Microwave-Assisted Synthesis and Characterization of Solar-Light-Active Copper–Vanadium Oxide: Evaluation of Antialgal and Dye Degradation Activity
Abstract
1. Introduction
2. Results
2.1. Synthesis
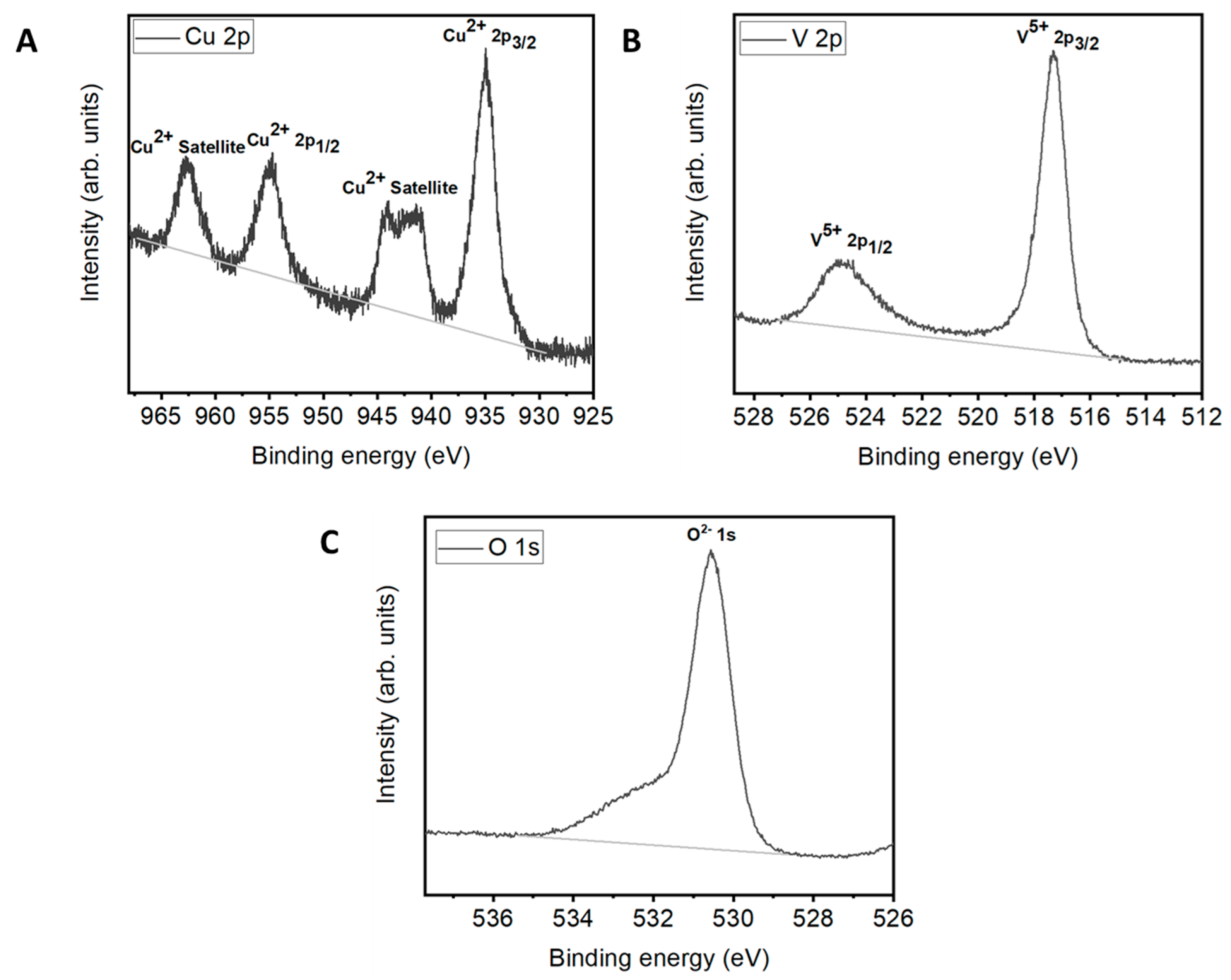
2.2. Characterization
2.3. Antialgal Assay
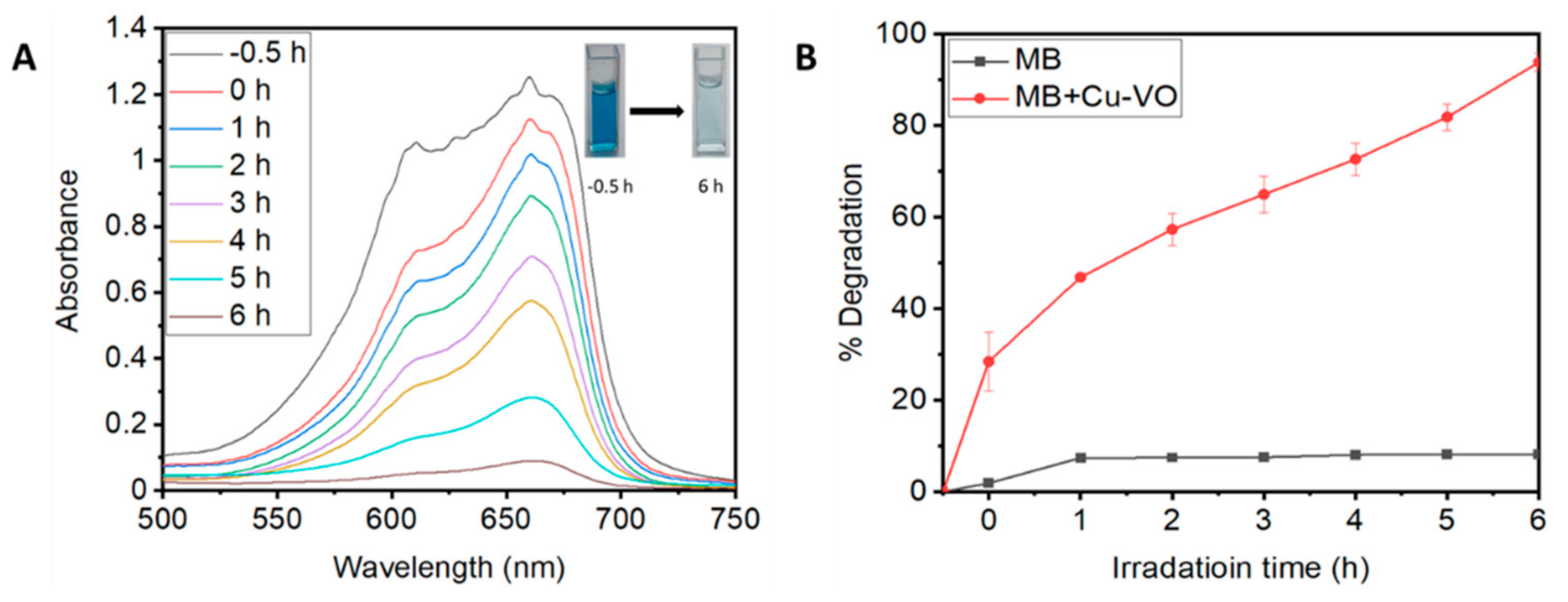
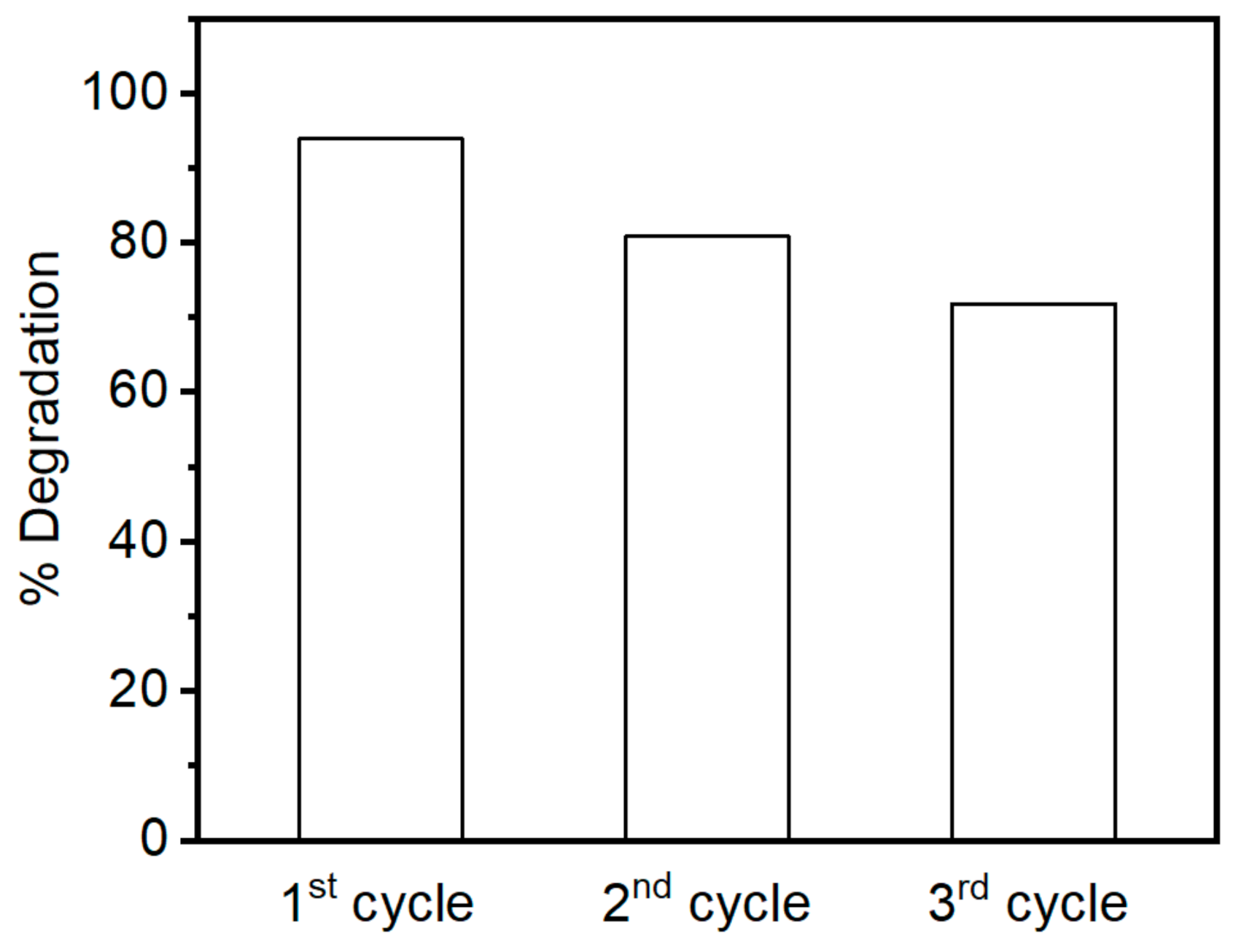
2.4. Dye Degradation Assay
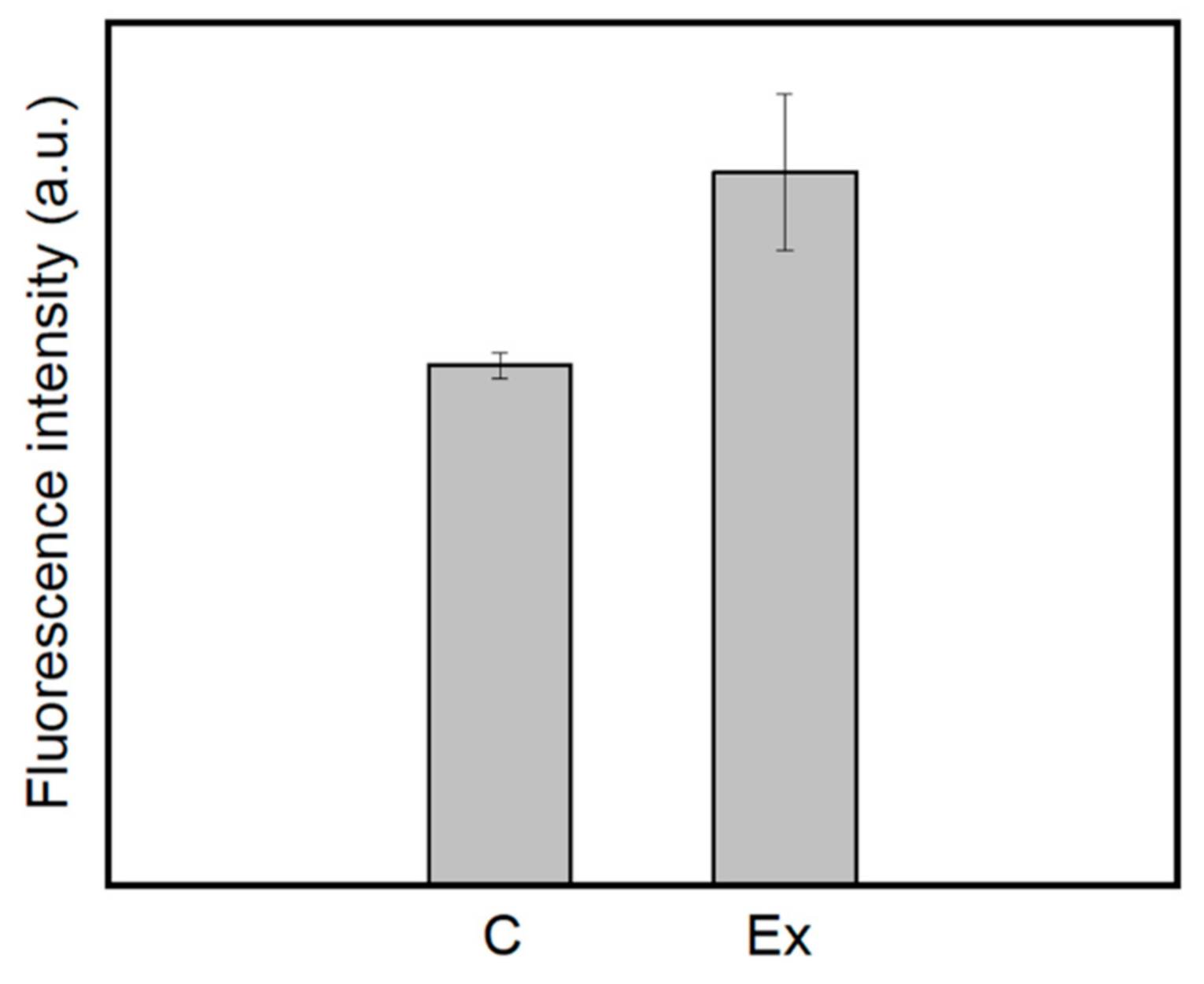
2.5. Mechanism of Action
3. Discussion
4. Materials and Methods
4.1. Synthesis of Cu-VO
4.2. Characterization
4.3. Photocatalytic Experiments
4.3.1. Algae Culture
4.3.2. Algae Growth Inhibition Assay
4.3.3. Dye Degradation Assay
4.4. Photocatalysis Mechanism
4.4.1. ·OH Assay
4.4.2. ROS Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wang, P.; Jia, P.; Zhang, Y.; Chu, X.; Wang, Y. A review on factors affecting microcystins production by algae in aquatic environments. World J. Microbiol. Biotechnol. 2016, 32, 51. [Google Scholar] [CrossRef] [PubMed]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Surface-Modified Zinc Oxide Nanoparticles for Antialgal and Antiyeast Applications. ACS Appl. Nano Mater. 2020, 3, 440–451. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Haroon, A.; Sabae, S. Activity of some Nile River aquatic macrophyte extracts against the cyanobacterium Microcystis aeruginosa. Afr. J. Aquat. Sci. 2017, 42, 271–277. [Google Scholar] [CrossRef]
- Begum, R.; Najeeb, J.; Sattar, A.; Naseem, K.; Irfan, A.; Al-Sehemi, A.G.; Farooqi, Z.H. Chemical reduction of methylene blue in the presence of nanocatalysts: A critical review. Rev. Chem. Eng. 2020, 36, 749–770. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef]
- Cao, X.; Yue, L.; Lian, F.; Wang, C.; Cheng, B.; Lv, J.; Wang, Z.; Xing, B. CuO nanoparticles doping recovered the photocatalytic antialgal activity of graphitic carbon nitride. J. Hazard. Mater. 2021, 403, 123621. [Google Scholar] [CrossRef]
