Effect of Dietary Methionine Deficiency Followed by a Re-Feeding Phase on the Hepatic Antioxidant Activities of Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Methionine Intake
2.3. Serum Profiles
2.4. Determination of Methionine and Metabolites
2.5. Total RNA Extraction and Quantitative RT-PCR
2.6. Western Blot Analysis
2.7. Statistical Analyses
3. Results
3.1. Methionine Intake
3.2. Methionine Metabolite Content
3.3. Serum Antioxidant Enzyme Activities
3.4. Quantitative Real-Time PCR Analysis
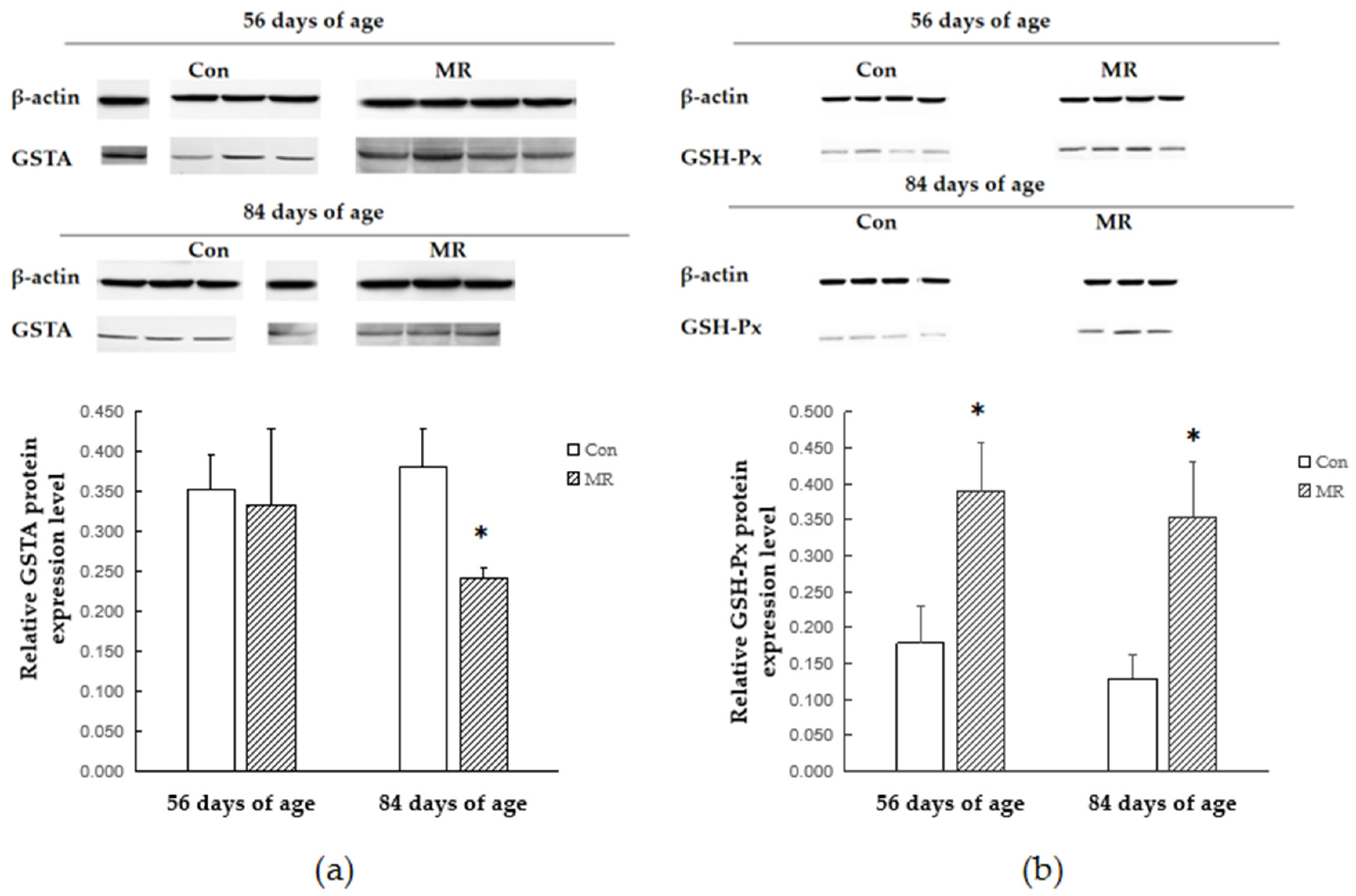
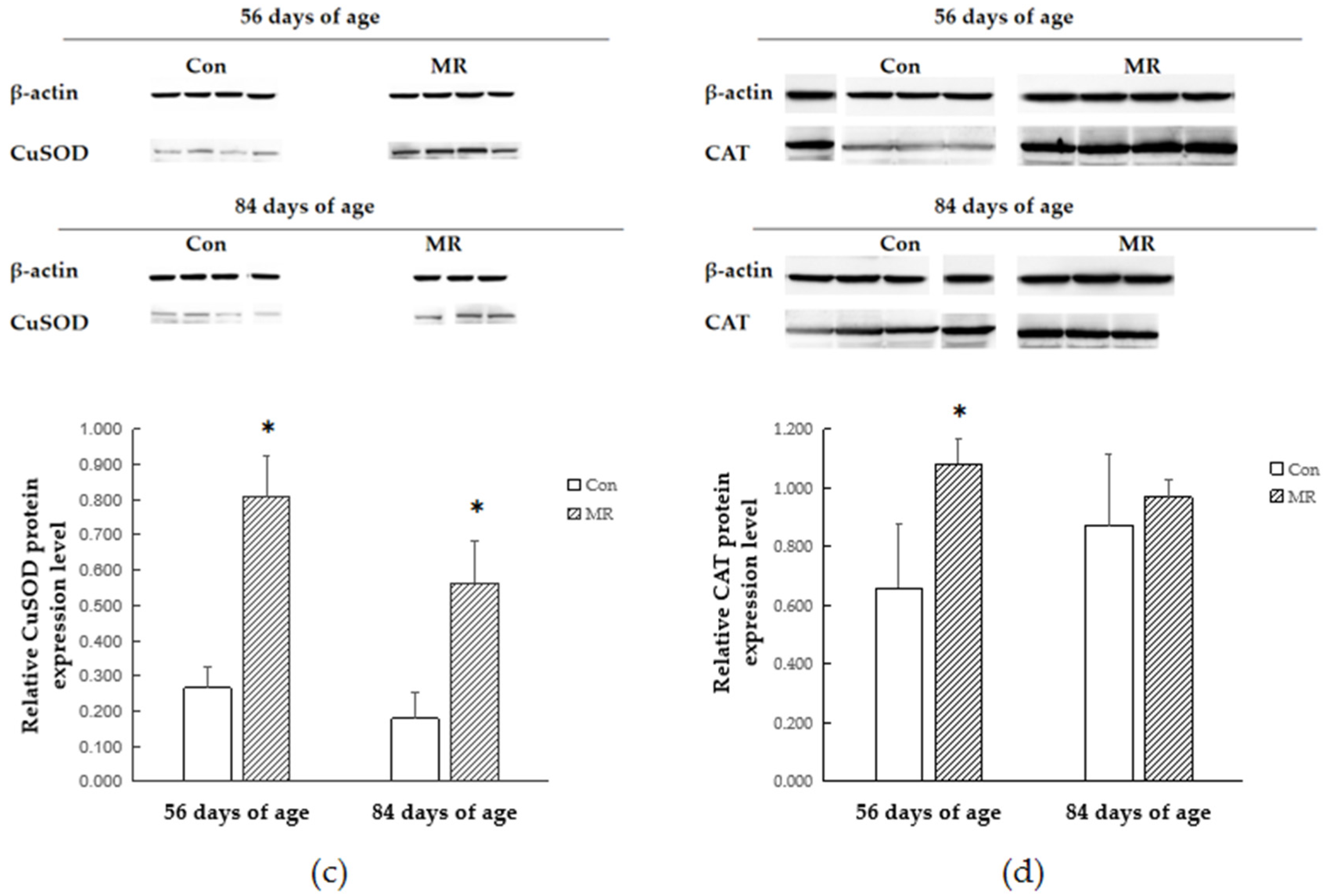
3.5. Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P., Jr.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Mercken, E.M.; Carboneau, B.A.; Krzysik-Walker, S.M.; de Cabo, R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012, 11, 390–398. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378. [Google Scholar] [CrossRef]
- Capó, X.; Martorell, M.; Ferrer, M.D.; Sureda, A.; Pons, V.; Domingo, J.C.; Drobnic, F.; Martínez-Rodríguez, A.; Leyva-Vela, B.; Sarabia, J.M.; et al. Calorie restriction improves physical performance and modulates the antioxidant and inflammatory responses to acute exercise. Nutrients 2020, 12, 930. [Google Scholar] [CrossRef]
- Sofi, F. Fasting-mimicking diet a clarion call for human nutrition research or an additional swan song for a commercial diet? Int. J. Food Sci. Nutr. 2020, 71, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Ables, G.P.; Johnson, J.E. Pleiotropic responses to methionine restriction. Exp. Gerontol. 2017, 94, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, D.; Gehring, M.; Tran, R.K.; Ballinger, T.; Henikoff, S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat. Genet. 2007, 39, 61–69. [Google Scholar] [CrossRef]
- Guerra, B.A.; Brando, B.B.; Pinto, S.S.; Salgueiro, W.G.; De-Souza, E.A.; Reis, F.C.G.; Batista, T.M.; Cavalcante-Silva, V.; D’Almeida, V.; Castilho, B.A.; et al. Dietary sulfur amino acid restriction upregulates DICER to confer beneficial effects. Mol. Metab. 2019, 29, 124–135. [Google Scholar] [CrossRef]
