Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use
Abstract
:1. Introduction
2. Materials and Methods/Data Collection and Selection
3. Results and Discussion
3.1. Scientific Papers Related to Silphium perfoliatum
3.2. Description and Origin
3.3. Utilization and Benefits/Advantages of Silphium perfoliatum
3.4. Silphium perfoliatum Yields
3.4.1. Biomass Yields
3.4.2. Biogas and Biomethane Yields
3.5. Composition of Biomass and Chemical Characteristics
4. Economic Aspects and Energy Efficiency of Biomass Production
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paolini, V.; Petracchini, F.; Segreto, M.; Tomassetti, L.; Naja, N.; Cecinato, A. Environmental impact of biogas: A short review of current knowledge. J. Environ. Sci. Health Part A 2018, 53, 899–906. [Google Scholar] [CrossRef] [Green Version]
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Herrmann, A. Biogas production from maize: Current state, challenges and prospects. 2. Agronomic and environmental aspects. Bioenergy Res. 2013, 6, 372–387. [Google Scholar] [CrossRef]
- Wu, X.; McLaren, J.; Madl, R.; Wang, D. Biofuels from Lignocellulosic Biomass. In Sustainable Biotechnology: Sources of Renewable Energy; Singh, O.V., Harvey, S.P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 19–41. [Google Scholar]
- Stolarski, M.J.; Krzyzaniak, M.; Snieg, M.; Slominska, E.; Piórkowski, M.; Filipkowski, R. Thermophysical and chemical properties of perennial energy crops depending on harvest period. Int. Agrophysics 2014, 28, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Romanowska-Duda, Z.; Grzesik, M.; Pszczółkowski, W.; Piotrowski, K.; Pszczółkowska, A. The didactic and environmental functions of the collection of energy crops in the transfer technology center in Konstantynów Łódzki. Acta Innov. 2014, 13, 37–48. [Google Scholar]
- Van Tassel, D.L.; Albrecht, K.A.; Bever, J.D.; Boe, A.A.; Brandvain, Y.; Crews, T.E.; Gansberger, M.; Gerstberger, P.; González-Paleo, L.; Hulke, B.S. Accelerating Silphium domestication: An opportunity to develop new crop ideotypes and breeding strategies informed by multiple disciplines. Crop Sci. 2017, 57, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Landis, D.A.; Gratton, C.; Jackson, R.D.; Gross, K.L.; Duncan, D.S.; Liang, C.; Meehan, T.D.; Robertson, B.A.; Schmidt, T.M.; Stahlheber, K.A. Biomass and biofuel crop effects on biodiversity and ecosystem services in the North Central US. Biomass Bioenergy 2018, 114, 18–29. [Google Scholar] [CrossRef]
- Langhammer, M.; Grimm, V. Mitigating bioenergy-driven biodiversity decline: A modelling approach with the European brown hare. Ecol. Model. 2020, 416, 108914. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D.A. Attractiveness of Michigan native plants to arthropod natural enemies and herbivores. Environ. Entomol. 2007, 36, 751–765. [Google Scholar] [CrossRef]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crop. Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Gansberger, M.; Stüger, H.-P.; Weinhappel, M.; Moder, K.; Liebhard, P.; von Gehren, P.; Mayr, J.; Ratzenböck, A. Germination characteristic of Silphium perfoliatum L. seeds. Bodenkult. J. Land Manag. Food Environ. 2017, 68, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Schoo, B.; Kage, H.; Schittenhelm, S. Radiation use efficiency, chemical composition, and methane yield of biogas crops under rainfed and irrigated conditions. Eur. J. Agron. 2017, 87, 8–18. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crop. Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Ustak, S.; Munoz, J. Cup-plant potential for biogas production compared to reference maize in relation to the balance needs of nutrients and some microelements for their cultivation. J. Environ. Manag. 2018, 228, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, H.; Li, Z.; Zhuang, P.; Gao, B. Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour. Technol. 2010, 101, 2063–2066. [Google Scholar] [CrossRef]
- Jasinskas, A.; Simonavičiūtė, R.; Šiaudinis, G.; Liaudanskienė, I.; Antanaitis, Š.; Arak, M.; Olt, J. The assessment of common mugwort (Artemisia vulgaris L.) and cup plant (Silphium perfoliatum L.) productivity and technological preparation for solid biofuel. Zemdirb. Agric. 2014, 101, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Short rotation coppices, grasses and other herbaceous crops: Productivity and yield energy value versus 26 genotypes. Biomass Bioenergy 2018, 119, 109–120. [Google Scholar] [CrossRef]
- Reinert, S.; Hulke, B.S.; Prasifka, J.R. Pest potential of Neotephritis finalis (Loew) on Silphium integrifolium Michx., Silphium perfoliatum L., and interspecific hybrids. Agron. J. 2020, 112, 1462–1465. [Google Scholar] [CrossRef]
- Boe, A.; Albrecht, K.A.; Johnson, P.J.; Wu, J. Biomass Production of Cup Plant (Silphium perfoliatum L.) in Response to Variation in Plant Population Density in the North Central USA. Am. J. Plant Sci. 2019, 10, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Douglas, J.A.; Follett, J.M.; Halliday, I.R.; Hughes, J.W.; Parr, C.R. Silphium: Preliminary research on a possible new forage crop for New Zealand. Proc. Agron. Soc. N. Z. 1987, 17, 51–53. [Google Scholar]
- Rakhmetov, D.B.; Vergun, O.M.; Stadnichuk, N.O.; Shymanska, O.V.; Rakhmetova, S.O.; Fishchenko, V.V. Biochemical study of plant raw material of Silphium L. SPP. in M.M. Gryshko National Botanical Garden of the NAS of Ukraine. Plant Introd. 2019, 83, 80–86. [Google Scholar] [CrossRef]
- Han, K.J.; Albrecht, K.A.; Mertens, D.R.; Kim, D.A. Comparison of in vitro digestion kinetics of cup-plant and alfalfa. Asian-Australas. J. Anim. Sci. 2000, 13, 641–644. [Google Scholar] [CrossRef]
- Han, K.J.; Albrecht, K.A.; Muck, R.E.; Kim, D.A. Moisture effect on fermentation characteristics of cup-plant silage. Asian-Australas. J. Anim. Sci. 2000, 13, 636–640. [Google Scholar] [CrossRef]
- Puia, I.; Szabo, A.T. Experimental cultivation of a new forage species-Silphium perfoliatum L.-in the Agrobotanical Garden from Cluj-Napoca. Not. Bot. Horti Agrobot. Cluj-Napoca 1985, 15, 15–20. [Google Scholar]
- Pastukhova, M.A.; Sheliuto, B.V. Вoзделывание сильфии прoнзеннoлистнoй пoд пoкрoвoм сельскoхoзяйственных культур [Cultivation of Silphium perfoaliatum under agricultural crops cover]. Bull. Belarus. State Agric. Acad. 2019, 3, 83–87. [Google Scholar]
- Pichard, G. Management, production, and nutritional characteristics of cup-plant (Silphium perfoliatum) in temperate climates of southern Chile. Cienc. Investig. Agrar. 2012, 39, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Guoyan, P.; Ouyang, Z.; Qunying, L.; Qiang, Y.; Jishun, W. Water consumption of seven forage cultivars under different climatic conditions in the North China plain. J. Resour. Ecol. 2011, 2, 74–82. [Google Scholar] [CrossRef]
