Intermittent Fasting, Dietary Modifications, and Exercise for the Control of Gestational Diabetes and Maternal Mood Dysregulation: A Review and a Case Report
Abstract
:1. Introduction
2. Pathophysiology of GDM
3. Mood Dysregulations in GDM
4. Dietary Interventions for Gestational Diabetes
5. Case Report
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| AOR | Adjusted odds ratio |
| BDNF | Brain-derived neurotrophic factor |
| BMI | Body mass index |
| CREB | Cyclic adenosine mono phosphate-response element-binding protein |
| C-section | Cesarean surgery |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DAPK3 | Death-associated protein kinase 3 |
| DM | Diabetes mellitus |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FMs | Fetal membranes |
| FTO | Fat mass and obesity associated gene |
| GDM | Gestational diabetes mellitus |
| GH-V | Placental variant growth hormone |
| GI | Glycemic index |
| HMGB1 | High mobility group box 1 |
| HMG20A | High mobility group protein 20A |
| IF | Intermittent fasting |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| MC4R | Melanocortin 4 receptor |
| MDs | Mood dysregulations |
| ncRNAs | Non-coding RNAs |
| NF-κB | Nuclear factor-kappa B |
| NLRP3 | Nod-like receptor family pyrin domain containing 3 |
| OGTT | Oral glucose tolerance test |
| OSA | Obstructive sleep apnea |
| Pax | Paired domain homeobox |
| PL | Placental lactogen |
| PUFA | Polyunsaturated fatty acids |
| RAGE | Receptor of advanced glycation end products |
| RCT | Randomized control trial |
| REM | Rapid eye movement |
| SHBG | Sex hormone-binding globulin |
| SNRIs | Serotonin-norepinephrine reuptake inhibitors |
| sRAGE | Soluble RAGE |
| SSRIs | Serotonin reuptake inhibitors |
| TGF-β | Tumor growth factor β |
| TLR4 | Toll-like receptor 4 |
References
- Lorenzo, P.I.; Martín-Montalvo, A.; Cobo Vuilleumier, N.; Gauthier, B.R. Molecular Modelling of Islet β-Cell Adaptation to Inflammation in Pregnancy and Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 6171. [Google Scholar] [CrossRef] [Green Version]
- Allman, B.R.; Andres, A.; Børsheim, E. The Association of Maternal Protein Intake during Pregnancy in Humans with Maternal and Offspring Insulin Sensitivity Measures. Curr. Dev. Nutr. 2019, 3, nzz055. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.E.; Curry, E.; Laabs, S.B.; Manhas, M.; Angstman, K. Impact of gestational diabetes diagnosis on concurrent depression in pregnancy. J. Psychosom. Obstet. Gynaecol. 2020, 1–4. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Brand-Miller, J.C. Nutrition Therapy in Gestational Diabetes Mellitus: Time to Move Forward. Diabetes Care 2018, 41, 1343–1345. [Google Scholar] [CrossRef] [Green Version]
- Santangelo, C.; Filardi, T.; Perrone, G.; Mariani, M.; Mari, E.; Scazzocchio, B.; Masella, R.; Brunelli, R.; Lenzi, A.; Zicari, A.; et al. Cross-talk between fetal membranes and visceral adipose tissue involves HMGB1-RAGE and VIP-VPAC2 pathways in human gestational diabetes mellitus. Acta Diabetol. 2019, 56, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filardi, T.; Panimolle, F.; Crescioli, C.; Lenzi, A.; Morano, S. Gestational Diabetes Mellitus: The Impact of Carbohydrate Quality in Diet. Nutrients 2019, 11, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalmbach, D.A.; Cheng, P.; Sangha, R.; O’Brien, L.M.; Swanson, L.M.; Palagini, L.; Bazan, L.F.; Roth, T.; Drake, C.L. Insomnia, Short Sleep, And Snoring in Mid-To-Late Pregnancy: Disparities Related To Poverty, Race, And Obesity. Nat. Sci. Sleep 2019, 11, 301–315. [Google Scholar] [CrossRef] [Green Version]