- Lebedev, A.; Anariba, F.; Tan, J.C.; Li, X.; Wu, P. A review of physiochemical and photocatalytic properties of metal oxides against Escherichia coli. J. Photochem. Photobiol. A Chem. 2018, 360, 306–315. [Google Scholar] [CrossRef]
- Jayaraj, S.K.; Sadishkumar, V.; Thirumurugan, A.; Thangadurai, P. Enhanced photocatalytic activity of V2O5 nanorods for the photodegradation of organic dyes: A detailed understanding of the mechanism and their antibacterial activity. Mater. Sci. Semicond. Process. 2018, 85, 122–133. [Google Scholar] [CrossRef]
- Zeleke, M.A.; Kuo, D.-H. Synthesis and application of V2O5-CeO2 nanocomposite catalyst for enhanced degradation of methylene blue under visible light illumination. Chemosphere 2019, 235, 935–944. [Google Scholar] [CrossRef]
- Simo, A.; Drah, M.; Sibuyi, N.; Nkosi, M.; Meyer, M.; Maaza, M. Hydrothermal synthesis of cobalt-doped vanadium oxides: Antimicrobial activity study. Ceram. Int. 2018, 44, 7716–7722. [Google Scholar] [CrossRef]
- Suresh, R.; Giribabu, K.; Manigandan, R.; Munusamy, S.; Kumar, S.P.; Muthamizh, S.; Stephen, A.; Narayanan, V. Doping of Co into V2O5 nanoparticles enhances photodegradation of methylene blue. J. Alloys Compd. 2014, 598, 151–160. [Google Scholar] [CrossRef]
- He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L. Sodium Ion Stabilized Vanadium Oxide Nanowire Cathode for High-Performance Zinc-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Ni, S.; Ma, J.; Zhang, J.; Yang, X.; Zhang, L. Electrochemical performance of cobalt vanadium oxide/natural graphite as anode for lithium ion batteries. J. Power Sources 2015, 282, 65–69. [Google Scholar] [CrossRef]
- Seabold, J.A.; Neale, N.R. All First Row Transition Metal Oxide Photoanode for Water Splitting Based on Cu3V2O8. Chem. Mater. 2015, 27, 1005–1013. [Google Scholar] [CrossRef]
