Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass
Abstract
1. Introduction
2. Kinetic Models of Enzymatic Cellulose Hydrolysis
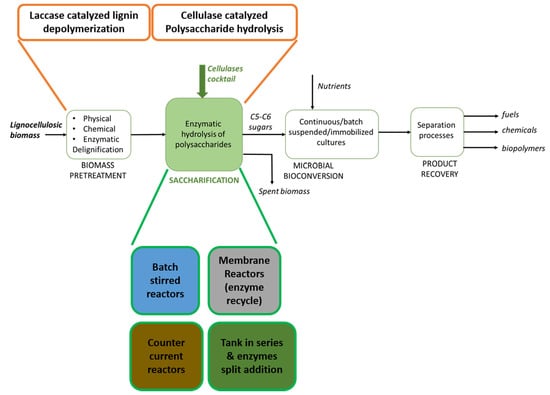
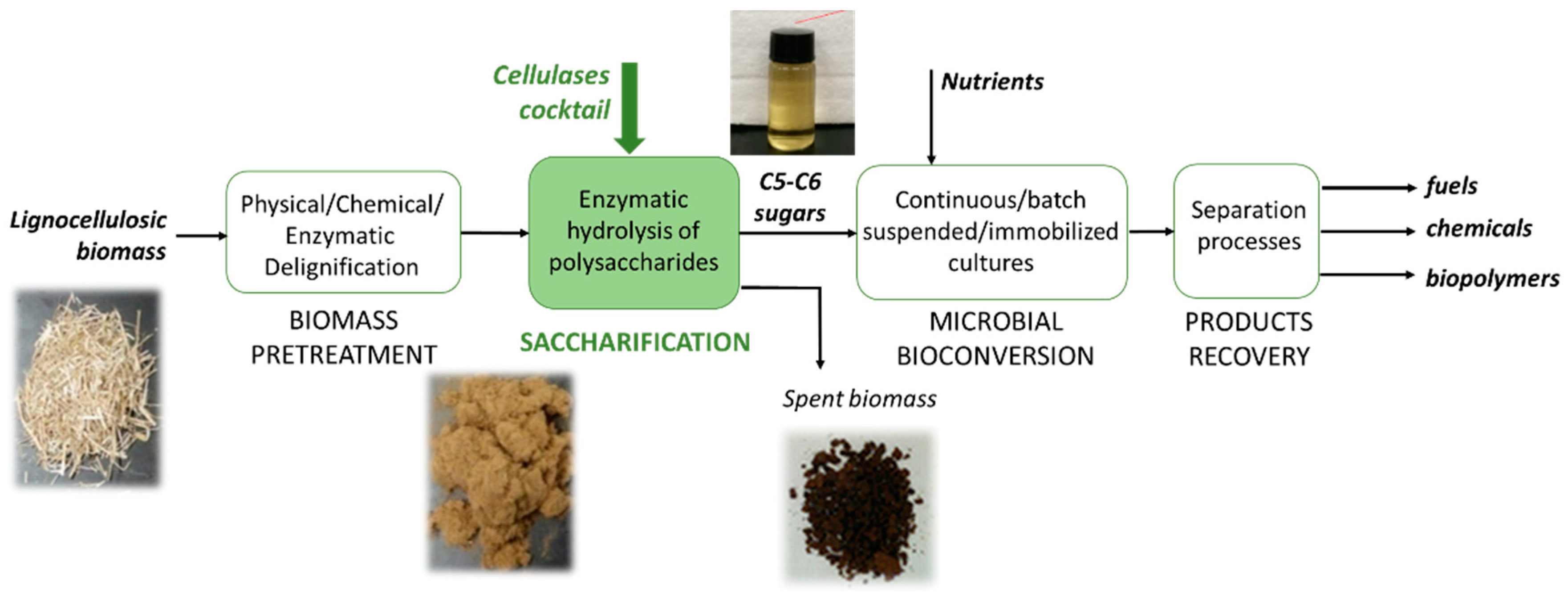
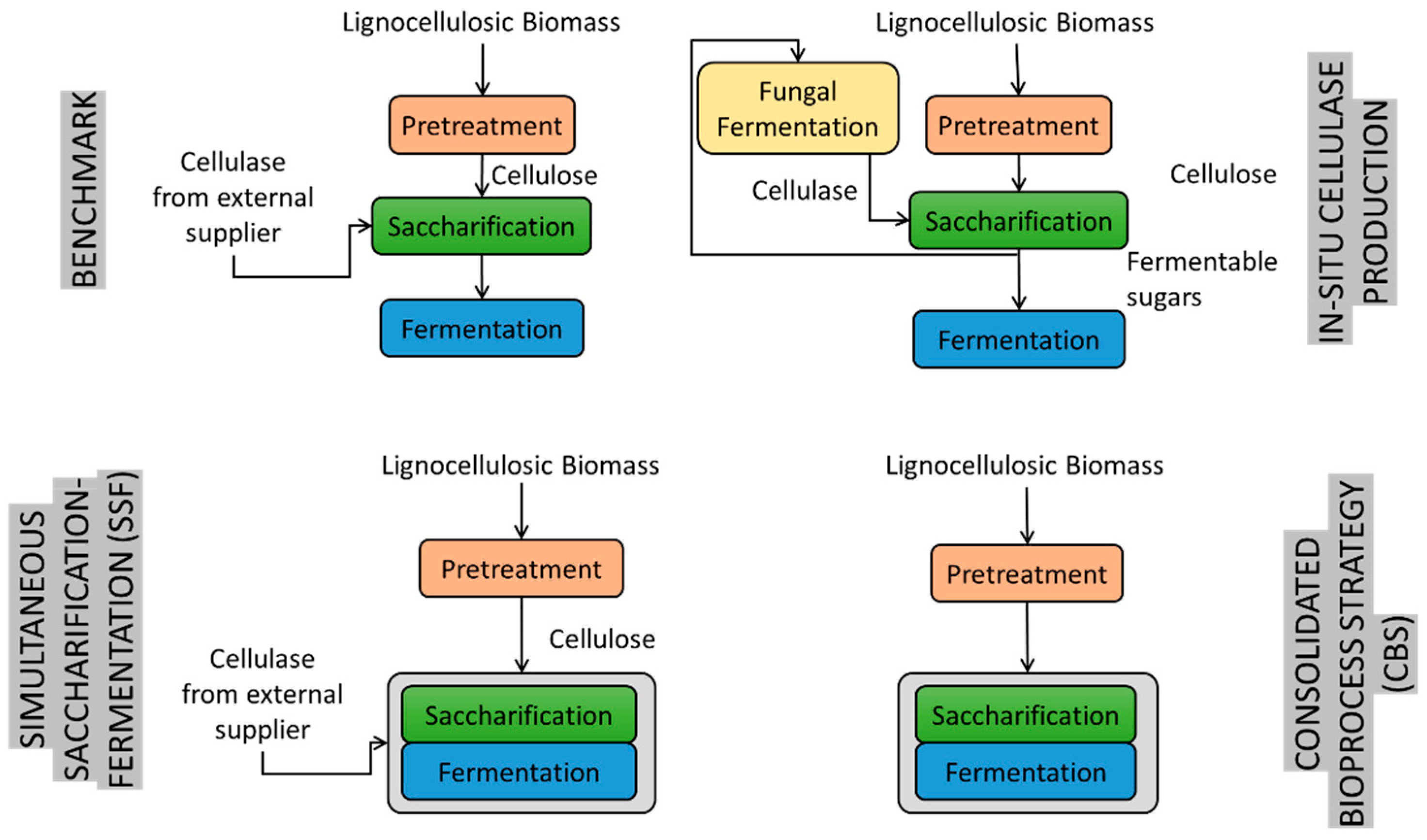
3. Process Design
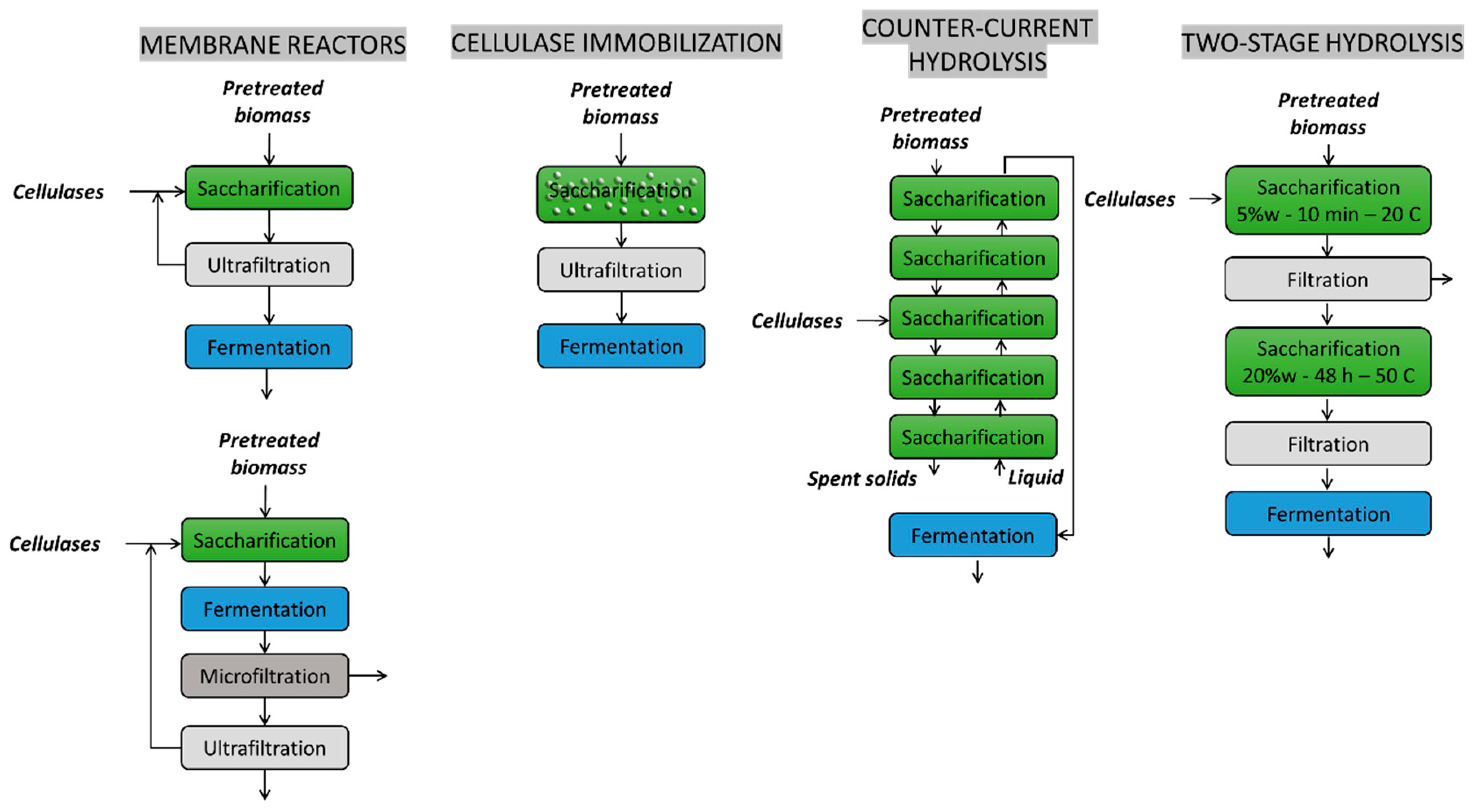
4. Bioreactor Design
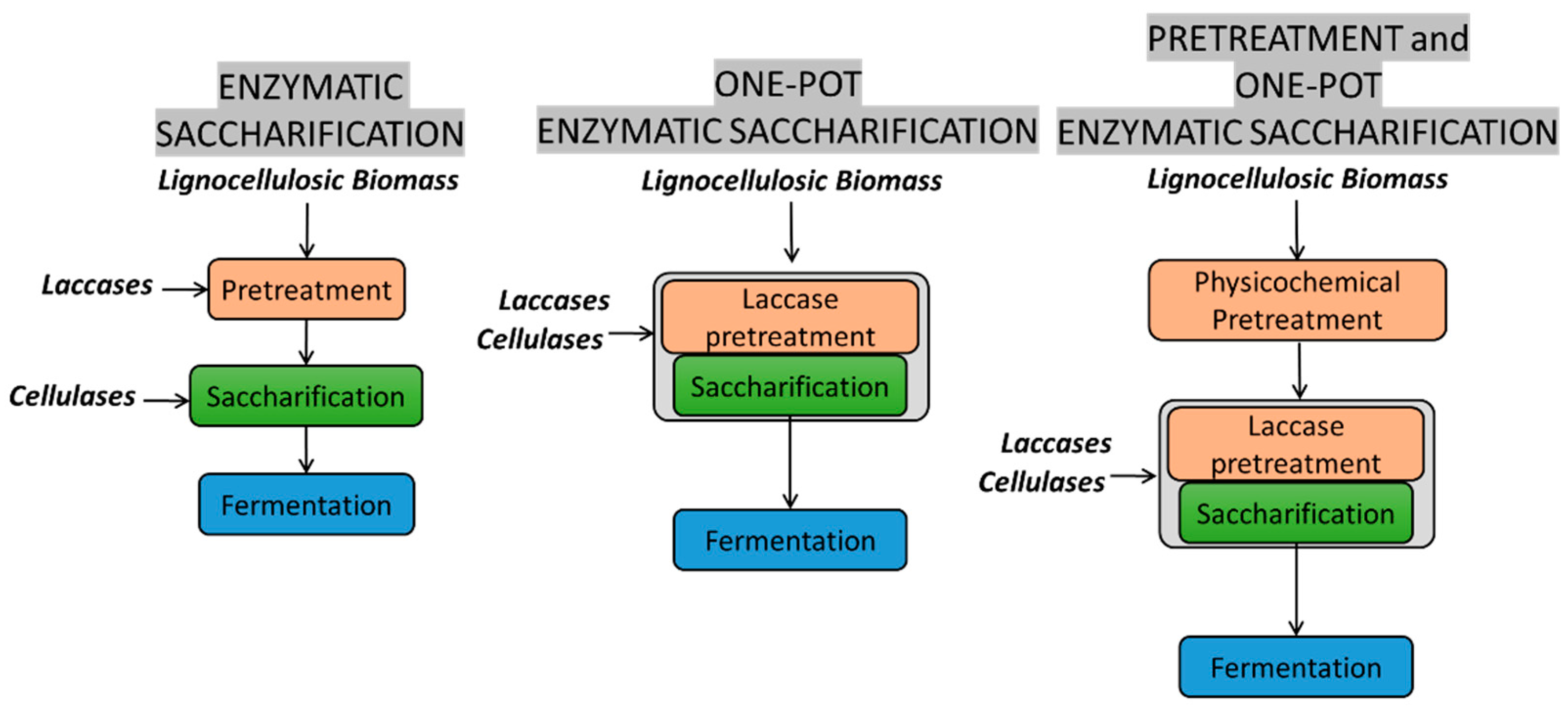
5. Enzymatic Pretreatment of Lignocellulosic Biomass: Laccase Catalyzed Delignification
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Østby, H.; Hansen, L.D.; Horn, S.J.; Eijsink, V.G.H.; Várnai, A. Enzymatic processing of lignocellulosic biomass: Principles, recent advances and perspectives. J. Ind. Microbiol. Biotechnol. 2020, 47, 623–657. [Google Scholar] [CrossRef] [PubMed]
- Brethauer, S.; Wyman, C.E. Review: Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour. Technol. 2010, 101, 4862–4874. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings: A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: An overview and future prospect. Bull. Natl. Res. Cent. 2019, 43, 51. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment of Lignocellulosic Materials for Efficient Bioethanol Production. Blue Biotechnol. 2007, 108, 41–65. [Google Scholar] [CrossRef]
- EUBIA. WikiBiomass. Available online: https://www.eubia.org/cms/wiki-biomass (accessed on 23 May 2021).
- Sheng, Y.; Lam, S.S.; Wu, Y.; Ge, S.; Wu, J.; Cai, L.; Huang, Z.; Van Le, Q.; Sonne, C.; Xia, C. Enzymatic conversion of pretreated lignocellulosic biomass: A review on influence of structural changes of lignin. Bioresour. Technol. 2021, 324, 124631. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Spiess, A.C. Laccases for biorefinery applications: A critical review on challenges and perspectives. Bioprocess Biosyst. Eng. 2015, 38, 2285–2313. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. MMBT 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol. Adv. 2009, 27, 833–848. [Google Scholar] [CrossRef]
- Olson, D.G.; E McBride, J.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef]
- Ferreira, R.; Azzoni, A.R.; Freitas, S. On the production cost of lignocellulose-degrading enzymes. Biofuels Bioprod. Biorefining 2021, 15, 85–99. [Google Scholar] [CrossRef]
- Jeoh, T.; Cardona, M.J.; Karuna, N.; Mudinoor, A.R.; Nill, J. Mechanistic kinetic models of enzymatic cellulose hydrolysis-A review. Biotechnol. Bioeng. 2017, 114, 1369–1385. [Google Scholar] [CrossRef] [PubMed]
- Gusakov, A.; Sinitsyn, A.; Klyosov, A. Kinetics of the enzymatic hydrolysis of cellulose: 1. A mathematical model for a batch reactor process. Enzym. Microb. Technol. 1985, 7, 346–352. [Google Scholar] [CrossRef]
