Abstract
Consumers are very concerned with following a healthy diet, along with some precautions that may influence environmental impact. Solanum melongena L. is one of the most consumed vegetables due to its excellent nutritional value and antioxidant action. Associated with its high consumption, considerable amounts of agro-food wastes are produced. This work targets the valorization of this matrix, through the use of its bio-residues to study the obtention of coloring pigments, applying innovative technologies. Its nutritional value, chemical composition, and bioactive potential were evaluated, and the ultrasound-assisted extraction to obtain coloring pigments of high industrial interest was optimized. Considering the results, low contents of fat and carbohydrates and energy value were evident, as well as the presence of compounds of interest (free sugars, organic acids, unsaturated fatty acids, and phenolic acids). In addition, the antioxidant and antimicrobial potential was detected. Response surface methodology was performed to optimize the extraction of natural pigments, showing a concentration of 11.9 mg/g of anthocyanins/g of extract, applying optimal conditions of time, solvent, and solid/liquid ratio of 0.5 min, 68.2% (v/v) and 5 g/L, respectively. S. melongena proved to be a good source of bioactive compounds and natural pigments, which can generate great interest in the food industry.
1. Introduction
The food industry is one of the sectors in Europe that generates great amounts of bio-waste. Annually, in Europe, food waste is around 1.3 billion tons, of which approximately 700 million tons are from agriculture. These residues result essentially from the processing of grains, fruits, and vegetables and have a high potential for reuse [1,2]. These residues can be transformed into resources, using intensified conversion processes that can produce potentially sustainable bio-products, such as energy, fertilizers, materials, and molecules. In this sense, the valorization of residues and agri-food by-products is currently presented not only as a necessity, but also as an opportunity to obtain new products of high value and of great impact on the economy of the industrial sector [3].
Solanum melongena L. (Solanaceae), known by its fruit, eggplant, is a non-tuberous species of great agronomic and economic value [4]; however, mature eggplant fruits are considered unmarketable due to their unpleasant fruit color, texture, pith, and bitter taste as well as the large amount of mature seeds, thus becoming a bio-waste of the food industry [5].
This matrix is considered a versatile food, as it is part of the gastronomy of many countries and different sorts of diets, namely Mediterranean and vegetarian [6]. In addition to their culinary interest, several authors describe the use of eggplants in traditional medicine, acting therapeutically on several health problems. This curative potential is manifested by its rich composition of ascorbic acid and phenolic compounds, both with high antioxidant potential [4,7].
Some of the main phenolic compounds found in eggplant are anthocyanins, responsible for the majority of red, purple, and blue colors of flowers and fruits [8,9]. Anthocyanins are increasingly valued by the industrial sector, due to their application as natural colorant ingredients. However, in addition to their coloring potential, they also exhibit bioactive properties related to their high antioxidant activity [10]. The potential use of anthocyanins as food colorants has attracted the attention of the scientific community, for combining the pigmentation capacity with health benefits [11,12]. In the case of eggplant, most of these anthocyanic compounds are concentrated in the epicarp [13].
Considering the nutritional and bioactive potential of Solanum melongena L. fruit pulp and the coloring compounds present in the epicarp, this study aims to optimize ultrasound-assisted extraction methodologies to obtain an anthocyanin-rich extract from S. melongena epicarp, to be used as a natural coloring ingredient, and thus, contributing to the sector sustainability and improvement of environmental impact. It also targets the nutritional and chemical characterization of the fruit, as well as at its bioactive properties’ assessment.
In this sense, this work addresses a subject with high relevance for several sectors, such as industrial, environmental, scientific, economic, and social. This wide relevance is justified by the proposal to develop strategies that reduce food waste, improving the quality of some commercial products. Furthermore, it leads to the application of innovative extraction technologies, an increase in the quality of products available on the market, an expansion in consumers’ preference for these products and, consequently, to an improvement in the environmental impact, economic growth, and the development of a sustainable economy.
2. Materials and Methods
2.1. Samples
The Solanum melongena L. fruits (eggplant) were purchased in April 2019, in a local market in Bragança (northeast Portugal). After the acquisition of the samples (15 fruits; approximately 3 kg in fresh weight), the epicarp and the pulp were separated by mechanical methods, and the different parts of samples were selected (whole fruit, pulp, and peel) in order to perform the different analyses. Subsequently, the samples were frozen, dehydrated by lyophilization (FreeZone 4.5, Labconco, Kansas City, MO, USA), and reduced to a fine dried powder. The samples were then stored in a dry place away from light and heat, until further analysis.
2.2. Determination of Color in Eggplant Epicarp
Color evaluation was performed in the epicarp of the fruit, in its fresh and lyophilized state, using a colorimeter (model CR-400, Konica Minolta Sensing, Inc., Osaka, Japan) (model CR-A50). For that, methodology previously described by Roriz et al. [14] was used. The values of the CIE (international lighting commission) L* a* b* coordinates were obtained on a computer system using the illuminant C and an 8 mm diameter diaphragm. For data processing, the “Spectra Magic Nx” software (version CM-S100W 2.03.0006, Konica Minolta, Japan) was used.
2.3. Determination of Nutritional Composition of Eggplant (Pulp and Whole Fruit)
The nutritional composition was determined in pulp and whole fruits, separately, considering their form of consumption. Approximately 10 g of dry sample were used for these analyses. For this purpose, the official analysis methodologies AOAC (association of official analytical chemists) [15] were used. The crude protein (N × 6.25) was evaluated using the macro-Kjeldahl method (AOAC 991.02); fat content was determined applying a soxhlet extraction of the dry sample with petroleum ether (AOAC 989.05); the ash was estimated by incineration at 550 ± 15 °C (AOAC 935.42); total carbohydrates were calculated by difference, and the energy was estimated using the following equation: Energy (kcal) = 4 × (g protein + g carbohydrates) + 9 × (g fat).
2.4. Free Sugars, Organic Acids, and Fatty Acids Profile of Eggplant (Pulp and Whole Fruit)
The chemical composition was also determined in pulp and whole fruits, separately, also according to their form of consumption. Approximately 5 g of dry sample were used for these analyses. Free sugars were analyzed by high performance liquid chromatography coupled to a refraction index detector (HPLC-RI, Knauer, Smartline system 1000), according to a practice earlier explained by other authors [16]. The compounds were identified by chromatographic comparison with authentic standards (D(−)-fructose, D(+)-sucrose, D(+)-glucose, D(+)-trehalose and D(+)-raffinose pentahydrate), purchased from Sigma-Aldrich (St. Louis, MO, USA). Melezitose was used as the internal standard and the results of sugars were expressed in g/100 g of fresh weight (fw).
Organic acids were evaluated following a procedure previously described by Barros et al. [16]. Approximately 5 g of dry sample were used for these analyses. Ultra-Fast Liquid Chromatography (UFLC, Shimadzu 20A series, Kyoto, Japan) coupled to a diode array detector was used, and the quantification was performed through a calibration curve obtained from commercial standards (L(+)-ascorbic acid, citric acid, malic acid, oxalic acid, shikimic acid, succinic acid, fumaric acid, and quinic acid; purchased from Sigma-Aldrich; St. Louis, MO, USA). The results were expressed in g/100 g of fresh weight (fw).
The fatty acids profile was determined using chromatographic methods, namely using gas chromatography coupled with a flame ionization detector (GC-FID, DANI model GC 1000, Contone, Switzerland) and the separation was achieved with a Macherey–Nagel (Düren, Germany) column (50% cyanopropyl-methyl-50% phenylmethylpolysiloxane, 30 m × 0.32 mm i.d. × 0.25 μm df). The compounds were identified by comparing the relative retention times of FAME (Fatty Acid Methyl Esters) peaks from samples with commercial standards (FAME reference standard mixture, standard 47885-U, Sigma-Aldrich, St. Louis, MO, USA) [16]. Approximately 9 g of dry sample were used for these analyses. The results were expressed in relative percentages (%).
2.5. Determination of Phenolic Compounds of Eggplant
The analysis of non-anthocyanin phenolic compounds was carried out on the epicarp, pulp, and whole fruit of S. melongena, whereas the determination of anthocyanin phenolic compounds was performed only in the epicarp, due to its evident purple color. For that purpose, methodology described in previous works was applied [17,18,19,20]. Approximately 3 g of dry sample were used for these analyses.
2.5.1. Non-Anthocyanin Compounds
Extraction procedure. An extract was prepared from the lyophilized samples (fruits of S. melongena; 1 g) and a maceration with ethanol/water solution (80:20, v/v; 30 mL) at room temperature was used. The alcoholic fraction of the extracts was evaporated under reduced pressure (Büchi R-210, Flawil, Switzerland) and the aqueous fraction was lyophilized (47 °C, 0.045 bar; FreeZone 4.5, Labconco, Kansas City, MO, USA) for further analysis. A quantity of the obtained dry extract (10 mg) was subsequently re-dissolved in an ethanol/water solution for further HPLC analysis [18].