- Zelenski, M.E.; Zubkova, N.V.; Pekov, I.V.; Boldyreva, M.M.; Pushcharovsky, D.Y.; Nekrasov, A.N. Pseudolyonsite, Cu3(VO4)2, a new mineral species from the Tolbachik volcano, Kamchatka Peninsula, Russia. Eur. J. Miner. 2011, 23, 475–481. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Li, C.; Ci, L. Cu3V2O8 hollow spheres in photocatalysis and primary lithium batteries. Solid State Sci. 2013, 25, 15–21. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Salavati-Niasari, M. Facile synthesis, characterization and optical properties of copper vanadate nanostructures for enhanced photocatalytic activity. J. Mater. Sci. Mater. Electron. 2016, 27, 4871–4878. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, B.; Thakur, N.; Mishra, S.; Sarma, T.K. Copper Pyrovanadate Nanoribbons as Efficient Multienzyme Mimicking Nanozyme for Biosensing Applications. ACS Appl. Nano Mater. 2020, 3, 7917–7929. [Google Scholar] [CrossRef]
- Karim, N.; Singh, M.; Weerathunge, P.; Bian, P.; Zheng, R.; Dekiwadia, C.; Ahmed, T.; Walia, S.; Della Gaspera, E.; Singh, S.; et al. Visible-Light-Triggered Reactive-Oxygen-Species-Mediated Antibacterial Activity of Peroxidase-Mimic CuO Nanorods. ACS Appl. Nano Mater. 2018, 1, 1694–1704. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Liu, J.; Zhou, M.; Xu, H.; Xie, M.; Li, H.; Xie, J. Direct Z-scheme red carbon nitride/rod-like lanthanum vanadate composites with enhanced photodegradation of antibiotic contaminants. Appl. Catal. B Environ. 2020, 277, 119245. [Google Scholar] [CrossRef]
- Kadam, R.L.; Kim, Y.; Gailkwad, S.; Chang, M.; Tarte, N.H.; Han, S. Catalytic Decolorization of Rhodamine B, Congo Red, and Crystal Violet Dyes, with a Novel Niobium Oxide Anchored Molybdenum (Nb–O–Mo). Catalysts 2020, 10, 491. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Yu, J.; Zhang, Y.; Yu, F.; Jia, L.; Tan, Y.; Zhu, Y.; Hou, B. Enhancement in the photocatalytic antifouling efficiency over cherimoya-like InVO4/BiVO4 with a new vanadium source. J. Colloid Interface Sci. 2019, 533, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sun, S.; Hu, B.; Wang, X.; Lu, W.; Shi, W. Enhanced photocatalytic activity induced by surface plasmon resonance on Ag-loaded strontium titanate nanoparticles. Micro Nano Lett. 2013, 8, 504–507. