Effects of Production Region, Production Systems and Grape Type/Variety on Nutritional Quality Parameters of Table Grapes; Results from a UK Retail Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retail Survey Strategy
2.2. Grape Sample Storage and Preparation
2.3. Chemical Reagents
2.4. Extraction of Secondary Metabolites from Table Grapes
2.5. Determination of Total Phenolic Content (TPC) and Total Antioxidant Activity (TAA)
2.6. Extraction and Determination of Total Anthocyanin Content (TAC)
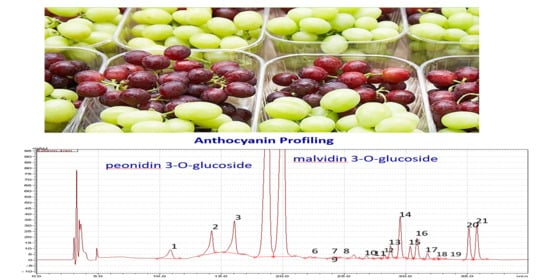
2.7. Identification and Quantification of Individual Anthocyanins by HPLC
2.8. Statistical Analysis
3. Results
3.1. Effects of Production Region and Year on Grape Composition
3.2. Effect of Grape Type (White vs. Red vs. Black) on Grape Composition
3.3. Effect of Production System (Organic vs. Conventional) on Grape Composition
3.4. Effects of Variety Choice on Grape Composition
4. Discussion
4.1. Effect of Year and Production Region on the Nutritional Composition of Table Grapes
4.2. Effect of Grape Type and Variety on the Nutritional Composition of Table Grapes
4.3. Effect of Production System on the Nutritional Composition of Table Grapes
4.4. Potential Nutritional/Health Impacts of Consuming Different Table Grape Products
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faostat, F. Statistical Databases. Food and Agriculture Organization of the United Nations. 2009. Available online: http://www.fao.org/statistics (accessed on 1 November 2020).
- Snoei, G. Overview Global Table Grape Market. Fresh Plaza. 2017. Available online: https://www.freshplaza.com/article/2174675/overview-global-table-grape-market/ (accessed on 1 November 2020).
- Li, S.; Pu, X.P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmez, I.; Ozyurt, H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem. Int. 2012, 60, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Kondrashov, A.; Ševčík, R.; Benáková, H.; Koštířová, M.; Štípek, S. The key role of grape variety for antioxidant capacity of red wines. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, e41–e46. [Google Scholar] [CrossRef] [Green Version]
- Venturini, C.D.; Merlo, S.; Souto, A.A.; Fernandes, M.d.C.; Gomez, R.; Rhoden, C.R. Resveratrol and red wine function as antioxidants in the nervous system without cellular proliferative effects during experimental diabetes. Oxid. Med. Cell. Longev. 2010, 3, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.D.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Vrček, I.V.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. Phenol content, antioxidant activity and metal composition of Croatian wines deriving from organically and conventionally grown grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Adams, D.O. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar]
- Marenghi, M. Biologico, una questione di cultura. Vignevini Riv. Ital. Vitic. Enol. 2002, 29, 51–53. [Google Scholar]
- Granato, D.; Margraf, T.; Brotzakis, I.; Capuano, E.; van Ruth, S.M. Characterization of conventional, biodynamic, and organic purple grape juices by chemical markers, antioxidant capacity, and instrumental taste profile. J. Food Sci. 2015, 80, C55–C65. [Google Scholar] [CrossRef]
- IFOAM EU Group. Organic in Europe, Prospects and Developments 2016; IFOAM EU Group: Bonn, Germany, 2016. [Google Scholar]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [Green Version]
- Rempelos, L.; Almuayrifi, A.M.; Baranski, M.; Tetard-Jones, C.; Eyre, M.; Shotton, P.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N. Effects of agronomic management and climate on leaf phenolic profiles, disease severity, and grain yield in organic and conventional wheat production systems. J. Agric. Food Chem. 2018, 66, 10369–10379. [Google Scholar] [CrossRef] [PubMed]
- Vian, M.A.; Tomao, V.; Coulomb, P.O.; Lacombe, J.M.; Dangles, O. Comparison of the anthocyanin composition during ripening of Syrah grapes grown using organic or conventional agricultural practices. J. Agric. Food Chem. 2006, 54, 5230–5235. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Haas, I.C.; Toaldo, I.M.; de Gois, J.S.; Borges, D.L.; de Oliveira Petkowicz, C.L.; Bordignon-Luiz, M.T. Phytochemicals, monosaccharides and elemental composition of the non-pomace constituent of organic and conventional grape juices (Vitis labrusca L.): Effect of drying on the bioactive content. Plant Foods Hum. Nutr. 2016, 71, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Bunea, C.-I.; Pop, N.; Babeş, A.C.; Matea, C.; Dulf, F.V.; Bunea, A. Carotenoids, total polyphenols and antioxidant activity of grapes (Vitis vinifera) cultivated in organic and conventional systems. Chem. Cent. J. 2012, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, C.; Oliboni, L.; Vanderlinde, R.; Bonatto, D.; Salvador, M.; Henriques, J. Phenolic content and antioxidant activities of white and purple juices manufactured with organically-or conventionally-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Mulero, J.; Pardo, F.; Zafrilla, P. Antioxidant activity and phenolic composition of organic and conventional grapes and wines. J. Food Compos. Anal. 2010, 23, 569–574. [Google Scholar] [CrossRef]