- Gusakov, A.; Sinitsyn, A.; Klyosov, A. Kinetics of the enzymatic hydrolysis of cellulose: 2. A mathematical model for the process in a plug-flow column reactor. Enzym. Microb. Technol. 1985, 7, 383–388. [Google Scholar] [CrossRef]
- Drissen, R.E.T.; Maas, R.H.W.; Van Der Maarel, M.J.E.C.; Kabel, M.A.; Schols, H.A.; Tramper, J.; Beeftink, H.H. A generic model for glucose production from various cellulose sources by a commercial cellulase complex. Biocatal. Biotransform. 2007, 25, 419–429. [Google Scholar] [CrossRef]
- Chrastil, J. Enzymic product formation curves with the normal or diffusion limited reaction mechanism and in the presence of substrate receptors. Int. J. Biochem. 1988, 20, 683–693. [Google Scholar] [CrossRef]
- Pratto, B.; Alencar de Souza, R.B.; Sousa, R., Jr.; Gonçalves da Cruz, A.J. Enzymatic Hydrolysis of Pretreated Sugarcane Straw: Kinetic Study and Semi-Mechanistic Modeling. Appl. Biochem. Biotechnol. 2016, 178, 1430–1444. [Google Scholar] [CrossRef]
- Procentese, A.; Russo, M.E.; Di Somma, I.; Marzocchella, A. Kinetic Characterization of Enzymatic Hydrolysis of Apple Pomace as Feedstock for a Sugar-Based Biorefinery. Energies 2020, 13, 1051. [Google Scholar] [CrossRef]
- Sousa, R.S., Jr.; Carvalho, M.L.; Giordano, R.L.C. Recent trends in the modeling of cellulose hydrolysis. Braz. J. Chem. Eng. 2011, 28, 545–564. [Google Scholar] [CrossRef]
- Gan, Q.; Allen, S.; Taylor, G. Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: An overview, an experimental study and mathematical modelling. Process. Biochem. 2003, 38, 1003–1018. [Google Scholar] [CrossRef]
- Gan, Q.; Allen, S.; Taylor, G. Analysis of process integration and intensification of enzymatic cellulose hydrolysis in a membrane bioreactor. J. Chem. Technol. Biotechnol. 2005, 80, 688–698. [Google Scholar] [CrossRef]
- Johnson, E. Integrated enzyme production lowers the cost of cellulosic ethanol. Biofuels Bioprod. Biorefining 2016, 10, 164–174. [Google Scholar] [CrossRef]
- Liu, C.G.; Xiao, Y.; Xia, X.X.; Zhao, X.Q.; Peng, L.; Srinophakun, P.; Bai, F.W. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef]
- Lynd, L.R.; Laser, M.S.; Bransby, D.; E Dale, B.; Davison, B.; Hamilton, R.; Himmel, M.; Keller, M.; McMillan, J.D.; Sheehan, J.; et al. How biotech can transform biofuels. Nat. Biotechnol. 2008, 26, 169–172. [Google Scholar] [CrossRef]
- Sinitsyn, A.P.; Sinitsyna, O.A. Bioconversion of Renewable Plant Biomass. Second-Generation Biofuels: Raw Materials, Biomass Pretreatment, Enzymes, Processes, and Cost Analysis. Biochemistry 2021, 86, S166–S195. [Google Scholar] [CrossRef]