Analytical method. The chromatographic data were acquired using a Dionex Ultimate 3000 UPLC (Thermo Scientific, San Jose, CA, USA), coupled to a diode array detector (280, 330, and 370 nm) and an electrospray ionization mass detector (Linear Ion Trap LTQ XL, Thermo Finnigan, San Jose, CA, USA), working in the negative mode. The chromatographic separation was performed using a Waters Spherisorb S3 ODS-2 C18 (3 μm, 4.6 mm × 150 mm, Waters, Milford, MA, USA) column at 35 °C. The compounds were identified considering the retention time, UV-Vis, and mass spectra in comparison with available standards (caffeic acid (y = 388,345x + 406,369), protocatequic acid (y = 214,168x + 27,102), and chlorogenic acid (y = 168,823x − 161,172)) and with literature data. Calibration curves of the available phenolic standards were constructed based on the UV-Vis signal to obtain quantitative analysis. In the case of unavailable commercial standards, the compounds were quantified via a calibration curve of the most similar standard available. The results were expressed as mg/g of extract [17].
2.5.2. Anthocyanin Compounds
Extraction procedure. The lyophilized samples (1 g) were extracted using the conventional methodology applied by this research group [20]; ethanol/water (80:20, v/v) acidified with 0.5% of citric acid was used as extraction solvent.
Analytical method. The chromatographic analysis was made using a system previously described in Section 2.5.1 and the separation was achieved using an AQUA® (Phenomenex) reverse phase C18 column (5 µm, 150 × 4.6 mm i.d.) thermostatted at 35 °C, using a UPLC-DAD-ESI/MSn system (Ultra-Performance Liquid Chromatography coupled with Diode Array Detection and an Electrospray Ionization Mass Spectrometry) previously mentioned. Detection was carried out using a DAD (520 nm) and a mass spectrometer (Linear Ion Trap LTQ XL Thermo Finnigan) equipped with an ESI source, operating in positive mode. Compounds identification was performed using the retention time, UV-Vis, and mass spectra data in comparison with available standards and literature review. For quantitative analysis, calibration curves were obtained by injection of standard solutions with known concentrations (0.25–50 µg/mL): cyanidin-3-O-glucoside (y = 97,787x − 743,469; R2 = 0.99993), peonidine (y = 110,391x − 1 × 106; R2 = 0.9999), and pelargonidin-3-O-glucoside (y = 43,781x − 275,315; R2 = 0.9989). The results were expressed in mg of anthocyanin/g of extract [19,20].
2.6. Evaluation of the Bioactive Properties of Solanum melongena L. Fruits: Epicarp, Pulp, and Whole Fruit, through In Vitro Tests
The lyophilized extract, provided by the extraction procedures (Section 2.5.1), was re-dissolved: (i) in culture medium (10 mg/mL) for antimicrobial activity assay; (ii) in distilled water at a concentration of 8 mg/mL for the evaluation of cytotoxic, hepatotoxic, and anti-inflammatory activity; and (iii) in a hydroethanolic solution (ethanol/water; 80:20, v/v), in a concentration of 5 mg/mL, for antioxidant activity evaluation. These solutions were diluted successively in order to obtain the working concentrations.
2.6.1. Antimicrobial Activity
The antibacterial activity was evaluated using Gram-negative (Enterobacter cloacae (ATCC—American type culture collection 35030), Escherichia coli (ATCC 35210), and Salmonella Typhimurium (ATCC 13311)) and Gram-positive bacteria strains (Listeria monocytogenes (NCTC—National collection of type cultures 7973) and Staphylococcus aureus (ATCC 6538)). The minimum inhibitory (MIC) and minimum bactericidal (MBC) concentrations were used to estimate antimicrobial potential; the microdilution method was applied, and the results were expressed in mg/mL [21].
For the antifungal activity, Aspergillus fumigatus (ATCC 1022), Aspergillus niger (ATCC 6275), Aspergillus versicolor (ATCC 11730), Penicillium funiculosum (ATCC 36839), Penicillium ochrochloron (ATCC 9112), and Trichoderma viride (IAM 5061) strains were used. Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were determined using a modified microdilution method, and the results were also expressed in mg/mL [21].
2.6.2. Cytotoxic, Hepatotoxic, and Anti-Inflammatory Activity
In order to evaluate the cytotoxicity of the extracts, the sulforhodamine B assay was applied using methodology previously described by Guimarães et al. [22]. For that, four human tumor cell lines, acquired from Leibniz-Institut DSMZ, HeLa (cervical carcinoma), HepG2 (hepatocellular carcinoma), MCF-7 (breast adenocarcinoma), and NCI-H460 (non-small cell lung cancer) were used, as well as a primary cell culture, PLP2, obtained from a freshly harvested porcine liver (purchased from a local slaughter house) to test the hepatotoxic activity. Ellipticine was used as a positive control and the results were expressed as GI50 values (the extract concentration that inhibits 50% of the net cell growth).
For the anti-inflammatory activity evaluation, a process previously mentioned in Jabeur et al. [18] was performed. For that purpose, a mouse macrophage-like cell line RAW 264.7 stimulated with LPS (Lipopolysaccharides) was used, and the extracts concentration ranged between 400–125 μg/mL. Nitric oxide (NO) production was studied with a Griess reagent system kit. Dexamethasone (50 μM) was used as a positive control and the results were expressed as EC50 values (μg/mL) equal to the sample concentration providing a 50% inhibition of NO production.
2.6.3. Antioxidant Activity
For the antioxidant activity evaluation, two colorimetric assays were used, using methodologies previously described [23,24,25]: the cell-based assays of thiobarbituric acid reactive substances (TBARS) formation inhibition and oxidative hemolysis inhibition (OxHLIA). The extracts capacity to inhibit the formation of TBARS was assessed using porcine brain cells as oxidizable biological substrates and the results were expressed as EC50 values (µg/mL), which translate the extract concentration responsible for 50% of antioxidant activity. In turn, the extracts capacity to inhibit the oxidative hemolysis was tested using sheep blood erythrocytes as ex vivo models and the extract concentration able to promote a Δt hemolysis delay of 60 min was calculated based on the Ht50 values of the hemolytic curves of each extract concentration. The results were expressed as IC50 values (μg/mL), which translate the extract concentration required to keep 50% of the erythrocyte population intact for 60 min. Trolox was used as a positive control in both assays.
2.7. Optimization of the Extraction Process to Obtain a Natural Colorant Rich in Anthocyanins, from the Epicarp of Solanum melongena L. Fruits
In order to optimize the extraction of anthocyanin compounds from the eggplant epicarp, ultrasound-assisted extraction was used and an experimental design called the central composite design (CCD) was developed, applying response surface methodology (RSM), aiming to simplify and reduce the operational costs of the process, shorten the extraction process times, and reduce the energy spent as well as the solvent consumption [14,26]. In order to obtain the extract rich in anthocyanins, it was essential to consider several factors that affect its stability to achieve the maximum extraction efficiency [27]. Hence, RSM methodology was used to optimize relevant factors (time, percentage of solvent, and solid/liquid ratio) simultaneously, obtaining graphic models and polynomial equations that allow one to describe the ideal conditions that maximize the response criteria [14].
2.7.1. Experimental Design and Extraction Procedure Assisted by Ultrasound
Screening tests were performed based on individual analysis of the variables and those that caused significant effects were selected along with the relevant intervals (Table 1). The effects of the three defined variables were studied using a CCD, associated with five levels [28], which generated 20 run combinations, performed randomly in order to obtain better predictive capacity of the model. The extraction procedure was performed according to Lopez et al. [29], using ultrasound equipment (sonicators QSonica, model CL-334, Newtown, Connecticut, USA). The samples were extracted with a fix volume of 25 mL of solvent (acidified with 0.05% citric acid). Variables and intervals employed were: time (t or X1 0.5 to 5.5 min), the ethanol/water extraction solvent (X2 8 to 92%), and the solid/liquid ratio (X3 5 to 65 g/L), particular levels analyzed within the ranges mentioned before are described in Table 1. Temperature was monitored and controlled below 30 °C.

Table 1.
Coded experimental design of the independent variables used in the 5-level CCD design.
2.7.2. Preparation of Extracts Obtained by Ultrasound-Assisted Extraction
After the extraction procedure, the samples were centrifuged (5000 rpm for 20 min at 10 °C) and filtered through filter paper (Whatman n° 4). The supernatant was collected and divided into two fractions: one for HPLC-DAD analysis and the second for determining the extraction yield. The fraction separated for HPLC analysis (2 mL) was filtered through an LC syringe filter (0.22 μm) and then injected; the second fraction, used to determine the extraction yield (5 mL), was subjected to drying at a temperature of 105 °C for 48 h, for subsequent weighing of the solid extract.
2.7.3. Identification and Quantification of Anthocyanin Compounds by HPLC-DAD
The extracts obtained in the previous process were analyzed using a high-performance liquid chromatography system (HPLC, Dionex UltiMate 3000 UPLC, Thermo Scientific). The anthocyanins present in the samples were characterized according to their UV-Vis spectra and mass and retention times compared to the identification obtained in Section 2.5.2. The quantitative analysis of anthocyanin compounds was obtained through a calibration curve with the injection of a standard of known concentrations (200–0.25 μg/mL) of the compound cyanidin-3-O-glucoside (y = 97787x − 743469; R2 = 0.993).
