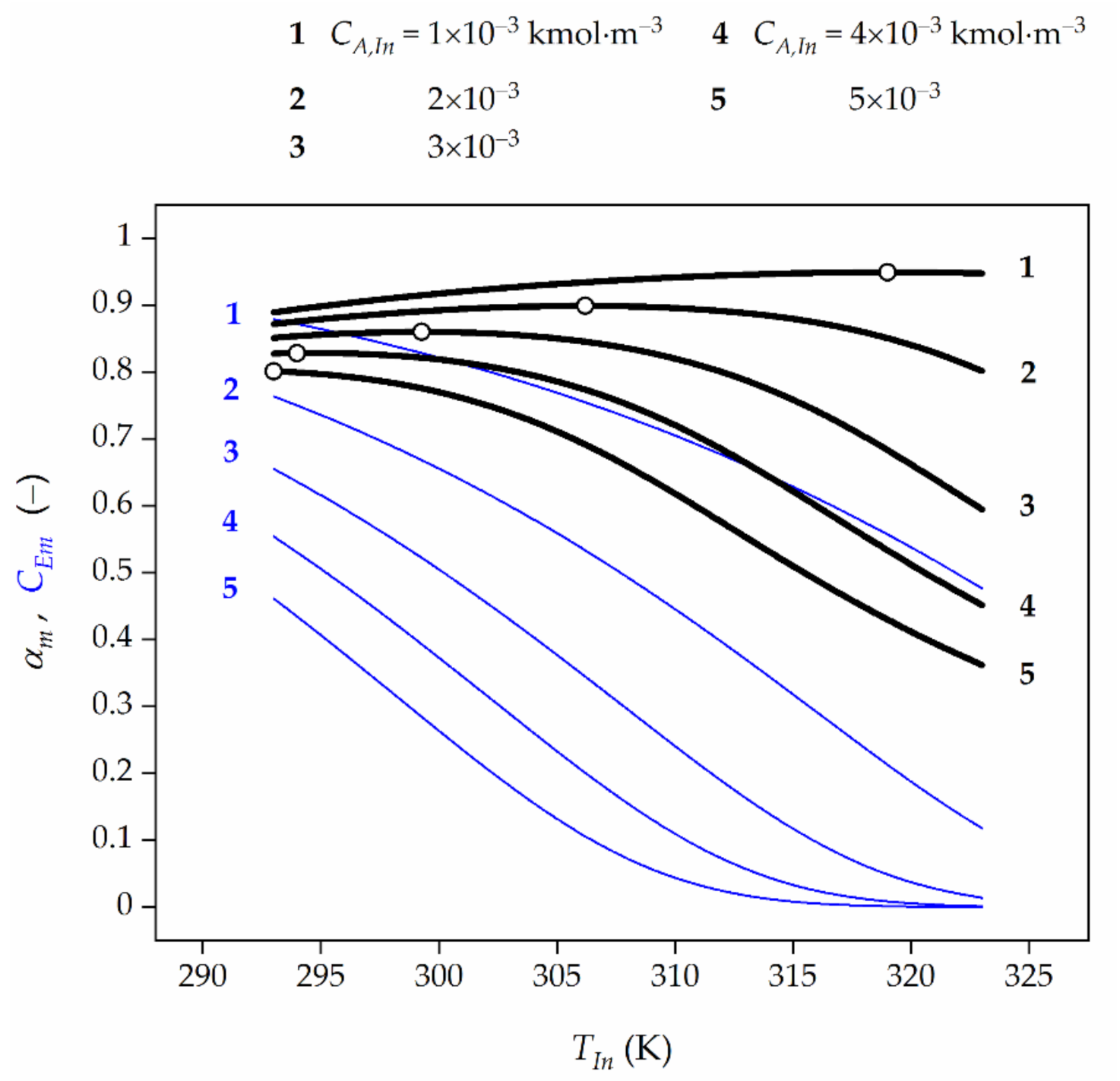
2. Results and Discussion
3. Materials and Methods
- Catalyst particles are spherical, symmetrical and uniformly packed inside the reactor;
- volume and density of the reacting medium are constant;
- the effective diffusivity does not change throughout the particles and is independent of the HP concentration.