- Gimbatov, A.S.; Alimirzaeva, G.A. Optimization of technology for the cultivation of new feed crops in the irrigated conditions of the lowland zone of Dagestan. Probl. Dev. Agric. Ind. Apk Reg. 2011, 5, 8–11. [Google Scholar]
- Zaynullina, K.S.; Ruban, G.A.; Mikhovich, J.E.; Portnyagina, N.V.; Potapov, A.A.; Punegov, V.V.; Fomina, M.G. Prospects of introduction in culture in the North some resource plants (Komi Republic). Bull. Samara Sci. Cent. Russ. Acad. Sci. 2015, 17, 121–126. [Google Scholar]
- Novichikhin, A.M.; Piscareva, L.A. The study of elements of the cultivation technology of Silphium Perfoliatum. Symb. Sci. 2016, 10, 38–41. [Google Scholar]
- Semerenko, M.V.; Chupina, M.P.; Stepanov, A.F. The influence of compacting crops on the productivity of perfoliate sylphs. Bull. Omsk State Agric. Univ. 2016, 2, 61–65. [Google Scholar]
- Chupina, M.P.; Stepanov, A.F. The application of Silphium perfoliatum in the system of green and raw material conveyors. Agric. Sci. J. Omsk 2017, 4, 92–97. [Google Scholar]
- Kowalski, R. Analysis of lipophilic fraction from leaves, inflorescences and rhizomes of Silphium perfoliatum L. Acta Soc. Bot. Pol. 2005, 74, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Wierciński, J. Evaluation of chemical composition of some Silphium L. species seeds as alternative foodstuff raw materials. Pol. J. Food Nutr. Sci. 2004, 13, 349–354. [Google Scholar]
- Danilov, K.P. The effect of the method and sowing rate on the yield of Silphium perfoliatum. News Orenbg. State Agrar. Univ. 2013, 4, 37–39. [Google Scholar]
- Klímek, P.; Meinlschmidt, P.; Wimmer, R.; Plinke, B.; Schirp, A. Using sunflower (Helianthus annuus L.), topinambour (Helianthus tuberosus L.) and cup-plant (Silphium perfoliatum L.) stalks as alternative raw materials for particleboards. Ind. Crop. Prod. 2016, 92, 157–164. [Google Scholar] [CrossRef]
- Pastukhova, M.A. The place of Silphium perfoliatum in the nectariferous conveyor in the conditions of the south-west of Belarus. Bull. Belarus. State Agric. Acad. 2019, 3, 88–92. [Google Scholar]
- Mueller, A.L.; Berger, C.A.; Schittenhelm, S.; Stever-Schoo, B.; Dauber, J. Water availability affects nectar sugar production and insect visitation of the cup plant Silphium perfoliatum L. (Asteraceae). J. Agron. Crop Sci. 2020, 206, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Mueller, A.L.; Biertümpfel, A.; Friedritz, L.; Power, E.F.; Wright, G.A.; Dauber, J. Floral resources provided by the new energy crop, Silphium perfoliatum L. (Asteraceae). J. Apic. Res. 2020, 59, 232–245. [Google Scholar] [CrossRef]
- Savin, A.P.; Gudimova, N.A. The influence of mineral fertilizers on the nectar, forage and seed productivity sylphs standardized. Сoвременные прoблемы пчелoвoдства и апитерапии: мoнoграфия/пoд, Рыбнoе: ФГБНУ «ФНЦ пчелoвoдства. In Modern Problem of Beekeeping and Apitherapy; Brandorf, A.Z., Lebedev, V.I., Kharitonova, M.N., Savina, A.P., Savushkina, L.N., Lizunova, A.S., Eds.; Publisher: Rybnoe, Russia, 2019; pp. 186–191. [Google Scholar]
- Clevinger, J.A. New combinations in Silphium (Asteraceae: Heliantheae). Novon 2004, 14, 275–277. [Google Scholar]
- Clevinger, J.A.; Panero, J.L. Phylogenetic analysis of Silphium and subtribe Engelmanniinae (Asteraceae: Heliantheae) based on ITS and ETS sequence data. Am. J. Bot. 2000, 87, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.; Kędzia, B. Antibacterial activity of Silphium perfoliatum. Extracts. Pharm. Biol. 2007, 45, 494–500. [Google Scholar] [CrossRef]
- Assefa, T.; Wu, J.; Albrecht, K.A.; Johnson, P.J.; Boe, A. Genetic variation for biomass and related morphological traits in cup plant (Silphium perfoliatum L.). Am. J. Plant Sci. 2015, 6, 1098–1108. [Google Scholar] [CrossRef] [Green Version]
- Ţîţei, V.; Teleuţă, A.; Muntea, A. The perspective of cultivation and utilization of the species Silphium perfoliatum L. and Helianthus tuberosus L. Mold. Bull. UASMV Agric. 2013, 70, 160–166. [Google Scholar]
- Țîței, V. Biological peculiarities of cup plant (Silphium perfoliatum L.) and utilization possibilities in the Republic of Moldova. Agron. Ser. Sci. Res. Lucr. Stiintifice Ser. Agron. 2014, 57, 289–293. [Google Scholar]
- Feng, W.-S.; Pei, Y.-Y.; Zheng, X.-K.; Li, C.-G.; Ke, Y.-Y.; Lv, Y.-Y.; Zhang, Y.-L. A new kaempferol trioside from Silphium perfoliatum. J. Asian Nat. Prod. Res. 2014, 16, 393–399. [Google Scholar] [CrossRef]
- Franzaring, J.; Holz, I.; Kauf, Z.; Fangmeier, A. Responses of the novel bioenergy plant species Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L. to CO2 fertilization at different temperatures and water supply. Biomass Bioenergy 2015, 81, 574–583. [Google Scholar] [CrossRef]
- Stanford, G. Silphium perfoliatum (cup-plant) as a new forage. In Proceedings of the Twelfth North American Prairie Conference: Recapturing a Vanishing Heritage, Cedar Falls, Iowa, 5–9 August 1990; pp. 33–37. [Google Scholar]
- Ţîţei, V. The evaluation of biomass of the Sida hermaphrodita and Silphium perfoliatum for renewable energy in Moldova. Sci. Pap. Ser. A Agron. 2017, 60, 534–540. [Google Scholar]
- Wever, C.; Höller, M.; Becker, L.; Biertümpfel, A.; Köhler, J.; van Inghelandt, D.; Westhoff, P.; Pude, R.; Pestsova, E. Towards high-biomass yielding bioenergy crop Silphium perfoliatum L.: Phenotypic and genotypic evaluation of five cultivated populations. Biomass Bioenergy 2019, 124, 102–113. [Google Scholar] [CrossRef]
- Shalyuta, B.V.; Kostitskaya, E.V. The yield of Silphium perfoliatum L. depending on the conditions of cultivation. Agric. Eng. 2018, 22, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Šiaudinis, G.; Jasinskas, A.; Šlepetienė, A.; Karčauskienė, D. The evaluation of biomass and energy productivity of common mugwort (Artemisia vulgaris L.) and cup plant (Silphium perfoliatum L.) in Albeluvisol. Agriculture 2012, 99, 357–362. [Google Scholar]
- Šiaudinis, G.; Skuodienė, R.; Repšienė, R. The investigation of three potential energy crops: Common mugwort, cup plant and Virginia mallow on Western Lithuania’s Albeluvisol. Appl. Ecol. Environ. Res. 2017, 15, 611–620. [Google Scholar] [CrossRef]
- Assefa, T.; Wu, J.; Boe, A. Genetic variation for achene traits in cup plant (Silphium perfoliatum L.). Open J. Genet. 2015, 5, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S.; Graban, Ł.; Lajszner, W. Short rotation coppices, grasses and other herbaceous crops: Biomass properties versus 26 genotypes and harvest time. Ind. Crop. Prod. 2018, 119, 22–32. [Google Scholar] [CrossRef]
- Stankevich, S.I.; Kiselev, A.A.; Nesterenko, T.K. The influence of the method of spreading on productivity of cup plant. Bull. Belarusian State Agric. Acad. 2017, 3, 77–80. [Google Scholar]
- Schäfer, A.; Meinhold, T.; Damerow, L.; Lammers, P.S. Crop establishment of Silphium perfoliatum by precision seeding. Agric. Eng. 2015, 70, 254–261. [Google Scholar] [CrossRef]