- Toaldo, I.M.; Cruz, F.A.; de Lima Alves, T.; de Gois, J.S.; Borges, D.L.; Cunha, H.P.; da Silva, E.L.; Bordignon-Luiz, M.T. Bioactive potential of Vitis labrusca L. grape juices from the Southern Region of Brazil: Phenolic and elemental composition and effect on lipid peroxidation in healthy subjects. Food Chem. 2015, 173, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Tassoni, A.; Tango, N.; Ferri, M. Comparison of biogenic amine and polyphenol profiles of grape berries and wines obtained following conventional, organic and biodynamic agricultural and oenological practices. Food Chem. 2013, 139, 405–413. [Google Scholar] [CrossRef]
- Tassoni, A.; Tango, N.; Ferri, M. Polyphenol and biogenic amine profiles of Albana and Lambrusco grape berries and wines obtained following different agricultural and oenological practices. Food Nutr. Sci. 2014, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Tarara, J.; Lee, J.; Spayd, S.; Scagel, C. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar]
- González, D.M.; Martín, P. Interaction effects of nitrogen and potassium fertilization on anthocyanin composition and chromatic features of Tempranillo grapes. Int. J. Vine Wine Sci. 2006, 40, 141–150. [Google Scholar]
- Morlat, R.; Symoneaux, R. Long-term additions of organic amendments in a Loire Valley vineyard on a calcareous sandy soil. III. Effects on fruit composition and chemical and sensory characteristics of Cabernet franc wine). Am. J. Enol. Vitic. 2008, 59, 375–386. [Google Scholar]
- Koureh, O.K.; Bakhshi, D.; Pourhayoumi, M.; Majidan, M. Comparison of yield, fruit quality, antioxidant activity, and some phenolic compounds of white seedless grape obtained from organic, conventional, and integrated fertilization. Int. J. Fruit Sci. 2019, 19. [Google Scholar] [CrossRef]
- Peterlunger, E.; Sivilotti, P.; Bonetto, C.; Paladin, M. Water stress induces changes in polyphenol concentration in Merlot grapes and wines. Riv. Vitic. Enol. 2002, 1, 51–66. [Google Scholar]
- Kennedy, J.; Matthews, M.; Waterhouse, A. Effect of maturity and vine water status on grape skin and wine flavonoids. Am. J. Enol. Vitic. 2002, 53, 268–274. [Google Scholar]
- Tardaguila, J.; Petrie, P.; Poni, S.; Diago, M.; Martinez-de-Toda, F. Effects of mechanical thinning on yield and fruit composition of Tempranillo and Grenache grapes trained to a vertical shoot-positioned canopy. Am. J. Enol. Vitic. 2008, 59, 412–417. [Google Scholar]
- Tian, S.-F.; Wang, Y.; Du, G.; Li, Y.-X. Changes in contents and antioxidant activity of phenolic compounds during gibberellin-induced development in Vitis vinifera L. ‘Muscat’. Acta Physiol. Plant. 2011, 33, 2467–2475. [Google Scholar] [CrossRef]
- Košmerl, T.; Bertalanič, L.; Maras, V.; Kodžulović, V.; Šućur, S.; Abramovič, H. Impact of Yield on Total Polyphenols, Anthocyanins, Reducing Sugars and Antioxidant Potential in White and Red Wines Produced from Montenegrin Autochthonous Grape Varieties. Food Sci. Technol. 2013, 1, 7–15. [Google Scholar] [CrossRef]
- Garrido, I.; Uriarte, D.; Hernández, M.; Llerena, J.L.; Valdés, M.E.; Espinosa, F. The Evolution of Total Phenolic Compounds and Antioxidant Activities during Ripening of Grapes (Vitis vinifera L., cv. Tempranillo) Grown in Semiarid Region: Effects of Cluster Thinning and Water Deficit. Int. J. Mol. Sci. 2016, 17, 1923. [Google Scholar] [CrossRef] [Green Version]
- Cuniff, P. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2012. [Google Scholar]
- Jayasena, V.; Cameron, I. Brix/Acid ratio as a predictor of consumer acceptability of crimson seedless Table grapes. J. Food Qual. 2008, 31, 736–750. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chiou, A.; Panagopoulou, E.A.; Gatzali, F.; De Marchi, S.; Karathanos, V.T. Anthocyanins content and antioxidant capacity of Corinthian currants (Vitis vinifera L., var. Apyrena). Food Chem. 2014, 146, 157–165. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org/ (accessed on 1 November 2020).