- process is diffusion-controlled; and
- substrate (HP) transport rate (rm) from the bulk liquid (CA) to the outer surface of the immobilized bead (CAs) is equimolar diffusion described previously ([23], Equation (1));
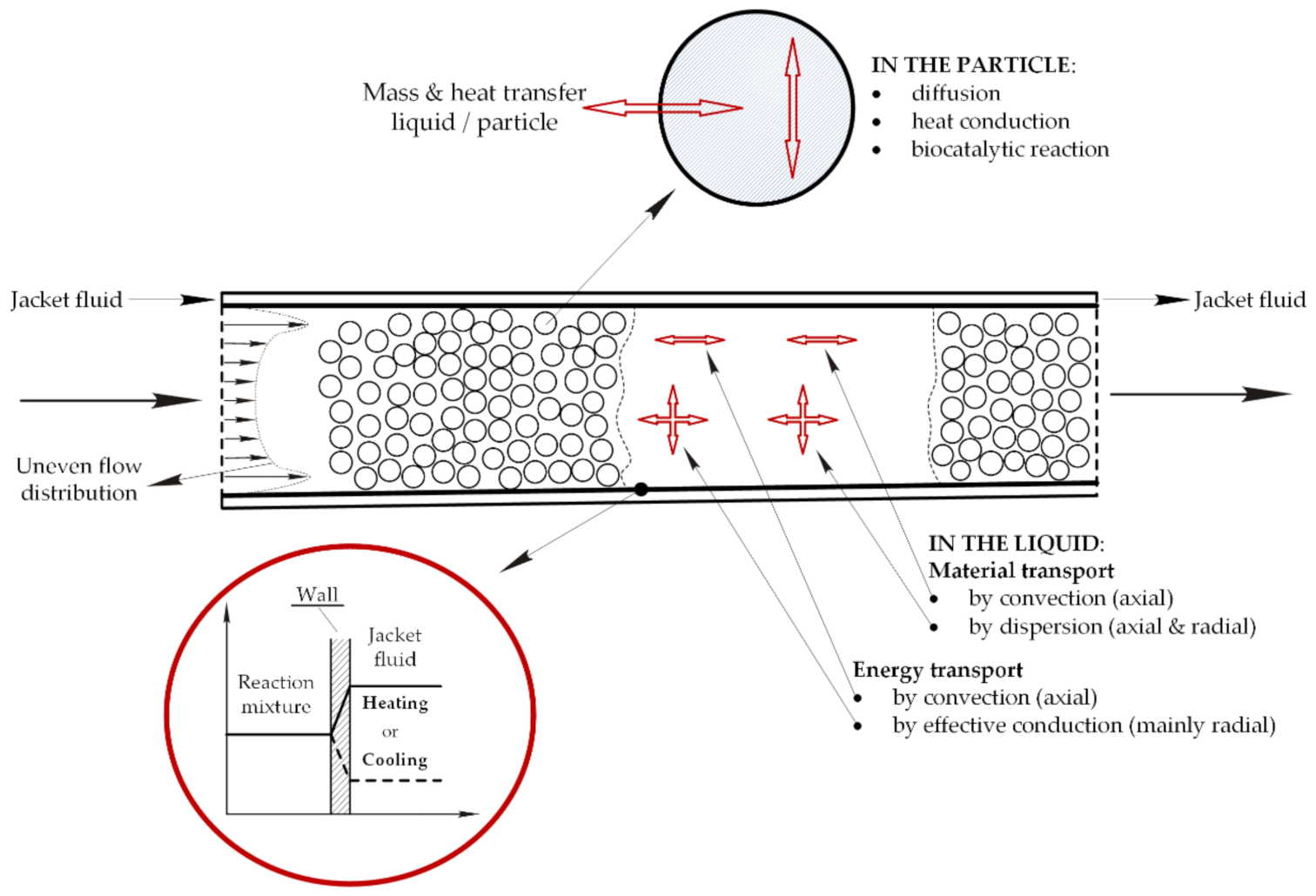
3.1. Development of the Fixed-Bed Reactor Problem
3.1.1. Kinetic Rate Equations for Reaction and Enzyme Deactivation
3.1.2. Mathematical Model Approach
3.1.3. Evaluation of the Effectiveness Factor
3.2. Expressions for Distributions of HP Concentration and TUC Activity
3.3. Optimization Problem Formulation
3.3.1. Performance Index
3.3.2. Equation Describing OFT
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| a, b, c | Experimental constants appearing in Equation (3) |
| am | External surface area for mass transfer, m2⋅m−3 |
| BiM | Biot number (=(dP/2)kL/Deff) |
| CE | Enzyme activity, kg⋅m−3 |
| CA | Bulk substrate concentration, kmol⋅m−3 |
| CA,In, | HP concentration at the reactor inlet, kmol⋅m−3 |
| CAm | Time-averaged HP concentration, kmol⋅m−3 |
| CAs | Surface HP concentration, kmol⋅m−3 |
| dP, DR | Particle diameter, and reactor diameter, respectively, m |
| Deff | Effective diffusion coefficient, m2⋅s−1 |
| Ei | Activation energy for reaction (i = R) and deactivation (i = D), J⋅mol−1 |
| h, H | Distance from reactor inlet, and bed depth, respectively, m |
| kD, | Modified rate constant for deactivation (=νD/KD), m3⋅kmol−1⋅s−1 |
| kD0 | Pre-exponential factor for deactivation rate constant, m3⋅kmol−1⋅s−1 |
| kL | Mass transfer coefficient, m⋅s−1 |
| kR* | Modified rate constant for reaction (=νR/KM), m3⋅kg−1⋅s−1 |
| kR0 | Pre-exponential factor for enzymatic reaction rate constant, m⋅s−1 |
| kR | Modified rate constant for reaction (=kR*CE0/am), m⋅s−1 |
| Ki | Michaelis constant for reaction (i = R) and deactivation (i = D), respectively, kmol⋅m−3 |
| Q | Feed flow rate, m3⋅s−1 |
| (-rA) | Reaction rate, kmol⋅m−3⋅s−1 |
| t | Biocatalyst age, s |
| T | Temperature generally, K |
| TIn | Feed temperature, K |
| Tmin, Tmax | Lower and upper temperature constraints, K |
| TOFT | Optimal feed temperature value, K |
| US | Superficial velocity, m⋅s−1 |
| z | Dimensionless distance from reactor inlet (=h/H) |
| Greek letters | |