- Schäfer, A.; Leder, A.; Graff, M.; Damerow, L.; Lammers, P.S. Determination and sorting of cup plant seeds to optimize crop establishment. Agric. Eng. 2018, 73, 97–105. [Google Scholar]
- Schäfer, A.; Damerow, L.; Lammers, P.S. Determination of the seed geometry of cup plant as requirement for precision seeding. Agric. Eng. 2017, 72, 122–129. [Google Scholar]
- Tomaszewska-Sowa, M.; Figas, A. Optimization of the processes of sterilization and micropropagation of cup plant (Silphium perfoliatum L.) from apical explants of seedlings in in vitro cultures. Acta Agrobot. 2011, 64, 3–10. [Google Scholar] [CrossRef]
- Figas, A.; Rolbiecki, R.; Tomaszewska-Sowa, M. Influence of drip irrigation on the height of cup plant (Silphium perfoliatum L.) cultivated on the very light soil from the micropagation seedlings. Infrastruct. Ecol. Rural Areas 2011, 10, 245–253. [Google Scholar]
- Figas, A.; Rolbiecki, R.; Tomaszewska-Sowa, M. Influence of drip irrigation on the height of the biennial cup plant (Silphium perfoliatum L.) from the micropropagation seedlings. Infrastruct. Ecol. Rural Areas 2015, 3, 779–786. [Google Scholar] [CrossRef]
- Figas, A.; Sawilska, A.K.; Rolbiecki, R.; Tomaszewska-Sowa, M. Morphological characteristics of achenes and fertility plants of cup plant (Silphium perfoliatum L.) obtained from micropropagation growing under irrigation. Infrastruct. Ecol. Rural Areas 2016, 4, 1363–1372. [Google Scholar] [CrossRef]
- Figas, A.; Siwik-Ziomek, A.; Rolbiecki, R. Effect of irrigation on some growth parameters of cup plant and dehydrogenase activity in soil. Ann. Wars. Univ. Life Sci. Land Reclam. 2015, 47, 279–288. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Oleszek, W. Phytochemicals in bioenergy crops. Phytochem. Rev. 2019, 18, 893–927. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Wolski, T. The chemical composition of essential oils of (Silphium perfoliatum L.). Flavour Fragr. J. 2005, 20, 306–310. [Google Scholar] [CrossRef]
- Von Gehren, P.; Gansberger, M.; Mayr, J.; Liebhard, P. The effect of sowing date and seed pretreatments on establishment of the energy plant Silphium perfoliatum by sowing. Seed Sci. Technol. 2016, 44, 310–319. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, H.; Zhao, J.; Zhang, H.; Chen, S. Enzyme-assisted extraction of a cup plant (Silphium perfoliatum L.) Polysaccharide and its antioxidant and hypoglycemic activities. Process Biochem. 2020, 92, 17–28. [Google Scholar] [CrossRef]
- Piszczek, P.; Kuszewska, K.; Błaszkowski, J.; Sochacka-Obruśnik, A.; Stojakowska, A.; Zubek, S. Associations between root-inhabiting fungi and 40 species of medicinal plants with potential applications in the pharmaceutical and biotechnological industries. Appl. Soil Ecol. 2019, 137, 69–77. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M.; Figas, A. Environmental and ecological aspects of cultivation of selected energy and herbal plants propagated by in vitro culture. Infrastruct. Ecol. Rural Areas 2014, 4, 1457–1465. [Google Scholar] [CrossRef]
- Davidyants, E.S. The effect of the purified amount of triterpene glycosides and their enriched extract from leaves of Silphium perfoliatum L. on the growth and activity of nitrate reductase of winter wheat plants. Chem. Plant Raw Mater. 2019, 4, 441–448. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, N.H.; Wojcińska, M.; Drost-Karbowska, K.; Matławska, I.; Williams, J.; Mabry, T.J. Kaempferol triosides from Silphium perfoliatum. Phytochemistry 2002, 60, 835–838. [Google Scholar] [CrossRef]
- Jemiołkowska, A.; Kowalski, R. In vitro estimate of influence of Silphium perfoliatum L. leaves extract on some fungi colonizing the pepper plants. Acta Sci. Pol. Hort. Cult 2012, 11, 43–55. [Google Scholar]
- Wu, H.; Shang, H.; Guo, Y.; Zhang, H.; Wu, H. Comparison of different extraction methods of polysaccharides from cup plant (Silphium perfoliatum L.). Process Biochem. 2020, 90, 241–248. [Google Scholar] [CrossRef]
- Kowalski, R.; Wolski, T. Evaluation of phenolic acid content in Silphium perfoliatum L. leaves, inflorescences and rhizomes. Electron. J. Pol. Agric. Univ. 2003, 6, 1–10. [Google Scholar]
- Shang, H.-M.; Zhou, H.-Z.; Li, R.; Duan, M.-Y.; Wu, H.-X.; Lou, Y.-J. Extraction optimization and influences of drying methods on antioxidant activities of polysaccharide from cup plant (Silphium perfoliatum L.). PLoS ONE 2017, 12, e0183001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolski, T.; Kowalski, R.; Mardarowicz, M. Chromatographic analysis of essential oil occurring in inflorescences, leaves and rhizomes of Silphium perfoliatum L. Herba Pol. 2000, 46, 235–242. [Google Scholar]
- Kowalski, R. Silphium L. extracts-composition and protective effect on fatty acids content in sunflower oil subjected to heating and storage. Food Chem. 2009, 112, 820–830. [Google Scholar] [CrossRef]
- Kowalska, G.; Pankiewicz, U.; Kowalski, R. Evaluation of Chemical Composition of Some Silphium L. Species as Alternative Raw Materials. Agriculture 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Ţîţei, V. The potential growth and the biomass quality of some herbaceous species for the production of renewable energy in Moldova. Rev. Bot. Bot. J. 2019, 18, 83–91. [Google Scholar]
- Mueller, A.L.; Dauber, J. Hoverflies (Diptera: Syrphidae) benefit from a cultivation of the bioenergy crop Silphium perfoliatum L. (Asteraceae) depending on larval feeding type, landscape composition and crop management. Agric. For. Entomol. 2016, 18, 419–431. [Google Scholar] [CrossRef]
- Gerstberger, P.; Asen, F.; Hartmann, C. Economy and ecology of cup plant (Silphium perfoliatum L.) compared with silage maize. J. Kult. 2016, 68, 372–377. [Google Scholar] [CrossRef]
- Schorpp, Q.; Schrader, S. Earthworm functional groups respond to the perennial energy cropping system of the cup plant (Silphium perfoliatum L.). Biomass Bioenergy 2016, 87, 61–68. [Google Scholar] [CrossRef]
- Schorpp, Q.; Schrader, S. Dynamic of nematode communities in energy plant cropping systems. Eur. J. Soil Biol. 2017, 78, 92–101. [Google Scholar] [CrossRef]
- Ruf, T.; Emmerling, C. Site-adapted production of bioenergy feedstocks on poorly drained cropland through the cultivation of perennial crops. A feasibility study on biomass yield and biochemical methane potential. Biomass Bioenergy 2018, 119, 429–435. [Google Scholar] [CrossRef]
- Ruf, T.; Makselon, J.; Udelhoven, T.; Emmerling, C. Soil quality indicator response to land-use change from annual to perennial bioenergy cropping systems in Germany. GCB Bioenergy 2018, 10, 444–459. [Google Scholar] [CrossRef]
- Chmelíková, L.; Wolfrum, S. Mitigating the biodiversity footprint of energy crops—A case study on arthropod diversity. Biomass Bioenergy 2019, 125, 180–187. [Google Scholar] [CrossRef]