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2006. [Google Scholar]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; Chapman and Hall/CRC: London, UK, 2011. [Google Scholar]
- Zoffoli, J.P.; Latorre, B.A. Table grape (Vitis vinifera L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits—Cocona to Mango; Yahia, E.M., Ed.; Woodhouse Publishing: Cambridge, UK, 2011; pp. 179–212. [Google Scholar] [CrossRef]
- Greasy, G.L.; Greasy, L.L. Grapes, 2nd ed.; CABI Publishing: Wallingford, UK, 2018. [Google Scholar]
- Valero, D.; Serrano, M. Growth and ripening stage at harvest modulates postharvest quality and bioactive compounds with antioxidant activity. Stewart Postharvest Rev. 2013, 3. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Vojnoski, B.; Dörnyei, Á.; Márk, L.; Dimovska, V.; Stafilov, T.; Kilár, F. Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Res. Int. 2011, 44, 2851–2860. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Robledo, P.; Manríquez Molina, R.; Defilippi, B.G. Characterization of sugars and organic acids in commercial varieties of table grapes. Chil. J. Agric. Res. 2011, 71, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Padilha, C.V.; dos Santos Lima, M.; Toaldo, I.M.; Pereira, G.E.; Bordignon-Luiz, M.T. Effects of successive harvesting in the same year on quality and bioactive compounds of grapes and juices in semi-arid tropical viticulture. Food Chem. 2019, 301125170. [Google Scholar] [CrossRef]
- Artés-Hdez, F.; Artés, F.; Allende, A. Sugar composition in ‘autumn seedless’ table grapes during long term cold storage. Acta Hortic. 2003, 628, 363–366. [Google Scholar] [CrossRef]
- Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J.O.; Dommes, J.; Pincemail, J. Evolution of antioxidant capacity during storage of selected fruits and vegetables. J. Agric. Food Chem. 2007, 55, 8596–8603. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest treatments of fresh produce. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef] [Green Version]
- Eshghi, S.; Salehi, L.; Karami, M.J. Antioxidant activity, total phenolic compounds and anthocyanin contents in 35 different grapevine (Vitis vinifera L.) cultivars grown in Fars Province. Int. J. Hortic. Sci. Technol. 2014, 1, 151–161. [Google Scholar] [CrossRef]
- Derradji-Benmeziane, F.; Djamai, R.; Cadot, Y. Antioxidant capacity, total phenolic, carotenoid, and vitamin C contents of five table grape varieties from Algeria and their correlations. OENO One 2014, 48, 153–162. [Google Scholar] [CrossRef]
- Niu, S.; Hao, F.; Mo, H.; Jiang, J.; Wang, H.; Liu, C.; Fan, X.; Zhang, Y. Phenol profiles and antioxidant properties of white skinned grapes and their coloured genotypes during growth. Biotechnol. Biotechnol. Equip. 2017, 31, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Dafermos, N.G.; Kasselaki, A.M.; Goumas, D.E.; Eyre, M.D.; Spandidakis, K.; Leifert, C. Integration of Elicitors and Less Susceptible Hybrids for the Control of Powdery Mildew in Organic Tomato Crops. Plant Dis. 2012, 96, 1506–1512. [Google Scholar] [CrossRef] [Green Version]
- Rempelos, L.; Almuayrifi, M.S.B.; Baranski, M.; Tetard-Jones, C.; Barkla, B.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; Hall, G.; et al. The effect of agronomic factors on crop health and performance of winter wheat varieties bred for the conventional and the low input farming sector. Field Crops Res. 2020, 254, 107822. Available online: https://authors.elsevier.com/sd/article/S0378429019315990 (accessed on 1 November 2020). [CrossRef]