| αm | Time-averaged substrate conversion |
| β1, β2 | Dimensionless numbers expressed by Equation (8) |
| εb | Porosity of the porous medium (=0.3) |
| φ | Thiele modulus (=dP/2(kRam/Deff)0.5) |
| η | Fluid viscosity, kg⋅m−1⋅s−1 |
| ηk | Effectiveness factor generally (k = eff), under EDR (k = EDR), and global effectiveness factor (k = G) |
| νD | Rate constant for deactivation, s−1 |
| νR | Rate constant for reaction, kmol⋅kg−1⋅s−1 |
| ρ | Fluid density, kg⋅m−3 |
| τ | Dimensionless time (=tUS/H) |
| Abbreviations | |
| COD | Coefficient of Determination |
| FXBR | Fixed-Bed Reactor |
| EDR | External Diffusional Resistances |
| HP | Hydrogen Peroxide |
| HPD | Hydrogen Peroxide Decomposition |
| IDR | Internal Diffusional Resistances |
| OFT | Optimal Feed Temperature |
| RMSE | Root Mean Square Error |
| SSE | Sum of Squares Error |
| TUC | Terminox Ultra Catalase |
Appendix A. Derivation of Expression Describing the Distributions of HP Concentration and Catalase Activity
References
- Zhang, H.; Li, H.; Xu, C.C.; Yang, S. Heterogeneously Chemo/Enzyme-Functionalized Porous Polymeric Catalysts of High-Performance for Efficient Biodiesel Production. ACS Catal. 2019, 9, 10990–11029. [Google Scholar] [CrossRef]
- Gennari, A.; Mobayed, F.H.; Da Rolt, N.B.; Benvenutti, E.V.; Nicolodi, S.; da Silveira, N.P.; Volpato, G.; Volken de Souza, C.F. Immobilization of β-Galactosidases on Magnetic Nanocellulose: Textural, Morphological, Magnetic, and Catalytic Properties. Biomacromolecules 2019, 20, 2315–2326. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117 Pt B, 1–20. [Google Scholar] [CrossRef]
- García-García, P.; Rocha-Martin, J.; Guisan, J.M.; Fernandez-Lorente, G. Co-Immobilization and Co-Localization of Oxidases and Catalases: Catalase from Bordetella Pertussis Fused with the Zbasic Domain. Catalysts 2020, 10, 810–828. [Google Scholar] [CrossRef]
- Li, M.; Christofides, P.D. Optimal control of diffusion-convection-reaction processes using reduced-order models. Comput. Chem. Eng. 2008, 32, 2123–2135. [Google Scholar] [CrossRef]
- Maria, G.; Crisan, M. Evaluation of optimal operation alternatives of reactors used for d-glucose oxidation in a bi-enzymatic system with a complex deactivation kinetics. Asia-Pac. J. Chem. Eng. 2015, 10, 22–44. [Google Scholar] [CrossRef]
