The Incorporation of Amplified Metal-Enhanced Fluorescence in a CMOS-Based Biosensor Increased the Detection Sensitivity of a DNA Marker of the Pathogenic Fungus Colletotrichum gloeosporioides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
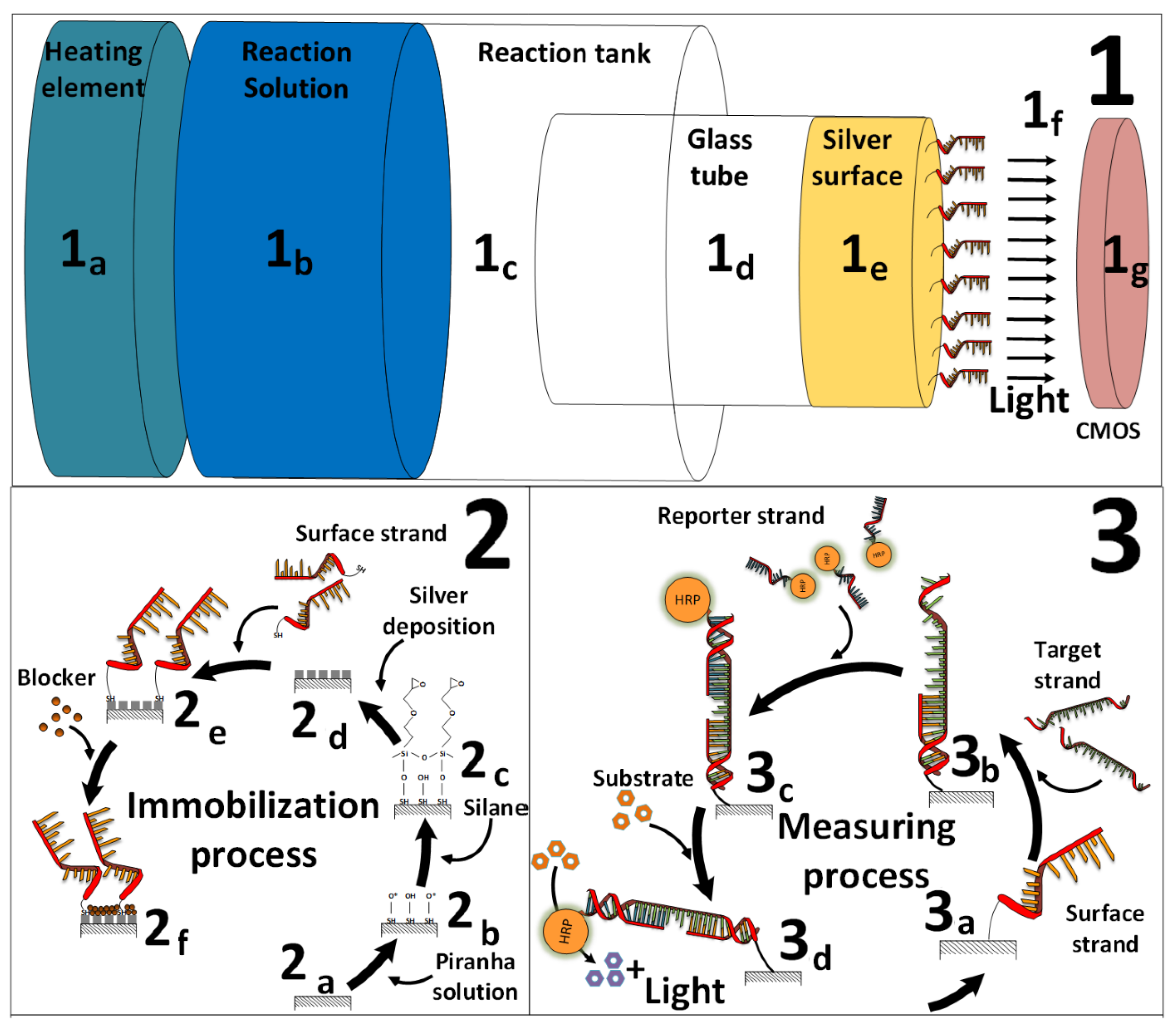
2.2. Surface Activation and Silver-Deposition Procedure
2.3. DNA Immobilization
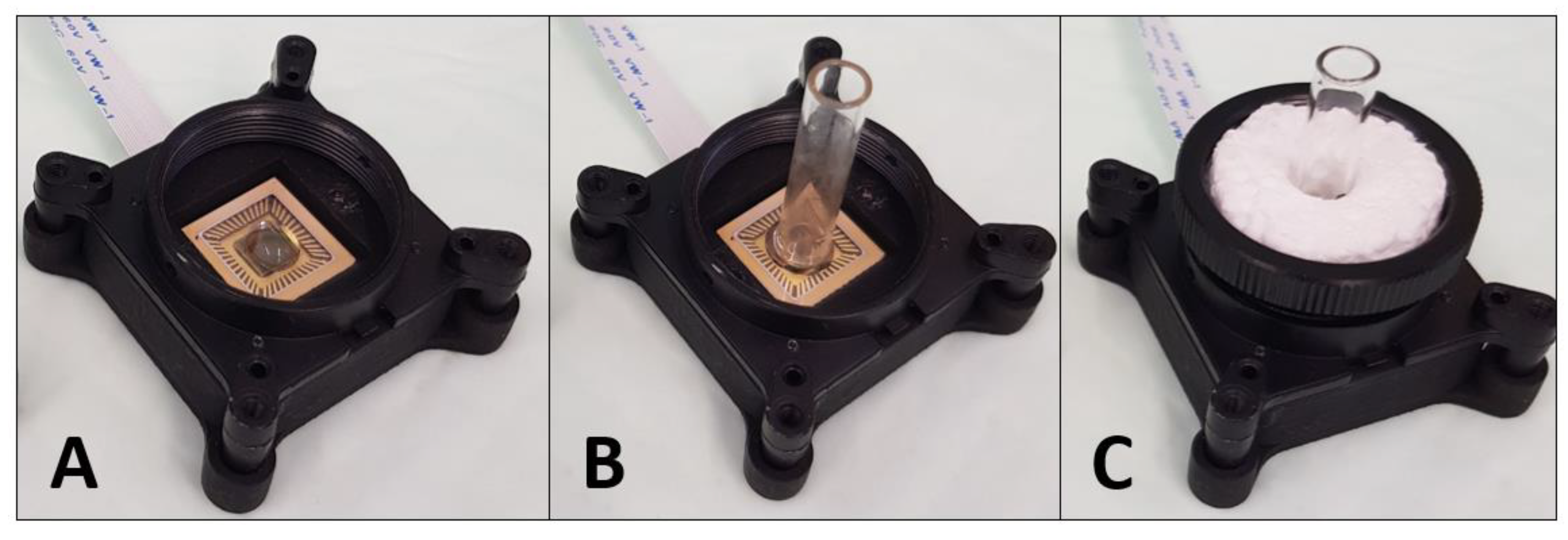
2.4. CMOS-Based Biosensor-System Setup
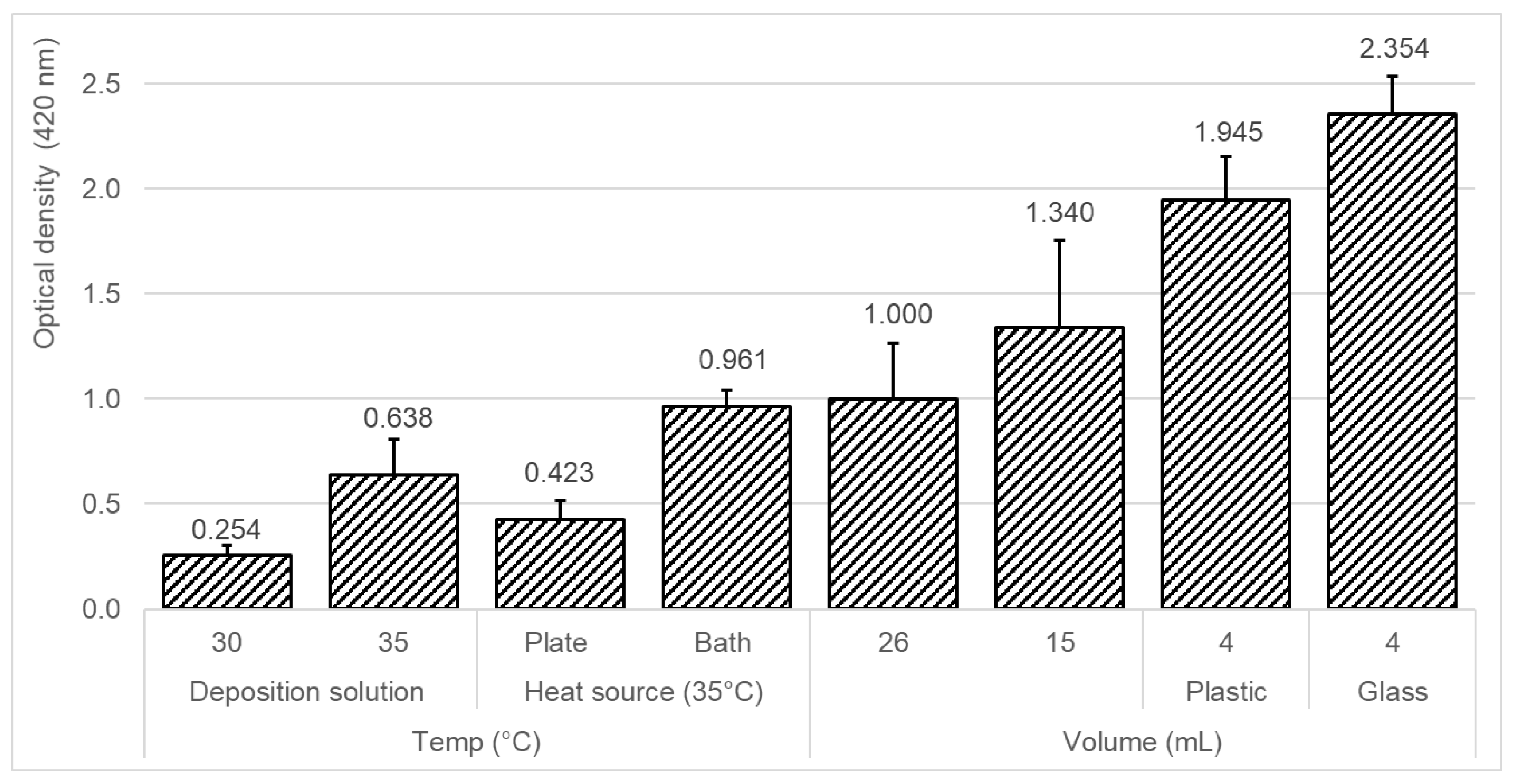
2.5. Parameters that Influence the Silver-Deposition Procedure Efficiency
2.6. Sensitivity and Reproducibility of the System
3. Results and Discussion
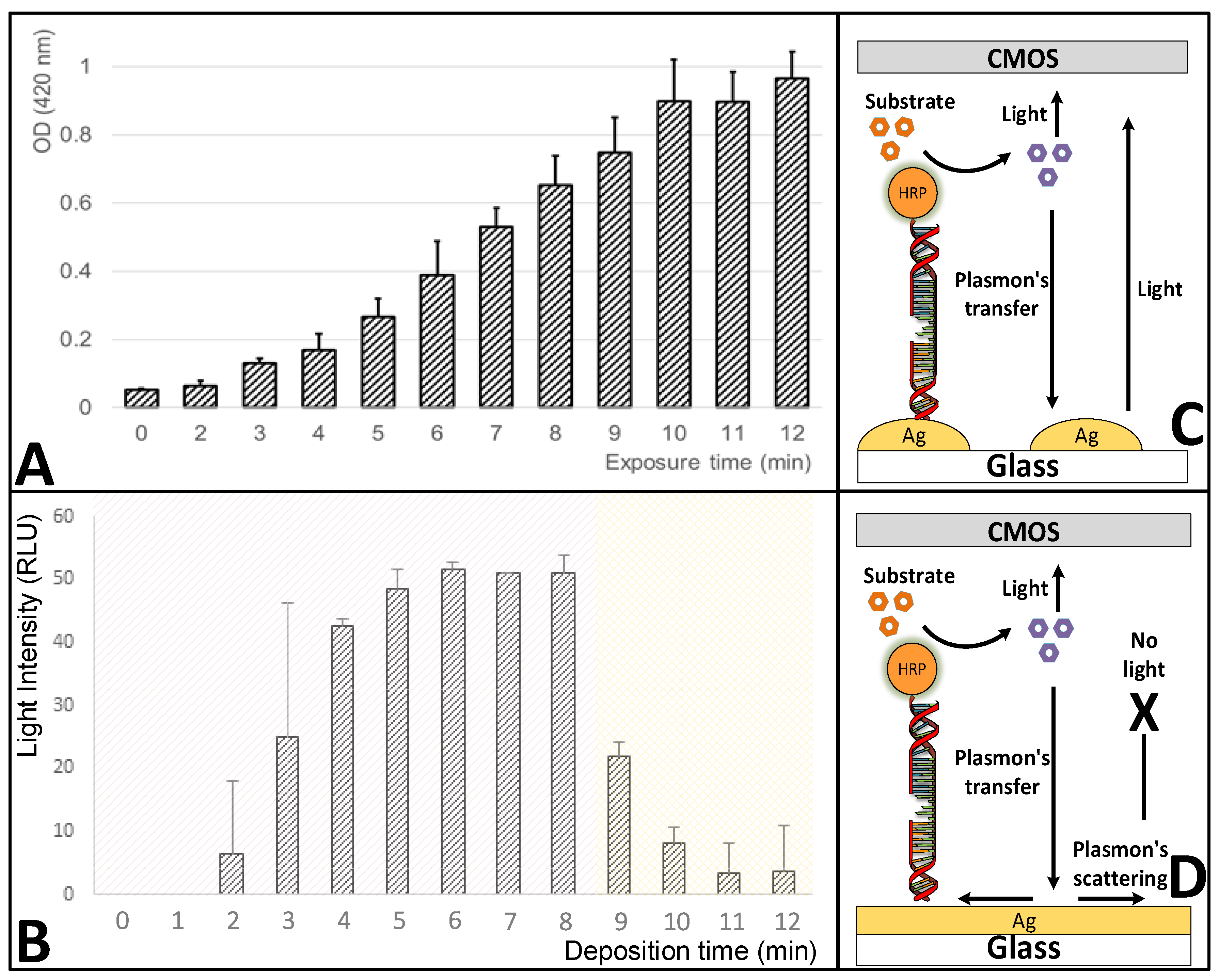
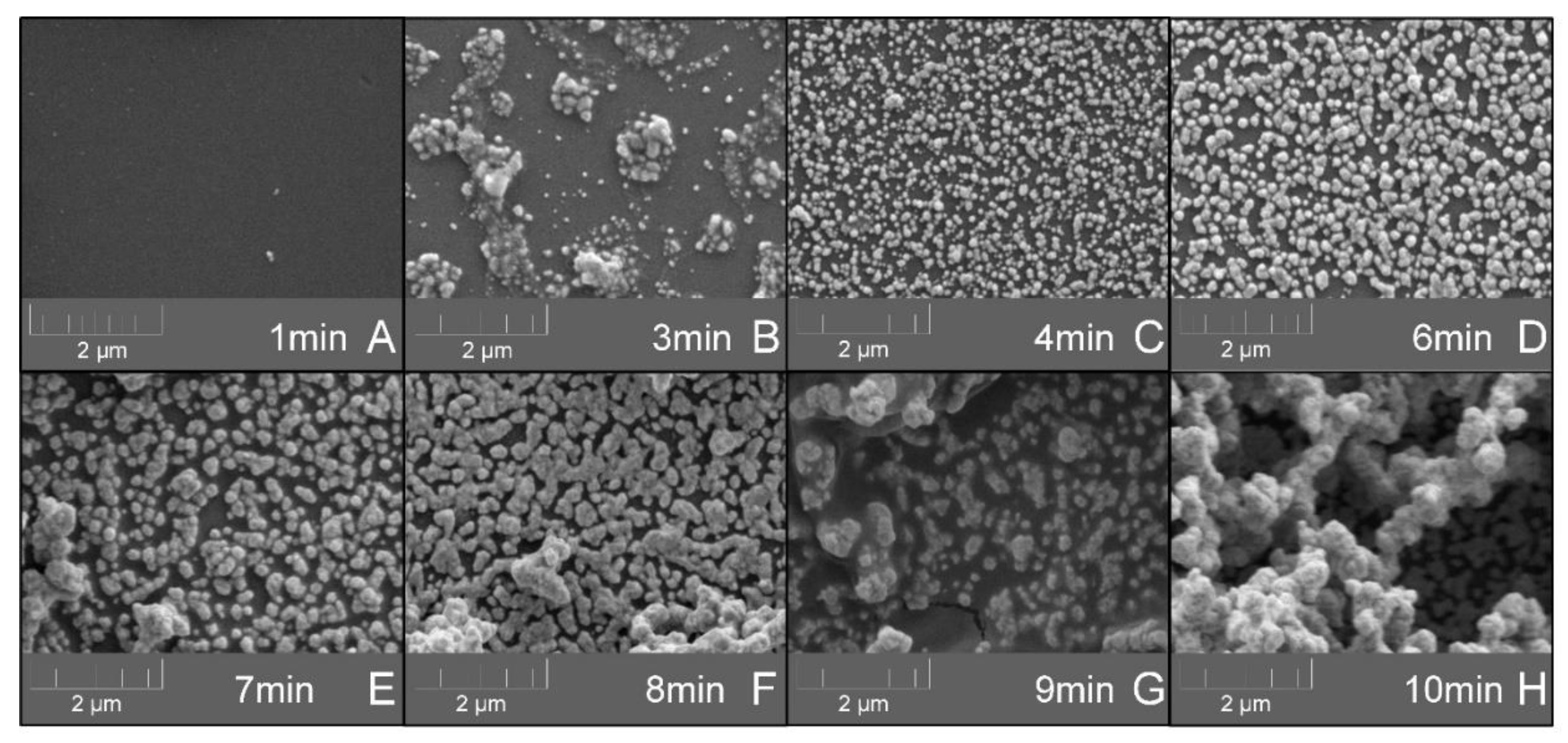
3.1. Effect of Different Deposition Conditions on the Efficiency of the Silver-Layer Formation
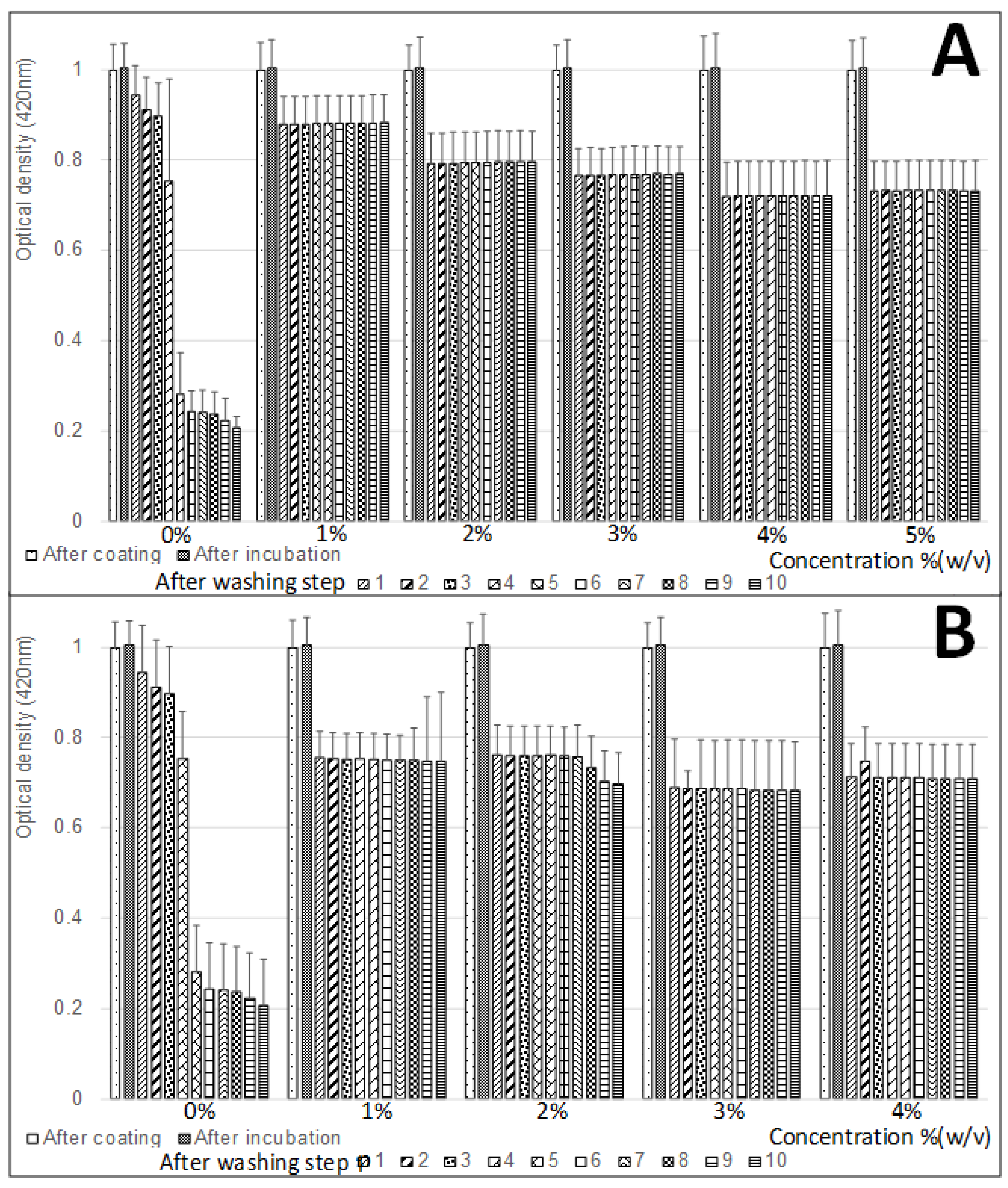
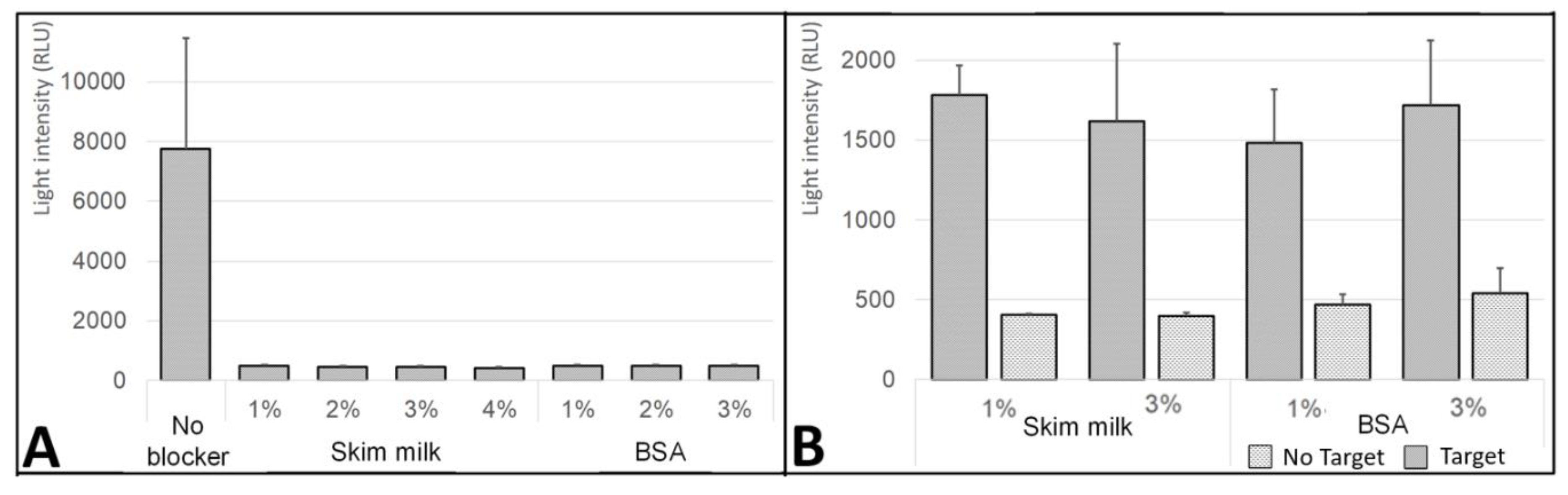
3.2. Effect of Blocking Treatment on the Silver-Layer Stability
3.3. Effect of the Blocking Step on the Nonspecific DNA Absorption
3.4. Effect of the Deposition-Reaction Duration on the Silver-Layer Formation and the Metal-Enhanced Fluorescence (MEF) Amplification
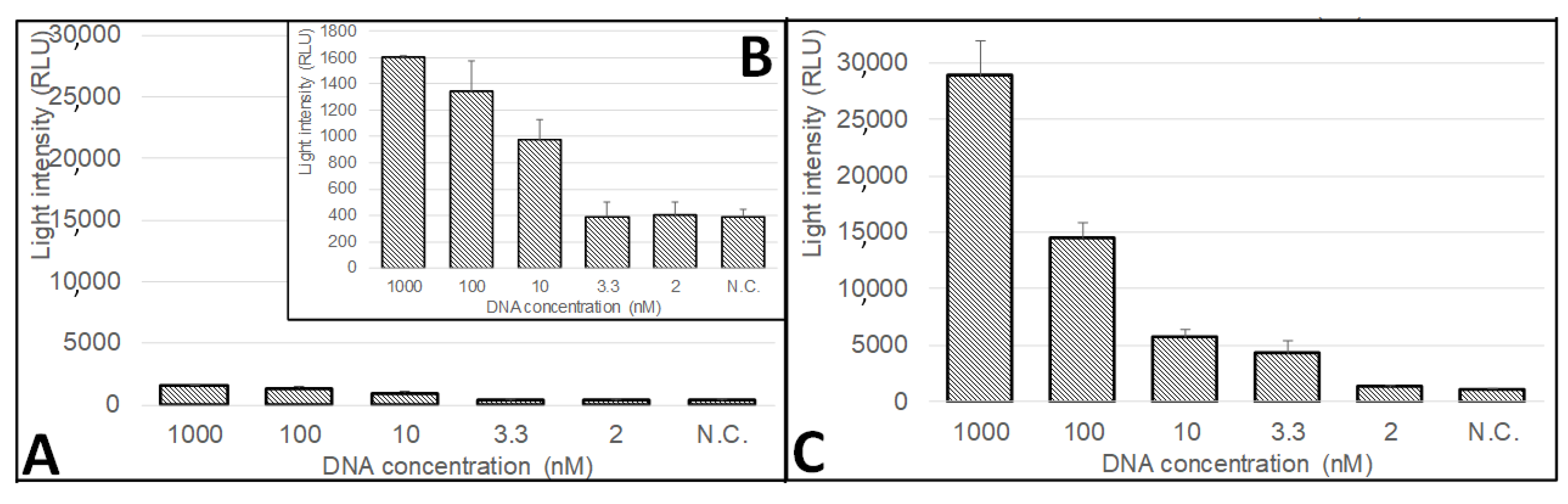
3.5. The Sensitivity of the CMOS-Based Biosensor System to Colletotrichum gloeosporioides Fungi
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saravanakumar, V.; Malaiarasan, U.; Balasubramanian, R. Sustainable Agriculture, Poverty, Food Security and Improved Nutrition. In Sustainable Development Goals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 13–39. [Google Scholar]