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika, S.R.R.; Kirubagaran, R. Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015, 113, 23–30. [Google Scholar] [CrossRef]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Y.; Chen, N.; Meng, X.; Wang, C.; Zhang, Y.; Zhang, D.; Wei, Y.; Du, F.; Chen, G. Cu3V2O8 Nanoparticles as Intercalation-Type Anode Material for Lithium-Ion Batteries. Chem. A Eur. J. 2016, 22, 11405–11412. [Google Scholar] [CrossRef]
- Saud, S.N.; Hamzah, E.; Abu Bakar, T.A.; Farahany, S. Structure-Property Relationship of Cu-Al-Ni-Fe Shape Memory Alloys in Different Quenching Media. J. Mater. Eng. Perform. 2014, 23, 255–261. [Google Scholar] [CrossRef]
- Shannon, R.D.; Calvo, C. Crystal Structure of a New Form of Cu3V2O8. Can. J. Chem. 1972, 50, 3944–3949. [Google Scholar] [CrossRef]
- Boukhachem, A.; Ouni, B.; Karyaoui, M.; Madani, A.; Chtourou, R.; Amlouk, M. Structural, opto-thermal and electrical properties of ZnO:Mo sprayed thin films. Mater. Sci. Semicond. Process. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Akgul, F.A.; Akgul, G.; Yildirim, N.; Unalan, H.E.; Turan, R. Influence of thermal annealing on microstructural, morphological, optical properties and surface electronic structure of copper oxide thin films. Mater. Chem. Phys. 2014, 147, 987–995. [Google Scholar] [CrossRef]
- Svintsitskiy, D.; Kardash, T.Y.; Boronin, A.I. Surface dynamics of mixed silver-copper oxide AgCuO2 during X-ray photoelectron spectroscopy study. Appl. Surf. Sci. 2019, 463, 300–309. [Google Scholar] [CrossRef]
- Sivakumar, V.; Suresh, R.; Giribabu, K.; Manigandan, R.; Munusamy, S.; Kumar, S.P.; Muthamizh, S.; Narayanan, V. Copper vanadate nanoparticles: Synthesis, characterization and its electrochemical sensing property. J. Mater. Sci. Mater. Electron. 2014, 25, 1485–1491. [Google Scholar] [CrossRef]