- Carrazco-Escalante, M.; Caro-Corrales, J.; Iribe-Salazar, R.; Ríos-Iribe, E.; Vázquez-López, Y.; Gutiérrez-Dorado, R.; Hernández-Calderón, O. A new approach for describing and solving the reversible Briggs-Haldane mechanism using immobilized enzyme. Can. J. Chem. Eng. 2020, 98, 316–329. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Illanes, A. Enzyme Reactor Design and Operation under Mass-Transfer Limitations. In Problem Solving in Enzyme Biocatalysis; John Wiley and Sons Ltd.: Chichester, UK, 2013; pp. 181–202. [Google Scholar]
- Sonwani, R.K.; Giri, B.S.; Jaiswal, R.P.; Singh, R.S.; Rai, B.N. Performance evaluation of a continuous packed bed bioreactor: Bio-kinetics and external mass transfer study. Ecotox. Environ. Safe 2020, 201, 110860. [Google Scholar] [CrossRef]
- Badillo-Hernandez, U.; Alvarez, J.; Alvarez-Icaza, L. Efficient modeling of the nonlinear dynamics of tubular heterogeneous reactors. Comput. Chem. Eng. 2019, 123, 389–406. [Google Scholar] [CrossRef]
- Haugwitz, S.; Hagander, P. Challenges in Start-Up control of a Heat Exchange Reactor with Exothermic Reactions. A Hybrid Approach. In Analysis and Design of Hybrid Systems 2006; Cassandras, C., Giua, A., Seatzu, C., Zaytoon, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 185–190. [Google Scholar] [CrossRef]
- Mohammadi, L.; Aksikas, I.; Dubljevic, S.; Forbes, J.F. Optimal boundary control of coupled parabolic PDE–ODE systems using infinite-dimensional representation. J. Process. Contr. 2015, 33, 102–111. [Google Scholar] [CrossRef]
- Ranzi, E.; Corbetta, M.; Manenti, F.; Pierucci, S. Kinetic modeling of the thermal degradation and combustion of biomass. Chem. Eng. Sci. 2014, 110, 2–12. [Google Scholar] [CrossRef]
- Grubecki, I. How to run biotransformations—At the optimal temperature control or isothermally? Mathematical assessment. J. Process Control 2016, 44, 79–91. [Google Scholar] [CrossRef]
- Fenila, F.; Shastri, Y. Optimization of cellulose hydrolysis in a non-ideally mixed reactors. Comput. Chem. Eng. 2019, 128, 340–351. [Google Scholar] [CrossRef]
- Kilpiö, T.; Aho, A.; Murzin, D.; Salmi, T. Experimental and Modeling Study of Catalytic Hydrogenation of Glucose to Sorbitol in a Continuously Operating Packed-Bed Reactor. Ind. Eng. Chem. Res. 2013, 52, 7690–7703. [Google Scholar] [CrossRef]