- Bahar, N.H.A.; Lo, M.; Sanjaya, M.; Van Vianen, J.; Alexander, P.; Ickowitz, A.; Sunderland, T. Meeting the food security challenge for nine billion people in 2050: What impact on forests? Glob. Environ. Chang. 2020, 62, 102056. [Google Scholar] [CrossRef]
- Faostat, F. Agriculture Organization of the United Nations Statistics Division; Economic and Social Development Department: Rome, Italy, 2016. [Google Scholar]
- Okawa, K. Market and trade impacts of food loss and waste reduction. OECD Food Agric. Fish. Pap. 2015. [Google Scholar] [CrossRef]
- Mbow, C.; Rosenzweig, C.; Barioni, L.G.; Benton, T.G.; Herrero, M.; Krishnapillai, M.; Liwenga, E.; Pradhan, P.; Rivera-Ferre, M.G.; Sapkota, T.B.; et al. Food security. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Searchinger, T.; Waite, R.; Hanson, C.; Ranganathan, J.; Dumas, P.; Matthews, E.; Klirs, C. Creating a Sustainable Food Future: A Menu of Solutions to Feed Nearly 10 Billion People by 2050. Final Report; WRI: Washington, DC, USA, 2019. [Google Scholar]
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. Trac. Trends Anal. Chem. 2020, 124, 115814. [Google Scholar] [CrossRef]
- Rahul, S.N.; Khilari, K.; Sagar, S.; Chaudhary, S.; Kumar, S.; Vihan, N.; Tomar, A. Challenges in postharvest management of fungal diseases in fruits and vegetables-a review. South Asian J. Food Technol. Environ. 2015, 1, 126–130. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Fallik, E.; Ilić, Z. Control of Postharvest Decay of Fresh Produce by Heat Treatments; the Risks and the Benefits. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Droby, S.; Lichter, A. Post-harvest Botrytis infection: Etiology, development and management. In Botrytis: Biology, Pathology and Control; Springer: Berlin/Heidelberg, Germany, 2007; pp. 349–367. [Google Scholar]
- Palou, L. Chapter 2—Penicillium digitatum, Penicillium italicum (Green Mold, Blue Mold). In Postharvest Decay; Bautista-Baños, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 45–102. [Google Scholar]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef]
- Alkan, N.; Friedlander, G.; Ment, D.; Prusky, D.; Fluhr, R. Simultaneous transcriptome analysis of Colletotrichum gloeosporioides and tomato fruit pathosystem reveals novel fungal pathogenicity and fruit defense strategies. New Phytol. 2015, 205, 801–815. [Google Scholar] [CrossRef]
- Fang, Y.; Ramasamy, R.P. Current and Prospective Methods for Plant Disease Detection. Biosensors 2015, 5, 537–561. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Marco, M.P.; Barcelo, D. Biosensors for environmental monitoring—A global perspective. Talanta 2005, 65, 291–297. [Google Scholar] [CrossRef]
- Algaar, F.; Eltzov, E.; Vdovenko, M.M.; Sakharov, I.Y.; Fajs, L.; Weidmann, M.; Mirazimi, A.; Marks, R.S. Fiber-Optic Immunosensor for Detection of Crimean-Congo Hemorrhagic Fever IgG Antibodies in Patients. Anal. Chem. 2015, 87, 8394–8398. [Google Scholar] [CrossRef]