- Liu, G.; Duan, X.; Li, H.; Dong, H. Hydrothermal synthesis, characterization and optical properties of novel fishbone-like LaVO4:Eu3+ nanocrystals. Mater. Chem. Phys. 2009, 115, 165–171. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W.; Yao, J. Hydrogen-interstitial CuWO4 nanomesh: A single-component full spectrum-active photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2018, 227, 35–43. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in Heterogeneous Photocatalytic Degradation of Phenols and Dyes in Wastewater: A Review. Water Air Soil Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Schiffer, S.; Liber, K. Estimation of vanadium water quality benchmarks for the protection of aquatic life with relevance to the Athabasca Oil Sands region using species sensitivity distributions. Environ. Toxicol. Chem. 2017, 36, 3034–3044. [Google Scholar] [CrossRef]
- Phongarthit, K.; Amornpitoksuk, P.; Suwanboon, S. Synthesis, characterization, and photocatalytic properties of ZnO nanoparticles prepared by a precipitation-calcination method using a natural alkaline solution. Mater. Res. Express 2019, 6, 045501. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Badjah, A.Y. Kinetics, Thermodynamics, and Modeling of Amido Black Dye Photo-degradation in Water Using Co/TiO2 Nanoparticles. Photochem. Photobiol. 2018, 94, 935–941. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Ghanbari, D.; Bagheri, S.; Salavati-Niasari, M. Novel chemical synthesis and characterization of copper pyrovanadate nanoparticles and its influence on the flame retardancy of polymeric nanocomposites. Sci. Rep. 2016, 6, 25231. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, M.; Ferey, G. Room-temperature structures of oxocopper(II) vanadate(V) hydrates, Cu3V2O8(H2O) and CuV2O6(H2O)2. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 15–18. [Google Scholar] [CrossRef]