- Wang, J.; Chatzidimitriou, E.; Wood, L.; Hasanalieva, G.; Markellou, E.; Iversen, P.O.; Seal, C.; Baranski, M.; Vigar, V.; Ernst, L.; et al. Effect of wheat species (Triticum aestivum vs. T. spelta), farming system (organic vs conventional) and flour type (wholegrain vs white) on composition of wheat flour—Results of a retail survey in the UK and Germany—2. Antioxidant activity, and phenolic and mineral content. Food Chem. 2020, 6, 100091. [Google Scholar] [CrossRef]
- Hasanalieva, G. Comparison of Antioxidant Activity and Concentrations in Organically and Conventionally Produced Grapes, Grape Juice and Wine: Results from Meta-Analyses, Farm and Retail Surveys; Newcastle University: Newcastle upon Tyne, UK, 2018. [Google Scholar]
- Yang, J.; Xiao, Y.-Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013, 53, 1202–1225. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: An update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Corrales, M.; Fernandez, A.; Pinto, M.G.V.; Butz, P.; Franz, C.M.; Schuele, E.; Tauscher, B. Characterization of phenolic content, in vitro biological activity, and pesticide loads of extracts from white grape skins from organic and conventional cultivars. Food Chem. Toxicol. 2010, 48, 3471–3476. [Google Scholar] [CrossRef]
| Season Grape Type | Varieties Available (No. of Samples) | |
|---|---|---|
| Organic | Conventional | |
| Winter 2015 | ||
| White | Early Sweet (n = 2) | Early Sweet (n = 4) |
| Prime (n = 7) | Prime (n = 8) | |
| Sugraone (n = 11) | Sugraone (n = 11) | |
| Sweet Sunshine (n = 2) | Sweet Sunshine (n = 1) | |
| Thompson (n = 7) | Thompson (n = 21) | |
| Muscat (n = 1) | ||
| Regal (n = 12) | ||
| Sundance (n = 4) | ||
| Red | Allison (n = 6) | Allison (n = 2) |
| Crimson (n = 6) | Crimson (n = 19) | |
| Flame (n = 4) | Flame (n = 6) | |
| Sweet Celebration (n = 12) | Sweet Celebration (n = 2) | |
| Jack’s Sallute (n = 8) | Ralli (n = 4) | |
| Sunred (n = 2) | Scarlotta (n = 4) | |
| Timco (n = 1) | ||
| Black | Autumn Royal (n = 3) | Autumn Royal (n = 5) |
| IFG16 (n = 5) | Desert (n = 1) | |
| IFG17 (n = 1) | ||
| Sweet Surrender (n = 3) | ||
| Summer 2015 | ||
| White | Sugraone (n = 14) | Sugraone (n = 13)) |
| Superior (n = 3) | Superior (n = 1) | |
| Timpson (n = 3) | Timpson (n = 4) | |
| Regal (n = 13) | Mellisa (n = 1) | |
| Princess (n = 1) | ||
| Thompson (n = 13) | ||
| Red | Allison (n = 2) | Allison (n = 2) |
| Crimson (n = 13) | Crimson (n = 20) | |
| Flame (n = 5) | Flame (n = 5) | |
| Scarlotta (n = 10) | Scarlotta (n = 3) | |
| Black | Autumn Royal (n = 4) | Autumn Royal (n = 4) |
| Midnight Beauty (5) | Midnight Beauty (n = 7) | |
| Summer Royal (n = 4) | Summer Royal (n = 1) | |
| Melody (n = 1) | ||
| Winter 2016 | ||
| White | Thompson (n = 9) | Thompson (n = 10) |
| Early Sweet (n = 1) | Sugraone (n = 3) | |
| Regal (n = 1) | ||
| Sundance (n = 5) | ||
| Sweet Globe (n = 3) | ||
| Sweet Sunshine (n = 1) | ||
| Red | Flame (n = 1) | Flame (n = 1) |
| Jack’s Sallute (n = 2) | Crimson (n = 8) | |
| Sweet Celebration (n = 8) | Ralli (n = 1) | |
| Black | Autumn Royal (n = 1) | |