- Mendez, D.D.; Thorpe, R.J.; Amutah, N.; Davis, E.M.; Walker, R.E.; Chapple-McGruder, T.; Bodnar, L. Neighborhood racial composition and poverty in association with pre-pregnancy weight and gestational weight gain. SSM-Popul. Health 2016, 2, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Abdel Wahed, W.Y.; Hassan, S.K.; Eldessouki, R. Malnutrition and Its Associated Factors among Rural School Children in Fayoum Governorate, Egypt. J. Environ. Public Health 2017, 2017, 4783791. [Google Scholar] [CrossRef] [Green Version]
- Amer, H.M.; Abd El Baky, R.S.; Nasr, M.S.; Hendawy, L.M.; Ibrahim, W.A.; Taha, M.O. Anti-islet Cell Antibodies in a Sample of Egyptian Females with Gestational Diabetes and its Relation to Development of Type 1 Diabetes Mellitus. Curr. Diabetes Rev. 2018, 14, 389–394. [Google Scholar] [CrossRef]
- Saif Elnasr, I.; Ammar, H. Ultrasound markers for prediction of gestational diabetes mellitus in early pregnancy in Egyptian women: Observational study. J. Matern. Fetal Neonatal Med. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- El Sagheer, G.; Hamdi, L. Prevalence and risk factors for gestational diabetes mellitus according to the Diabetes in Pregnancy Study Group India in comparison to International Association of the Diabetes and Pregnancy Study Groups in El-Minya, Egypt. Egypt. J. Intern. Med. 2018, 30, 131–139. [Google Scholar] [CrossRef]
- Salem, M.L.; Zeid, W.A.; Ismail, M.A. Prevalence and Predictors of Gestational Diabetes Mellitus among Pregnant Women Attending Fanara Family Center, in Egypt. Suez Canal Univ. Med. J. 2019, 22, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Khalil, N.A.; Fathy, W.M.; Mahmoud, N.S. Screening for Gestational Diabetes Among Pregnant Women Attending a Rural Family Health Center- Menoufia Governorate- Egypt. J. Fam. Med. Health Care 2017, 3, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Muche, A.A.; Olayemi, O.O.; Gete, Y.K. Prevalence and determinants of gestational diabetes mellitus in Africa based on the updated international diagnostic criteria: A systematic review and meta-analysis. Arch. Public Health 2019, 77, 36. [Google Scholar] [CrossRef] [Green Version]
- Filardi, T.; Catanzaro, G.; Mardente, S.; Zicari, A.; Santangelo, C.; Lenzi, A.; Morano, S.; Ferretti, E. Non-Coding RNA: Role in Gestational Diabetes Pathophysiology and Complications. Int. J. Mol. Sci. 2020, 21, 4020. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Jayabalan, N.; Haghvirdizadeh, P.; Salomon, C.; Lappas, M. Role of adipose tissue in regulating fetal growth in gestational diabetes mellitus. Placenta 2020, 102, 39–48. [Google Scholar] [CrossRef]
- Filardi, T.; Tavaglione, F.; Di Stasio, M.; Fazio, V.; Lenzi, A.; Morano, S. Impact of risk factors for gestational diabetes (GDM) on pregnancy outcomes in women with GDM. J. Endocrinol. Investig. 2018, 41, 671–676. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Mlodzik, M.A.; Radziejewska, A.; Szwengiel, A.; Malinowska, A.M.; Nowacka-Woszuk, J. Caloric restriction can affect one-carbon metabolism during pregnancy in the rat: A transgenerational model. Biochimie 2018, 152, 181–187. [Google Scholar] [CrossRef]
- Hernandez, T.L.; Friedman, J.E.; Barbour, L.A. Insulin Resistance in Pregnancy: Implications for Mother and Offspring. In Insulin Resistance: Childhood Precursors of Adult Disease; Zeitler, P.S., Nadeau, K.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 67–94. [Google Scholar]
- Pintaudi, B.; Fresa, R.; Dalfrà, M.; Dodesini, A.R.; Vitacolonna, E.; Tumminia, A.; Sciacca, L.; Lencioni, C.; Marcone, T.; Lucisano, G.; et al. The risk stratification of adverse neonatal outcomes in women with gestational diabetes (STRONG) study. Acta Diabetol. 2018, 55, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Panimolle, F.; Lenzi, A.; Morano, S. Bisphenol A and Phthalates in Diet: An Emerging Link with Pregnancy Complications. Nutrients 2020, 12, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soubry, A.; Murphy, S.K.; Huang, Z.; Murtha, A.; Schildkraut, J.M.; Jirtle, R.L.; Wang, F.; Kurtzberg, J.; Demark-Wahnefried, W.; Forman, M.R.; et al. The effects of depression and use of antidepressive medicines during pregnancy on the methylation status of the IGF2 imprinted control regions in the offspring. Clin. Epigenetics 2011, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Meers, J.M.; Nowakowski, S. Sleep, premenstrual mood disorder, and women’s health. Curr. Opin. Psychol. 2020, 34, 43–49. [Google Scholar] [CrossRef]