- Harish, K.N.; Naik, H.B.; Kumar, P.N.P.; Viswanath, P. Optical and Photocatalytic Properties of Solar Light Active Nd-Substituted Ni Ferrite Catalysts: For Environmental Protection. ACS Sustain. Chem. Eng. 2013, 1, 1143–1153. [Google Scholar] [CrossRef]
- Sankar, R.; Prasath, B.B.; Nandakumar, R.; Santhanam, P.; Shivashangari, K.S.; Ravikumar, V. Growth inhibition of bloom forming cyanobacterium Microcystis aeruginosa by green route fabricated copper oxide nanoparticles. Environ. Sci. Pollut. Res. 2014, 21, 14232–14240. [Google Scholar] [CrossRef]
- Fan, G.; Bao, M.; Zheng, X.; Hong, L.; Zhan, J.; Chen, Z.; Qu, F. Growth inhibition of harmful cyanobacteria by nanocrystalline Cu-MOF-74: Efficiency and its mechanisms. J. Hazard. Mater. 2019, 367, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Gupta, V.; Mosquera, E.; Gracia, F. Preparation and characterization of V2O5/ZnO nanocomposite system for photocatalytic application. J. Mol. Liq. 2014, 198, 409–412. [Google Scholar] [CrossRef]
- Zhang, M.; Niu, Y.; Xu, Y. Heterogeneous Fenton-like magnetic nanosphere coated with vanadium oxide quantum dots for enhanced organic dyes decolorization. J. Colloid Interface Sci. 2020, 579, 269–281. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, Z.; Shi, J.; Li, M.; He, Z. Rapid Photocatalytic Decolorization of Methyl Orange under Visible Light Using VS4/Carbon Powder Nanocomposites. ACS Sustain. Chem. Eng. 2017, 5, 7690–7699. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Police, A.K.R.; Shima, J.; Byonb, C. In Situ fabrication of the Bi2O3-V2O5 hybrid embedded with graphitic carbon nitride nanosheets: Oxygen vacancies mediated enhanced visible-light—Driven photocatalytic degradation of organic pollutants and hydrogen evolution. Appl. Surf. Sci. 2018, 447, 740–756. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Di, L.; Dai, J. Enhanced photocatalytic activity of BaTiO3@ g-C3N4 for the degradation of methyl orange under simulated sunlight irradiation. J. Alloys Compd. 2015, 622, 1098–1104. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, S.; Cai, R.; Li, M.; He, Z. Highly-efficient photocatalytic disinfection of Escherichia coli under visible light using carbon supported Vanadium Tetrasulfide nanocomposites. Appl. Catal. B Environ. 2018, 224, 383–393. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Regmi, C.; Kshetri, Y.K.; Kim, T.-H.; Pandey, R.P.; Ray, S.K.; Lee, S.W. Fabrication of Ni-doped BiVO4 semiconductors with enhanced visible-light photocatalytic performances for wastewater treatment. Appl. Surf. Sci. 2017, 413, 253–265. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, C.; Wang, K.; Fang, Z. Efficient oxidative degradation of fluconazole by a heterogeneous Fenton process with Cu-V bimetallic catalysts. Chem. Eng. J. 2020, 380, 122516. [Google Scholar] [CrossRef]
- Malka, E.; Perelshtein, I.; Lipovsky, A.; Shalom, Y.; Naparstek, L.; Perkas, N.; Patick, T.; Lubart, R.; Nitzan, Y.; Banin, E.; et al. Eradication of Multi-Drug Resistant Bacteria by a Novel Zn-doped CuO Nanocomposite. Small 2013, 9, 4069–4076. [Google Scholar] [CrossRef]
- Nandanwar, S.K.; Lee, M.W.; Borkar, S.B.; Cho, J.H.; Tarte, N.H.; Kim, H.J. Synthesis, Characterization, and Anti-Algal Activity of Molybdenum-Doped Metal Oxides. Catalysts 2020, 10, 805. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.; Zhou, H.; Jin, P.; Cheung, K.M.C.; Chu, P.K.; Yeung, K.W. Vanadium Dioxide Nanocoating Induces Tumor Cell Death through Mitochondrial Electron Transport Chain Interruption. Glob. Chall. 2018, 3, 1800058. [Google Scholar] [CrossRef]