| Summer 2016 | ||
| White | Sugraone (n = 11) | Sugraone (n = 9) |
| Superior (n = 2) | Superior (n = 2) | |
| Thompson (n = 1) | Thompson (n = 5) | |
| Timpson (n = 4) | Timpson (n = 2) | |
| Sophia (n = 3) | Cotton Candy (n = 1) | |
| Sublima (n = 3) | Early Sweet (n = 1) | |
| ITUM (n = 2) | ||
| Red | Allison (n = 3) | Allison (n = 2) |
| Scarlotta (n = 1) | ||
| Crimson (n = 4) | Crimson (n = 5) | |
| Sweet Celebration (n = 2) | Sweet Celebration (n = 1) | |
| Black | Midnight Beauty (n = 6) | Midnight Beauty (n = 6) |
| Summer Royal (n = 6) | Summer Royal (n = 1) | |
| Vitroblack (n = 3) | Autumn Royal (n = 5) | |
| Melody (n = 2) | ||
| Factors | Dry Matter Content % | Sugar Content (SC) | Total Phenolic Content (TPC) mg GAE kg−1 | Antioxidant Activity (TAA) | Total Anthocyanin Content (TAC) | |||
|---|---|---|---|---|---|---|---|---|
| (Pulp) Brix° | (Juice) Brix° | DPPH µmol TE g−1 | TEAC µmol TE g−1 | mg cyan kg−1 | mg mal kg−1 | |||
| Year (Yr) | ||||||||
| 2015 (n = 292) | 19.5 ± 0.1 | 17.6 ± 0.1 | 17.8 ± 0.1 | 1583 ± 36 | 78 ± 2 | 7.7 ± 0.3 | 48 ± 4 | 51 ± 4 |
| 2016 (n = 118) | 19.7 ± 0.3 | 17.7 ± 0.2 | 17.9 ± 0.2 | 1551 ± 44 | 77 ± 3 | 5.1 ± 0.4 | 45 ± 6 | 48 ± 7 |
| Production system (PS) | ||||||||
| ORG (n = 210) | 19.8 ± 0.2 | 17.7 ± 0.1 | 17.9 ± 0.2 | 1689 ± 42 | 78 ± 2 | 7.7 ± 0.4 | 39 ± 3 | 41 ± 3 |
| CON (n = 200) | 19.3 ± 0.2 | 17.6 ± 0.1 | 17.8 ± 0.1 | 1452 ± 37 | 77 ± 2 | 6.2 ± 0.3 | 56 ± 6 | 59 ± 6 |
| Production region (PR) | ||||||||
| RSA (n = 220) | 19.8 ± 0.2 | 17.9 ± 0.1 | 18.0 ± 0.1 | 1481 ± 32 | 72 ± 2 | 6.3 ± 0.3 | 48 ± 4 | 51 ± 5 |
| MED. (n = 190) | 19.3 ± 0.2 | 17.3 ± 0.2 | 17.7 ± 0.2 | 1681 ± 49 | 85 ± 3 | 7.7 ± 0.4 | 46 ± 5 | 49 ± 5 |
| Grape type (GT) | ||||||||
| red (n = 174) | 20.6 ± 0.2 | 18.3 ± 0.1 | 18.5 ± 0.1 | 1827 ± 42 | 104 ± 2 | 7.1 ± 0.4 | 98 ± 6 | 103 ± 6 |
| white (n = 236) | 18.8 ± 0.2 | 17.2 ± 0.1 | 17.3 ± 0.1 | 1386 ± 34 | 59 ± 1 | 6.9 ± 0.4 | 10 ± 1 | 11 ± 1 |
| ANOVA p-values | ||||||||
| Main effects | ||||||||
| Yr | NS | NS | NS | NS | NS | <0.0001 | NS | NS |
| PS | 0.0179 | NS | NS | <0.0001 | NS | 0.0008 | 0.0001 | 0.0001 |
| PR | 0.0270 | 0.0014 | NS | 0.0002 | <0.0001 | 0.0002 | NS | NS |
| GT | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | NS | <0.0001 | <0.0001 |
| Interactions * | ||||||||
| Yr:PS | NS | NS | 0.0797 | 0.0005 | NS | 0.0363 | NS | NS |
| Yr:PR | NS | NS | NS | 0.0327 | NS | <0.0001 | 0.0244 | 0.0237 |
| Yr:GT | 0.0453 | NS | NS | 0.0306 | <0.0001 | NS | NS | NS |
| PS:PR | NS | 0.0233 | 0.0305 | NS | NS | NS | NS | NS |
| GT:PS | NS | NS | NS | NS | NS | NS | <0.0001 3 | <0.0001 3 |
| GT:PR | NS | NS | NS | 0.0004 1 | <0.0001 1 | NS | NS | NS |
| Yr:PR:GT | 0.0858 | NS | 0.0112 2 | NS | 0.0155 2 | <0.0001 2 | 0.0003 | 0.0004 |
| PS:PR:GT | NS | 0.0300 4 | 0.0204 4 | NS | NS | 0.0036 4 | 0.0438 | 0.0434 |
| Yr:PS:PR:GT | NS | NS | NS | NS | NS | NS | 0.0187 5 | 0.0188 5 |
| Parameter | Factor 1 | Factor 2 | |
|---|---|---|---|
| Production Region | |||
| Grape Type | South Africa | Mediterranean | |
| Total phenolic content (mg GAE kg−1) | White | 1365 ± 44 a B | 1409 ± 52 a B |
| Red | 1629 ± 41 b A | 2070 ± 72 a A | |
| Antioxidant activity (DPPH, µmol TE g−1) | White | 55 ± 1 a B | 62 ± 2 a B |
| Red | 93 ± 2 b A | 117 ± 4 a A | |
| Parameter | Factor 3 | |||