2.7.4. Response Format Used for Analytical Processes
The yield (%) and the content of the detected anthocyanin compound were used as responses. The results were expressed in three response formats (Y): Y1, in mg of anthocyanins per gram of dry weight (mg/g dw), Y2 in mg of anthocyanins per g of extracted residue (mg/g E), which was used to evaluate the purity of anthocyanins in the extracts, and Y3 the amount of residue extracted expressed by yield (%).
2.7.5. Analysis of the Mathematical Model, Procedure for Optimization of Variables and Numerical Methods, Statistical Analysis, and Graphic Illustrations
The RSM data were adjusted by calculating least squares, using the following second order polynomial equation with complex interaction terms, as described by Pinela et al. [30]. The responses (Y) applied were the extraction yield (yield, %), the content of anthocyanins per gram of dry sample (mg/g dw), and the content of anthocyanins per g of extracted residue (mg/g E).
In order to optimize the forecasting model and, consequently, maximize the extraction yield, a simplex method was used, which allows solving non-linear problems [31]. In order to avoid variables with unnatural and unrealistic physical conditions, some limitations were imposed on the coded variables (namely t ≥ 0, 0 > S > 100).
The adjustment procedures, coefficient estimates, and statistical calculations of the experimental results were performed according to a procedure previously described by Melgar et al. [32]. The experimental data were fitted to the second-order polynomial model (Equation (1)) to obtain the regression coefficients (b) using Statgraphics Centurion XVI (StatPoint Technologies, Inc. Warrenton, VA, USA) and Design expert 12.0.1. (Stat-Ease, Inc., Minneapolis, MN, USA) softwares. The generalized second-order polynomial model used in the response surface analysis was the following:
where Y is the dependent variable (response variable) to be modelled, b0 is a constant coefficient (intercept); bi, bii and bij are the coefficients of the linear, quadratic, and interactive terms, respectively; k is the number of tested variables (k = 3); and Xi and Xj are the independent variables.
2.8. Preparation of the Optimal Extract Rich in Anthocyanins from the Epicarp of Solanum melongena L. Fruit
After the optimization studies, an ultrasound-assisted extraction was performed, from the epicarp of the S. melongena fruits, applying the optimum variable values, obtained in Section 2.7.1.
The sample was extracted with ethanol/water solvent (68:32; v/v) acidified with 0.05% citric acid (until pH = 3), in the proportion of 5 g/L, during 5.5 min. Then, the samples were centrifuged (Centurion K24OR, West Sussex, United Kingdom) at 5000 rpm for 5 min at 10 °C and filtered through paper filter (Whatman n° 4). The supernatant was evaporated on a rotary evaporator at 35 °C (Büchi R-210, Flawil, Switzerland) in order to remove the ethanolic fraction. Finally, the aqueous fraction obtained was lyophilized (FreeZone 4.5, Labconco), obtaining a pigmented extract.
2.9. Statistical Analysis
The described experiments, except for RSM, were performed in triplicate and the results were expressed as mean ± standard deviation (SD). The data for the evaluation of S. melongena fruit with regard to color parameters, nutritional parameters, chemical composition, bioactive potential, and optimization of anthocyanin extraction were analyzed through the application of different statistical tests. The Student t-test was applied in order to determine the significant differences between two different types of eggplant samples (whole fruit and pulp); in addition to that, the statistical analysis for the evaluation of the results of the three different types of eggplant samples (epicarp, whole fruit, and pulp), was carried out through the application of one-way analysis of variance (ANOVA) with p-value = 0.05 (SPSS v. 23.0; IBM Corp., Armonk, New York, NY, USA).
The tests of the optimization of the extraction process to obtain an extract rich in anthocyanins were analyzed using ANOVA and cluster analysis through the software Design-Expert 12.0.1 and Statgraphics 18.
3. Results and Discussions
3.1. Color Evaluation of the Epicarp, Pulp, and Whole Fruit of S. melongena
The color evaluation in the CIE L* a* b* color space was performed on fresh and lyophilized eggplant epicarp and the results are shown in Table 2. The luminosity scale, represented by the parameter L*, varies between 0 and 100, and the parameters a* (green to red) and b* (blue to yellow) vary between −120 and 120 [33]. In the color evaluation of the fresh epicarp, for the parameters L*, a*, and b*, were obtained the mean values of 26.2 ± 0.5; 5.1 ± 0.2 and 1.5 ± 0.1, respectively. On the other hand, in the lyophilized eggplant epicarp the parameter L* showed a value of 34 ± 2, and the values in parameters a* and b* were 2.8 ± 0.1 and −0.17 ± 0.01, respectively.

Table 2.
Physical parameters (color-CIE L* a* b*) of the fresh and lyophilized fruit epicarp of Solanum melongena L. (mean ± SD).
Through the statistical analysis of the results, it was verified that the process of dehydration (by lyophilization) of the eggplant epicarp had significant influence (p < 0.05) in all the evaluated color parameters. The L* parameter had lower values in the fresh epicarp, indicating a lighter hue when compared to the lyophilized sample. In contrast, the parameter a* showed higher values in the fresh epicarp, showing a color nearest to the red hue. The same decrease, after the lyophilization process, was verified in parameter b*, which revealed that the lyophilized epicarp presents a close hue to the blue color.
According to several results available in the literature, it is possible to verify that different dehydration processes can modify the physicochemical properties of the fruits [34]. In the present study, it was evident that the lyophilization process led to a decrease in the purple color of the epicarp. This may be associated with the degradation of anthocyanins present in the sample, mainly verified by the decrease in the value presented in parameter a* [35]. Other authors [36,37,38] also studied this process and demonstrated that the color change after dehydration processes is associated with the degradation of anthocyanin pigments.
However, it is important to mention that the applied dehydration process must be chosen considering the degree of degradation of anthocyanins, in order to preserve the original physical and chemical characteristics of the samples as much as possible. According to literature, one of the methodologies that shows less associated degradation is the lyophilization process [39]. In fact, Badulescu et al. [40] studied the effect of dehydration by lyophilization and hot-air drying on the content of anthocyanins in raspberries and the results showed that the lyophilization process was more efficient in preserving the anthocyanin compounds in the samples.
3.2. Nutritional Characterization of Eggplant (Whole Fruit and Pulp)
The results obtained in the nutritional characterization of the whole fruit and the pulp of Solanum melongena L. are shown in Table 3.

Table 3.
Nutritional parameters of the whole fruit and pulp of Solanum melongena L. (mean ± SD).
The moisture content showed values of 91.3 ± 0.3 g/100 g fw (fresh weight) for the whole fruit samples and 92.9 ± 0.4 g/100 g fw for the pulp samples, indicating a high percentage of water in its composition. On the other hand, considerably lower levels were evident in the values of the remaining nutrients and non-nutrients evaluated in these samples. In the evaluation of the carbohydrate content, values of 3.0 ± 0.1 g/100 g fw were obtained for the whole fruit and 2.89 ± 0.01 g/100 g fw for the pulp; in fat content, lower concentrations were obtained, ranging from 0.050 ± 0.002 g/100 g fw (whole fruit) to 0.040 ± 0.001 g/100 g fw (pulp); the protein concentration was 0.86 ± 0.02 g/100 g fw (whole fruit) and 0.78 ± 0.03 g/100 g fw (pulp) and, finally, the ash content was 0.56 ± 0.01 g/100 g fw and 0.56 ± 0.02 g/100 g fw for the whole fruit and the pulp, respectively.
The energy value showed satisfactory values of 32.6 ± 1.1 kcal/100 g fw in the whole fruit and 27.5 ± 0.6 kcal/100 g fw in the pulp. The values obtained in the present study explain the high interest in the consumption of this food, namely, in diets with caloric restriction.
Through the statistical analysis performed, there was an absence of significant difference (p > 0.05) in most of the tested parameters (moisture, ash, proteins, carbohydrates, and energy), with the exception of the fat content, which showed a significant difference (p < 0.05), being higher in whole eggplant samples.
Regarding the data available in the literature, the United States Department of Agriculture (USDA) [41] presented in a report 11,209 similar concentrations to those achieved in the present study, namely, in the moisture (92.3 g/100 g fw), ash (0.66 g/100 g fw), and protein content (0.98 g/100 g fw). However, the remaining macronutrients showed diverse values, such as fat and carbohydrate content with values of 0.18 g/100 g fw and 5.88 g/100 g fw, respectively; while the amount of energy presented by the USDA was 25 kcal/100 g fw.
Kandoliya et al. [42] studied the composition of six eggplant varieties and found that the moisture content obtained in the pulp was 91.1 and 93.0 ± 0.6%, while the protein content was 0.66 to 1.28 ± 0.05%, these concentrations being in agreement with the values obtained in the present study.
In addition, Agoreyo et al. [43] evaluated the nutritional content of two unripened varieties of Solanum melongena (round and oval). The results obtained revealed moisture contents of 78.4 ± 0.49% and 72.93 ± 0.76%, protein contents of 5.79 ± 0.22 g/100 g fw and 4.58 ± 0.40 g/100 g fw, ash contents of 1.96 ± 0.12 g/100 g fw and 3.15 ± 1.54 g/100 g fw, and, finally, a carbohydrate concentration of 11.77 ± 1.55 g/100 g fw and 15.42 ± 0.69 g/100 g fw. This disparity in results, compared to the present study, may be related to several factors that directly interfere with the nutritional composition of foods, particularly: geographical location of production, cultivation conditions, climatic conditions, harvest conditions, or state of maturity of the raw material, among others [44].