- Barta, Z.; Kovacs, K.; Reczey, K.; Zacchi, G. Process Design and Economics of On-Site Cellulase Production on Various Carbon Sources in a Softwood-Based Ethanol Plant. Enzym. Res. 2010, 2010, 1–8. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; Technical Report NREL/TP-5100-47764; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2011.
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27. [Google Scholar] [CrossRef]
- Davis, R.; Tao, L.; Tan, E.C.D.; Biddy, M.J.; Beckham, G.T.; Scarlata, C.; Jacobson, J.; Cafferty, K.; Ross, J.; Lukas, J.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons; National Renewable Energy Laboratory: Golden, CO, USA, 2013.
- Taha, M.; Foda, M.; Shahsavari, E.; Aburto-Medina, A.; Adetutu, E.; Ball, A. Commercial feasibility of lignocellulose bio-degradation: Possibilities and challenges. Curr. Opinion Biotechnol. 2016, 38, 190–197. [Google Scholar] [CrossRef]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuels Bioprod. Biorefineries 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Bhati, N.; Shreya; Sharma, A.K. Cost-effective cellulase production, improvement strategies, and future challenges. J. Food Process. Eng. 2021, 44, 1–11. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Rodrigues, C.; Vandenberghe, L.P.D.S.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, B.; Feng, Y.; Cui, Q. Consolidated bio-saccharification: Leading lignocellulose bioconversion into the real world. Biotechnol. Adv. 2020, 40, 107535. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.A.; Alam, Z.; Moniruzzaman, M.; Goto, M. Ionic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochem. Eng. J. 2016, 109, 252–267. [Google Scholar] [CrossRef]
- Bose, S.; Armstrong, D.W.; Petrich, J.W. Enzyme-Catalyzed Hydrolysis of Cellulose in Ionic Liquids: A Green Approach Toward the Production of Biofuels. J. Phys. Chem. B 2010, 114, 8221–8227. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Low-energy biomass pretreatment with deep eutectic solvents for bio-butanol production. Bioresour. Technol. 2017, 243, 464–473. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Deep Eutectic Solvents pretreatment of agro-industrial food waste. Biotechnol. Biofuels 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Wahlström, R.M.; Suurnakki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Arbain, D.; Gumba, E.R.; Jong, B.C.; Jamal, P. Potential halophilic cellulases for in situ enzymatic saccharification of ionic liquids pretreated lignocelluloses. Biores. Technol. 2014, 155, 177–181. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Arbain, D.; Nashef, E.M.; Jamal, P. Applicability evaluation of Deep Eutectic Solvents–Cellulase system for lignocellulose hydrolysis. Bioresour. Technol. 2015, 181, 297–302. [Google Scholar] [CrossRef]