- Ruf, T.; Audu, V.; Holzhauser, K.; Emmerling, C. Bioenergy from periodically waterlogged cropland in Europe: A first assessment of the potential of five perennial energy crops to provide biomass and their interactions with soil. Agronomy 2019, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Schoo, B.; Schroetter, S.; Kage, H.; Schittenhelm, S. Root traits of cup plant, maize and lucerne grass grown under different soil and soil moisture conditions. J. Agron. Crop Sci. 2017, 203, 345–359. [Google Scholar] [CrossRef]
- Grunwald, D.; Panten, K.; Schwarz, A.; Bischoff, W.A.; Schittenhelm, S. Comparison of maize, permanent cup plant and a perennial grass mixture with regard to soil and water protection. GCB Bioenergy 2020, 12, 694–705. [Google Scholar] [CrossRef]
- Schorpp, Q.; Riggers, C.; Lewicka-Szczebak, D.; Giesemann, A.; Well, R.; Schrader, S. Influence of Lumbricus terrestris and Folsomia candida on N2O formation pathways in two different soils—With particular focus on N2 emissions. Rapid Commun. Mass Spectrom. 2016, 30, 2301–2314. [Google Scholar] [CrossRef]
- Šiaudinis, G.; Liaudanskiene, I.; Slepetiene, A. Changes in soil carbon, nitrogen and sulphur content as influenced by liming and nitrogen fertilization of three energy crops. Icel. Agric. Sci. 2017, 30, 43–50. [Google Scholar] [CrossRef]
- Bufe, C.; Korevaar, H. Evaluation of Additional Crops for Dutch List of Ecological Focus Area: Evaluation of Miscanthus, Silphium perfoliatum, Fallow Sown in with Melliferous Plants and Sunflowers in Seed Mixtures for Catch Crops; Report/WPR, no. 793; Wageningen Research Foundation (WR) Business Unit Agrosystems Research: Lelystad, The Netherlands, 2018; p. 34. [Google Scholar] [CrossRef] [Green Version]
- Bedlan, G. Ascochyta silphii sp. nov.—A new Ascochyta species on Silphium perfoliatum. J. Kult. 2014, 66, 281–283. [Google Scholar] [CrossRef]
- Balezentiene, L.; Streimikiene, D.; Balezentis, T. Fuzzy decision support methodology for sustainable energy crop selection. Renew. Sustain. Energy Rev. 2013, 17, 83–93. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Jasiewicz, C. Effect of Soil Contamination with Heavy Metals on Element Contents in Silphium perfoliatum (Silphium perfoliatum L.). Chem. Ecol. Eng. 2003, 10, 205–210. [Google Scholar]
- Du, J.; Zhang, L.; Ali, A.; Li, R.; Xiao, R.; Guo, D.; Liu, X.; Zhang, Z.; Ren, C.; Zhang, Z. Research on thermal disposal of phytoremediation plant waste: Stability of potentially toxic metals (PTMs) and oxidation resistance of biochars. Process Saf. Environ. Prot. 2019, 125, 260–268. [Google Scholar] [CrossRef]
- Šiaudinis, G.; Jasinskas, A.; Šarauskis, E.; Steponavičius, D.; Karčauskienė, D.; Liaudanskienė, I. The assessment of Virginia mallow (Sida hermaphrodita Rusby) and cup plant (Silphium perfoliatum L.) productivity, physico-mechanical properties and energy expenses. Energy 2015, 93, 606–612. [Google Scholar] [CrossRef]
- Wrobel, M.; Frączek, J.; Francik, S.; Slipek, Z.; Mudryk, K. Influence of degree of fragmentation on chosen quality parameters of briquette made from biomass of cup plant Silphium perfoliatum L. In Proceedings of the 12th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 23–24 May 2013; pp. 653–657. [Google Scholar]
- Styks, J.; Wróbel, M.; Frączek, J.; Knapczyk, A. Effect of Compaction Pressure and Moisture Content on Quality Parameters of Perennial Biomass Pellets. Energies 2020, 13, 1859. [Google Scholar] [CrossRef] [Green Version]
- Bury, M.; Facciotto, G.; Chiocchini, F.; Cumplido-Marín, L.; Graves, A.; Kitczak, T.; Martens, R.; Morhart, C.; Możdżer, E.; Nahm, M. Preliminary results regarding yields of Virginia mallow (Sida hermaphrodita L. Rusby) and cup plant (Silphium perfoliatum L.) in different condition of Europe. In Proceedings of the 27th European Biomass Conference and Exhibition, Lisbon, Portugal, 27–30 May 2019; pp. 101–104. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; Van Eupen, M. Prospects of bioenergy cropping systems for a more social-ecologically sound bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef] [Green Version]
- Jucsor, N.; Sumalan, R. Researches concerning the potential of biomass accumulation in cup plant (Silphium perfoliatum L.). J. Hortic. For. Biotechnol. 2018, 22, 34–39. [Google Scholar]
- Stolarski, M.J.; Krzyżaniak, M.; Warmiński, K.; Olba-Zięty, E.; Penni, D.; Bordiean, A. Energy efficiency indices for lignocellulosic biomass production: Short rotation coppices versus grasses and other herbaceous crops. Ind. Crop. Prod. 2019, 135, 10–20. [Google Scholar] [CrossRef]
- Franzaring, J.; Schmid, I.; Bäuerle, L.; Gensheimer, G.; Fangmeier, A. Investigations on plant functional traits, epidermal structures and the ecophysiology of the novel bioenergy species Sida hermaphrodita Rusby and Silphium perfoliatum L. J. Appl. Bot. Food Qual. 2014, 87, 36–45. [Google Scholar] [CrossRef]
- Šiaudinis, G.; Karčauskienė, D.; Aleinikovienė, J. Assessment of a single application of sewage sludge on the biomass yield of Silphium perfoliatum and changes in naturally acid soil properties. Agriculture 2019, 106, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Šiaudinis, G.; Skuodienė, R.; Katutis, K. Biomass and energy productivity of different plant species under western Lithuania conditions. In Proceedings of the 25th Nordic View to Sustainable Rural Development Congress, Riga, Latvia, 16–18 June 2015; pp. 232–237. [Google Scholar]
- Siwek, H.; Włodarczyk, M.; Możdżer, E.; Bury, M.; Kitczak, T. Chemical Composition and Biogas Formation potential of Sida hermaphrodita and Silphium perfoliatum. Appl. Sci. 2019, 9, 4016. [Google Scholar] [CrossRef] [Green Version]
- Boe, A.; Albrecht, K.A.; Johnson, P.J.; Wu, J. Biomass production of monocultures and mixtures of cup plant and native grasses on prime and marginal cropland. Am. J. Plant Sci. 2019, 10, 911. [Google Scholar] [CrossRef] [Green Version]
- Heneman, P.; Červinka, J. Energy crops and bioenergetics in the Czech Republic. Agric. (Agric. Eng.) 2007, 51, 73–78. [Google Scholar]
- Skorupskaitė, V.; Makarevičienė, V.; Šiaudinis, G.; Zajančauskaitė, V. Green energy from different feedstock processed under anaerobic conditions. Agron. Res. 2015, 13, 420–429. [Google Scholar]
- Slepetys, J.; Kadziuliene, Z.; Sarunaite, L.; Tilvikiene, V.; Kryzeviciene, A. Biomass potential of plants grown for bioenergy production. In Proceedings of the Renewable Energy and Energy Efficiency, Growing and Processing Technologies of Energy Crops, Jelgava, Latvia, 2012; pp. 66–72. Available online: https://www.semanticscholar.org/paper/Biomass-potential-of-plants-grown-for-bioenergy-%C5%A0lepetys-Kadz%CC%8Ciuliene%CC%87/3d0f87eb3d601ee7472c9eca3abfe8f895c0c009?p2df (accessed on 4 December 2020).