Ossamulu et al. [45] also compared the nutritional values of four species of eggplants fruits, namely Solanum marcrocarpon (round), Solanum aetheopicum, Solanum marcrocapon (oval), and Solanum gilo. The results presented similarity of values in some parameters, among them the moisture (88.31 ± 0.23% to 91.94 ± 0.11%), ash content (0.41 ± 0.01 g/100 g fw to 0.56 ± 0.02 g/100 g fw), and energy value (34.02 ± 0.95 kcal/100 g fw to 22.90 ± 0.46 kcal/100 g fw). Otherwise, there were some discrepancies in protein (1.21 ± 0.02 g/100 g fw to 2.36 ± 0.03 g/100 g fw), fat (0.24 ± 0.01 g/100 g fw to 0.42 ± 0.02 g/100 g fw), and carbohydrates concentration (4.06 ± 0.19 g/100 g fw to 6.03 ± 0.19 g/100 g fw).
3.3. Chemical Composition of Whole Fruit and Pulp of S. melongena
3.3.1. Content of Sugars, Organic Acids, and Fatty Acids
The chemical composition of the whole fruit and pulp of Solanum melongena L. was evaluated and the results of hydrophilic compounds (free sugars and organic acids) and lipophilic compounds (fatty acids) are represented in Table 4.

Table 4.
Free sugars, organic acids, and fatty acids profile of in Solanum melongena L. whole fruit and pulp (mean ± SD).
Concerning the composition in free sugars, the studied samples revealed the presence of two monosaccharides (fructose and glucose) and two disaccharides (sucrose and trehalose). In both samples, the monosaccharides were found in higher quantities, with fructose concentrations of 1.26 g/100 g fw in the whole fruit and pulp; and glucose concentrations of 1.25 ± 0.03 and 1.29 ± 0.01 g/100 g fw in the whole fruit and pulp, respectively.
Regarding the statistical analysis of the data, it was clear that the whole fruit and the pulp showed the absence of significant differences in the concentration of fructose (p = 0.093) and trehalose (p = 0.201), contrary to the molecules of glucose and sucrose, and total sugars content, where significant differences were evident (p < 0.05), exhibiting the whole fruit with higher values.
Other authors [46] also studied the individual sugar profile in the pulp of seven eggplant cultivars (Aydin Siyahi, Pala 49, Süper pala, Kemer 27, Kadife Kemer, Topan, and Kadife) and the results showed the presence of fructose (1.242 ± 0.028 g/100 g fw to 1.379 ± 0.051 g/100 g fw), glucose (1.275 ± 0.015 g/100 g fw to 1.327 ± 0.047 g/100 g fw), and sucrose (0.109 ± 0.036 g/100 g fw to 0.494 ± 0.048 g/100 g fw) in similar concentrations compared to the present study.
The composition in organic acids was also evaluated, and revealed, in both samples, the presence of several molecules of interest, particularly oxalic, quinic, and malic acids. In general, it was evident that the whole fruit and the pulp showed the presence of the same molecules, with the exception of fumaric acid, which was only detected in the whole fruit, but in trace amounts. Whole fruit samples showed a higher amount of oxalic, quinic, and malic acids, and total concentration of organic acids, compared to the pulp samples, where oxalic, quinic, and malic acid values, and total organic acid concentration obtained were lower.
In both samples, oxalic acid stood out as the major organic acid. According to Kayashima and Katayama [47], this molecule is present in several plants and is characterized by its antioxidant action and its ability to preserve oxidized materials. In addition, its potential in inducing resistance against pathogens [48] and the ability to exercise different functions in plants and fungi, including protection against insects, has been proven. Its use covers several areas, being widely used in the food sector as a preservative of fruits and vegetables, in order to extend the shelf life [49].
Furthermore, the malic acid appears in similar concentrations. This molecule stands out for its therapeutic capacity, namely in helping muscle recovery (being used in the treatment of fibromyalgia), fighting fatigue, and increasing energy (when acting on the Krebs cycle). Moreover, it is also commonly used by the food industry as an acidulant, flavoring, and stabilizer, and by the pharmaceutical industry in cleaning and regenerating wounds and burns [50].
Quinic acid, although present in lower concentration, is also characterized by its therapeutic properties, namely by its radioprotector, antioxidant, and anti-inflammatory activity [51].
Regarding the statistical evaluation of the results, significant variations were visible in all the detected molecules (p < 0.05), except for oxalic acid (p = 0.966).
Other studies have been carried out for the valorization of this food matrix, evaluating the composition in functional compounds. Ayas et al. [46] studied seven varieties of eggplant fruit (Aydin Siyahi, Pala 49, Süper pala, Kemer 27, Kadife Kemer, Topan, and Kadife) and, in addition to malic acid (129.87 ± 6.78 mg/100 g fw to 181.06 ± 19.46 mg/100 g fw), they also identified the presence of other organic acids that were not detected in the present study, namely ascorbic (7.69 ± 1.21 mg/100 g fw to 11.74 ± 1.33 mg/100 g fw) and citric acids (18.98 ± 0.80 mg/100 g fw at 21.63 ± 0.35 mg/100 g fw).
As with other molecules, the discrepancy in results obtained in the detection of organic acids can be explained due to differences in fruit varieties, cultivation conditions, and the geographical area where the S. melongena varieties were produced.
In the evaluation of the fatty acid composition, the presence of 17 molecules was evident, with C16:0 standing out as the major fatty acid, with values of 40.5 ± 0.9% and 44.8 ± 0.2% in the whole fruit and pulp samples, respectively. C18:0 also presented relevant concentrations.
Saturated fatty acids were the main group present, with values varying between 83.8 ± 0.6% in the whole fruit and 89.6 ± 0.3% in the pulp; followed by polyunsaturated fatty acids (11.8 ± 0.4% whole fruit and 4.89 ± 0.04% in the pulp), and finally, monounsaturated fatty acids with concentrations of 4.5 ± 0.2% for the whole fruit and 5.5 ± 0.3% for the pulp. However, it is important to note that, although this group stands out with more pronounced concentrations, they are still low values that are unable to cause adverse effects on consumers’ health [52].
Regarding the statistical analysis, a significant variation (p < 0.05) was noticed in C6:0, C8:0, C10:0, C11:0, C:14, C16:0, C17:0, C18: 2n6c, C18: 3n3, and C21:0. Contrarily, in the molecules of C12:0, C15:0, C18:0, C18:1n9c, C20:0, C22:0, and C23: 0, small oscillations were observed between both studied samples, verifying the absence of statistical significance (p > 0.05).
Other authors have also studied the fatty acids profile of eggplant fruit samples. In a study conducted by Hanifah et al. [53], eggplant fruits of 21 different morphological varieties (color and shape) were evaluated and the results revealed the presence of some fatty acid molecules similar to the present work but the composition was mostly different. This can be justified by the different varieties of the fruit assessed and the application of another extraction method.
The fatty acids content was also studied by Ayas et al. [46], when evaluating seven eggplant cultivars. The presence of six fatty acid molecules was detected (C16:0; C18:0; C18:1; C18:2; C18:3, and C20:0), as well as the composition in SFA (Saturated Fatty Acids) of 31.44% to 36.92%, in MUFA (monounsaturated fatty acids) of 3.04% to 11.71%, and in PUFA (Polyunsaturated Fatty Acids) of 53, 66% to 62.71%, presenting, therefore, different percentages comparing with the present study.
3.3.2. Profile in Non-Anthocyanin and Anthocyanin Phenolic Compounds
The detailed profile of the phenolic compounds present in the eggplant samples (whole fruit, pulp, and epicarp) is shown in Table 5. Four phenolic compounds were identified, three being non-anthocyanin and one anthocyanin. Considering the non-anthocyanin compounds (Figure 1A), three phenolic acids were identified, namely caffeic acid hexoxide, protocatechuic acid, and 5-O-caffeoylquinic acid. Concerning the anthocyanin compound, a glycosylated derivative of delphinidin was identified only in the epicarp of the fruit, as expected considering its purple color.

Table 5.
Retention time (Tr), maximum absorption wavelengths in the UV-Vis region (λmax), attempt to identify and quantify phenolic compounds in the hydroethanolic extract of the pulp, epicarp, and the whole fruit of Solanum melongena L. (mean ± SD).
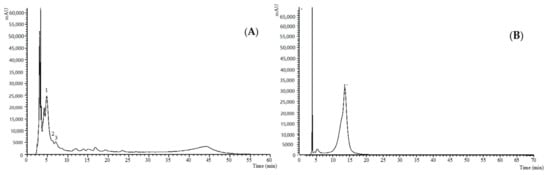
Figure 1.
Profile of non-anthocyanin (A) and anthocyanin (B) phenolic compounds from the hydroethanolic extracts of the S. melongena L. (whole fruit and epicarp, respectively) recorded at 280 nm (i) and 520 nm (ii). The peak number correspondence is shown in Table 6.