- Gunny, A.A.N.; Arbain, D.; Javed, M.; Baghaei-Yazdi, N.; Gopinath, S.C.; Jamal, P. Deep eutectic solvents-halophilic cellulase system: An efficient route for in situ saccharification of lignocellulose. Process. Biochem. 2019, 81, 99–103. [Google Scholar] [CrossRef]
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Biores. Technol. 2018, 265, 334–339. [Google Scholar] [CrossRef]
- Carere, C.R.; Sparling, R.; Cicek, N.; Levin, D.B. Third Generation Biofuels via Direct Cellulose Fermentation. Int. J. Mol. Sci. 2008, 9, 1342–1360. [Google Scholar] [CrossRef]
- Lynd, L.R.; Van Zyl, W.H.; E McBride, J.; Laser, M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.J.; Feng, Y.; Li, B.; Cui, Q. Construction of consolidated bio-saccharification biocatalyst and process optimization for highly efficient lignocellulose solubilization. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zhang, Q.; Liu, Z.Y.; Li, F.L.; Lu, M.; Fang, X.C. Emerging technologies for the pretreatment of lignocellulosic materials for bio-based products. Appl. Microbiol. Biotechnol. 2020, 104, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, T.; Okazaki, F.; Okai, N.; Hara, K.Y.; Ishii, J.; Kondo, A. A review of enzymes and microbes for lignocellulosic biorefinery and the possibility of their application to consolidated bioprocessing technology. Bioresour. Technol. 2013, 135, 513–522. [Google Scholar] [CrossRef]
- Xin, F.; Dong, W.; Zhang, W.; Ma, J.; Jiang, M. Biobutanol Production from Crystalline Cellulose through Consolidated Bioprocessing. Trends Biotechnol. 2019, 37, 167–180. [Google Scholar] [CrossRef]
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B.; Montague, L.; Slayton, A.; Lukas, J. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; Technical Report NREL/TP-510-32438; National Renewable Energy Laboratory: Golden, CO, USA, 2002.
- Jørgensen, H.; Pinelo, M. Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod. Biorefining 2017, 11, 150–167. [Google Scholar] [CrossRef]
- Alfani, F.; Albanesi, D.; Cantarella, M.; Scardi, V.; Vetromile, A. Kinetics of enzymatic saccharification of cellulose in a flat-membrane reactor. Biomass 1982, 2, 245–253. [Google Scholar] [CrossRef]
- Kinoshita, S.; Chua, J.W.; Kato, N.; Yoshida, T.; Taguchi, H. Hydrolysis of cellulose by cellulases of Sporotrichum cellulophilum in an ultrafilter membrane reactor. Enzym. Microb. Technol. 1986, 8, 691–695. [Google Scholar] [CrossRef]