- Šiaudinis, G.; Šlepetienė, A.; Karčauskienė, D. The evaluation of dry mass yield of new energy crops and their energetic parameters. In Proceedings of the Renewable Energy and Energy Efficiency, Growing and Processing Technologies of Energy Crops, Jelgava, Latvia, 2012; pp. 24–28. Available online: https://www.db-thueringen.de/receive/dbt_mods_00024401 (accessed on 4 December 2020).
- Von Cossel, M.; Amarysti, C.; Wilhelm, H.; Priya, N.; Winkler, B.; Hoerner, L. The replacement of maize (Zea mays L.) by cup plant (Silphium perfoliatum L.) as biogas substrate and its implications for the energy and material flows of a large biogas plant. Biofuels Bioprod. Biorefining 2020, 14, 152–179. [Google Scholar] [CrossRef] [Green Version]
- Von Cossel, M.; Steberl, K.; Hartung, J.; Pereira, L.A.; Kiesel, A.; Lewandowski, I. Methane yield and species diversity dynamics of perennial wild plant mixtures established alone, under cover crop maize (Zea mays L.), and after spring barley (Hordeum vulgare L.). GCB Bioenergy 2019, 11, 1376–1391. [Google Scholar] [CrossRef] [Green Version]
- Schittenhelm, S.; Schoo, B.; Schroetter, S. Yield physiology of biogas plants: Comparison of streaky Silphie, maize and Luzernegra. J. Kult. 2016, 68, 378–384. [Google Scholar] [CrossRef]
- Herrmann, C.; Idler, C.; Heiermann, M. Biogas crops grown in energy crop rotations: Linking chemical composition and methane production characteristics. Bioresour. Technol. 2016, 206, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Haag, N.L.; Nägele, H.-J.; Reiss, K.; Biertümpfel, A.; Oechsner, H. Methane formation potential of cup plant (Silphium perfoliatum). Biomass Bioenergy 2015, 75, 126–133. [Google Scholar] [CrossRef]
- Majtkowski, W.; Piłat, J.; Szulc, P.M. Prospects of cultivation and utilization of Silphium perfoliatum L. in Poland. Biul. Inst. Hod. I Aklim. Roślin 2009, 251, 283–291. [Google Scholar]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Tyśkiewicz, K.; Olba-Zięty, E.; Graban, Ł.; Lajszner, W.; Załuski, D.; Wiejak, R.; Kamiński, P. How does extraction of biologically active substances with supercritical carbon dioxide affect lignocellulosic biomass properties? Wood Sci. Technol. 2020, 54, 519–546. [Google Scholar] [CrossRef]
- Stolarski, M.; Szczukowski, S.; Tworkowski, J. Biofuels from biomass of perennial energy crops. Energetyka Ekol. 2008, 1, 77–80. [Google Scholar]
- Trölenberg, S.D.; Kruse, M.; Jonitz, A. Improvement of the seed quality in the Streaky Silphie (Silphium perfoliatum L.). In Nachhaltigkeitsindikatoren für die Landwirtschaft: Bestimmung und Eignung, VDLUFA-Schriftenreihe. Presented at the 124th VDLUFA-Kongress; VDLUFA-Verlag: Darmstadt, Germany, 2012; pp. 926–933. [Google Scholar]