- Illanes, A.; Wilson, L. Enzyme Reactor Design Under Thermal Inactivation. Crit. Rev. Biotechnol. 2003, 23, 61–93. [Google Scholar] [CrossRef]
- Farkye, N.Y. Cheese technology. Int. J. Dairy Technol. 2004, 57, 91–98. [Google Scholar] [CrossRef]
- Fruhwirth, G.; Paar, A.; Gudelj, M.; Cavaco-Paulo, A.; Robra, K.H.; Gübitz, G. An immobilised catalase peroxidase from the alkalothermophilic Bacillus SF for the treatment of textile-bleaching effluents. Appl. Microbiol. Biotechnol. 2002, 60, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Reis, C.Z.; Fogolari, O.; Oliveira, D.; de Arruda Guelli Ulson de Souza, S.M.; de Souza, A.A.U. Bioscouring and bleaching of knitted cotton fabrics in one-step process using enzymatically generated hydrogen peroxide. Can. J. Chem. Eng. 2017, 95, 2048–2055. [Google Scholar] [CrossRef]
- Soares, J.C.; Moreira, P.R.; Queiroga, A.C.; Morgado, J.; Malcata, F.X.; Pintado, M.E. Application of immobilized enzyme technologies for the textile industry: A review. Biocatal. Biotransform. 2011, 29, 223–237. [Google Scholar] [CrossRef]
- Grubecki, I. Optimal feed temperature for an immobilized enzyme fixed-bed reactor: A case study on hydrogen peroxide decomposition by commercial catalase. Chem. Process Eng. 2018, 39, 39–57. [Google Scholar] [CrossRef]
- Miłek, J. Thermal deactivation of catalase from Saccharomyces cerevisiae. Przem. Chem. 2020, 1, 128–130. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Li, D.; Wang, C.; Liu, Y.; Qu, S.; Zou, G. Thermokinetic models of enzyme-catalyzed reactions in batch and plug-flow reactors. Thermochim. Acta 1997, 307, 149–153. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1999. [Google Scholar]
- Gupta, R.; Bansal, A. Effect of Bed Configuration on Dispersion in a Packed-Bed Reactor. Ind. Eng. Chem. Res. 2010, 49, 9525–9528. [Google Scholar] [CrossRef]
- Grubecki, I. External mass transfer model for hydrogen peroxide decomposition by Terminox Ultra catalase in a packed-bed reactor. Chem. Process. Eng. 2017, 38, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Mathpati, A.C.; Kalghatgi, S.G.; Mathpati, C.S.; Bhanage, B.M. Immobilized lipase catalyzed synthesis of n-amyl acetate: Parameter optimization, heterogeneous kinetics, continuous flow operation and reactor modeling. Chem. Technol. Biotechnol. 2018, 93, 2906–2916. [Google Scholar] [CrossRef]