|---|---|---|---|---|
| Factor 1 | Factor 2 | Grape Type | ||
| Year | Production Region | White | Red | |
| Sugar content (juice) Brix° | 2015 | South Africa | 17.2 ± 0.2 b A | 18.8 ± 0.2 a A |
| Mediterranean | 17.5 ± 0.3 a A | 17.9 ± 0.2 a B | ||
| 2016 | South Africa | 17.6 ± 0.3 b A | 18.7 ± 0.4 a AB | |
| Mediterranean | 17.1 ± 0.4 b A | 19.3 ± 0.4 a A | ||
| Antioxidant activity (DPPH) (µmol TE g−1) | 2015 | South Africa | 56.7 ± 0.8 b B | 91.4 ± 2.6 a B |
| Mediterranean | 63.5 ± 2.2 b A | 110.0 ± 4.3 a A | ||
| 2016 | South Africa | 51.6 ± 2.2 b B | 98.7 ± 2.3 a B | |
| Mediterranean | 60.1 ± 3.5 b A B | 140.0 ± 1.2 a A | ||
| Antioxidant activity (TEAC) (µmol TE g−1) | 2015 | South Africa | 5.9 ± 0.4 a B | 6.4 ± 0.5 a B |
| Mediterranean | 10.1 ± 0.8 a A | 9.4 ± 0.7 a A | ||
| 2016 | South Africa | 8.8 ± 1.3 a A | 3.8 ± 0.3 b C | |
| Mediterranean | 2.8 ± 0.2 b C | 5.8 ± 0.3 a B C | ||
| Factors | Dry Matter Content % | Sugar Content (SC) | Total Phenolic Content | Antioxidant Activity (TAA) | Total Anthocyanin Content (TAC) | |||
|---|---|---|---|---|---|---|---|---|
| (Pulp) Brix° | (Juice) Brix° | (TPC) mg GAE kg−1 | DPPH µmol TE g−1 | TEAC µmol TE g−1 | mg cyan kg−1 | mg mal kg−1 | ||
| Year (Yr) | ||||||||
| 2015 (n = 152) | 19.1 ± 0.2 | 17.1 ± 0.2 | 17.6 ± 0.2 | 1858 ± 63 | 93 ± 3 | 11.3 ± 0.6 | 116 ± 17 | 123 ± 18 |
| 2016 (n = 93) | 19.2 ± 0.3 | 17.4 ± 0.3 | 17.7 ± 0.3 | 1735 ± 60 | 98 ± 4 | 4.3 ± 0.2 | 234 ± 33 | 247 ± 35 |
| Production system (PS) | ||||||||
| ORG (n = 124) | 19.4 ± 0.3 | 17.4 ± 0.2 | 17.9 ± 0.2 | 1891 ± 67 | 96 ± 4 | 9.5 ± 0.7 | 158 ± 25 | 167 ± 27 |
| CON (n = 121) | 18.8 ± 0.2 | 17.0 ± 0.2 | 17.3 ± 0.2 | 1730 ± 62 | 94 ± 4 | 7.8 ± 0.5 | 164 ± 22 | 173 ± 23 |
| Grape Type (GT) | ||||||||
| black (n = 55) | 18.5 ± 0.3 b | 16.8 ± 0.3 b | 17.3 ± 0.3 b | 2262 ± 90 a | 130 ± 3 a | 11.9 ± 1.2 a | 557 ± 41 a | 588 ± 43 a |
| red (n = 78) | 20.2 ± 0.2 a | 17.9 ± 0.2 a | 18.2 ± 0.2 a | 2070 ± 72 a | 117 ± 4 b | 8.6 ± 0.6 b | 95 ± 10 b | 100 ± 11 b |
| white (n = 112) | 18.7 ± 0.3 b | 17.0 ± 0.2 b | 17.3 ± 0.2 b | 1410 ± 52 b | 62 ± 2 c | 7.1 ± 0.6 b | 13 ± 1 c | 13 ± 1 c |
| ANOVA | ||||||||
| p-values | ||||||||
| Main effects | ||||||||
| Yr | NS | NS | NS | NS | NS | <0.0001 | <0.0001 | <0.0001 |
| PS | 0.0875 | 0.0837 | 0.0253 | 0.0294 | NS | 0.007 | NS | NS |
| GT | 0.0001 | 0.0014 | 0.0047 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Interactions * | ||||||||
| Yr:PS | NS | NS | NS | 0.0005 | NS | 0.0109 | NS | NS |
| Yr:GT | 0.0456 1 | NS | NS | NS | 0.0003 1 | <0.0001 1 | 0.0013 1 | 0.0013 1 |
| PS:GT | NS | NS | NS | NS | NS | 0.0247 2 | NS | NS |
| Parameter | Factor 1 | Factor 2 | |
|---|---|---|---|
| Year | |||
| Grape Type | 2015 | 2016 | |
| Dry matter content (%) | White | 18.7 ± 0.3 a A | 18.6 ± 0.5 a B |
| Red | 19.8 ± 0.2 b A | 21.6 ± 0.6 a A | |
| Black | 18.6 ± 0.5 a A | 18.4 ± 0.5 a B | |
| Antioxidant activity (DPPH, µmol TE g−1) | White | 127 ± 6.5 a A | 60 ± 3.5 a B |
| Red | 110 ± 4.3 b B | 140 ± 1.2 a A | |
| Black | 63 ± 2.2 a C | 132 ± 2.5 a A | |
| Antioxidant activity (TEAC, µmol TE g−1) | White | 10.1 ± 0.8 a B | 2.8 ± 0.2 b B |
| Red | 9.4 ± 0.7 a B | 5.8 ± 0.3 b A | |
| Black | 18.6 ± 1.7 a A | 5.9 ± 0.5 b A | |
| Anthocyanin content (mg cyan kg−1) | White | 12 ± 1 a C | 15 ± 1 a C |