- Biertümpfel, A.; Conrad, M. (Eds.) Joint Project: Increase of the Performance Potential and the Competitiveness of the Streaky Silphie as an Energy Plant through Breeding and Optimization of the Cultivation Method: Subproject 2: Optimization of the Cultivation Method and Provision of Selection Material; Abschlussbericht; Project no. 99.05; FKZ; no.: 22012809; Friedrich-Schiller-Universität Jena: Jena, Germany, 2014. [Google Scholar]
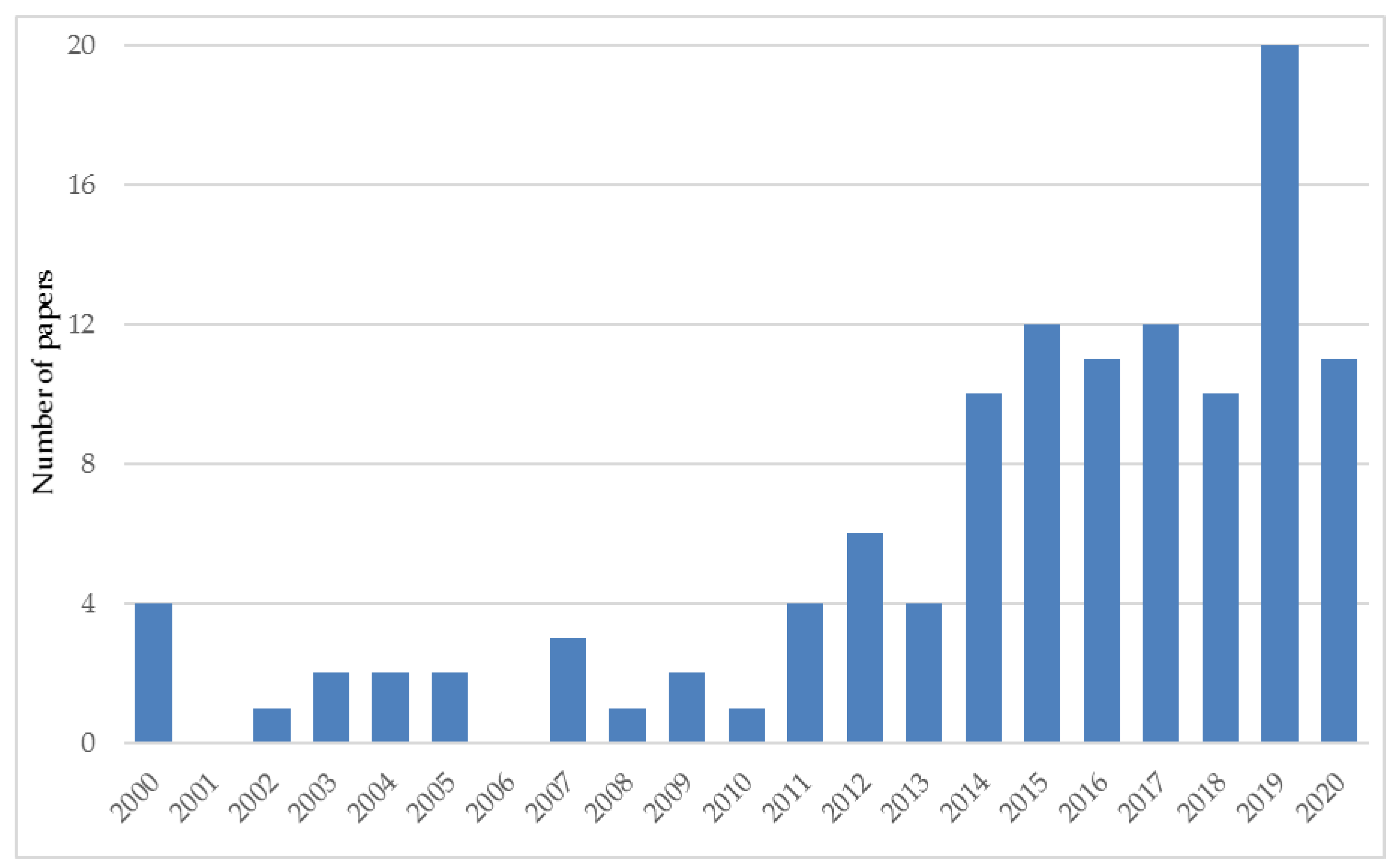
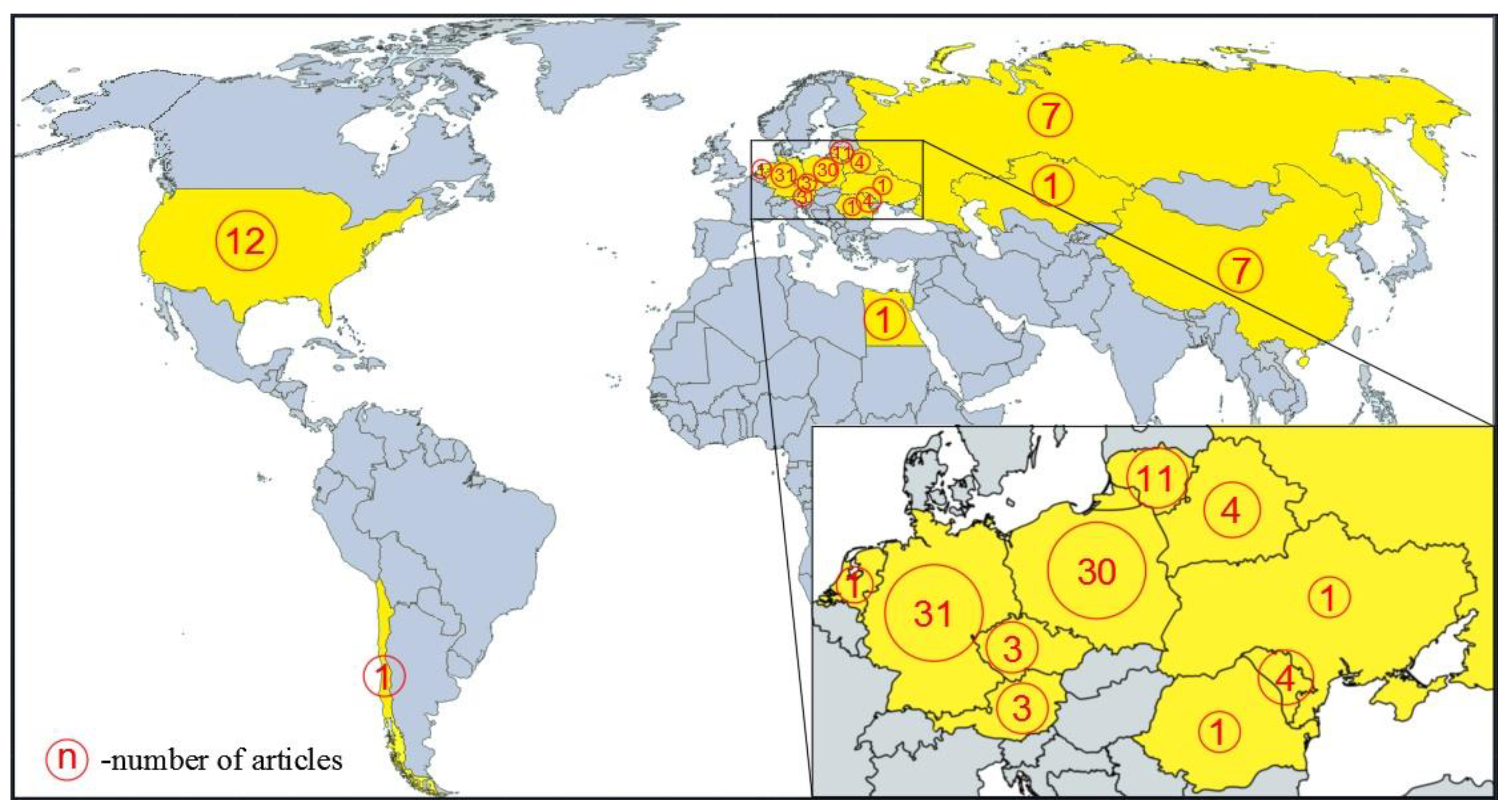
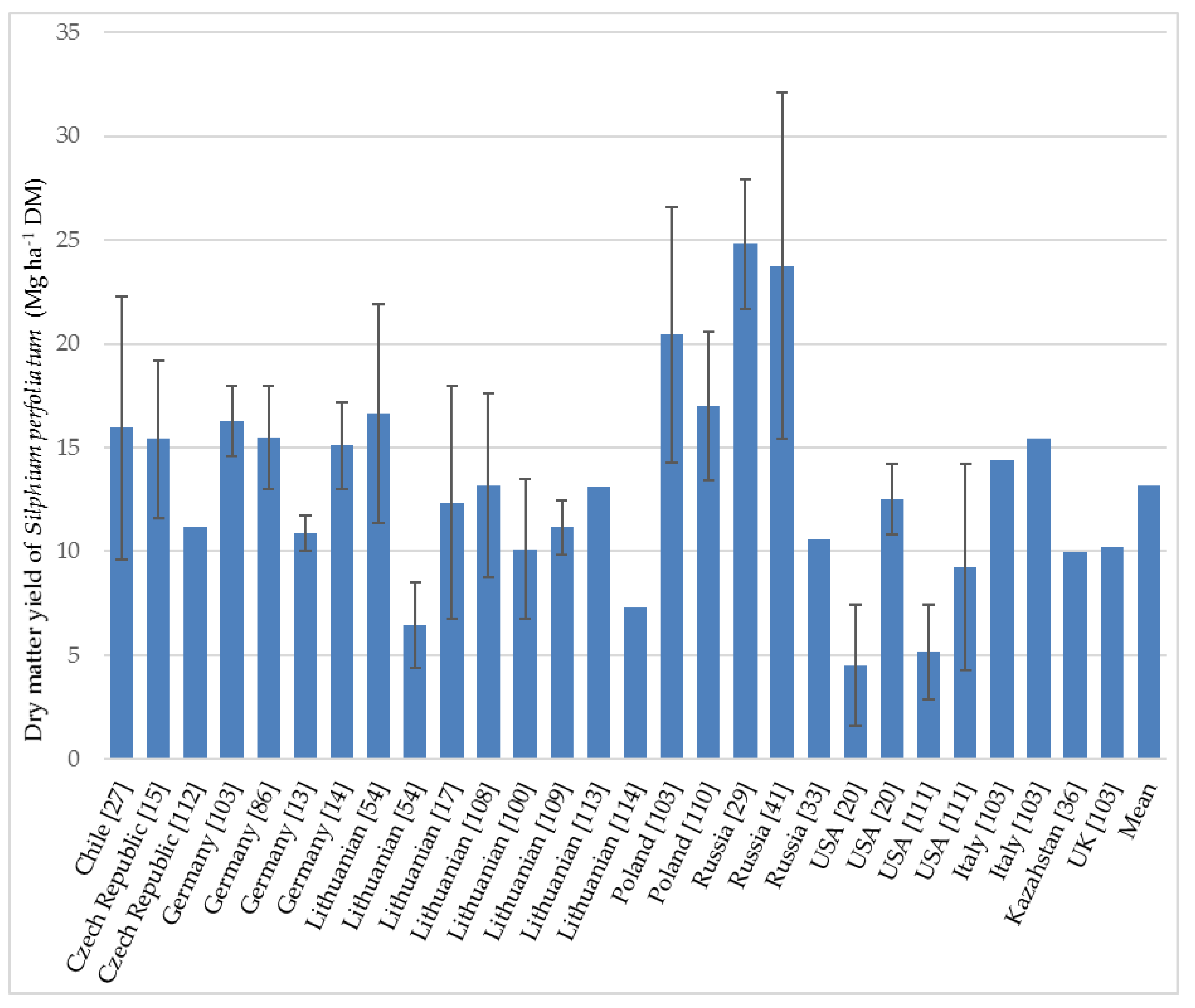
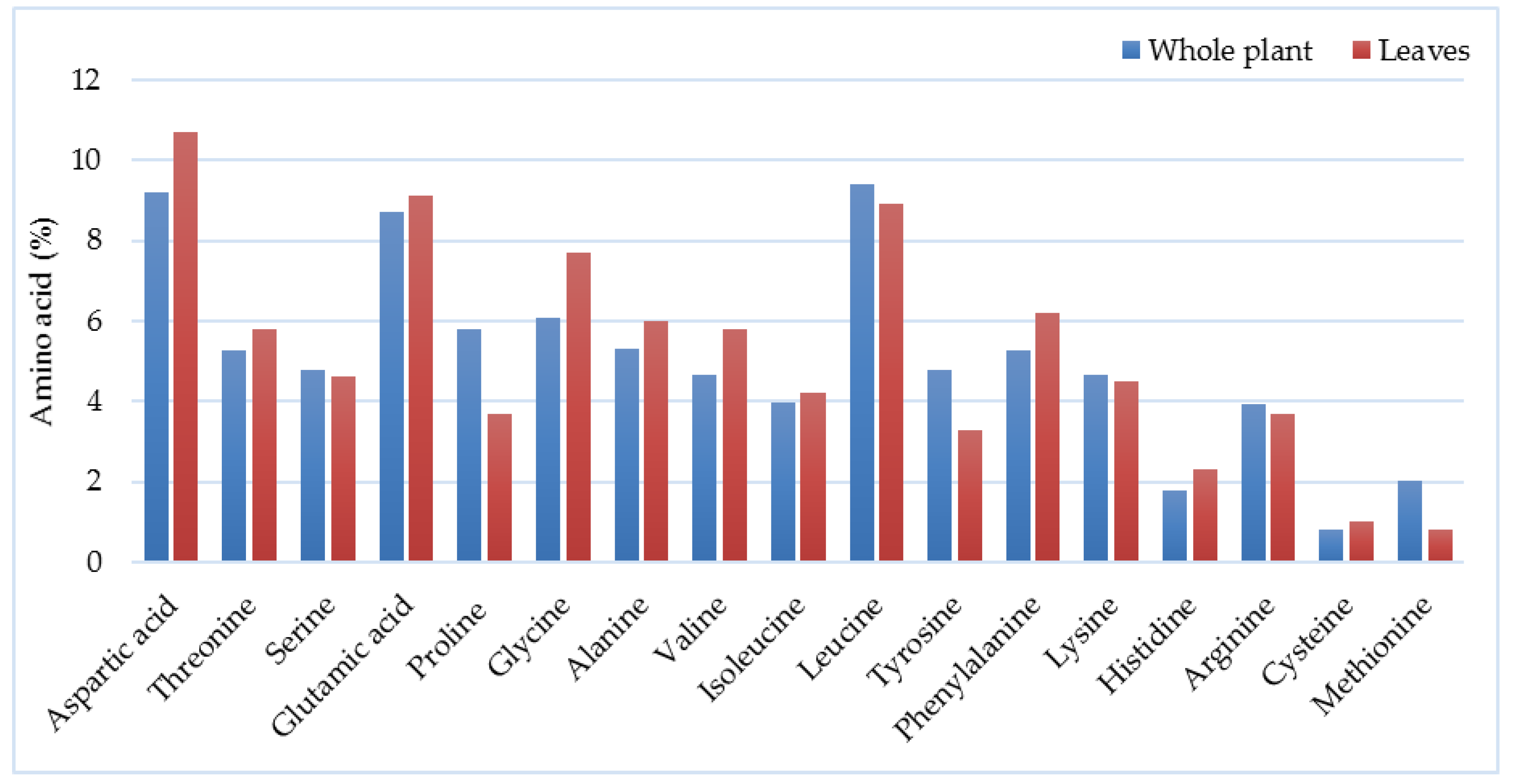
| Year of Harvesting | Yield (Mg ha−1 DM) | Country | Observations and Harvesting Details | Reference |
|---|---|---|---|---|
| 1st year | 22.3 | Chile | Chahuilco site (Trumao soils): very fertile acidic soils, pH 5.6, high Al and Mn concentration, 18% organic matter. Average rainfall 1303 mm. Harvested at flower initiation. | [27] |
| 9.6 | Chile | La Unión site (Red clay soils): pH 5.2, organic matter 8.0%, suffered extreme water deficit during the summer season. Average precipitation was 1277 mm. Harvested at flower initiation. | [27] | |
| 10.0 *–15.5 ** | Germany | Basic fertilization and nitrogen fertilizers. The values depend on the watering regime. Harvest on different dates, depending on watering: August. | [13] | |
| 13.0 ***–17.2 ⁂ | Germany | Soil of experimental fields was Cambisol with a heavy loam texture overlaid with loess loam. Average precipitation 591 mm (2011) and 727 mm (2012). | [14] | |
| 4.4–8.5 | Lithuania | The values of DM yields differ due to the amount and types of fertilization: by liming and nitrogen treatments. Average precipitation during the vegetation period 437 mm. Harvest at maturity stage on September 16. | [54] | |
| 7.5 | USA | Mean biomass for three planting densities (17,000 34,000 and 680,000 plants ha−1) at Brookings on soil considered marginal for conventional crop production due to poor drainage. No fertilizer, previous planted crop was soybean (Glycine max L. Merr.) with long-term rotation with wheat (Triticum aestivum L.). Planting was executed one year earlier in June by seedlings (2010). Harvesting time: in October. | [20] | |
| 2nd year | 19.4 | Chile | Chahuilco site (see above). | [27] |
| 14.6 | Chile | La Unión site (see above). | [27] | |
| 17.8 | Chile | Nochaco site (Ñadi soils): iron and aluminum hardpan layer between the soil, pH 5.4. Average rainfall 1420 mm, dry period 1–2 months. Harvested at flower initiation. | [27] | |
| 11.7 *–16.8 ** | Germany | The values depend on the watering regime (see from the first year). | [13] | |
| 13.4–15.7 ****; 19.4–21.7 ⁂⁑ | Poland | The experiment was conducted on light rust-brown sandy soil of poor rye complex. Average rainfall during the growing season was 359 mm. | [110] | |