- Li, H.; Cai, L.; Liang, M.; Wang, Z. Methionine augments endogenous antioxidant capacity of rice protein through stimulating MSR antioxidant system and activating Nrf2-ARE pathway in growing and adult rats. Eur. Food Res. Technol. 2020, 246, 1051–1063. [Google Scholar] [CrossRef]
- Lee, B.C.; Kaya, A.; Gladyshev, V.N. Methionine restriction and life-span control. Ann. N. Y. Acad. Sci. 2016, 1363, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, V.P.; Conde, R.D.; Guillemot, J.C.; Sanllorenti, P.M. The mouse liver content of carbonic anhydrase III and glutathione S-tranferases A3 and P1 depend on dietary supply of methionine and cysteine. Int. J. Biochem. Cell Biol. 2004, 36, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Kim, Y.G.; Lee, M.G.; Kim, M.G. The effect of cysteine on the altered expression of class alpha and mu glutathione S-transferase genes in the rat liver during protein-calorie malnutrition. Biochim. Biophys. Acta 2000, 1502, 235–246. [Google Scholar] [CrossRef]
- Sanchez-Roman, I.; Gomez, A.; Gomez, J.; Suarez, H.; Sanchez, C.; Naudi, A.; Ayala, V.; Portero-Otin, M.; Lopez-Torres, M.; Pamplona, R.; et al. Forty percent methionine restriction lowers DNA methylation, complex I ROS generation, and oxidative damage to mtDNA and mitochondrial proteins in rat heart. J. Bioenerg. Biomembr. 2011, 43, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Yun, J.; Guoyao, W.; Kaiji, S.; Zhaolai, D.; Zhenlong, W. Dietary L-methionine restriction decreases oxidative stress in porcine liver mitochondria. Exp. Gerontol. 2015, 65, 35–41. [Google Scholar] [CrossRef]
- Séité, S.; Mourier, A.; Camougrand, N.; Salin, B.; Figueiredo-Silva, A.C.; Fontagné-Dicharry, S.; Panserat, S.; Seiliez, I. Dietary methionine deficiency affects oxidative status, mitochondrial integrity and mitophagy in the liver of rainbow trout (Oncorhynchus mykiss). Sci. Rep. 2018, 8, 10151. [Google Scholar] [CrossRef]
- Maddineni, S.; Nichenametla, S.; Sinha, R.; Wilson, R.P.; Richie, J.P., Jr. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med. 2013, 238, 392–399. [Google Scholar] [CrossRef]
- Richie, J.P., Jr.; Komninou, D.; Leutzinger, Y.; Kleinman, W.; Orentreich, N.; Malloy, V.; Zimmerman, J.A. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition 2004, 20, 800–805. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Ghosh, S.; Forney, L.A.; Wanders, D.; Stone, K.P.; Gettys, T.W. An integrative analysis of tissue-specific transcriptomic and metabolomic responses to short-term dietary methionine restriction in mice. PLoS ONE 2017, 12, e0177513. [Google Scholar] [CrossRef]