- Sadurni, C.; Schneider, B.; Perez Delgadillo, P.; Rodriguez, M.; Tourgerman, I. A-72The Effects of Maternal Prenatal Mental Health Stress on Neurodevelopmental Deficits. Arch. Clin. Neuropsychol. 2016, 31, 611. [Google Scholar] [CrossRef]
- Aoyagi, S.S.; Tsuchiya, K.J. Does maternal postpartum depression affect children’s developmental outcomes? J. Obstet. Gynaecol. Res. 2019, 45, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Ahmed, A.H.; Smail, L. Psychological Climacteric Symptoms and Attitudes toward Menopause among Emirati Women. Int. J. Environ. Res. Public Health 2020, 17, 5028. [Google Scholar] [CrossRef]
- Ecklund-Flores, L.; Myers, M.M.; Monk, C.; Perez, A.; Odendaal, H.J.; Fifer, W.P. Maternal depression during pregnancy is associated with increased birth weight in term infants. Dev. Psychobiol. 2017, 59, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, A.H.; Al Khedair, K.; Al-Jeheiman, R.; Al-Turki, H.A.; Al Qahtani, N.H. Anxiety and depression during pregnancy in women attending clinics in a University Hospital in Eastern province of Saudi Arabia: Prevalence and associated factors. Int. J. Women Health 2018, 10, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Green, J. Factor structure of the depression anxiety stress Scale-21 (DASS-21): Unidimensionality of the Arabic version among Egyptian drug users. Subst. Abus. Treat. Prev. Policy 2019, 14, 40. [Google Scholar] [CrossRef]
- Howdeshell, K.L.; Ornoy, A. Depression and Its Treatment during Pregnancy: Overview and Highlights. Birth Defects Res. 2017, 109, 877–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galbally, M.; Watson, S.J.; Boyce, P.; Nguyen, T.; Lewis, A.J. The mother, the infant and the mother-infant relationship: What is the impact of antidepressant medication in pregnancy. J. Affect. Disord. 2020, 272, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Meltzer-Brody, S.; Propper, C.; Pearson, B.; Beiler, P.; Elam, M.; Walker, C.; Mills-Koonce, R.; Grewen, K. The Mood, Mother, and Infant Study: Associations between Maternal Mood in Pregnancy and Breastfeeding Outcome. Breastfeed. Med. 2019, 14, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Hassan, A.A.; Hendawy, A.O. The Adverse Effects of Antidepressant Medication Treatments on the Offspring of Women with Perinatal Depression. Sci. J. Res. Rev. 2019, 1, SJRR.MS.ID.000508. [Google Scholar] [CrossRef] [Green Version]
- Malheiros, R.T.; Delgado, H.O.; Felber, D.T.; Kraus, S.I.; Dos Santos, A.R.S.; Manfredini, V.; da Silva, M.D. Mood disorders are associated with the reduction of brain derived neurotrophic factor in the hypocampus in rats submitted to the hipercaloric diet. Metab. Brain Dis. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Viana, L.V.; Gross, J.L.; Azevedo, M.J. Dietary Intervention in Patients with Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis of Randomized Clinical Trials on Maternal and Newborn Outcomes. Diabetes Care 2014, 37, 3345–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, L.; Gross, J.; Lanzi, S.; Quansah, D.Y.; Puder, J.; Horsch, A. How diet, physical activity and psychosocial well-being interact in women with gestational diabetes mellitus: An integrative review. BMC Pregnancy Childbirth 2019, 19, 60. [Google Scholar] [CrossRef]
- Ali, A.M.; Hendawy, A.O. So, Antidepressant Drugs have Serious Adverse Effects, but what are the Alternatives? Nov. Appro Drug Des. Dev. 2018, 4, 555636. [Google Scholar] [CrossRef] [Green Version]