- Caneira, C.R.F.; Soares, R.R.G.; Pinto, I.F.; Mueller-Landau, H.S.; Azevedo, A.M.; Chu, V.; Conde, J.P. Development of a rapid bead-based microfluidic platform for DNA hybridization using single- and multi-mode interactions for probe immobilization. Sens. Actuators B Chem. 2019, 286, 328–336. [Google Scholar] [CrossRef]
- Mann, M.; Hojrup, P.; Roepstorff, P. Use of Mass-Spectrometric Molecular-Weight Information to Identify Proteins in Sequence Databases. Biol. Mass Spectrom. 1993, 22, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Nemoto, J.; Shiba, S.; Yamane, Y.; Kawai, G.; Hashimoto, K. Immobilization of DNA on Quartz Crystal Microbalance Sensor Modified with Self-Assembled Monolayer of Thiol Derivative. J. Oleo Sci. 2020, 69, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Marx, K.A. Quartz crystal microbalance: A useful tool for studying thin polymer films and complex biomolecular systems at the solution−surface interface. Biomacromolecules 2003, 4, 1099–1120. [Google Scholar] [CrossRef]
- Baker, R.J. Institute of Electronics. CMOS Circuit Design, Layout, and Simulation; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Zhang, P. Chapter 3—Sensors and actuators. In Advanced Industrial Control Technology; Zhang, P., Ed.; William Andrew Publishing: Oxford, UK, 2010; pp. 73–116. [Google Scholar]