| 11.4–21.9 | Lithuania | Different types and rates of fertilization. Average precipitation during the growing season was 620 mm. Plants planted in 2008, the first harvest was in 2009, the second harvest was on 30 September 2010. | [54] | |
| 1.6 | USA | Brookings site (see above). | [20] | |
| 10.8–14.3 | USA | Soil at Arlington location: Huntsville silt loam (fine–silty, mixed, mesic Cumulic Hapludoll in a low-lying area with a capability class of II because of potential flood damage from water retention. Nitrogen fertilizer (180 kg N ha−1). The previous crop was alfalfa (Medicago sativa L.) Cultivation was in June 2010. Harvesting time: in September 2011. | [20] | |
| 1.8 7.4–10.8 | USA | The experiment was conducted at two different sites on prime and marginal cropland (Brookings and Arlington) with a well-detailed description of soil. Lower value was obtained on marginal cropland at Brookings (2013–second year) due to severe drought during the 2012 growing season. Hand-harvesting in November. | [111] | |
| 3rd year | 19.1–20.6 | Poland | Harvesting was conducted in the second (2017) and third years (2018) of growth. | [110] |
| 8.8–17.6 | Lithuania | The experiment was started in 2013. Soil of the experimental site was naturally acid moraine loam Bathygleyic Dystric Glossic Retisol. Different treatments were tested: not fertilized, fertilized N60P60K60, and fertilized with different amounts of granulated sewage sludge (45 and 90 Mg ha−1). The precipitation was 526 mm and temperatures were close to perennial values. Harvest by a rotary mower at the end of September. | [108] | |
| 15.4 | Czech Republic | This average value of cup-plant is achieved in long-term experiments under the same agro-ecological conditions and conventional agro-techniques. Harvested at the end of the flowering period. | [15] | |
| 11.2 | Czech Republic | Different plants were tested, only under experimental conditions, and only on a very small scale; only several species have been tested under field conditions. | [112] | |
| Mean | 15.5 | Germany | The experimental fields were situated in two different locations with N and P fertilizers applied. Mean for six biomass traits over three years (2014–2016). Plants were harvested each year in August. | [52] |
| 13.2 | Lithuania | Three different concentrations of fertilization were used. Mean for the period between 2009 and 2014. Harvesting time: end of September. | [113] |
| Country | Specific Methane Yield SMY (NL kg−1 VS) | Methane Hectare Yield MHY (Nm3 ha−1) | Observations | Reference |
|---|---|---|---|---|
| Southern Germany | 232 * 275 *** | 4301 a 3318 b | The highest and the lowest yields obtained from four different harvested batches, two doses of N fertilizer: 80 and 100 kg ha−1. | [14] |