- Hong, J.; Tsao, G.T.; Wankat, P.C. Membrane reactor for enzymatic hydrolysis of cellobiose. Biotechnol. Bioeng. 1981, 23, 1501–1506. [Google Scholar] [CrossRef]
- Gan, Q.; Allen, S.; Taylor, G. Design and operation of an integrated membrane reactor for enzymatic cellulose hydrolysis. Biochem. Eng. J. 2002, 12, 223–229. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Al-Hosany, M.; Zooba, Y.; Al-Hammadi, A.; Al-Kaabi, S. Development of a membrane bioreactor for enzymatic hydrolysis of cellulose. Renew. Energy 2013, 56, 85–89. [Google Scholar] [CrossRef]
- Chen, G.; Song, W.; Qi, B.; Lu, J.; Wan, Y. Recycling cellulase from enzymatic hydrolyzate of acid treated wheat straw by electroultrafiltration. Bioresour. Technol. 2013, 144, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. A review of bioreactor technology used for enzymatic hydrolysis of cellulosic materials. Cellulose 2018, 25, 6279–6304. [Google Scholar] [CrossRef]
- Gomes, D.G.; Serna-Loaiza, S.; Cardona, C.A.; Gama, M.; Domingues, L. Insights into the economic viability of cellulases recycling on bioethanol production from recycled paper sludge. Bioresour. Technol. 2018, 267, 347–355. [Google Scholar] [CrossRef]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: II. Quantification of inhibition and suitability of membrane reactors. Biotechnol. Adv. 2010, 28, 407–425. [Google Scholar] [CrossRef]
- Sillu, D.; Agnihotri, S. Cellulase Immobilization onto Magnetic Halloysite Nanotubes: Enhanced Enzyme Activity and Stability with High Cellulose Saccharification. ACS Sustain. Chem. Eng. 2020, 8, 900–913. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Neo, K.R.S.; Yang, K.-L. Continuous hydrolysis of carboxymethyl cellulose with cellulase aggregates trapped inside membranes. Enzym. Microb. Technol. 2015, 78, 34–39. [Google Scholar] [CrossRef]
- Hwangbo, M.; Tran, J.L.; Chu, K.H. Effective one-step saccharification of lignocellulosic biomass using magnet-ite-biocatalysts containing saccharifying enzymes. Sc. Total Environ. 2019, 647, 806–813. [Google Scholar] [CrossRef]
- Dong, R.; Zheng, D.; Yang, D.; Qiu, X. pH-responsive lignin-based magnetic nanoparticles for recovery of cellulase. Bioresour. Technol. 2019, 294, 122133. [Google Scholar] [CrossRef]
- Zhou, Z.; Ju, X.; Zhou, M.; Xu, X.; Fu, J.; Li, L. An enhanced ionic liquid-tolerant immobilized cellulase system via hydrogel microsphere for improving in situ saccharification of biomass. Bioresour. Technol. 2019, 294, 122146. [Google Scholar] [CrossRef]