- Tamanna, N.; Kroeker, K.; Braun, K.; Braun, K.; Banh, S.; Treberg, J.R. The effect of short-term methionine restriction on glutathione synthetic capacity and antioxidant responses at the whole tissue and mitochondrial level in the rat liver. Exp. Gerontol. 2019, 127, 110712. [Google Scholar] [CrossRef]
- Lin, A.H.; Chen, H.W.; Liu, C.T.; Tsai, C.W.; Lii, C.K. Activation of Nrf2 is required for up-regulation of the π class of glutathione S-transferase in rat primary hepatocytes with l-methionine starvation. J. Agric. Food Chem. 2012, 60, 6537–6545. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Chen, H.W.; Yang, J.J.; Liu, K.L.; Lii, C.K. Sulfur amino acid restriction induces the pi class of glutathione S-transferase expression in primary rat hepatocytes. J. Nutr. 2005, 135, 1034–1039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Statistical Analysis System (SAS). 9.2 Software; Institute Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Perrone, C.E.; Mattocks, D.A.L.; Hristopoulos, G.; Plummer, J.D.; Krajcik, R.A.; Orentreich, N. Methionine restriction effects on 11β-HSD1 activity and lipogenic/lipolytic balance in F344 rat adipose tissue. J. Lipid Res. 2008, 49, 12–23. [Google Scholar] [CrossRef]
- Stone, K.P.; Wanders, D.; Orgeron, M.; Cortez, C.C.; Gettys, T.W. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 2014, 63, 3721–3733. [Google Scholar] [CrossRef]
- Hasek, B.E.; Stewart, L.K.; Henagan, T.M.; Boudreau, A.; Lenard, N.R.; Black, C.; Shin, J.; Huypens, P.; Malloy, V.L.; Plaisance, E.P.; et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R728–R739. [Google Scholar] [CrossRef]
- Wanders, D.; Forney, L.A.; Stone, K.P.; Burk, D.H.; Pierse, A.; Gettys, T.W. FGF21 mediates the thermogenic and insulin-sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes 2017, 66, 858–867. [Google Scholar] [CrossRef]
- Lees, E.K.; Król, E.; Grant, L.; Shearer, K.; Wyse, C.; Moncur, E.; Bykowska, A.S.; Mody, N.; Gettys, T.W.; Delibegovic, M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 2014, 13, 817–827. [Google Scholar] [CrossRef]