- Xi, W.-S.; Tang, H.; Liu, Y.-Y.; Liu, C.-Y.; Gao, Y.; Cao, A.; Liu, Y.; Chen, Z.; Wang, H.-F. Cytotoxicity of vanadium oxide nanoparticles and titanium dioxide-coated vanadium oxide nanoparticles to human lung cells. J. Appl. Toxicol. 2019, 40, 567–577. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Z.; Pu, Y.; Dou, W.; Wang, C.; Wang, W.; Hao, X.; Chen, S.; Shao, Q.; Dong, M.; et al. Zinc oxide/vanadium pentoxide heterostructures with enhanced day-night antibacterial activities. J. Colloid Interface Sci. 2019, 547, 40–49. [Google Scholar] [CrossRef]
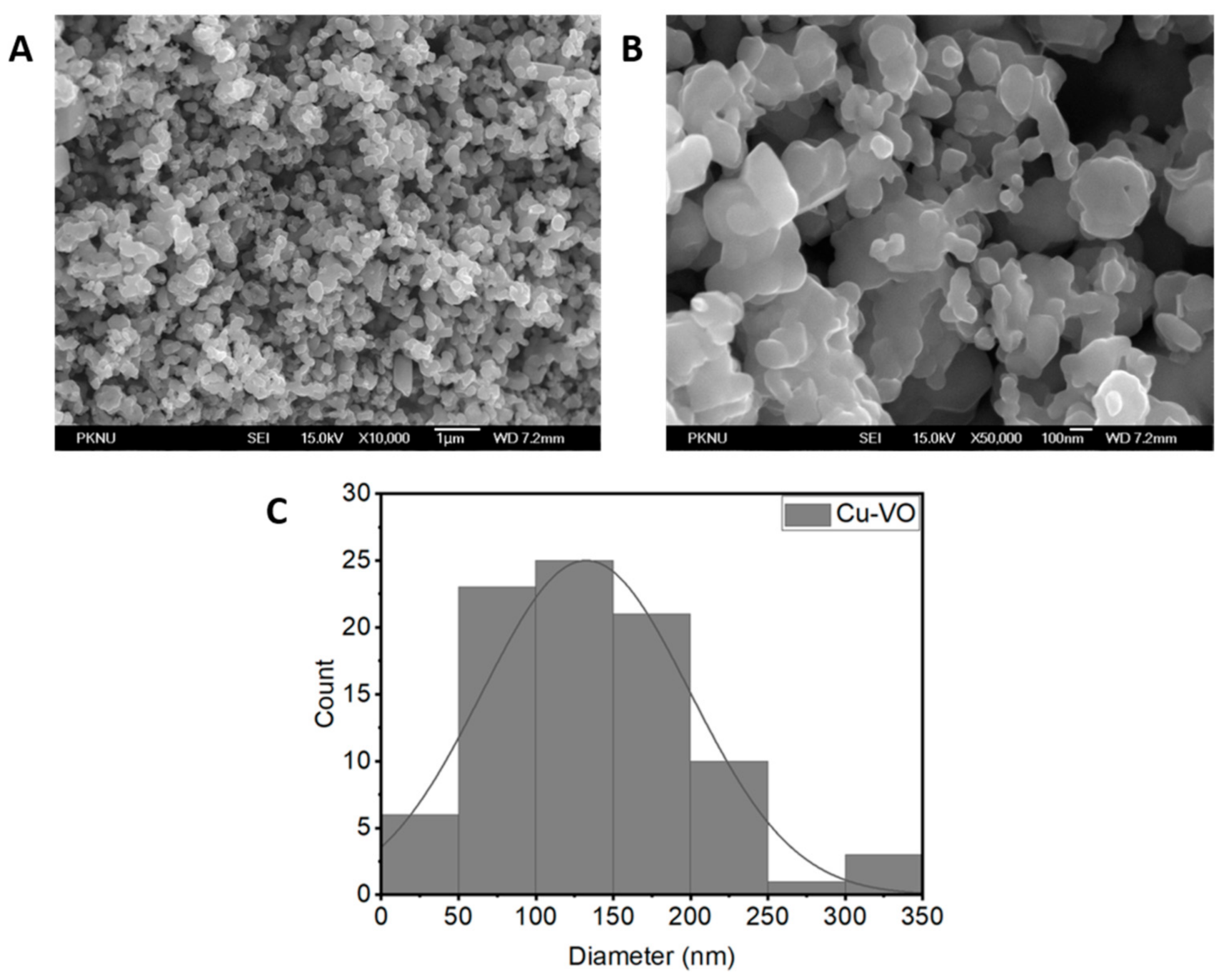
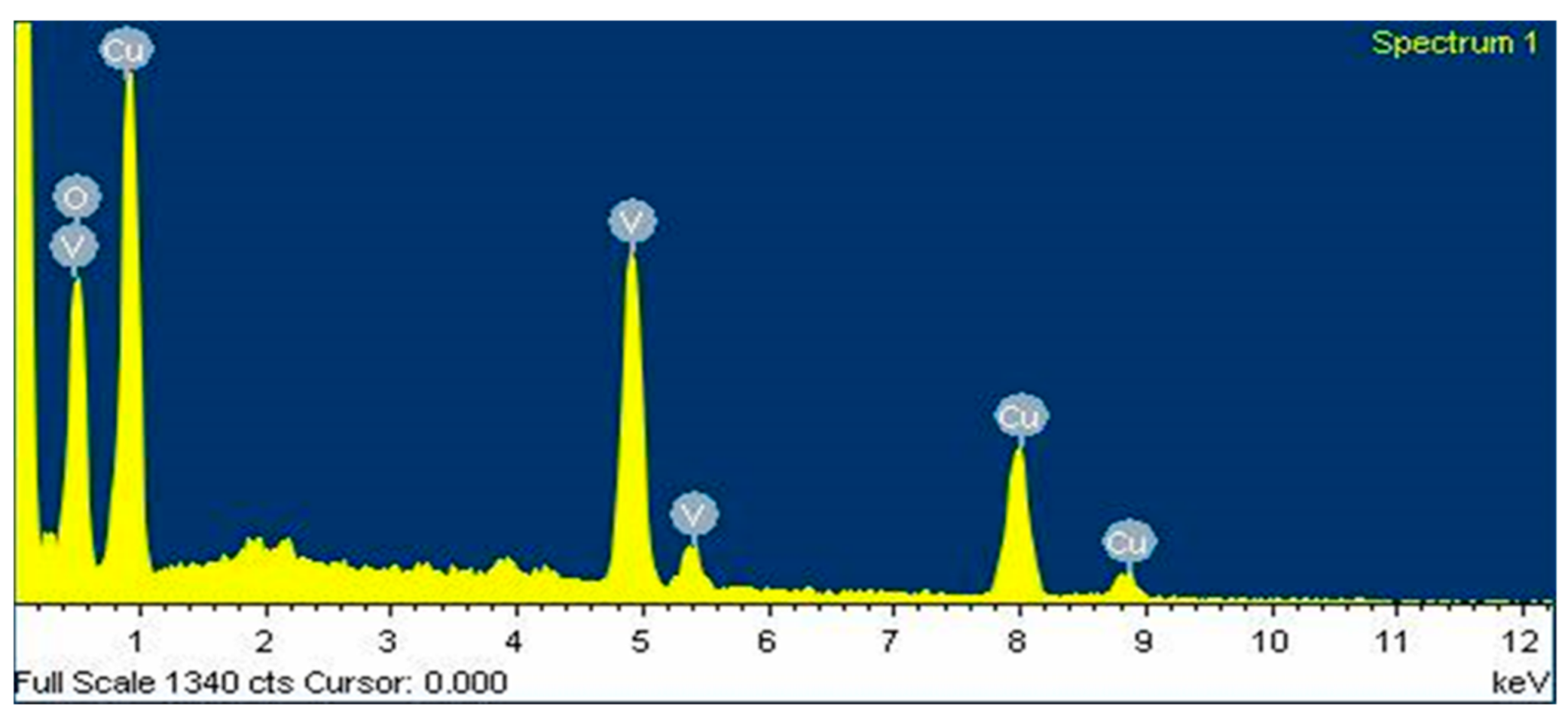





| Element | Weight% | Atomic% |
|---|---|---|
| O | 27.90 | 58.64 |
| V | 24.41 | 16.12 |
| Cu | 47.69 | 25.24 |
| Material | Crystallite Size (nm) | Atomic% | Lattice Constant (Å) | Lattice Strain (ε) | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| Cu-VO | 58 ± 10 | 58.64 | 6.28 | 7.99 | 6.39 | 0.13 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandanwar, S.; Borkar, S.; Cho, J.H.; Kim, H.J. Microwave-Assisted Synthesis and Characterization of Solar-Light-Active Copper–Vanadium Oxide: Evaluation of Antialgal and Dye Degradation Activity. Catalysts 2021, 11, 36. https://doi.org/10.3390/catal11010036
Nandanwar S, Borkar S, Cho JH, Kim HJ. Microwave-Assisted Synthesis and Characterization of Solar-Light-Active Copper–Vanadium Oxide: Evaluation of Antialgal and Dye Degradation Activity. Catalysts. 2021; 11(1):36. https://doi.org/10.3390/catal11010036
Chicago/Turabian StyleNandanwar, Sondavid, Shweta Borkar, Jeong Hyung Cho, and Hak Jun Kim. 2021. "Microwave-Assisted Synthesis and Characterization of Solar-Light-Active Copper–Vanadium Oxide: Evaluation of Antialgal and Dye Degradation Activity" Catalysts 11, no. 1: 36. https://doi.org/10.3390/catal11010036
APA StyleNandanwar, S., Borkar, S., Cho, J. H., & Kim, H. J. (2021). Microwave-Assisted Synthesis and Characterization of Solar-Light-Active Copper–Vanadium Oxide: Evaluation of Antialgal and Dye Degradation Activity. Catalysts, 11(1), 36. https://doi.org/10.3390/catal11010036