- Zhou, M.; Ju, X.; Li, L.; Yan, L.; Xu, X.; Chen, J. Immobilization of cellulase in the non-natural ionic liquid environments to enhance cellulase activity and functional stability. Appl. Microbiol. Biotechnol. 2019, 103, 2483–2492. [Google Scholar] [CrossRef]
- Zhou, M.J.X.; Zhou, Z.; Yan, L.; Chen, J.; Yao, X.; Xu, X.; Li, L.Z. Development of an Immobilized Cellulase System Based on Metal–Organic Frameworks for Improving Ionic Liquid Tolerance and In Situ Saccharification of Bagasse. ACS Sustain. Chem. Eng. 2019, 7, 19185–19193. [Google Scholar] [CrossRef]
- Abraham, R.E.; Puri, M. Nano-immobilized cellulases for biomass processing with application in biofuel production. Methods Enzymol. 2020, 630, 327–346. [Google Scholar] [CrossRef]
- Cherian, E.; Baskar, G.G. Immobilized Biocatalysts in Bioethanol Production: Scale-up Opportunities for Commercialization. In Horizons in Bioprocess. Engineering 2021; Pogaku, R., Ed.; Springer Nature: Basingstoke, UK, 2019. [Google Scholar]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol. Adv. 2010, 28, 308–324. [Google Scholar] [CrossRef]
- Yang, B.; Willies, D.M.; Wyman, C.E. Changes in the Enzymatic Hydrolysis Rate of Avicel Cellulose with Conversion. Biotechnol. Bioeng. 2006, 94, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Zentay, A.N.; Liang, C.; Lonkar, S.; Holtzapple, M.T. Countercurrent enzymatic saccharification of cellulosic biomass. Biomass Bioenergy 2016, 90, 122–130. [Google Scholar] [CrossRef]
- Liang, C.; Gu, C.; Karim, M.N.; Holtzapple, M. Kinetic modeling of countercurrent saccharification. Biotechnol. Biofuels 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Jameel, H.; Phillips, R.; Chang, H.-M. Split addition of enzymes in enzymatic hydrolysis at high solids concentration to increase sugar concentration for bioethanol production. J. Ind. Eng. Chem. 2012, 18, 707–714. [Google Scholar] [CrossRef]
- Quiroga, A.G.; Silvera, A.B.; Padilla, R.V.; Da Costa, A.C.; Filho, R.M. Continuous and semicontinuous reaction systems for high-solids enzymatic hydrolysis of lignocellulosics. Braz. J. Chem. Eng. 2015, 32, 805–819. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Narron, R.H.; Kim, H.; Chang, H.-M.; Jameel, H.; Park, S. Biomass pretreatments capable of enabling lignin valorization in a biorefinery process. Curr. Opin. Biotechnol. 2016, 38, 39–46. [Google Scholar] [CrossRef]
- Crestini, C.; Argyropoulos, D.S. The early oxidative biodegradation steps of residual kraft lignin models with laccase. Bioorg. Med. Chem. 1998, 6, 2161–2169. [Google Scholar] [CrossRef]
- Barreca, A.M.; Fabbrini, M.; Galli, C.; Gentili, P.; Ljunggren, S. Laccase/mediated oxidation of a lignin model for improved delignification procedures. J. Mol. Catal. B Enzym. 2003, 26, 105–110. [Google Scholar] [CrossRef]