| Germany | 236–2450 * 273–282 ** | n.d. | Different excess and non-excess soil moisture were tested. Higher values for excess moisture. | [87] |
| Germany | 290 *–303 ** 310 *–321 ** | 2889 *–3543 ** 4789 *–5399 ** | Rainfed and irrigated, first and second-year harvest, respectively. | [13] |
| Southwest Germany | 260 | 4856 | Average for four years of harvesting (2015–2018). The first year (2014, n.d.) was excluded from the calculation of average. | [117] |
| Republic of Moldova | 275 | 4235 | The local variety Vital was tested for biomethane productivity. | [51] |
| Czech Republic | 276 | 3921 | Study based on long-term experiments and mixed samples with different fertilization rates. | [15] |
| Germany | 258 c–273 d | 3697 d–4634 c | Study of five countries of origin: USA, East Germany, Russia, Northern Europe, Ukraine over three years without fertilizer and the application of 100–150 kg ha−1 N. | [52] |
| Fertilization Rate | Energy Input (GJ ha−1) | Energy Accumulated in Biomass (GJ ha−1) | Energy Gain (GJ ha−1) | Energy Ratio |
|---|---|---|---|---|
| N0 (not limed) | 7.4 | 187.6 | 180.2 | 25.3 |
| N120 (not limed) | 17.2 | 299.4 | 282.2 | 17.4 |
| N0 + 0.5 liming rate | 12.8 | 267.1 | 254.3 | 20.9 |
| N120 + 0.5 liming rate | 22.6 | 290.5 | 267.9 | 12.9 |
| N0 + 1.0 liming rate | 18.2 | 332.6 | 314.4 | 18.3 |
| N120 + 1.0 liming rate | 28.0 | 361.9 | 333.9 | 12.9 |
| Year/Plantation Age | Energy Input (GJ ha−1) | Energy Accumulated in Biomass (GJ ha−1) | Energy Gain (GJ ha−1) | Energy Ratio |
|---|---|---|---|---|
| 2012/2 | 7.6 | 82.3 | 74.7 | 10.8 |
| 2013/3 | 8.2 | 93.2 | 85.0 | 11.4 |
| 2014/4 | 7.6 | 84.0 | 76.4 | 11.0 |
| 2015/5 | 7.9 | 99.3 | 91.5 | 12.7 |
| 2016/6 | 8.2 | 119.8 | 111.7 | 14.6 |
| 2017/7 | 9.1 | 184.4 | 175.3 | 20.3 |
| Mean | 8.1 | 110.5 | 102.4 | 13.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peni, D.; Stolarski, M.J.; Bordiean, A.; Krzyżaniak, M.; Dębowski, M. Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture 2020, 10, 640. https://doi.org/10.3390/agriculture10120640
Peni D, Stolarski MJ, Bordiean A, Krzyżaniak M, Dębowski M. Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture. 2020; 10(12):640. https://doi.org/10.3390/agriculture10120640
Chicago/Turabian StylePeni, Dumitru, Mariusz Jerzy Stolarski, Anna Bordiean, Michał Krzyżaniak, and Marcin Dębowski. 2020. "Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use" Agriculture 10, no. 12: 640. https://doi.org/10.3390/agriculture10120640
APA StylePeni, D., Stolarski, M. J., Bordiean, A., Krzyżaniak, M., & Dębowski, M. (2020). Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture, 10(12), 640. https://doi.org/10.3390/agriculture10120640