| Red | 81 ± 10 a B | 139 ± 27 a B | |
| Black | 462 ± 61 b A | 642 ± 50 a A | |
| Anthocyanin content (mg mal kg−1) | White | 12 ± 1 a C | 16 ± 1 a C |
| Red | 86 ± 10 a B | 147 ± 28 a B | |
| Black | 487 ± 65 b A | 677 ± 52 a A | |
| Factor | Concentrations of Individual Anthocyanins (mg FW kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Delphindin 3-O-Glucoside | Cyanindin 3-O-Glucoside | Petunidin 3-O-Glucoside | Peonidin 3-O-Glucoside | Malvidin 3-O-Glucoside | Peonidin 3-O-p-Coumaroyl Glucoside | Malvidin 3-O-p-Coumaroyl Glucoside | |
| Year (Yr) | |||||||
| 2015 (n = 37) | 29 ± 7 | 23 ± 5 | 51 ± 12 | 241 ± 39 | 567 ± 113 | 31 ± 6 | 264 ± 65 |
| 2016 (n = 15) | 40 ± 22 | 24 ± 10 | 75 ± 37 | 273 ± 73 | 759 ± 321 | 37 ± 14 | 272 ± 126 |
| Production system (PS) | |||||||
| ORG (n = 26) | 37 ± 11 | 21 ± 6 | 65 ± 18 | 207 ± 36 | 673 ± 183 | 34 ± 8 | 270 ± 82 |
| CON (n = 26) | 28 ± 13 | 25 ± 7 | 52 ± 20 | 294 ± 59 | 572 ± 162 | 32 ± 9 | 262 ± 84 |
| Grape type (GT) | |||||||
| black (n = 22) | 67 ± 17 | 21 ± 8 | 128 ± 25 | 279 ± 64 | 1415 ± 181 | 67 ± 10 | 627 ± 93 |
| red (n = 30) | 7 ± 3 | 25 ± 5 | 7 ± 2 | 229 ± 39 | 41 ± 7 | 8 ± 2 | 2 ± 0.4 |
| ANOVA p-values | |||||||
| Main effects | |||||||
| Yr | NS | NS | NS | NS | NS | NS | NS |
| PS | NS | NS | NS | NS | NS | NS | NS |
| GT | <0.0001 | NS | <0.0001 | NS | <0.0001 | <0.0001 | <0.0001 |
| Interactions * | |||||||
| Yr:PS | 0.0360 | NS | 0.0846 | NS | NS | NS | NS |
| Yr:GT | 0.0088 | NS | 0.0030 | NS | 0.0010 | NS | NS |
| Yr:PS:GT | 0.01101 | NS | 0.0238 1 | 0.0142 1 | NS | NS | NS |
| Factor 1 | Factor 2 | ||
|---|---|---|---|
| Production System | |||
| Parameter | Grape Type | Organic | Conventional |
| TAC (mg cyan kg−1) | White | 11 ± 1 a B | 8 ± 1 a B |
| Red | 77 ± 5 b A | 118 ± 20 a A | |
| TAC (mg mal kg−1) | White | 12 ± 1 a B | 9 ± 1 a B |
| Red | 82 ± 5 b A | 124 ± 10 a A | |
| Parameter | Factor 3 | |||
|---|---|---|---|---|
| Factor 1 | Factor 2 | Grape Type | ||
| Production Region | Production System | White | Red | |
| Sugar content (pulp) Brix° | South Africa | Organic | 17.0 ± 0.2 b A B | 19.0 ± 0.2 a A |
| Conventional | 17.7 ± 0.2 b A | 18.7 ± 0.2 a A | ||
| Mediterranean | Organic | 17.3 ± 0.4 a A | 18.0 ± 0.3 a B | |
| Conventional | 16.6 ± 0.3 b B | 17.8 ± 0.3 a B | ||
| Sugar content (juice) Brix° | South Africa | Organic | 17.1 ± 0.2 b AB | 18.9 ± 0.2 a A |
| Conventional | 17.6 ± 0.2 b AB | 18.6 ± 0.2 a A | ||
| Mediterranean | Organic | 17.8 ± 0.4 a A | 18.3 ± 0.3 a A | |
| Conventional | 16.9 ± 0.3 b B | 18.2 ± 0.3 a A | ||
| Antioxidant activity (TEAC) (µmol TE g−1) | South Africa | Organic | 7.0 ± 0.7 a AB | 6.8 ± 0.7 a AB |
| Conventional | 6.2 ± 0.6 a B | 4.8 ± 0.5 a B | ||
| Mediterranean | Organic | 8.6 ± 1.0 a A | 8.5 ± 0.8 a A | |
| Conventional | 5.6 ± 0.5 b B | 8.7 ± 0.9 a A | ||
| Parameter | Factor 4 | ||||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Grape Type | ||
| Year | Production Region | Production System | White | Red | |
| TAC mg cyan kg−1 | 2015 | South Africa | Organic | 6 ± 2 b A | 89 ± 8 a C |
| Conventional | 5 ± 2 b A | 119 ±12 a B | |||
| Mediterranean | Organic | 13 ± 1 b A | 62 ± 7 a D | ||
| Conventional | 9 ± 2 b A | 101 ± 17 a C | |||
| 2016 | South Africa | Organic | 14 ± 3 b A | 78 ± 15 a C D | |
| Conventional | 10 ± 2 b A | 98 ± 18 a C | |||