- Caro, P.; Gomez, J.; Sanchez, I.; Naudi, A.; Ayala, V.; López-Torres, M.; Pamplona, R.; Barja, G. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenation Res. 2009, 12, 421–434. [Google Scholar] [CrossRef]
- Sanz, A.; Caro, P.; Ayala, V.; Portero-Otin, M.; Pamplona, R.; Barja, G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006, 20, 1064–1073. [Google Scholar] [CrossRef]
- Zimmerman, J.A.; Malloy, V.; Krajcik, R.; Orentreich, N. Nutritional control of aging. Exp. Gerontol. 2003, 38, 47–52. [Google Scholar] [CrossRef]
- Tamanna, N.; Mayengbam, S.; House, J.D.; Treberg, J.R. Methionine restriction leads to hyperhomocysteinemia and alters hepatic H2S production capacity in Fischer-344 rats. Mech. Ageing Dev. 2018, 176, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Espe, M.; Andersen, S.M.; Holen, E.; Rønnestad, I.; Veiseth-Kent, E.; Zerrahn, J.E.; Aksnes, A. Methionine deficiency does not increase polyamine turnover through depletion of hepatic S-adenosylmethionine in juvenile Atlantic salmon. Br. J. Nutr. 2014, 112, 1274–1285. [Google Scholar] [CrossRef]
- Castellano, R.; Perruchot, M.H.; Conde-Aguilera, J.A.; van Milgen, J.; Collin, A.; Tesseraud, S.; Mercier, Y.; Gondret, F. A methionine deficient diet enhances adipose tissue lipid metabolism and alters anti-oxidant pathways in young growing pigs. PLoS ONE 2015, 10, e0130514. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Yamamoto, M. The KEAP1-NRF2 System in Cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xu, T.; Li, S.; Ruan, H. GPX1, A biomarker for the diagnosis and prognosis of kidney cancer, promotes the progression of kidney cancer. Aging 2019, 11, 12165–12176. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 532–553. [Google Scholar] [CrossRef]
- Cyrne, L.; Martins, L.; Fernandes, L.; Marinho, H.S. Regulation of antioxidant enzymes gene expression in the yeast Saccharomyces cerevisiae during stationary phase. Free Radic. Biol. Med. 2003, 34, 385–393. [Google Scholar] [CrossRef]
- Limaye, P.V.; Raghuram, N.; Sivakami, S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol. Cell Biochem. 2003, 243, 147–152. [Google Scholar] [CrossRef]