- Mudry, J.M.; Lassiter, D.G.; Nylen, C.; Garcia-Calzon, S.; Naslund, E.; Krook, A.; Zierath, J.R. Insulin and Glucose Alter Death-Associated Protein Kinase 3 (DAPK3) DNA Methylation in Human Skeletal Muscle. Diabetes 2017, 66, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Donoso, C.; Sánchez-Villegas, A.; Martínez-González, M.A.; Gea, A.; Mendonça, R.D.; Lahortiga-Ramos, F.; Bes-Rastrollo, M. Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: The SUN Project. Eur. J. Nutr. 2020, 59, 1093–1103. [Google Scholar] [CrossRef]
- Olmedo-Requena, R.; Gómez-Fernández, J.; Amezcua-Prieto, C.; Mozas-Moreno, J.; Khan, K.S.; Jiménez-Moleón, J.J. Pre-Pregnancy Adherence to the Mediterranean Diet and Gestational Diabetes Mellitus: A Case-Control Study. Nutrients 2019, 11, 1003. [Google Scholar] [CrossRef] [Green Version]
- Vall Castelló, J.; Tubianosa, C. Linking Mediterranean Diet and Lifestyle with Cardio Metabolic Disease and Depressive Symptoms: A Study on the Elderly in Europe. Int. J. Environ. Res. Public Health 2020, 17, 7053. [Google Scholar] [CrossRef]
- Kołomańska, D.; Zarawski, M.; Mazur-Bialy, A. Physical Activity and Depressive Disorders in Pregnant Women-A Systematic Review. Medicina 2019, 55, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Age-related skeletal muscle failure (sarcopenia)—A detrimental challenge during the Coronavirus Disease 2019 (COVID-19) era: A review. 2020. in progress. [Google Scholar]
- Ali, A.M.; Kunugi, H. Corona Virus Disease 2019 (COVID-19): A pandemic that threatens physical and mental health by promoting physical inactivity. Sports Med. Health Sci. 2020. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Skeletal Muscle Injury in Corona Virus Disease 2019 (COVID-19): A brief mechanistic review. 2020. in progress. [Google Scholar]
- Zhai, L.; Zhang, Y.; Zhang, D. Sedentary behaviour and the risk of depression: A meta-analysis. Br. J. Sports Med. 2015, 49, 705–709. [Google Scholar] [CrossRef]
- Hill, D.J. Placental control of metabolic adaptations in the mother for an optimal pregnancy outcome. What goes wrong in gestational diabetes? Placenta 2018, 69, 162–168. [Google Scholar] [CrossRef]
- Qiao, L.; Saget, S.; Lu, C.; Hay, W.W., Jr.; Karsenty, G.; Shao, J. Adiponectin Promotes Maternal β-Cell Expansion Through Placental Lactogen Expression. Diabetes 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kondegowda, N.G.; Filipowska, J.; Hampton, R.F.; Leblanc, S.; Garcia-Ocana, A.; Vasavada, R.C. Lactogens Reduce Endoplasmic Reticulum Stress-Induced Rodent and Human β-Cell Death and Diabetes Incidence in Akita Mice. Diabetes 2020, 69, 1463–1475. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging—A focus on cognitive aging and Alzheimer’s disease: A review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. On the interaction between malnutrition and COVID-19 in older adults—Exploration of the most suitable nutritional screening and nutritional management approaches: A review. Nutrients 2020. in progress. [Google Scholar]
- Sonaglioni, A.; Esposito, V.; Caruso, C.; Nicolosi, G.L.; Bianchi, S.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Association between neutrophil to lymphocyte ratio and carotid artery wall thickness in healthy pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Ali, E.M.; Ahmed, M.S.; Hendawy, A.O. Targeting gut microbiome and the recovery of muscle loss associated with cancer (cachexia): An overview of the possible effect of bee products. Med. Legal Update 2020. accepted. [Google Scholar]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s disease: A focus on the effects of propolis and royal jelly. Oxid. Med. Cell Longev. 2020, 2020, 1727142. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Shiels, P.G. The role of the microbiota in sedentary lifestyle disorders and ageing: Lessons from the animal kingdom. J. Intern. Med. 2020, 287, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Bleau, C.; Karelis, A.D.; St-Pierre, D.H.; Lamontagne, L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab. Res. Rev. 2015, 31, 545–561. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, R.; Stupin, J.H.; Melchior, K.; Schellong, K.; Ziska, T.; Dudenhausen, J.W.; Henrich, W.; Rancourt, R.C.; Plagemann, A. Alterations of adiponectin gene expression and DNA methylation in adipose tissues and blood cells are associated with gestational diabetes and neonatal outcome. Clin. Epigenetics 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Egawa, T.; Ohno, Y.; Yokoyama, S.; Yokokawa, T.; Tsuda, S.; Goto, K.; Hayashi, T. The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle. Foods 2019, 8, 439. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Javaheri, A.; Godar, R.J.; Murphy, J.; Ma, X.; Rohatgi, N.; Mahadevan, J.; Hyrc, K.; Saftig, P.; Marshall, C.; et al. Intermittent fasting preserves beta-cell mass in obesity-induced diabetes via the autophagy-lysosome pathway. Autophagy 2017, 13, 1952–1968. [Google Scholar] [CrossRef]
- Liu, B.; Gan, X.; Zhao, Y.; Yu, H.; Gao, J.; Yu, H. Inhibition of HMGB1 Promotes Osseointegration under Hyperglycemic Condition through Improvement of BMSC Dysfunction. Oxid. Med. Cell. Longev. 2019, 2019, 1703709. [Google Scholar] [CrossRef]
- Lu, H.Y.; Ma, J.L.; Shan, J.Y.; Zhang, J.; Wang, Q.X.; Zhang, Q. High-mobility group box-1 and receptor for advanced glycation end products in preterm infants with brain injury. World J. Pediatr. 2017, 13, 228–235. [Google Scholar] [CrossRef]
- Thakur, V.; Sadanandan, J.; Chattopadhyay, M. High-Mobility Group Box 1 Protein Signaling in Painful Diabetic Neuropathy. Int. J. Mol. Sci. 2020, 21, 881. [Google Scholar] [CrossRef] [Green Version]
- Davegardh, C.; Hall Wedin, E.; Broholm, C.; Henriksen, T.I.; Pedersen, M.; Pedersen, B.K.; Scheele, C.; Ling, C. Sex influences DNA methylation and gene expression in human skeletal muscle myoblasts and myotubes. Stem Cell Res. Ther. 2019, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Giresi, P.G.; Stevenson, E.J.; Theilhaber, J.; Koncarevic, A.; Parkington, J.; Fielding, R.A.; Kandarian, S.C. Identification of a molecular signature of sarcopenia. Physiol. Genom. 2005, 21, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gensous, N.; Bacalini, M.G.; Franceschi, C.; Meskers, C.G.M.; Maier, A.B.; Garagnani, P. Age-Related DNA Methylation Changes: Potential Impact on Skeletal Muscle Aging in Humans. Front. Physiol. 2019, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qu, L.; Ni, H.; Wang, Y.; Li, L.; Yang, X.; Wang, X.; Hou, Y. Expression and function of lncRNA MALAT1 in gestational diabetes mellitus. Adv. Clin. Exp. Med. 2020, 29, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lan, X.; Zhang, Y.; Zhou, F.; Cai, C.; Zhang, J.; Pang, X.; Hao, L.; Li, R.; Zeng, G. Anxiety and depression on gestational diabetes mellitus in early pregnancy. Wei Sheng Yan Jiu 2020, 49, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta, A.; Zhou, A.; Vimaleswaran, K.S.; Dickson, C.; Hyppönen, E. Depression increases the genetic susceptibility to high body mass index: Evidence from UK Biobank. Depress. Anxiety 2019, 36, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Gao, H.; Zhang, H.; Zhang, H.; Wang, Q.; Sun, Z. P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci. 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.W.; Ching, S.M.; Hoo, F.K.; Ramachandran, V.; Chong, S.C.; Tusimin, M.; Mohd Nordin, N.; Devaraj, N.K.; Cheong, A.T.; Chia, Y.C. Neonatal outcomes and its association among gestational diabetes mellitus with and without depression, anxiety and stress symptoms in Malaysia: A cross-sectional study. Midwifery 2020, 81, 102586. [Google Scholar] [CrossRef]