Regarding the identified phenolic acids, compound 1 ((M−H)− at m/z 341) was identified as caffeic acid hexoside based on its UV spectrum (λmax of 322) and the production of the MS2 fragment at m/z 179 and 135, being detected only in the whole fruit and pulp. Compound 2 ((M−H)− at m/z 153) was positively identified as protocatechuic acid based on its UV spectrum (λmax of 294) and the production of the MS2 fragment at m/z 108, which presented similar characteristics to the commercial standard. This compound was detected only in the eggplant epicarp. Finally, compound 3 ((M−H)− at m/z 353) was also positively identified as 5-O-caffeoylquinic acid based on its UV spectrum (λmax of 325) and the production of the MS2 fragment at m/z 191, 179, and 135, in comparison with the commercial standard. The latter stands out as the major compound, as well as the only one present in all studied samples, with values of 0.0159 ± 0.0002, 0.0337 ± 0.0004, and 0.96 ± 0.01 mg/g of extract for the whole fruit, pulp, and epicarp, respectively. The remaining compounds showed lower concentrations, notably the caffeic acid hexoside, with levels of 0.0025 ± 0.0001 mg/g of extract (whole fruit), 0.0062 ± 0.0004 mg/g of extract (pulp), and protocatechuic acid with a concentration of 0.37 ± 0.02 mg/g of extract in the epicarp.
The phenolic acids detected are described in the literature as having properties of high therapeutic interest. Caffeic acid is a hydroxycinnamic acid, found in several fruits and vegetables, characterized mainly by its anti-inflammatory and antioxidant potential, as well as by its therapeutic capacity in the prevention of cardiovascular diseases, hypoglycemic, and anti-cancerous activity [54,55]. On the other hand, protocatechuic acid is one of the main bioactive compounds present in some medicinal plants, and is characterized by its cardioprotective, neuroprotective, antibacterial, antidiabetic, antiviral, analgesic, chemotherapeutic, anti-inflammatory, and antioxidant potential [56]. The 5-O-caffeoylquinic acid is used mainly in the pharmaceutical, cosmetic, and food industry, and is distinguished by its antimicrobial, antioxidant, anti-tumor, and antihistamine activity [57].
In addition to non-anthocyanin phenolic compounds, in the epicarp of the fruit an anthocyanin phenolic compound, identified as delphinidin-O-rutinoside (1′) ((M+H)+ at m/z 611), was also detected. This compound was identified based on its UV spectrum (λmax of 523) and the production of the MS2 fragment at m/z 303, in comparison to the commercial standard (Figure 1B) and presented a concentration of 18.4 ± 0.7 mg/g extract.
The delphinidins evidenced, in previous studies, several bioactive properties, being the anthocyanin compound with the greatest beneficial effect in preventing cancer, being related to the death of apoptotic or autophagic cells in various types of cancer. In addition, it has antioxidant and anti-inflammatory action [58,59].
Significant differences (p < 0.05) were found in all compounds, with the epicarp having a higher concentration in all the identified compounds.
Other authors have also studied the individual profile of phenolic compounds in eggplant samples. In a review study by Niño-Medina et al. [60], the authors reported that the phenolic acids, commonly present in eggplant fruits, are caffeic and ferulic acids, and their derivatives. Wu and Prior [61] studied the anthocyanin composition of the lyophilized eggplant fruit, and the results obtained allowed the identification of four derivatives of delphinidin in eggplant fruits: delphinidin-3-O-rutinoside, delphinidin-3-O-rutinoside-5-O-glucoside, delphinidin-3-O-glucoside, and delphinidin-3-O-rutinoside-5-O-galactoside.
García-Salas et al. [62] evaluated three eggplant cultivars (PSE: striped purple, LE: long, and RE: rounded), grown in Spain in two different seasons (spring and summer). The results showed the identification of 25 compounds, most of which are derived from caffeoylquinic acid. The detected anthocyanin compound was identified as delphinidin rutinoside. These authors also evaluated the effect of the growing season on the composition of the fruits, and it was concluded that the high summer temperatures negatively affected the composition of phenolic compounds in the eggplant fruits. The results obtained presented a higher concentration of phenolic compounds compared to the samples studied in the present study, which can be justified by the difference in the cultivation region, fruit varieties, and/or the extraction solvents used.
Salermo et al. [63] evaluated the content of phenolic compounds in samples of whole fruit, pulp, and epicarp of Solanum melongena L. grown in Italy. The results showed a higher concentration of phenolic compounds in the epicarp of the lyophilized fruit, using water: ethanol as extraction solvent (about 14.25 ± 0.06 mg GAE (Gallic Acid Equivalent) 1.5 g of extract).
Another work, performed by Ferarsa et al. [7], evaluated the anthocyanin composition in the epicarp of S. melongena L. fruits, grown in France. The results indicated that the use of acidified water (pH 2.0) showed greater extraction power of the phenolic compounds, and that the yield is better when using hydroethanolic extracts (50 and 75%). Concerning the temperature during extraction, it was observed that the increase in this parameter led to an increase in the content of phenolic compounds detected, with the optimum temperature being 75 °C. In this study, five anthocyanin molecules were identified in the epicarp: delphinidin-3-O-rutinoside, delphinidin-3-O-rutinoside-5-O-glucoside, petunidine-3-O-rutinoside, malvidine-3-O-rutinoside-5-O-glucoside, and cyanidin-3-O-rutinoside.
Finally, a different study carried out by Horincar et al. [64] evaluated the composition in phenolic compounds of the extract of the epicarp of the fruits of Solanum melongena L., targeting its application in the nutritional enrichment of beer. The results showed the presence of five anthocyanin compounds: delphinidin-3-O-rutinoside (82.39%), delphinidin-3-O-glucoside (11.36%), delphinidin-3-O-rutinoside-5-O-glucoside (2.37%), cyanidin-3-O-rutinoside, and petunidine-3-O-rutinoside.
As previously mentioned, there are several external factors that directly interfere with the nutritional and chemical composition of foods, changing not only the concentration of compounds, but also their variety and presence. The differences observed in the phenolic profile identified in the present study compared to previous studies can be explained by several factors, namely different cultivation conditions, storage conditions after harvest, climate, soil type, season, and geographic origin [59]. Differences in the extraction methods used and the type of solvent can also be an important factor. In addition, the content of phenolic compounds can also vary under the effect of stresses such as air pollution, exposure to extreme temperatures, periods of drought, and high light intensity, among others [65].
3.4. Antioxidant, Antimicrobial, Cytotoxic, and Hepatotoxic Activity of the Hydroethanolic Extract Obtained from the S. melongena Fruit by Conventional Method
In order to evaluate the antioxidant activity of the hydroethanolic extract obtained from S. melongena (whole fruit, pulp, and epicarp), two in vitro colorimetric methods were applied (inhibition of lipid peroxidation—TBARS and inhibition of oxidative hemolysis—OxHLIA) and the results are presented in Table 6. The extract showed antioxidant potential in all tests performed, however the lowest values (best antioxidant activity) were obtained in the OxHLIA assay.

Table 6.
Antioxidant activity of the hydroethanolic extracts of the whole fruit, pulp, and epicarp of S. melongena. (mean ± SD).
In the TBARS evaluation, the EC50 values ranged between 135 ± 6 and 8941 ± 284 µg/mL, and according to the statistical analysis, showed the best activity in the epicarp, followed by the pulp, and, finally, the whole fruit.
In the OxHLIA assay, the hydroethanolic extracts also showed better antioxidant capacity in the epicarp sample, followed by the pulp and, finally, the whole fruit.
Several studies [42,66,67] have been performed with the aim of studying the antioxidant potential (through in vitro tests) of different parts of eggplant and positive and promising results were observed.
Table 7 shows the results obtained for the antimicrobial activity of the hydroethanolic extract obtained from S. melongena (whole fruit, pulp, and epicarp). The results showed bacteriostatic (MIC) and bactericidal (MBC) activity in all bacterial cultures tested, highlighting the extracts obtained from the epicarp and the whole fruit with the best results. In all cases, Escherichia coli (E.c.) stood out as the most susceptible strain to the bactericidal and bacteriostatic potential of all extracts (whole fruit, pulp, and epicarp).

Table 7.
Antibacterial (MIC and MBC mg/mL) and antifungal (MIC and MFC mg/mL) activity of S. melongena extracts (whole fruit, epicarp, and pulp).
Otherwise, in the evaluation of the antifungal potential, only the pulp showed fungicidal (MIC) and fungiostatic (MFC) activity against some strains, namely Penicillium fumiculosum (P.f.) and Penicillium ochrochloron (P.o.). This sample showed MIC and MFC values of 8.00 and 1.00 mg/mL for P. fumiculosum and P. ochrochloron, respectively.
Other authors [10,67] studied the antimicrobial action of S. melongena (fruits and/or leaves) and the results evidenced antibacterial and antifungal potential.
Regarding the results obtained for the cytotoxicity and hepatotoxicity assays (Table 8), it was clear that the epicarp of S. melongena fruit was the only hydroethanolic extract that revealed an inhibitory potential (GI50 < 400 µg/mL), showing its anti-proliferative capacity in the HeLa, NCI H460, and HepG2 tumor lines. This potential was demonstrated by GI50 values of 337 ± 7 µg/mL for the HeLa cell line, 338 ± 16 µg/mL for the NCI H460 cell line and, finally, 284 ± 10 µg/mL for the HepG2 cell line. The absence of toxicity of all the studied samples (GI50 > 400 µg/mL) was also evident, through the assessment of hepatotoxicity where a culture of primary non-tumor cells (PLP2) was used.