- Nikkhoo, N.; Cumby, N.; Gulak, P.G.; Maxwell, K.L. Rapid Bacterial Detection via an All-Electronic CMOS Biosensor. PLoS ONE 2016, 11, e0162438. [Google Scholar] [CrossRef]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Sci. Rep. 2015, 5, 9152. [Google Scholar] [CrossRef]
- Axelrod, T.; Eltzov, E.; Marks, R.S. Bioluminescent bioreporter pad biosensor for monitoring water toxicity. Talanta 2016, 149, 290–297. [Google Scholar] [CrossRef]
- Eltzov, E.; Cohen, A.; Marks, R.S. Bioluminescent liquid light guide pad biosensor for indoor air toxicity monitoring. Anal. Chem. 2015, 87, 3655–3661. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; Chung, Y.; Ultralow-Light CMOS Bio-Optical Sensor Enables Low-Cost, Portable Molecular Testing. Photonics Spectra 2017. Available online: http://www.anitoa.com/docs/anitoa-whitepaper-l.pdf (accessed on 12 December 2020).
- Zhou, S.; Gou, T.; Hu, J.; Wu, W.; Ding, X.; Fang, W.; Hu, Z.; Mu, Y. A highly integrated real-time digital PCR device for accurate DNA quantitative analysis. Biosens. Bioelectron. 2019, 128, 151–158. [Google Scholar] [CrossRef]
- Tang, D.; Lin, Y.; Zhou, Q.; Lin, Y.; Li, P.; Niessner, R.; Knopp, D. Low-Cost and Highly Sensitive Immunosensing Platform for Aflatoxins Using One-Step Competitive Displacement Reaction Mode and Portable Glucometer-Based Detection. Anal. Chem. 2014, 86, 11451–11458. [Google Scholar] [CrossRef]