- Franco, A.A.; Odom, R.S.; Rando, T.A. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic. Biol. Med. 1999, 27, 1122–1132. [Google Scholar] [CrossRef]


| Items | Baseline Milk Replacer B | Baseline Starter |
|---|---|---|
| Ingredients (% of DM) | Content | |
| Corn meal | 65.07 | |
| Wheat bran | 15.00 | |
| Soybean meal | 5.58 | |
| Limestone | 1.90 | |
| Fat powder | 2.00 | |
| Dicalcium phosphate | 1.51 | |
| NaCl | 0.79 | |
| DL-methionine | 0.40 | |
| Compound amino acids C | 6.75 | |
| Premix D | 1.00 | |
| Total | 100.00 | |
| Nutritional composition E | ||
| DM (%, air-dried basis) | 95.69 | 88.20 |
| CP (%) | 21.66 | 16.16 |
| EE (%) | 6.44 | 4.68 |
| Ash (%) | 5.88 | 9.38 |
| Ca (%) | 1.02 | 1.23 |
| TP (%) | 0.51 | 0.54 |
| NDF (%) | 2.63 | 28.32 |
| ME (MJ/kg) | 15.10 | 10.75 |
| Met (%) | 0.91 | 0.60 |
| Try (%) | 0.29 | 0.18 |
| Lys (%) | 2.77 | 1.01 |
| Thr (%) | 1.17 | 0.60 |
| Gene | Forward (5′–3′) | Reverse (5′–3′) | GenBank Accession of mRNA |
|---|---|---|---|
| β-actin | CTCACGGAGCGTGGCTACA | GCCATCTCCTGCTCGAAGTC | NM_001009784.3 |
| Nrf2 | TCTGCCAACTACTCCCAGGT | AGGAGCATTGAAGACTGGGC | XM_012132956.3 |
| GSTA | GACCAGAGCCATTCTCAACTAC | CTTTTCTCGGATTAGGGTCAG | XM_027959005.1 |
| GSTP | ACGGAGACCTCACCCTTTAC | TTTGTCCTCCTCACGCTTG | XM_027959471.1 |
| CuSOD | AGGCAAAGGGAGATAAAGTCGTCG | TGCACTGGTACAGCCTTGTGTATTG | NM_001145185.2 |
| GSH-Px | CCAAGAACGAGGAGATCCTG | ACTTAGGGTCGGTCATGAGAG | XM_004018462.4 |
| CAT | CCTATCCTGACACTCACCGC | CATCGCTGGCACTGTTGAAG | XM_012096208.3 |
| Items | CON | MR | SEM | p-Value |
|---|---|---|---|---|
| Days 8 to 56 | ||||
| Milk replacer intake (g DM/d) | 131.41 | 131.19 | - | - |
| Starter intake (g DM/d) A | 91.64 | 97.45 | 2.90 | 0.071 |
| Methionine intake (g DM/d) A | 1.75 a | 0.47 b | 0.02 | <0.001 |
| Days 57 to 84 | ||||
| Starter intake (g DM/d) B | 517.32 | 532.77 | 8.70 | 0.136 |
| Methionine intake (g DM/d) B | 3.10 | 3.19 | 0.05 | 0.140 |
| Item | Groups | SEM | p-Value | |
|---|---|---|---|---|
| CON | MR | |||
| 56 days of age | ||||
| Met (ng/mL) | 264.17 a | 199.05 b | 15.10 | 0.0231 |
| SAM (ng/mL) | 11.99 a | 9.62 b | 0.64 | 0.0341 |
| SAH (ng/mL) | 9.16 | 8.24 | 0.66 | 0.2993 |