Table 8.
Cytotoxic activity of S. melongena extracts (whole fruit, epicarp, and pulp) (mean ± SD).
Akanitapichat et al. [66] also studied this matrix and evaluated the hepatoprotective potential of five varieties of S. melongena obtained in Thailand (SM1: uniform, purple, moderate size; SM2: white and green, moderate size; SM3: elongated and green; SM4: striped, green, moderate size; and SM5: uniform, light green, small size). For this purpose, the HepG2 cell line was used and the results demonstrated a hepatoprotective effect in all samples evaluated.
3.5. Optimization of the Process of Obtaining a Natural Colorant Extract Based on Anthocyanins from the S. melongena Epicarp
3.5.1. Effect of Solvent Concentration
In the optimization studies, considering the evaluation of the solvent concentration, it was possible to prove that the concentration of ethanol directly influences the polarity and viscosity of the extractor in physical-chemical terms. However, the percentage used will affect the mass transfer of the target compounds in the extraction process. In this context, the effects of ethanol concentration on the extraction yields of biomolecules were analyzed in a range of 8 to 92% concentration (v/v). From the visual representation of Figure 2A, the extraction yields of the desired constituents increased, with an increase in the concentration of ethanol in the range of 54–68% (v/v), but no more than that, since the decrease in recovery is evident in all cases. The reason for this behavior in the concentration of ethanol can be attributed to the polarity of the solvent, which eases the dissolution of the specific anthocyanins analyzed. As a result, 54–68% of the ethanol concentration achieved higher extraction yields, similar to the results shown in a study carried out by Muangrat et al. [68] in a different food matrix.
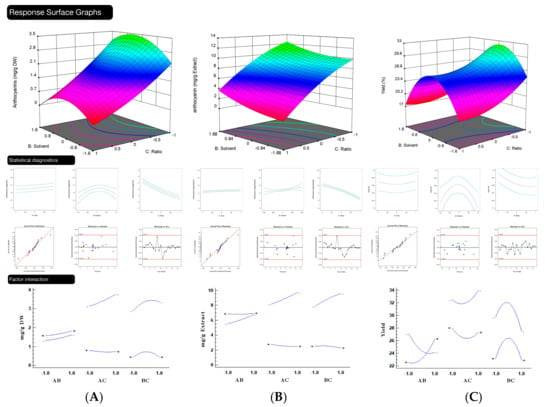
Figure 2.
Graphical representation of response surface methodology (RSM) for: (A) response Y1 (anthocyanins mg/g per dry mass). (From left to right and top to bottom: Modeling of the representation of the data obtained with time zero factor; single factorial behavior of time; single factorial behavior of the solvent; single factorial behavior of the ratio; normal residual plot; residuals versus predicted values; predicted values versus execution; and factorial interaction); for (B) the Y2 response (mg/g extract). (From left to right and top to bottom: Modeling data representation obtained with time zero factor; single factorial behavior of time; single factorial behavior of the solvent; single factorial behavior of the ratio; normal residual graph; residuals versus predicted values; predicted values versus execution; and factorial interaction); for (C) the Y3 response (yield). (From left to right and top to bottom: Modeling data representation obtained with time zero factor; single factorial behavior of time; single factorial behavior of the solvent; single factorial behavior of the ratio; normal residual graph; residuals versus predicted values; predicted values versus execution; and factorial interaction.).
3.5.2. Effect of the Solid-Liquid Ratio
Considering the evaluation of solid-liquid ratio, the effects of the ratio of powdered solid dissolved in a specific percentage of a fixed volume of liquid on the extraction yields of anthocyanins was investigated in the range of 5 to 65 g/L. The obtained results are illustrated in Figure 2B. The results indicated that lower amounts of solid employed had greater impact on the extraction yields of the biomolecules. The anthocyanins yield increased remarkably with the increase of the extractant volume. Lower amounts with higher volumes would accelerate the diffusion of compounds from the sample due to bigger contact surface area. In fact, the amounts of anthocyanins extracted by ultrasound assisted extraction decreased when the amount of matrix analyzed increased, almost in a linear manner, until reaching the saturation point. Large amounts of solid in respect to liquid would easily form aggregates and lead to contact surface decrease, resulting in an obstacle to mass transfer [69]. Thus, 5 g of matrix per liter of solvent was chosen as the optimal solid-liquid ratio in this optimization process.
3.5.3. Effect of Extraction Time
Finally, in the evaluation of the extraction time, it was possible to observe that this is a key parameter in the extraction yield of flavonoids, being thus also investigated in this work, in an interval of 0.5 to 5.5 min. In Figure 2C, it is important to note that, in addition to the interaction effect in the RSM graph, we can also observe that interactions were found between time and solvent and, although the time factor is not statistically significant, it is important to include the terms of the quadratic equation, at least for the Y3 response (yield), where this phenomenon is expressed widely. Initially, different extraction times were tested before the execution of this CCD, and longer times were discarded, showing non-significant differences. Although short times have been used, we can observe, as well as Melgar et al. [32], that the main effect of ultrasound-assisted extraction was produced within a few seconds. Apparently, the combination of the cavitation effect with the size of the fine particles and the percentage of solvent, has a very pronounced reaction on the extraction of the bioactive molecules. Thus, recognizing the relevance of ultrasound-assisted extraction, it is possible to save a lot of time, energy, and cost, increasing the importance of optimizing the extraction process.
3.5.4. Statistical Analysis and Model Adjustment
In Table 9, ANOVA analysis of the experimental data from the 20 extractions was executed, a second order polynomial equation was obtained from each response regression coefficient using the Design-Expert and Statgraphics software. The second order polynomial models for the extraction of epicarp (Y1), extract (Y2), and recovered yield (Y3) are presented by the following equations:

Table 9.
Statistical analysis (ANOVA) of the central composite design (CCD), including response terms for building the predictive models and optimal response values for the parametric response criteria.
The significance of each coefficient was determined in the regression models and in the analysis of variance (ANOVA), from which only the significant numerical terms were displayed in previous equations. The ANOVA results can be used to assess whether the regression models were successfully established by p-values (<0.05) at a confidence level along with the lack of fit analysis (p > 0.05), which implied that the lack of adjustment was not significant in relation to the pure error, revealing that the elaborated models are useful to travel the design within the analyzed values. In addition, the coefficients of determination of the second order model (R2) higher than 0.94 demonstrated that the models with high statistical significance were well adjusted to the experiments. Thus, based on the statistical analysis above, the models were adequate to predict the yields of anthocyanin extraction.
3.5.5. Optimization of the Analysis of the Response Surface Methodology
The optimal values obtained by optimizing the extraction using the ultrasound technique are divided into specific response values: Y1 (solvent = 64%; ratio = 5 g/L; and time = 0.5 min), Y2 = (solvent = 58%; ratio = 5 g/L; and time = 0.5 min), and Y3 (solvent = 54%; ratio = 5 g/L; and time = 0.5 min); and in general optimization values for the three responses involved: with an optimal disability value of 0.99, 68.2% ethanol concentration (v/v), 5 g of S. melongena per liter of solvent, and 0.5 min extraction time.
4. Conclusions
Considering the nutritional and bioactive potentialities of the pulp of the fruits of Solanum melongena L. and the coloring compounds present in the epicarp, the present study aimed to characterize, chemically and nutritionally, the pulp, epicarp, and whole fruit of S. melongena. An evaluation was also made of its bioactive properties and optimization of methodologies for obtaining an extract rich in anthocyanins from the epicarp of S. melongena, in order to be used as a natural coloring ingredient.
This study showed that the lyophilization process is an adequate dehydration process, due to the small changes it causes in the sample. Otherwise, the nutritional and chemical evaluation showed a low energy and fat content, the high moisture content, and the presence of bioactive molecules confirming the interest of this fruit in calorie restricted diets. In addition, the antioxidant, bacteriostatic, and bactericidal activities were also observed in all tested samples.
The optimization studies allow to understand the behavior of the main variables and also the strength of the interaction, which provided a visual representation that helped to select the ideal points of extraction. In general terms, factors ratio and solvent were the ones that showed a bigger magnitude in their responses, therefore, particular handling of these two is strongly advisable; additionally, not strong interaction within the stronger factor tested was detected in the responses analyzed, which suggests the independence of them and how we can be sure of modifying any of them within the range tested without affecting the predicted responses. Finally, the statistical diagnostic showed good fitting of data, hence, moving within the levels of the experiment would still draw great outputs if we wanted to modify parameters, because the equations provided will continue displaying strong predictions.
Therefore, these results confirm the excellent chemical and nutritional composition of the eggplant, which, together with the bioactive properties identified, make it an excellent option to incorporate into the daily diet. Moreover, considering the presence of anthocyanins in the epicarp, it also has potential application in the industrial sector, namely through the use of its natural coloring pigment. Thus, chemical and nutritional studies have shown the dietary benefits of this food, considering the different forms of consumption; and the optimization study carried out allows the industry to carry out the pigment extraction process, without preliminary studies and experiments, avoiding economic and operational expenses.