- Gao, Z.; Tang, D.; Xu, M.; Chen, G.; Yang, H. Nanoparticle-based pseudo hapten for target-responsive cargo release from a magnetic mesoporous silica nanocontainer. Chem. Commun. 2014, 50, 6256–6258. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Wei, Q.; Xu, M.; Zhuang, J.; Tang, D. Enzyme-controlled dissolution of MnO2 nanoflakes with enzyme cascade amplification for colorimetric immunoassay. Biosens. Bioelectron. 2017, 89, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Wei, Q.; Zhuang, J.; Lu, M.; Tang, D. Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens. Bioelectron. 2016, 80, 249–256. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, J.; Ye, Y.; Zhang, J.; Zhang, Y.; Sun, X. Loop-mediated isothermal amplification-based microfluidic chip for pathogen detection. Crit. Rev. Food Sci. Nutr. 2020, 60, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Chalupowicz, D.; Veltman, B.; Droby, S.; Eltzov, E. Evaluating the use of biosensors for monitoring of Penicillium digitatum infection in citrus fruit. Sens. Actuators B Chem. 2020, 311, 127896. [Google Scholar] [CrossRef]
- Kwon, O.; Park, T. Applications of smartphone cameras in agriculture, environment, and food: A review. J. Biosyst. Eng. 2017, 42, 330–338. [Google Scholar]
- Eltzov, E.; Prilutsky, D.; Kushmaro, A.; Marks, R.S.; Geddes, C.D. Metal-enhanced bioluminescence: An approach for monitoring biological luminescent processes. Appl. Phys. Lett. 2009, 94, 083901. [Google Scholar] [CrossRef]
- Anitoa Systems LLC. ULS24 Product Brief. In 149 Commonwealth Drive, Suite 1001, Menlo Park, CA 94025. 2020. Available online: http://www.anitoa.com/docs/uls24_brief.pdf (accessed on 1 October 2020).