- Ellis, M.; Durand, H.; Christofides, P.D. A tutorial review of economic model predictive control methods. J. Process Control 2014, 24, 1156–1178. [Google Scholar] [CrossRef]
- Grubecki, I. Optimal temperature control in a batch bioreactor with parallel deactivation of enzyme. J. Process Control 2010, 20, 573–584. [Google Scholar] [CrossRef]
- Grubecki, I. Comparison between Isothermal and Optimal Temperature Policy for Batch Process with Parallel (Bio-)Catalyst Deactivation. J. Chem. Eng. Jpn. 2010, 43, 1014–1019. [Google Scholar] [CrossRef]
- Arvin, E.; Pedersen, L.-F. Hydrogen peroxide decomposition kinetics in aquaculture water. Aquacult. Eng. 2015, 64, 1–7. [Google Scholar] [CrossRef]
- Ghadermarzi, M.; Moosavi-Movahedi, A.A. Determination of the Kinetic Parameters for the “Suicide Substrate” Inactivation of Bovine Liver Catalase by Hydrogen Peroxide. J. Enzyme Inhib. 1996, 10, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Trawczyńska, I. New Method of Determining Kinetic Parameters for Decomposition of Hydrogen Peroxide by Catalase. Catalysts 2020, 10, 323–335. [Google Scholar] [CrossRef] [Green Version]
- George, P. Reaction between catalase and hydrogen peroxide. Nature 1947, 160, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Do, D.D.; Weiland, R.H. Consistency between rate expressions for enzyme reactions and deactivation. Biotechnol. Bioeng. 1980, 22, 1087–1093. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Weiland, R.H. Deactivation of catalase by hydrogen peroxide. Biotechnol. Bioeng. 1990, 36, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Vasudeven, P.T.; Weiland, R.H. Immobilized catalase: Deactivation and reactor stability. Biotechnol. Bioeng. 1993, 41, 231–236. [Google Scholar] [CrossRef]
- Do, D.D.; Greenfield, P.F. The concept of an effectiveness factor for reaction problems involving catalyst deactivation. Chem. Eng. J. 1983, 27, 99–105. [Google Scholar] [CrossRef]