- Boisard, S.; Shahali, Y.; Aumond, M.-C.; Derbré, S.; Blanchard, P.; Dadar, M.; Le Ray, A.-M.; Richomme, P. Anti-AGE activity of poplar-type propolis: Mechanism of action of main phenolic compounds. Int. J. Food. Sci. 2020, 55, 453–460. [Google Scholar] [CrossRef]
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; van der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational Diabetes Mellitus and Diet: A Systematic Review and Meta-analysis of Randomized Controlled Trials Examining the Impact of Modified Dietary Interventions on Maternal Glucose Control and Neonatal Birth Weight. Diabetes Care 2018, 41, 1346–1361. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Lin, L.; Shan, N.; Ren, C.Y.; Long, X.; Sun, Y.H.; Wang, L. The impact of omega-3 fatty acid supplementation on glycemic control in patients with gestational diabetes: A systematic review and meta-analysis of randomized controlled studies. J. Matern. Fetal Neonatal Med. 2020, 33, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Donovan, L.E.; Vallee, R.; Yamamoto, J.M. Evidenced-Based Nutrition for Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2019, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Arqoub, A.M.S.; Flynn, K.G.; Martinez, L.A. Gestational exposure to a ketogenic diet increases sociability in CD-1 mice. Behav. Neurosci. 2020, 134, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, J.; Louie, J.C.Y.; Buso, M.E.C.; Atkinson, F.S.; Ross, G.P.; Markovic, T.P.; Brand-Miller, J.C. Effects of a modestly lower carbohydrate diet in gestational diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Manchishi, S.M.; Cui, R.J.; Zou, X.H.; Cheng, Z.Q.; Li, B.J. Effect of caloric restriction on depression. J. Cell. Mol. Med. 2018, 22, 2528–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [Green Version]
- Haritov, E.; Garalova, M.; Tivcheva, J.; Angelov, T.; Stamenov, V. Mechanisms and Effects of Dietary Restriction on CNS and Affective Disorders. Acta Med. Bulg. 2020, 47, 55–63. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [Green Version]
- AlHussain, F.; AlRuthia, Y.; Al-Mandeel, H.; Bellahwal, A.; Alharbi, F.; Almogbel, Y.; Awwad, O.; Dala’een, R.; Alharbi, F.A. Metformin Improves the Depression Symptoms of Women with Polycystic Ovary Syndrome in a Lifestyle Modification Program. Patient Prefer Adherence 2020, 14, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Domaszewski, P.; Konieczny, M.; Pakosz, P.; Bączkowicz, D.; Sadowska-Krępa, E. Effect of a Six-Week Intermittent Fasting Intervention Program on the Composition of the Human Body in Women over 60 Years of Age. Int. J. Environ. Res. Public Health 2020, 17, 4138. [Google Scholar] [CrossRef]
- Stone, V.; Maurmann, R.M.; Dal Magro, B.M.; Crestani, M.S.; Hozer, R.M.; Klein, C.P.; Matté, C. Gestational caloric restriction with micronutrients supplementation does not delay development and promotes feeding behavior benefits. Nutr. Neurosci. 2019, 1–11. [Google Scholar] [CrossRef]
- Powell, C.D.; Wilson, W.M.; Olesaningo, G.; Manyama, M.; Jamniczky, H.; Spritz, R.; Cross, J.C.; Lukowiak, K.; Hallgrimsson, B.; Gonzalez, P.N. Lack of head sparing following third-trimester caloric restriction among Tanzanian Maasai. PLoS ONE 2020, 15, e0237700. [Google Scholar] [CrossRef] [PubMed]
- International Fetal Growth Standards. Available online: https://media.tghn.org/medialibrary/2017/03/05_Fetal.pdf (accessed on 14 December 2020).
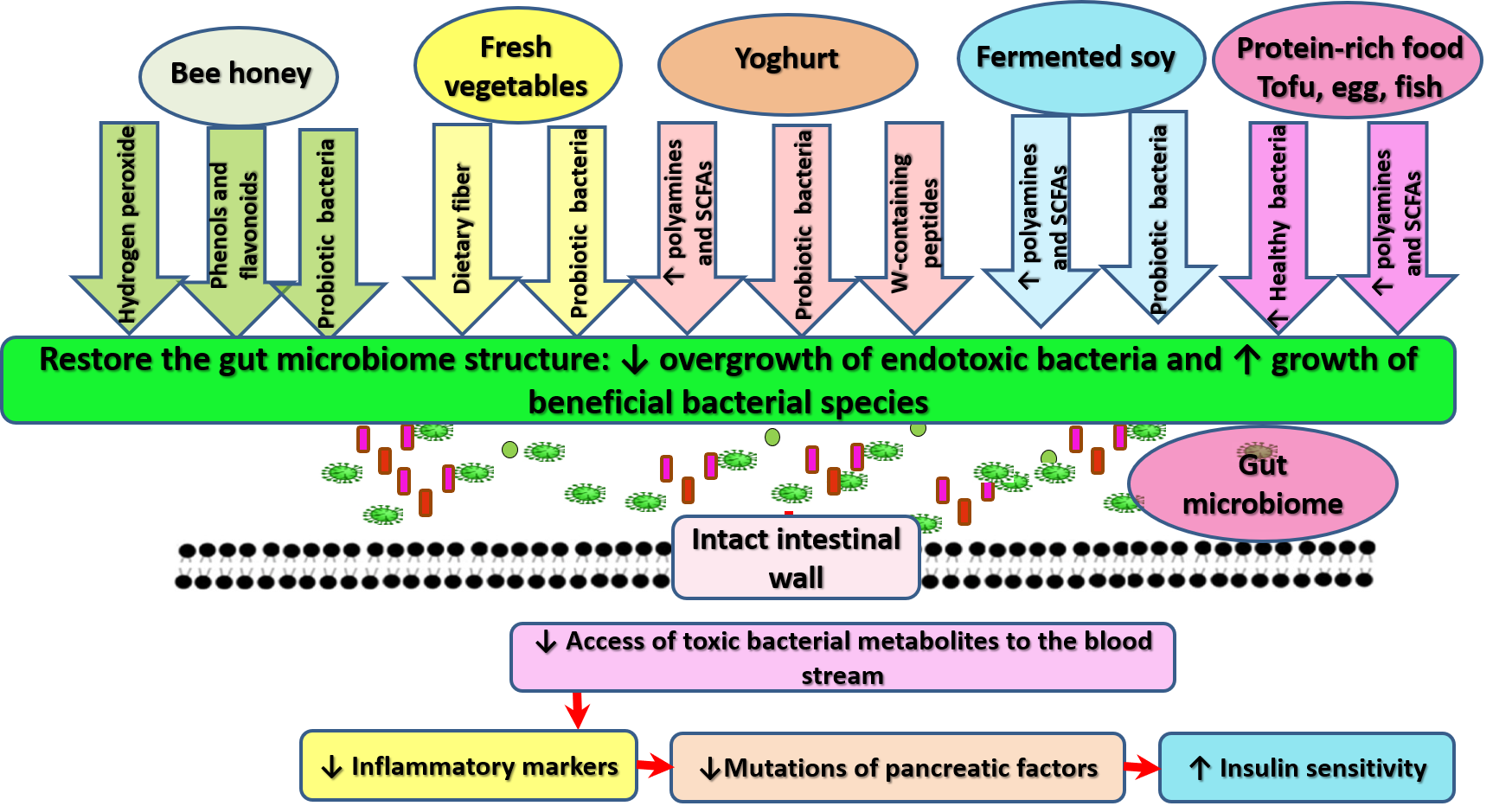