| Mediterranean | Organic | 16 ± 2 b A | 78 ± 7 a C D | ||
| Conventional | 13 ± 3 b A | 201 ± 45 a A | |||
| TAC mg mal kg−1 | 2015 | South Africa | Organic | 7 ± 2 b A | 94 ± 9 a C |
| Conventional | 6 ± 2 b A | 126 ± 13 a B | |||
| Mediterranean | Organic | 14 ± 1 b A | 66 ± 8 a D | ||
| Conventional | 10 ± 2 b A | 106 ± 18 a C | |||
| 2016 | South Africa | Organic | 15 ± 3 b A | 82 ± 16 a C D | |
| Conventional | 10 ± 2 b A | 97 ± 19 a C | |||
| Mediterranean | Organic | 17 ± 2 b A | 82 ± 7 a C D | ||
| Conventional | 14 ± 3 b A | 213 ± 47 a A | |||
| Parameter | Factor 1 | Factor 2 | |
|---|---|---|---|
| Production System | |||
| Grape Type | Organic | Conventional | |
| Antioxidant activity (TEAC, µmol TE g−1) | White | 8.6 ± 1.0 a B | 5.6 ± 0.5 b B |
| Red | 8.5 ± 0.8 a B | 8.7 ± 0.9 a A | |
| Black | 12.6 ± 2.0 a A | 11.1 ± 1.3 a A | |
| Parameter | Factor 3 | |||
|---|---|---|---|---|
| Factor 1 | Factor 2 | Grape Type | ||
| Year | Production System | Black | Red | |
| delphinidin 3-O-glucoside (mg FW kg−1) | 2015 | Organic | 74 ± 27 a B | 15.7 ± 7.0 b A |
| Conventional | 32 ± 8 a B | 5.3 ± 2.0 b A | ||
| 2016 | Organic | 69 ± 25 a B | 0.2 ± 0.1 b A | |
| Conventional | 193 ± 136 a A | 0.8 ± 0.6 b A | ||
| petunidin 3-O-glucoside (mg FW kg−1) | 2015 | Organic | 132 ± 392 a B C | 12.6 ± 5.6 b A |
| Conventional | 72 ± 15 a C | 6.2 ± 1.7 b A | ||
| 2016 | Organic | 163 ± 50 a B | 0.5 ± 0.2 b A | |
| Conventional | 314 ± 198 a A | 1.6 ± 0.9 b A | ||
| peonidin 3-O-glucoside (mg FW kg−1) | 2015 | Organic | 325 ± 105 a BA | 122 ± 20 a A |
| Conventional | 173 ± 50 a B | 355 ± 98 a B | ||
| 2016 | Organic | 217 ± 29 a B | 184 ± 24 a A B | |
| Conventional | 670 ± 570 a A | 238 ± 69 b A B | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanaliyeva, G.; Chatzidimitrou, E.; Wang, J.; Baranski, M.; Volakakis, N.; Seal, C.; Rosa, E.A.S.; Iversen, P.O.; Vigar, V.; Barkla, B.; et al. Effects of Production Region, Production Systems and Grape Type/Variety on Nutritional Quality Parameters of Table Grapes; Results from a UK Retail Survey. Foods 2020, 9, 1874. https://doi.org/10.3390/foods9121874
Hasanaliyeva G, Chatzidimitrou E, Wang J, Baranski M, Volakakis N, Seal C, Rosa EAS, Iversen PO, Vigar V, Barkla B, et al. Effects of Production Region, Production Systems and Grape Type/Variety on Nutritional Quality Parameters of Table Grapes; Results from a UK Retail Survey. Foods. 2020; 9(12):1874. https://doi.org/10.3390/foods9121874
Chicago/Turabian StyleHasanaliyeva, Gultakin, Eleni Chatzidimitrou, Juan Wang, Marcin Baranski, Nikolaos Volakakis, Chris Seal, Eduardo A. S. Rosa, Per Ole Iversen, Vanessa Vigar, Bronwyn Barkla, and et al. 2020. "Effects of Production Region, Production Systems and Grape Type/Variety on Nutritional Quality Parameters of Table Grapes; Results from a UK Retail Survey" Foods 9, no. 12: 1874. https://doi.org/10.3390/foods9121874
APA StyleHasanaliyeva, G., Chatzidimitrou, E., Wang, J., Baranski, M., Volakakis, N., Seal, C., Rosa, E. A. S., Iversen, P. O., Vigar, V., Barkla, B., Leifert, C., & Rempelos, L. (2020). Effects of Production Region, Production Systems and Grape Type/Variety on Nutritional Quality Parameters of Table Grapes; Results from a UK Retail Survey. Foods, 9(12), 1874. https://doi.org/10.3390/foods9121874