- Do, D.D.; Weiland, R.H. Enzyme deactivation in fixed bed reactors with michaelis-menten kinetics. Biotechnol. Bioeng. 1981, 23, 691–705. [Google Scholar] [CrossRef]
- Sun, B.; Zhu, H.; Jin, Y.; Qiao, K.; Xu, W.; Jiang, J. Rapid Hydrogen Peroxide Decomposition Using a Microreactor. Chem. Eng. Technol. 2019, 42, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Ricca, E.; Calabro, V.; Curcio, S.; Iorio, G. Optimization of inulin hydrolysis by inulinase accounting for enzyme time- and temperature-dependent deactivation. Biochem. Eng. J. 2009, 48, 81–86. [Google Scholar] [CrossRef]
- Leow, J.W.H.; Chan, E.C.Y. Atypical Michaelis-Menten kinetics in cytochrome P450 enzymes: A focus on substrate inhibition. Biochem. Pharmacol. 2019, 169, 113615. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, I.F.; Serrano, H.L.; Sphaier, L.A.; Peixoto, F.C.; Silva, V.N.H. Integral transform analysis of heat and mass diffusion in chemically reacting systems with Michaelis–Menten kinetics. Int. Commun. Heat Mass Transf. 2019, 100, 20–26. [Google Scholar] [CrossRef]
- Burghardt, A.; Bartelmus, G. Models of Heterogeneous Fixed-Bed Catalitic Reactors. In Chemical Reactors Engineering. Part II. Heterogeneous Reactors, 1st ed.; Scientific Publishing Company: Warsaw, Poland, 2001; pp. 170–277. [Google Scholar]
- Illanes, A.; Wilson, L.; Vera, C. Enzyme Kinetics in a Heterogeneous System. In Problem Solving in Enzyme Biocatalysis; John Wiley and Sons Ltd.: Chichester, UK, 2015; pp. 87–140. [Google Scholar]
- Dehkordi, A.M.; Safari, I.; Karima, M. Experimental and Modeling study of catalytic reaction of glucose isomerization: Kinetics and packed-bed dynamic modeling. AlChE J. 2008, 54, 1333–1343. [Google Scholar] [CrossRef]
- Palazzi, E.; Converti, A. Evaluation of diffusional resistances in the process of glucose isomerization to fructose by immobilized glucose isomerase. Enzyme Microb. Technol. 2001, 28, 246–252. [Google Scholar] [CrossRef]
- Harmand, J.; Rapaport, A.; Dochain, D.; Lobry, C. Microbial ecology and bioprocess control: Opportunities and challenges. J. Process Control 2008, 18, 865–875. [Google Scholar] [CrossRef]
- Guay, M.; Dochain, D.; Perrier, M. Adaptive extremum-seeking control of nonisothermal continuous stirred tank reactors. Chem. Eng. Sci. 2005, 60, 3671–3681. [Google Scholar] [CrossRef]