- Niglio, S.; Procentese, A.; Russo, M.E.; Piscitelli, A.; Marzocchella, A. Integrated enzymatic pretreatment and hydrolysis of apple pomace in a bubble column bioreactor. Biochem. Eng. J. 2019, 150, 107306. [Google Scholar] [CrossRef]
- Moilanen, U.; Kellock, M.; Várnai, A.; Andberg, M.; Viikari, L. Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol. Biofuels 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rencoret, J.; Cadena, E.M.; Rico, A.; Barth, D.; del Río, J.C.; Martínez, Á.T. Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour. Technol. 2012, 119, 114–122. [Google Scholar] [CrossRef]
- Rico, A.; Rencoret, J.; Del Río, J.C.; Martínez, A.T.; Gutiérrez, A. Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol. Biofuels 2014, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, S.; Pezzella, C.; Lettera, V.; Sannia, G.; Piscitelli, A. Laccase pretreatment for agrofood wastes valorization. Bioresour. Technol. 2018, 265, 59–65. [Google Scholar] [CrossRef]
- Giacobbe, S.; Piscitelli, A.; Raganati, F.; Lettera, V.; Sannia, G.; Marzocchella, A.; Pezzella, C. Butanol production from laccase-pretreated brewer’s spent grain. Biotechnol. Biofuels 2019, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Krishnaswamy, S.; Chandrababu, R.; Veerabagu, U.; Pugazhendhi, A.; Mathimani, T. Optimal immobilization of Trichoderma asperellum laccase on polymer coated Fe3O4@SiO2 nanoparticles for enhanced biohydrogen production from delignified lignocellulosic biomass. Fuel 2020, 273, 117777. [Google Scholar] [CrossRef]
- Steinmetz, V.; Villain-Gambier, M.; Klem, A.; Ziegler, I.; Dumarcay, S.; Trebouet, D. In-situ extraction of depolymerization products by membrane filtration against lignin condensation. Bioresour. Technol. 2020, 311, 123530. [Google Scholar] [CrossRef]

| Ref. | Rate of Glucose Production, g/L min | Type of Tests | Type of Model and Equations | Reactor/Volume | Biomass/Substrate | Enzymes |
|---|---|---|---|---|---|---|
| [18] | 0.055–0.3 | Initial rate (60 min) and long term (48 h) tests for kinetic model assessment | Semi-mechanistic (M&M product inhibition: Chrastil’s: k 2.9–7.4 10−5 L/(g min), n 0.41–0.63). | Stirred Erlenmeyer flasks/250 mL | Sugarcane straw (SCS)/raw and hydrothermally pretreated SCS | Cellic® CTec2 |
| [19] | 0.04–0.09 | Semi-mechanistic (M&M product inhibition: Vmax 0.067–0.3 g L−1min−1, Km 5.5–9 g L−1, Ki 0.16–0.31 g/L, Chrastil’s: k 2–1.1 10−3 Lg−1min−1, n 0.38–0.46). | Stirred cell reactor (Applikon minibio)/250 mL | Apple pomace | ||