In this sense, this study presented a topic with high relevance for several sectors, such as industrial, environmental, scientific, economic, and social. This wide relevance is justified by the proposal to develop strategies that reduce food waste, improving the quality of some commercial products. Furthermore, it leads to the application of innovative extraction technologies, an increase in the quality of products available on the market, an increase in consumer preference for these products, and, consequently, to an improvement in the environmental impact, economic growth, and the development of a sustainable economy.
Author Contributions
Conceptualization, I.C.F.R.F. and L.B.; formal analysis, G.F.P.S., E.P., R.C.C., C.P., R.M.V.A., and L.B.; funding acquisition, I.C.F.R.F. and L.B.; investigation, G.F.P.S., E.P., B.M., D.S., R.C.C., C.P., and R.M.V.A.; methodology, G.F.P.S., E.P., B.M., D.S., M.S., R.C.C., and C.P.; supervision, E.P., I.C.F.R.F., and L.B.; writing—original draft, G.F.P.S., E.P., B.M., C.P., and R.M.V.A.; writing—review and editing, D.S., M.S., I.C.F.R.F., and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) and European Regional Development Fund (ERDF) under Program PT2020 for financial support to CIMO (UID/AGR/00690/2020); this work was also funded by ERDF through the Regional Operational Program North 2020, within the scope of project “Mobilizador” Norte-01-0247-FEDER-024479: ValorNatural®. The authors are also grateful to Interreg España-Portugal for financial support through the project 0377_Iberphenol_6_E and TRANSCoLAB 0612_TRANS_CO_LAB_2_P; to Ministry of Education, Science and Technological Development, Republic of Serbia.
Acknowledgments
The authors are grateful to the national funding by FCT, P.I., through the institutional scientific employment program-contract for L. Barros and R. Calhelha’s contract; C. Pereira’s contract though the celebration of program-contract foreseen in No. 4, 5, and 6 of article 23° of Decree-Law No. 57/2016, of 29 August, amended by Law No. 57/2017, of 19 July.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Woiciechowski, A.L.; de Carvalho, J.C.; Spier, M.R.; Habu, S.; Yamaguishi, C.T.; Ghiggi, V.; Soccol, C.R. Use of Agro-Industrial Residues in Food Bioprocesses; Food Biotechnology, Science, Technology, Food Engineering and Nutrition Collection; Atheneu Publisher: Sao Paulo, Brazil; Rio de Janeiro, Brazil; Belo Horizonte, Brazil, 2013; Volume 12, pp. 143–172. [Google Scholar]
- Toop, T.A.; Ward, S.; Oldfield, T.; Hull, M.; Kirby, M.E.; Theodorou, M.K. AgroCycle—Developing a circular economy in agriculture. Energy Procedia 2017, 123, 76–78. [Google Scholar] [CrossRef]
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.W.; Verniquet, A.; Broeze, J.; et al. A Research Challenge Vision Regarding Management of Agricultural Waste in a Circular Bio-Based Economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health Benefits and Bioactive Compounds of Eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Radicetti, E.; Massantini, R.; Campiglia, E.; Mancinelli, R.; Ferri, S.; Moscetti, R. Yield and quality of eggplant (Solanum melongena L.) as affected bycover crop species and residue management. Sci. Hortic. 2016, 204, 161–171. [Google Scholar] [CrossRef]
- Rodriguez-Jimenez, J.R.; Amaya-Guerra, C.A.; Baez-Gonzalez, J.G.; Aguilera-Gonzalez, C.; Urias-Orona, V.; Nino-Medina, G. Physicochemical, Functional, and Nutraceutical Properties of Eggplant Flours Obtained by Different Drying Methods. Molecules 2018, 23, 3210. [Google Scholar] [CrossRef]
- Ferarsa, S.; Zhang, W.; Moulai-Mostefa, N.; Ding, L.; Jaffrin, M.Y.; Grimi, N. Recovery of Anthocyanins and Other Phenolic Compounds from Purple Eggplant Peels and Pulps Using Ultrasonic-Assisted Extraction. Food Bioprod. Process. 2018, 109, 19–28. [Google Scholar] [CrossRef]
- Bobbio, P.A.; Bobbio, F.O. Química do Processamento de Alimentos: Pigmentos., 2nd ed.; Livraria Varela: Sao Paulo, Brazil, 1995; pp. 105–120. ISBN 85-85519-12-6. [Google Scholar]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Das, S.; Raychaudhuri, U.; Falchi, M.; Bertelli, A.; Braga, P.C.; Das, D.K. Cardioprotective properties of raw and cooked eggplant (Solanum melongena L). Food Funct. 2011, 2, 395–399. [Google Scholar] [CrossRef]
- Jung, E.J.; Bae, M.S.; Jo, E.K.; Jo, Y.H.; Lee, S.C. Antioxidant Activity of Different Parts of Eggplant. J. Med. Plants Res. 2011, 5, 4610–4615. [Google Scholar]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Optimization of Ultrasound-Assisted Extraction of Total Monomeric Anthocyanin (TMA) and Total Phenolic Content (TPC) from Eggplant (Solanum melongena L.) Peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Morales, P.; Ferreira, I.C.F.R. Floral Parts of Gomphrena globosa L. as a Novel Alternative Source of Betacyanins: Optimization of the Extraction Using Response Surface Methodology. Food Chem. 2017, 229, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis of AOAC International; AOAC: Washinton, DC, USA, 2016; ISBN 0935584870. [Google Scholar]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and Chemical Characterization in Hydrophilic and Lipophilic Compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic Profile and Antioxidant Activity of Coleostephus myconis (L.) Rchb.f.: An Underexploited and Highly Disseminated Species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Jabeur, I.; Tobaldini, F.; Martins, N.; Barros, L.; Martins, I.; Calhelha, R.C.; Henriques, M.; Silva, S.; Achour, L.; Santos-Buelga, C.; et al. Bioactive Properties and Functional Constituents of Hypericum androsaemum L.: A Focus on the Phenolic Profile. Food Res. Int. 2016, 89, 422–431. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Marcato, A.C.; Rodrigues, A.C.B.; Pagano, A.P.E.; Freitas, B.C.; de Machado, C.O.; Nakashima, K.K.; Esteves, L.C.; Lopes, N.B.; Bastos, E.L. Betalaínas: Das cores das beterrabas à fluorescência das flores. Rev. Virtual Química 2015, 7, 292–309. [Google Scholar]
- Albuquerque, B.R.; Prieto, M.A.; Barreiro, M.F.; Rodrigues, A.E.; Curran, T.P.; Barros, L.; Ferreira, I.C.F.R. Catechin-Based Extract Optimization Obtained from Arbutus unedo L. Fruits Using Maceration/Microwave/Ultrasound Extraction Techniques. Ind. Crop. Prod. 2017, 95, 404–415. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Soković, M.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C.F.R. Melissa officinalis L. decoctions as functional beverages: A bioactive approach and chemical characterization. Food Funct. 2015, 6, 2240–2248. [Google Scholar]
- Guimarães, R.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Infusion and Decoction of Wild German Chamomile: Bioactivity and Characterization of Organic Acids and Phenolic Compounds. Food Chem. 2013, 136, 947–954. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Lipid Peroxidation and Antioxidants as Biomarkers of Tissue Damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidant activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical Features and Bioactivities of Cornflower (Centaurea cyanus L.) Capitula: The Blue Flowers and the Unexplored Non-Edible Part. Ind. Crop. Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Zhu, Z.; He, J.; Liu, G.; Barba, F.J.; Koubaa, M.; Ding, L.; Vorobiev, E. Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: A review. Innov. Food Sci. Emerg. Technol. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Jiménez, L.C.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. fruits. Food Chem. 2018, 264, 81–91. [Google Scholar]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of microwave-assisted extraction of ergosterol from Agaricus bisporus L. byproducts using response surface methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef]
- López, C.J. Desarrollo de un Aditivo Colorante Natural a Base de Cianidina Obtenido a Partir de Frutos de Arbutus unedo L.: Optimización de la Extracción y Estudio de su Aplicación en Gofres. Master’s Thesis, Polythecnic Institute of Bragança, Bragança, Portugal, 2017. [Google Scholar]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-Assisted Extraction of Phenolic Acids and Flavonoids and Production of Antioxidant Ingredients from Tomato: A Nutraceutical-Oriented Optimization Study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, O.; Ferreira, I.C.F.R. Optimization and Comparison of Maceration and Microwave Extraction Systems for the Production of Phenolic Compounds from Juglans regia L. for the Valorization of Walnut Leaves. Ind. Crop. Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M. Ultrasound and Microwave Assisted Extraction of Opuntia Fruit Peels Biocompounds: Optimization and Comparison Using RSM-CCD. Molecules 2019, 24, 3618. [Google Scholar] [CrossRef]
- Xu, M.; Du, C.; Zhang, N.; Shi, X.; Wu, Z.; Qiao, Y. Color Spaces of Safflower (Carthamus tinctorius L.) for Quality Assessment. J. Tradit. Chin. Med. Sci. 2016, 3, 168–175. [Google Scholar] [CrossRef]
- Russo, P.; Adiletta, G.; Di Matteo, M. The Influence of Drying Air Temperature on the Physical Properties of Dried and Rehydrated Eggplant. Food Bioprod. Process. 2013, 91, 249–256. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of Anthocyanins and Polymeric Color Formation during Heat Treatment of Purple Sweet Potato Extract at Different PH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. The Effect of Pigment Matrix, Temperature and Amount of Carrier on the Yield and Final Color Properties of Spray Dried Purple Corn (Zea mays L.) Cob Anthocyanin Powders. Food Chem. 2017, 227, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Boch, K.; Schieber, A. Influence of Copigmentation on the Stability of Spray Dried Anthocyanins from Blackberry. LWT Food Sci. Technol. 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Nemzer, B.; Vargas, L.; Xia, X.; Sintara, M.; Feng, H. Phytochemical and Physical Properties of Blueberries, Tart Cherries, Strawberries, and Cranberries as Affected by Different Drying Methods. Food Chem. 2018, 262, 242–250. [Google Scholar] [CrossRef]
- Lima-Corrêa, R.A.B.; Andrade, M.S.; da Silva, M.F.d.G.F.; Freire, J.T.; Ferreira, M.d.C. Thin-Layer and Vibrofluidized Drying of Basil Leaves (Ocimum basilicum L.): Analysis of Drying Homogeneity and Influence of Drying Conditions on the Composition of Essential Oil and Leaf Colour. J. Appl. Res. Med. Aromat. Plants 2017, 7, 54–63. [Google Scholar]
- Badulescu, L.; Dobrin, A.; Stan, A.; Mot, A.; Bujor, O.C. Drying treatment effects on anthocyanins of organic raspberry (cv. Heritage) fruit. In Proceedings of the Third Nordic Baltic Drying Conference, Saint Petersburg, Russia, 12–14 June 2019; pp. 48–53. [Google Scholar]
- USDA (United States Department of Agriculture Agricultural Research Service), 11209 Report, Raw Eggplant. Available online: https://ndb.nal.usda.gov/ndb/foods/show/2962 (accessed on 17 April 2019).