| Hcy (ng/mL) | 7.20 | 5.38 | 0.88 | 0.1736 |
| 84 days of age | ||||
| Met (ng/mL) | 259.92 a | 208.92 b | 18.91 | 0.0433 |
| SAM (ng/mL) | 11.13 a | 9.44 b | 0.49 | 0.0420 |
| SAH (ng/mL) | 11.75 | 10.61 | 0.47 | 0.0930 |
| Hcy (ng/mL) | 7.35 | 6.93 | 0.32 | 0.3223 |
| Item | Groups | SEM | p-Value | |
|---|---|---|---|---|
| CON | MR | |||
| 56 days of age | ||||
| Met (ng/mL) | 236.37 b | 252.23 a | 3.57 | 0.047 |
| SAM (ng/mL) | 3389.93 | 3455.49 | 192.03 | 0.750 |
| SAH (ng/mL) | 3768.22 | 3419.33 | 365.67 | 0.384 |
| Hcy (ng/mL) | 766.91 | 784.97 | 155.66 | 0.913 |
| 84 days of age | ||||
| Met (ng/mL) | 284.33 a | 175.74 b | 13.39 | 0.015 |
| SAM (ng/mL) | 4053.18 a | 2865.50 b | 236.35 | 0.007 |
| SAH (ng/mL) | 4639.28 a | 3603.02 b | 305.98 | 0.020 |
| Hcy (ng/mL) | 849.87 | 841.30 | 169.91 | 0.962 |
| Items | Groups | SEM | p-Value | |
|---|---|---|---|---|
| CON | MR | |||
| 56 days of age | ||||
| GST (U/mL) | 271.61 | 276.3 | 10.13 | 0.6631 |
| SOD (U/mL) | 362.02 a | 284.95 b | 17.4 | 0.0068 |
| GSH-Px (U/mL) | 21.87 | 20.36 | 1.14 | 0.2432 |
| CAT (U/mL) | 49.91 | 56.01 | 6.78 | 0.4097 |
| 84 days of age | ||||
| GST (U/mL) | 279.69 | 260.42 | 16.53 | 0.2964 |
| SOD (U/mL) | 365.08 | 338.53 | 31.15 | 0.433 |
| GSH-Px (U/mL) | 25.42 a | 20.11 b | 1.4 | 0.0128 |
| CAT (U/mL) | 56.99 | 54.8 | 7.76 | 0.7896 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Diao, Q.; Cui, K. Effect of Dietary Methionine Deficiency Followed by a Re-Feeding Phase on the Hepatic Antioxidant Activities of Lambs. Animals 2021, 11, 7. https://doi.org/10.3390/ani11010007
Liu R, Diao Q, Cui K. Effect of Dietary Methionine Deficiency Followed by a Re-Feeding Phase on the Hepatic Antioxidant Activities of Lambs. Animals. 2021; 11(1):7. https://doi.org/10.3390/ani11010007
Chicago/Turabian StyleLiu, Rong, Qiyu Diao, and Kai Cui. 2021. "Effect of Dietary Methionine Deficiency Followed by a Re-Feeding Phase on the Hepatic Antioxidant Activities of Lambs" Animals 11, no. 1: 7. https://doi.org/10.3390/ani11010007
APA StyleLiu, R., Diao, Q., & Cui, K. (2021). Effect of Dietary Methionine Deficiency Followed by a Re-Feeding Phase on the Hepatic Antioxidant Activities of Lambs. Animals, 11(1), 7. https://doi.org/10.3390/ani11010007