- Moon, J.H.; Kim, K.H.; Choi, H.W.; Lee, S.W.; Park, S.J. Electroless silver coating of rod-like glass particles. Ultramicroscopy 2008, 108, 1307–1310. [Google Scholar] [CrossRef]
- Chen, W.; Thoreson, M.D.; Ishii, S.; Kildishev, A.V.; Shalaev, V.M. Ultra-thin ultra-smooth and low-loss silver films on a germanium wetting layer. Opt. Express 2010, 18, 5124–5134. [Google Scholar] [CrossRef]
- Barthel, E.; Kerjan, O.; Nael, P.; Nadaud, N. Asymmetric silver to oxide adhesion in multilayers deposited on glass by sputtering. Thin Solid Film. 2005, 473, 272–277. [Google Scholar] [CrossRef]
- Perkas, N.; Amirian, G.; Applerot, G.; Efendiev, E.; Kaganovskii, Y.; Ghule, A.V.; Chen, B.J.; Ling, Y.C.; Gedanken, A. Depositing silver nanoparticles on/in a glass slide by the sonochemical method. Nanotechnology 2008, 19, 435604. [Google Scholar] [CrossRef] [PubMed]
- Caillosse, E.; Zaier, M.; Mezghani, M.; Hajjar-Garreau, S.; Vidal, L.; Lougnot, D.; Balan, L. Photo-Induced Self-Assembly of Silver Nanoparticles for Rapid Generation of First and Second Surface Mirrors. ACS Appl. Nano Mater. 2020, 3, 6531–6540. [Google Scholar] [CrossRef]
- Lončarić, M.; Sancho-Parramon, J.; Pavlović, M.; Zorc, H.; Dubček, P.; Turković, A.; Bernstorff, S.; Jakopic, G.; Haase, A. Optical and structural characterization of silver islands films on glass substrates. Vacuum 2009, 84, 188–192. [Google Scholar] [CrossRef]
- Pitts, J.R.; Thomas, T.M.; Czanderna, A.W. Method of Bonding Silver to Glass and Mirrors Produced according to This Method. U.S. Patents US 45,474,32A, 15 October 1985. [Google Scholar]
- Noethe, A.; Rissmann, M.; Paul, T. Coatings with a Silver Layer. U.S. Patents US 657,294,0B1, 3 June 2003. [Google Scholar]
- Novis, Y.; Depauw, J.-M.; Decroupet, D. Transparent Substrate Coated with a Silver Layer. U.S. Patents US 784,654,9B2, 7 December 2010. [Google Scholar]
- Montazer, M.; Alimohammadi, F.; Shamei, A.; Rahimi, M.K. In situ synthesis of nano silver on cotton using Tollens’ reagent. Carbohydr. Polym. 2012, 87, 1706–1712. [Google Scholar] [CrossRef]
- Ersoy, M.S.; Onder, E. Electroless silver coating on glass stitched fabrics for electromagnetic shielding applications. Text. Res. J. 2014, 84, 2103–2114. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, R.; Chen, H.; Hou, X.; Liu, G.; Liu, Y. Silver-coated glass fibers prepared by a simple electroless plating technique. J. Mater. Sci. Mater. Electron. 2014, 25, 4638–4642. [Google Scholar] [CrossRef]
- Chitvoranund, N.; Jiemsirilers, S.; Kashima, D.P. Effects of surface treatments on adhesion of silver film on glass substrate fabricated by electroless plating. J. Aust. Ceram. Soc. 2013, 49, 62–69. [Google Scholar] [CrossRef]
- Cruz-Pacheco, A.F.; Muñoz-Castiblanco, D.T.; Gómez Cuaspud, J.A.; Paredes-Madrid, L.; Parra Vargas, C.A.; Martínez Zambrano, J.J.; Palacio Gómez, C.A. Coating of Polyetheretherketone Films with Silver Nanoparticles by a Simple Chemical Reduction Method and Their Antibacterial Activity. Coatings 2019, 9, 91. [Google Scholar] [CrossRef]
- Chen, J.E.; Wang, Q.; Shull, K.R.; Richards, J.J. Control over Electroless Plating of Silver on Silica Nanoparticles with Sodium Citrate. J. Colloid Interface Sci. 2020, 576, 376–384. [Google Scholar] [CrossRef]
- Hussain, A.R.J.; Alahyari, A.A.; Eastman, S.A.; Thibaud-Erkey, C.; Johnston, S.; Sobkowicz, M.J. Review of polymers for heat exchanger applications: Factors concerning thermal conductivity. Appl. Therm. Eng. 2017, 113, 1118–1127. [Google Scholar] [CrossRef]
- Hafezian, S.; Beaini, R.; Martinu, L.; Kéna-Cohen, S. Degradation mechanism of protected ultrathin silver films and the effect of the seed layer. Appl. Surf. Sci. 2019, 484, 335–340. [Google Scholar] [CrossRef]
- Ando, E.; Miyazaki, M. Moisture degradation mechanism of silver-based low-emissivity coatings. Thin Solid Film. 1999, 351, 308–312. [Google Scholar] [CrossRef]
- Kurapati, R.; Maurice, V.; Seyeux, A.; Klein, L.H.; Mercier, D.; Chauveau, G.; Grèzes-Besset, C.; Berthod, L.; Marcus, P. Advanced protection against environmental degradation of silver mirror stacks for space application. J. Mater. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Khan, A.; Nguyen, V.H.; Muñoz-Rojas, D.; Aghazadehchors, S.; Jiménez, C.; Nguyen, N.D.; Bellet, D. Stability enhancement of silver nanowire networks with conformal ZnO coatings deposited by atmospheric pressure spatial atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 19208–19217. [Google Scholar] [CrossRef]
- Moussy, F.; Harrison, D.J. Prevention of the rapid degradation of subcutaneously implanted Ag/AgCl reference electrodes using polymer coatings. Anal. Chem. 1994, 66, 674–679. [Google Scholar] [CrossRef]
- Tracy, C.E.; Benson, D.K. Passivation Coating for Flexible Substrate Mirrors. U.S. Patents US 49,630,12A, 16 October 1990. [Google Scholar]
- Morales, A.; Duran, A. Sol-gel protection of front surface silver and aluminum mirrors. J. Sol.-Gel. Sci. Technol. 1997, 8, 451–457. [Google Scholar] [CrossRef]
- Tracy, C.E.; Benson, D.K. Silicon Nitride Protective Coatings for Silvered Glass Mirrors. U.S. Patents US 47,803,72A, 25 October 1988. [Google Scholar]