- Harmand, J.; Dochain, D. The optimal design of two interconnected (bio)chemical reactors revisited. Comput. Chem. Eng. 2005, 30, 70–82. [Google Scholar] [CrossRef]
- Grubecki, I.; Wójcik, M. How much of enzyme can be saved in the process with the optimal temperature control? J. Food Eng. 2013, 116, 255–259. [Google Scholar] [CrossRef]
- Rade, L.L.; Lemos, C.O.T.; Barrozo, M.A.d.S.; Ribas, R.M.; Monteiro, R.d.S.; Hori, C.E. Optimization of esterification reaction over niobium phosphate in a packed bed tubular reactor. Renew. Energy 2019, 131, 348–355. [Google Scholar] [CrossRef]


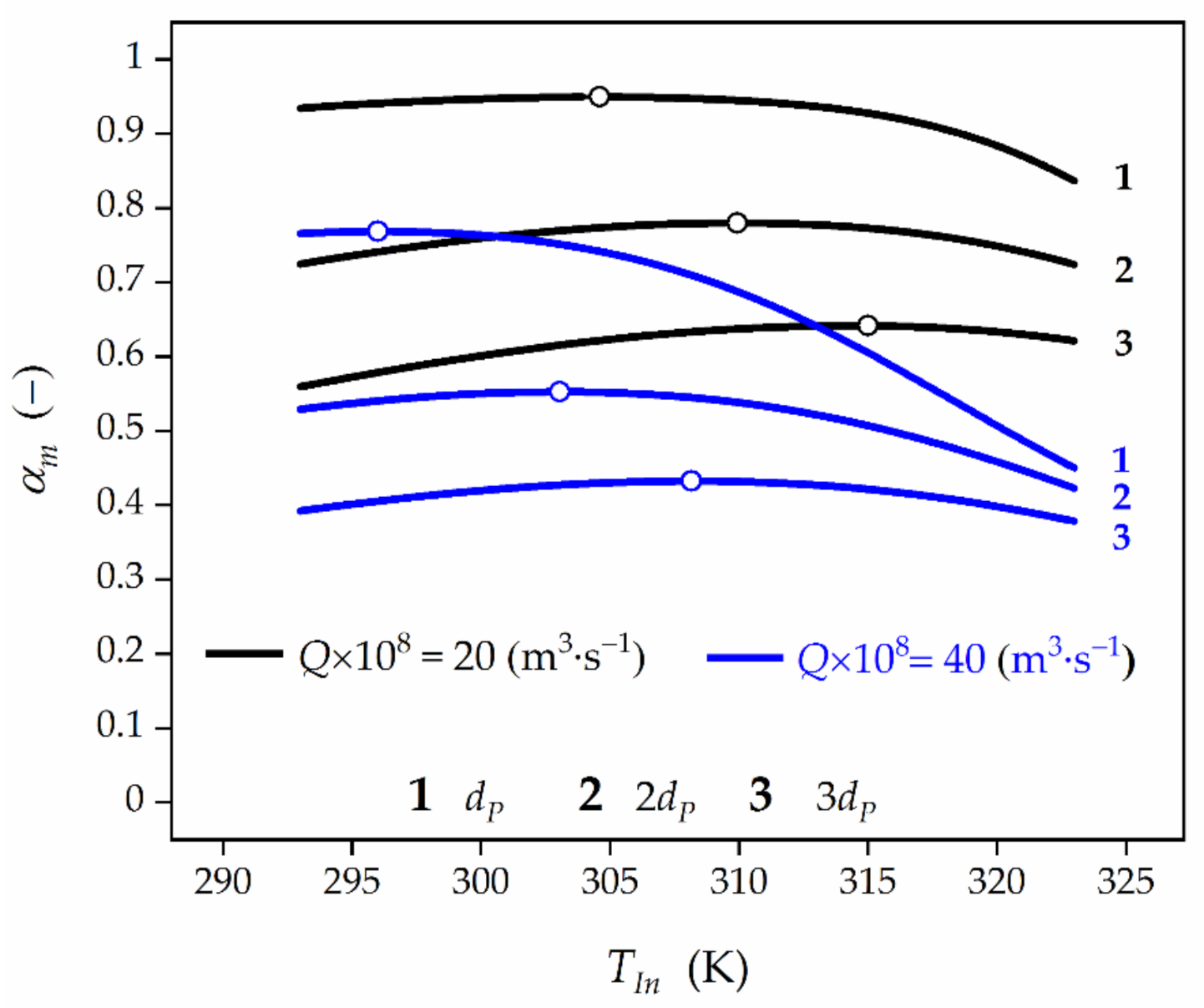

| Reaction of HPD | |
| Activation energy (kJ⋅mol−1) | ER = 12.6 ± 0.3 |
| Frequency factor (s−1) | kR0am = 48.00 ± 5.38 |
| Parallel deactivation of TUC | |
| Activation energy (kJ⋅mol−1) | ED = 49.7 ± 1.2 |
| Frequency factor (m3⋅kmol−1⋅s−1) | kD0 = (2.77 ± 5.38) × 107 |
| Q = 20 × 10−8 (m3⋅s−1) | Q = 40 × 10−8 (m3⋅s−1) | |||||
|---|---|---|---|---|---|---|
| dP × 104 (m) | 5 | 10 | 15 | 5 | 10 | 15 |
| ηEDR × 100 | 49.54 | 27.55 | 17.93 | 60.34 | 37.08 | 25.29 |
| ηG × 100 | 20.51 | 9.451 | 5.876 | 24.29 | 11.60 | 7.352 |
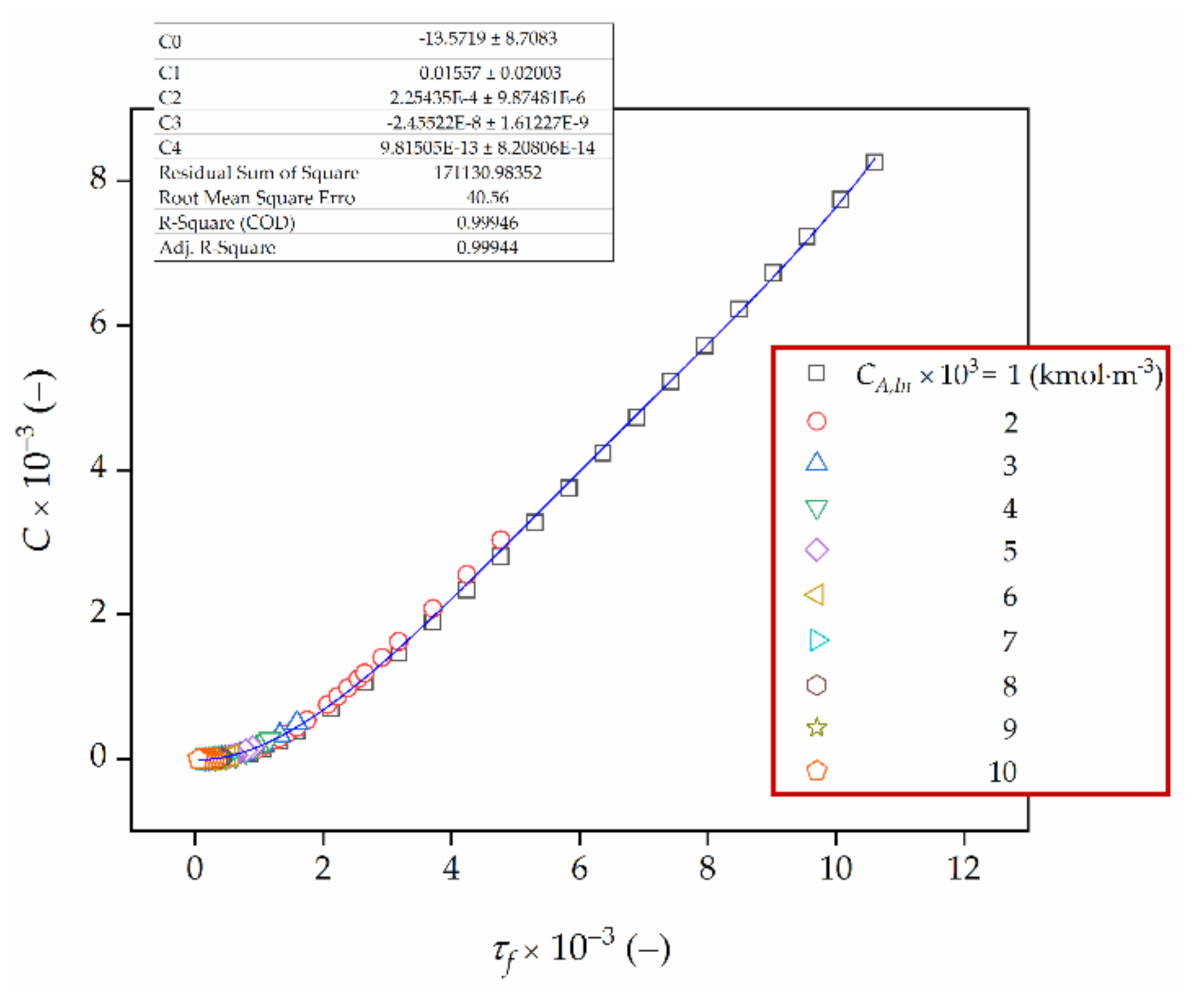
| Approximation | R2 (COD) | SSE | RMSE |
|---|---|---|---|
| Linear | 0.9760 | 7,644,101 | 271.1 |
| Quadratic | 0.9956 | 1,393,490 | 115.7 |
| Cubic | 0.9987 | 406,419 | 62.50 |
| Fourth degree | 0.9995 | 171,130 | 40.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grubecki, I. Analytical Determination of the Optimal Feed Temperature for Hydrogen Peroxide Decomposition Process Occurring in Bioreactor with a Fixed-Bed of Commercial Catalase. Catalysts 2021, 11, 35. https://doi.org/10.3390/catal11010035
Grubecki I. Analytical Determination of the Optimal Feed Temperature for Hydrogen Peroxide Decomposition Process Occurring in Bioreactor with a Fixed-Bed of Commercial Catalase. Catalysts. 2021; 11(1):35. https://doi.org/10.3390/catal11010035
Chicago/Turabian StyleGrubecki, Ireneusz. 2021. "Analytical Determination of the Optimal Feed Temperature for Hydrogen Peroxide Decomposition Process Occurring in Bioreactor with a Fixed-Bed of Commercial Catalase" Catalysts 11, no. 1: 35. https://doi.org/10.3390/catal11010035