- Fond, G.; Boukouaci, W.; Chevalier, G.; Regnault, A.; Eberl, G.; Hamdani, N.; Dickerson, F.; Macgregor, A.; Boyer, L.; Dargel, A.; et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. 2015, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bekebrede, A.F.; Keijer, J.; Gerrits, W.J.J.; Boer, V.C.J. The Molecular and Physiological Effects of Protein-Derived Polyamines in the Intestine. Nutrients 2020, 12, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Sasaki, H.; Shiga, K.; Miyakawa, H.; Shibata, S. The Timing Effects of Soy Protein Intake on Mice Gut Microbiota. Nutrients 2019, 12, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, K.; Wada, K.; Yamakawa, M.; Goto, Y.; Mizuta, F.; Koda, S.; Uji, T.; Tsuji, M.; Nagata, C. Dietary Soy Intake Is Inversely Associated with Risk of Type 2 Diabetes in Japanese Women but Not in Men. J. Nutr. 2019, 149, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.M.; Henley, E.C.; Tabor, A. Soy foods have low glycemic and insulin response indices in normal weight subjects. Nutr. J. 2006, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konya, J.; Sathyapalan, T.; Kilpatrick, E.S.; Atkin, S.L. The Effects of Soy Protein and Cocoa with or Without Isoflavones on Glycemic Control in Type 2 Diabetes. A Double-Blind, Randomized, Placebo-Controlled Study. Front. Endocrinol. 2019, 10, 296. [Google Scholar] [CrossRef] [Green Version]
- Hardee, J.P.; Lynch, G.S. Current pharmacotherapies for sarcopenia. Expert Opin. Pharmacother. 2019, 20, 1645–1657. [Google Scholar] [CrossRef]
- Walrand, S.; Gryson, C.; Salles, J.; Giraudet, C.; Migne, C.; Bonhomme, C.; Le Ruyet, P.; Boirie, Y. Fast-digestive protein supplement for ten days overcomes muscle anabolic resistance in healthy elderly men. Clin. Nutr. 2016, 35, 660–668. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. Milk proteins as a source of tryptophan-containing bioactive peptides. Food Funct. 2015, 6, 2115–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirian, A.; Rahnemaei, F.A.; Abdi, F. Role of C-reactive Protein (CRP) or high-sensitivity CRP in predicting gestational diabetes Mellitus:Systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Potential anti-fatigue effects of bee honey, royal jelly, propolis, and bee pollen: A review. Molecules 2020. in progress. [Google Scholar]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Hendawy, A.O. Bee Honey as a Potentially Effective Treatment for Depression: A Review of Clinical and Preclinical Findings. JOJ Nurse Health Care 2018, 9, 555764. [Google Scholar] [CrossRef] [Green Version]
- Chandran, H.C.; Keerthi, T.R. Probiotic Potency of Lactobacillus Plantarum KX519413 and KX519414 Isolated from Honey Bee Gut. 2018. Available online: https://academic.oup.com/femsle/advance-article-abstract/doi/10.1093/femsle/fnx285/4793248 (accessed on 1 September 2020).
- Khan, W.A.; Malik, A.; Khan, M.W.A. Estrogenization of insulin by catecholestrogen produced high affinity autoantibodies and altered the normal function of insulin in type 1 diabetes. Life Sci. 2020, 256, 117910. [Google Scholar] [CrossRef]
- Wang, X.; Chi, X.; Feng, C.; Zhang, X.; Jin, Z. Sex hormone-binding globulin regulates the activity of the ERK pathway in the placentas of patients with gestational diabetes mellitus. Biochem. Biophys. Res. Commun. 2020, 532, 613–619. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alsibai, J.; Clark, C.C.T.; Faris, M.E.A.-I.E. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur. J. Nutr. 2020, 1–26. [Google Scholar] [CrossRef]
- Reutrakul, S.; Van Cauter, E. Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Ann. N. Y. Acad. Sci. 2014, 1311, 151–173. [Google Scholar] [CrossRef]
- Luque-Fernandez, M.A.; Bain, P.A.; Gelaye, B.; Redline, S.; Williams, M.A. Sleep-disordered breathing and gestational diabetes mellitus: A meta-analysis of 9795 participants enrolled in epidemiological observational studies. Diabetes Care 2013, 36, 3353–3360. [Google Scholar] [CrossRef] [Green Version]