- Geddes, C.D.; Lakowicz, J.R. Metal-enhanced fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Aslan, K.; Leonenko, Z.; Lakowicz, J.R.; Geddes, C.D. Fast and Slow Deposition of Silver Nanorods on Planar Surfaces: Application to Metal-Enhanced Fluorescence. J. Phys. Chem. B 2005, 109, 3157–3162. [Google Scholar] [CrossRef]
- Aslan, K.; Gryczynski, I.; Malicka, J.; Matveeva, E.; Lakowicz, J.R.; Geddes, C.D. Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr. Opin. Biotechnol. 2005, 16, 55–62. [Google Scholar] [CrossRef]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal enhanced fluorescence biosensing: From ultra-violet towards second near-infrared window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef]
- Aslan, K.; Huang, J.; Wilson, G.M.; Geddes, C.D. Metal-enhanced fluorescence-based RNA sensing. J. Am. Chem. Soc. 2006, 128, 4206–4207. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kook, Y.-M.; Lee, K.; Koh, W.-G. Metal enhanced fluorescence (MEF) for biosensors: General approaches and a review of recent developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.I.; Bishop, E.S.; Casas-Finet, J.R.; Strouse, R.J.; McGivney, J.; Schenerman, M.A.; Geddes, C.D. Distance dependence of metal-enhanced fluorescence. Plasmonics 2012, 7, 739–744. [Google Scholar] [CrossRef]
- Pribik, R.; Dragan, A.I.; Zhang, Y.; Gaydos, C.; Geddes, C.D. Metal-Enhanced Fluorescence (MEF): Physical characterization of Silver-island films and exploring sample geometries. Chem. Phys. Lett. 2009, 478, 70–74. [Google Scholar] [CrossRef]
- Aslan, K.; Leonenko, Z.; Lakowicz, J.R.; Geddes, C.D. Annealed Silver-Island Films for Applications in Metal-Enhanced Fluorescence: Interpretation in Terms of Radiating Plasmons. J. Fluoresc. 2005, 15, 643. [Google Scholar] [CrossRef]
- Ray, M.; Ray, A.; Dash, S.; Mishra, A.; Achary, K.G.; Nayak, S.; Singh, S. Fungal disease detection in plants: Traditional assays, novel diagnostic techniques and biosensors. Biosens. Bioelectron. 2017, 87, 708–723. [Google Scholar] [CrossRef]
- Cecchini, F.; Manzano, M.; Mandabi, Y.; Perelman, E.; Marks, R.S. Chemiluminescent DNA optical fibre sensor for Brettanomyces bruxellensis detection. J. Biotechnol. 2012, 157, 25–30. [Google Scholar] [CrossRef]
- Mendes, R.K.; Carvalhal, R.F.; Stach-Machado, D.R.; Kubota, L.T. Surface plasmon resonance immunosensor for early diagnosis of Asian rust on soybean leaves. Biosens. Bioelectron. 2009, 24, 2483–2487. [Google Scholar] [CrossRef]
- Mendes, R.K.; Ferreira, D.C.M.; Carvalhal, R.F.; Peroni, L.A.; Stach-Machado, D.R.; Kubota, L.T. Development of an electrochemical immunosensor for Phakopsora pachyrhizi detection in the early diagnosis of soybean rust. J. Braz. Chem. Soc. 2009, 20, 795–801. [Google Scholar] [CrossRef]
- Wongkaew, P.; Poosittisak, S. Diagnosis of sugarcane white leaf disease using the highly sensitive DNA based voltammetric electrochemical determination. Am. J. Plant Sci. 2014, 5, 2256. [Google Scholar] [CrossRef]
- Eun, A.J.-C.; Huang, L.; Chew, F.-T.; Li, S.F.-Y.; Wong, S.-M. Detection of two orchid viruses using quartz crystal microbalance-based DNA biosensors. Phytopathology 2002, 92, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, G.; Skandalis, N.; Dimopoulou, A.; Glynos, P.; Gizeli, E. Bacteria Murmur: Application of an Acoustic Biosensor for Plant Pathogen Detection. PLoS ONE 2015, 10, e0132773. [Google Scholar] [CrossRef] [PubMed]
- MacKay, S.; Wishart, D.; Xing, J.Z.; Chen, J. Developing Trends in Aptamer-Based Biosensor Devices and Their Applications. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Thewes, R.; Hofmann, F.; Frey, A.; Holzapfl, B.; Schienle, M.; Paulus, C.; Schindler, P.; Eckstein, G.; Kassel, C.; Stanzel, M.; et al. Sensor arrays for fully-electronic DNA detection on CMOS. In Proceedings of the 2002 IEEE International Solid-State Circuits Conference. Digest of Technical Papers (Cat. No.02CH37315), San Francisco, CA, USA, 7 February 2002; Volume 1, pp. 350–473. [Google Scholar]
- Zhang, G.-J.; Chua, J.H.; Chee, R.-E.; Agarwal, A.; Wong, S.M. Label-free direct detection of MiRNAs with silicon nanowire biosensors. Biosens. Bioelectron. 2009, 24, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harpaz, D.; Alkan, N.; Eltzov, E. The Incorporation of Amplified Metal-Enhanced Fluorescence in a CMOS-Based Biosensor Increased the Detection Sensitivity of a DNA Marker of the Pathogenic Fungus Colletotrichum gloeosporioides. Biosensors 2020, 10, 204. https://doi.org/10.3390/bios10120204
Harpaz D, Alkan N, Eltzov E. The Incorporation of Amplified Metal-Enhanced Fluorescence in a CMOS-Based Biosensor Increased the Detection Sensitivity of a DNA Marker of the Pathogenic Fungus Colletotrichum gloeosporioides. Biosensors. 2020; 10(12):204. https://doi.org/10.3390/bios10120204
Chicago/Turabian StyleHarpaz, Dorin, Noam Alkan, and Evgeni Eltzov. 2020. "The Incorporation of Amplified Metal-Enhanced Fluorescence in a CMOS-Based Biosensor Increased the Detection Sensitivity of a DNA Marker of the Pathogenic Fungus Colletotrichum gloeosporioides" Biosensors 10, no. 12: 204. https://doi.org/10.3390/bios10120204
APA StyleHarpaz, D., Alkan, N., & Eltzov, E. (2020). The Incorporation of Amplified Metal-Enhanced Fluorescence in a CMOS-Based Biosensor Increased the Detection Sensitivity of a DNA Marker of the Pathogenic Fungus Colletotrichum gloeosporioides. Biosensors, 10(12), 204. https://doi.org/10.3390/bios10120204