| [15] | 0.017–0.083 (Productivity) | Enzyme adsorption/Continuous hydrolysis tests compared with predictive model results | Adsorption/desorption of cellulases kinetics/hydrolysis is a network of four parallel reactions: two substrates (crystalline and amorphous cellulose) and two products (cellobiose and glucose). M&M product inhibition + enzyme inactivation + enzyme desorption (see original paper). | Glass columns/4–80 mL | Cellulose (from cotton stalks) | Cellulase from Trichoderma longibrachiatum |
| [21,22] | Not reported | Batch/fed-batch/continuous tests compared with predictive model results | Mechanistic model: Two substrates (hydrolysable cellulose, inert cellulose), enzyme loss through inert binding, shear enzyme deactivation, competitive product inhibition. Primary rate constant of enzyme hydrolyzable cellulose complex formation/consumption: 0.2 s−1/0.05 M−1s−1; rate of product formation: 9.05 s−1 (see Gan et al. [21] for complete description of equations) | Strirred cell + ultrafiltration membrane/2.5 L | Purified and pulverized cellulose (38–106 10−6 m) | Cellulase from Trichoderma reesei (Sigma C8546) |
| [16] | 0.007 | Initial rate tests (15–60 min) for kinetic model assessment | Semi-mechanistic model: linear dependence on cellulose concentration, Langmuir type dependence of max rate of hydrolysis on enzyme concentration, glucose inhibition, enzyme deactivation by temperature, substrate recalcitrance (See Equation 10, 11, and 12 of the original reference for rate equations of conversion of cellulose into cellobiose, cellobiose into glucose, and cellulose into glucose, respectively) | Test tube (total working volume 6 mL) on vertical rotator | Cellulose (Avicel and Whatman, No. 1,001,090 filter Paper) and wheat straw | Cellubrix, Celluclast, Novozymes 188 (Novozymes Corp., Denmark) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivieri, G.; Wijffels, R.H.; Marzocchella, A.; Russo, M.E. Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass. Catalysts 2021, 11, 680. https://doi.org/10.3390/catal11060680
Olivieri G, Wijffels RH, Marzocchella A, Russo ME. Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass. Catalysts. 2021; 11(6):680. https://doi.org/10.3390/catal11060680
Chicago/Turabian StyleOlivieri, Giuseppe, René H. Wijffels, Antonio Marzocchella, and Maria Elena Russo. 2021. "Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass" Catalysts 11, no. 6: 680. https://doi.org/10.3390/catal11060680
APA StyleOlivieri, G., Wijffels, R. H., Marzocchella, A., & Russo, M. E. (2021). Bioreactor and Bioprocess Design Issues in Enzymatic Hydrolysis of Lignocellulosic Biomass. Catalysts, 11(6), 680. https://doi.org/10.3390/catal11060680