- Wanitcharoenkul, E.; Chirakalwasan, N.; Amnakkittikul, S.; Charoensri, S.; Saetung, S.; Chanprasertyothin, S.; Chailurkit, L.-O.; Panburana, P.; Bumrungphuet, S.; Ongphiphadhanakul, B.; et al. Obstructive sleep apnea and diet-controlled gestational diabetes. Sleep Med. 2017, 39, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.; Pavlović, J.M. Sleep Disorders and Migraine: Review of Literature and Potential Pathophysiology Mechanisms. Headache J. Head Face Pain 2018, 58, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Hendawy, A.O. Royal Jelly Acid, 10-Hydroxy-Trans-2-Decenoic Acid, for Psychiatric and Neurological Disorders: How helpful could it be?! Edelweiss J. Food Sci. Technol. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Rodriguez-Lozada, C.; Cuervo, M.; Cuevas-Sierra, A.; Goni, L.; Riezu-Boj, J.I.; Navas-Carretero, S.; Milagro, F.I.; Martinez, J.A. Changes in Anxiety and Depression Traits Induced by Energy Restriction: Predictive Value of the Baseline Status. Nutrients 2019, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
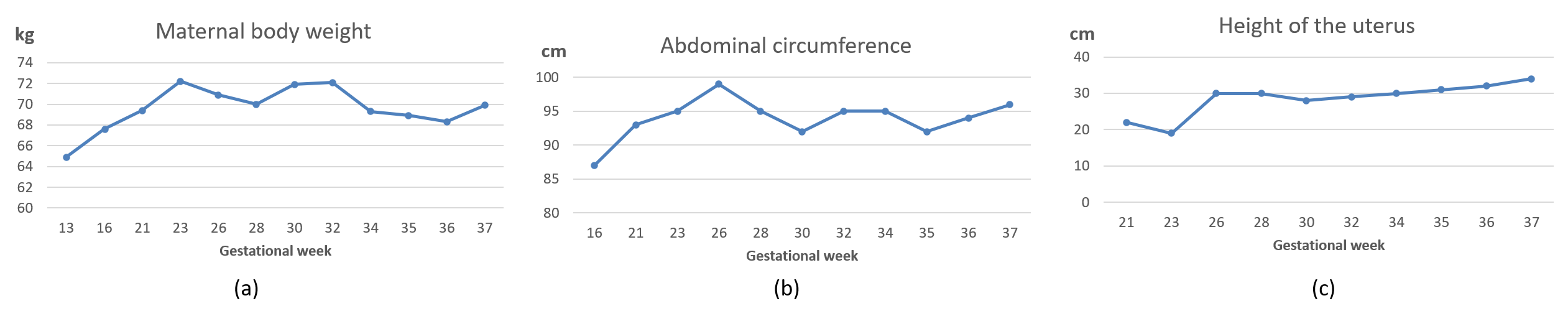
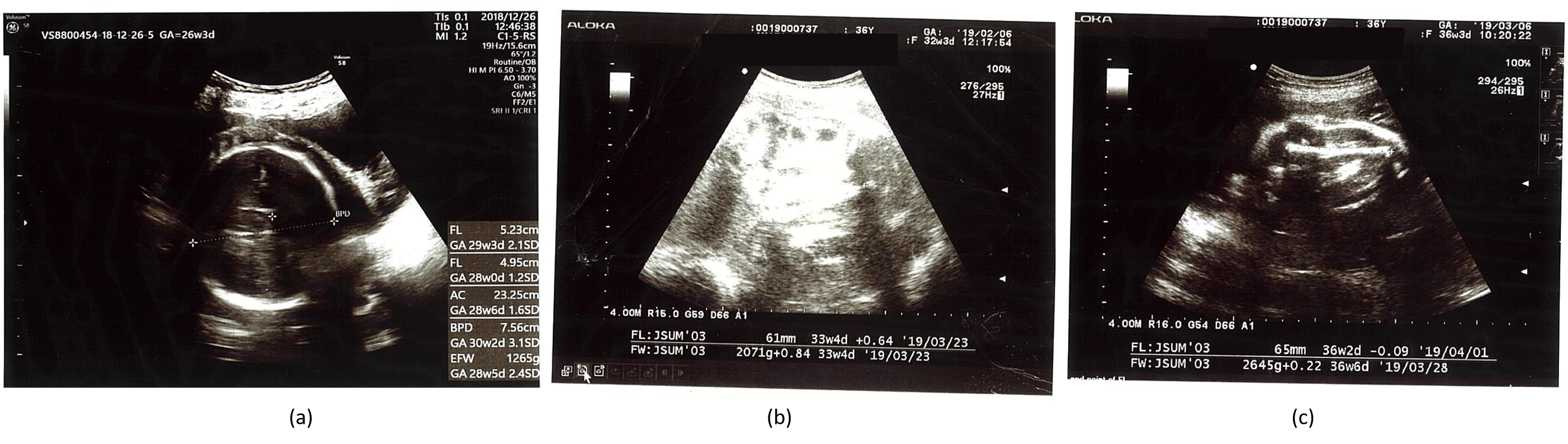
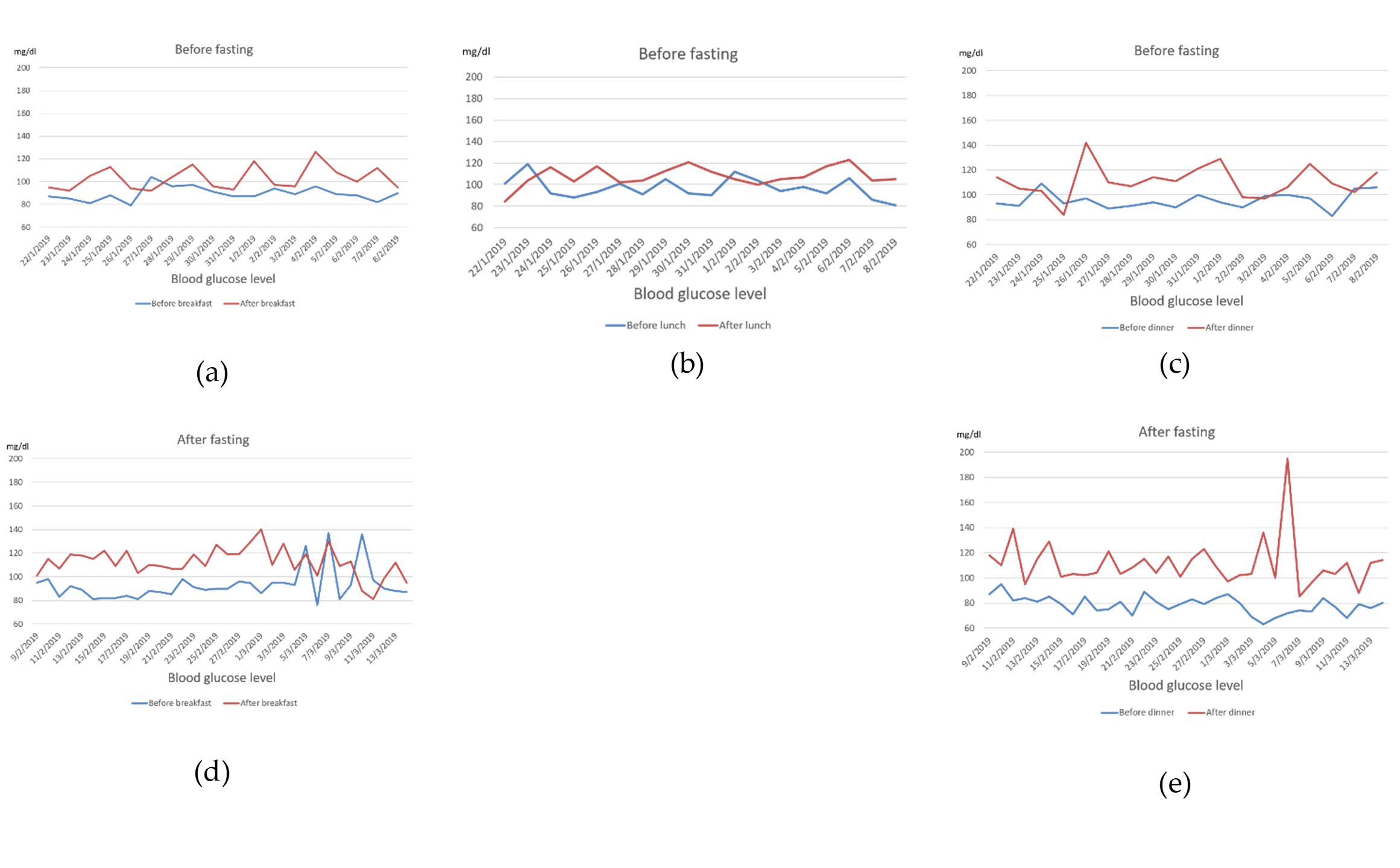

| Gestational Age | BPD (mm) | HC (mm) | AC (mm) | FL (mm) | EFBW (g) |
|---|---|---|---|---|---|
| 26w3d | 76 + 3.1 SD 97.5% | -- | 233 + 1.6 SD 95% | 52 + 2.1 SD 97% | 1265 + 2.4 SD 97% |
| 27w5d | 75 + 1.6 SD 90% | -- | 242 + 1.3 SD 90% | 56 + 2.3 SD 97% | 1421 + 2.0 SD 97% |
| 30w1d | 82 + 1.83 SD 90% | 301 ▲ 97% | 275 + 1.9 SD 95% | 60 + 1.9 SD 97% | 1947 + 2.4 SD 97% |
| 32w3d | 87 + 1.8 SD 90% | -- | 274 + 0.65 SD 50% | 61 + 0.64 SD 50% | 2071 + 0.84 SD 97% |
| 34w1d | 91▲ 50% | 339 ▲ 97% | 290 + 0.6 SD 10% | 67 + 1.5 SD 90% | 2480 + 1.2 SD 50% |
| 36w3d | 93 + 1.47 SD 50% | -- | 303 + 0.4 SD 10% | 65 − 0.09 SD 10% | 2645 + 0.22 SD 50% |
| 37w3d | 95 + 1.5 SD 50% | -- | 302 − 0.02 SD 10% | 73 + 1.75 SD 90% | 2886 + 0.44 SD 50% |
| Parameter | Patient’s Value | Reference |
|---|---|---|
| Fasting blood sugar | 87 mg/dL | 70–110 |
| Bilirubin | 0.43 mg/dL | 0.20–1.20 |
| Aspartate aminotransferase | 22 IU/L | 8–38 |
| Alanine aminotransferase | 14 IU/L | 4–44 |
| Total protein | 6.3 g/dL | 6.7–8.3 |
| Albumin | 3.2 g/dL | 3.6–5.2 |
| Creatine | 0.51 mg/dL | 0.40–0.80 |
| Sodium | 135 mEq/L | 137–147 |
| Potassium | 4.2 mEq/L | 3.5–5.5 |
| Chloride | 106 mEq/L | 97–108 |
| White blood cells | 83 × 102 | 40–85 |
| Red blood cells | 375 × 104 | 380–480 |
| Hemoglobin | 11.0 g/dL | 12.0–15.0 |
| Hematocrit | 32.4% | 34.0–45.0 |
| Activated partial thromboplastin clotting time | 23.4 s | 25.0–40.0 |
| C-reactive protein | 0.64 mg/dL | 0.00–0.30 |
| Creatine kinase | 264 IU/L | 43–165 |
| Urine ketone bodies | Negative | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.M.; Kunugi, H. Intermittent Fasting, Dietary Modifications, and Exercise for the Control of Gestational Diabetes and Maternal Mood Dysregulation: A Review and a Case Report. Int. J. Environ. Res. Public Health 2020, 17, 9379. https://doi.org/10.3390/ijerph17249379
Ali AM, Kunugi H. Intermittent Fasting, Dietary Modifications, and Exercise for the Control of Gestational Diabetes and Maternal Mood Dysregulation: A Review and a Case Report. International Journal of Environmental Research and Public Health. 2020; 17(24):9379. https://doi.org/10.3390/ijerph17249379
Chicago/Turabian StyleAli, Amira Mohammed, and Hiroshi Kunugi. 2020. "Intermittent Fasting, Dietary Modifications, and Exercise for the Control of Gestational Diabetes and Maternal Mood Dysregulation: A Review and a Case Report" International Journal of Environmental Research and Public Health 17, no. 24: 9379. https://doi.org/10.3390/ijerph17249379
APA StyleAli, A. M., & Kunugi, H. (2020). Intermittent Fasting, Dietary Modifications, and Exercise for the Control of Gestational Diabetes and Maternal Mood Dysregulation: A Review and a Case Report. International Journal of Environmental Research and Public Health, 17(24), 9379. https://doi.org/10.3390/ijerph17249379