- Kandoliya, U.K.; Bajaniya, V.K.; Bhadja, N.K.; Bodar, N.P.; Golakiya, B.A. Antioxidant and Nutritional of Egg Plant (Solanun melongena L.) Fruit Grown in Saurastra Region. Intern. J. Curr. Microbiol. Appl. Sci. 2015, 4, 806–813. [Google Scholar]
- Agoreyo, B.O.; Obansa, E.S.; Obanor, E.O. Comparative nutritional and phytochemical analyses of two varieties of Solanum melongena. Sci. World J. 2012, 7, 5–8. [Google Scholar]
- Raigón, M.D.; Prohens, J.; Muñoz-Falcón, J.E.; Nuez, F. Comparison of Eggplant Landraces and Commercial Varieties for Fruit Content of Phenolics, Minerals, Dry Matter and Protein. J. Food Compos. Anal. 2008, 21, 370–376. [Google Scholar] [CrossRef]
- Ossamulu, I.; Akanya, H.; Jigam, A.; Egwim, E. Evaluation of Nutrient and Phytochemical Constituents of Four Eggplant Cultivars. Elixir Food Sci. 2014, 73, 26424–26428. [Google Scholar]
- Ayas, F.A.; Colak, N.; Topuz, M.; Tarkowski, P.; Jaworek, P.; Seiler, G.; Inceer, H. Comparison of Nutrient Content in Fruit of Commercial Cultivars of Eggplant (Solanum melongena L.). Pol. J. Food Nutr. Sci. 2015, 65, 251–259. [Google Scholar]
- Kayashima, T.; Katayama, T. Oxalic acid is available as a natural antioxidant in some systems. Biochim. Biophys. Acta 2002, 1573, 1–3. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, T.; Qin, G.; Tian, S. Response of Jujube Fruits to Exogenous Oxalic Acid Treatment Based on Proteomic Analysis. Plant. Cell Physiol. 2009, 50, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Cefola, M.; Pace, B.; Azara, E.; Spissu, Y.; Serra, P.A.; Logrieco, A.F.; D’hallewin, G.; Fadda, A. Postharvest Application of Oxalic Acid to Preserve Overall Appearance and Nutritional Quality of Fresh-Cut Green and Purple Asparagus during Cold Storage: A Combined Electrochemical and Mass-Spectrometry Analysis Approach. Postharvest Biol. Technol. 2019, 148, 158–167. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, Y.; Lin, M.; Wei, P.; Yang, S.T. Polymalic Acid Fermentation by Aureobasidium Pullulans for Malic Acid Production from Soybean Hull and Soy Molasses: Fermentation Kinetics and Economic Analysis. Bioresour. Technol. 2017, 223, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.H.; Koo, H.J.; Kang, S.C. Quinic Acid Inhibits Vascular Inflammation in TNF-α-Stimulated Vascular Smooth Muscle Cells. Biomed. Pharm. 2017, 96, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.; del Mar Bibiloni, M.; Pons, A.; Tur, J.A. Total Fat and Fatty Acid Intakes and Food Sources in Mediterranean Older Adults Requires Education to Improve Health. Nutr. Res. 2020, 73, 67–74. [Google Scholar] [CrossRef]
- Hanifah, A.; Maharijaya, A.; Putri, S.P.; Laviña, W.A.; Sobir. Untargeted Metabolomics Analysis of Eggplant (Solanum melongena L.) Fruit and Its Correlation to Fruit Morphologies. Metabolites 2018, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Min, T.S. Caffeic Acid Phenethyl Ester Ameliorates Changes in IGFs Secretion and Gene Expression in Streptozotocin-Induced Diabetic Rats. Life Sci. 2006, 78, 1741–1747. [Google Scholar] [CrossRef]
- Araújo, V.M. Estudo do Potencial Terapêutico do Ácido Cafeico em Protocolos de Diabetes e Dislipidemia em Camundongos. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2014; 99p. [Google Scholar]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharm. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Forino, M.; Tenore, G.C.; Tartaglione, L.; Carmela, D.; Novellino, E.; Ciminiello, P. (1S,3R,4S,5R)5-O-Caffeoylquinic Acid: Isolation, Stereo-Structure Characterization and Biological Activity. Food Chem. 2015, 178, 306–310. [Google Scholar] [CrossRef]
- Song, S.E.; Jo, H.J.; Kim, Y.W.; Cho, Y.J.; Kim, J.R.; Park, S.Y. Delphinidin Prevents High Glucose-Induced Cell Proliferation and Collagen Synthesis by Inhibition of NOX-1 and Mitochondrial Superoxide in Mesangial Cells. J. Pharm. Sci. 2016, 130, 235–243. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, D.Y.; Park, Y.J.; Hwang, S.C.; Kim, K.D.; Moon, D.K.; Kim, D.H. Cytotoxic Effects of Delphinidin in Human Osteosarcoma Cells. Acta Orthop. Traumatol. Turc. 2018, 52, 58–64. [Google Scholar]
- Niño-Medina, G.; Urías-Orona, V.; Muy-Rangel, M.D.; Heredia, J.B. Structure and Content of Phenolics in Eggplant (Solanum melongena)-A Review. South. Afr. J. Bot. 2017, 111, 161–169. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and Characterization of Anthocyanins by High-Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry in Common Foods in the United States: Vegetables, Nuts, and Grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández Gutiérrez, A. Identification and quantification of phenolic compounds in diverse cultivars of eggplant grown in different seasons by high performance liquid chromatography coupled to diode array detector and electrospray-quadrupole-time of flight-mass spectrometry. Food Res. Int. 2014, 57, 114–122. [Google Scholar] [CrossRef]
- Salerno, L.; Modica, M.N.; Pittalà, V.; Romeo, G.; Siracusa, M.A.; Di Giacomo, C.; Sorrenti, V.; Acquaviva, R. Antioxidant Activity and Phenolic Content of Microwave-Assisted Solanum melongena Extracts. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Horincar, G.; Enachi, E.; Bolea, C.; Râpeanu, G.; Aprodu, I. Value-Added Lager Beer Enriched with Eggplant (Solanum melongena L.) Peel Extract. Molecules 2020, 25, 731. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Akanitapichat, P.; Phraibung, K.; Nuchklang, K.; Prompitakkul, S. Antioxidant and Hepatoprotective Activities of Five Eggplant Varieties. Food Chem. Toxicol. 2010, 48, 3017–3021. [Google Scholar] [CrossRef]
- Hong, H.; Lee, J.H.; Kim, S.K. Phytochemicals and Antioxidant Capacity of Some Tropical Edible Plants. Asian Australas. J. Anim. Sci. 2018, 31, 1677–1684. [Google Scholar] [CrossRef]
- Muangrat, R.; Pongsirikul, I.; Blanco, P.H. Ultrasound assisted extraction of anthocyanins and total phenolic compounds from dried cob of purple waxy corn using response surface methodology. J. Food Process. Pres. 2018, 42, 1–11. [Google Scholar] [CrossRef]
- Taofiq, O.; Corrêa, R.C.G.; Barros, L.; Prieto, M.A.; Bracht, A.; Peralta, R.M.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. A Comparative Study between Conventional and Non-Conventional Extraction Techniques for the Recovery of Ergosterol from Agaricus blazei Murrill. Food Res. Int. 2019, 125, 108541. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).