Abstract
COVID-19 is known as one of the deadliest pandemics of the century. The rapid spread of this deadly virus at incredible speed has stunned the planet and poses a challenge to global scientific and medical communities. Patients with COVID-19 are at an increased risk of co-morbidities associated with liver dysfunction and injury. Moreover, hepatotoxicity induced by antiviral therapy is gaining importance and is an area of great concern. Currently, alternatives therapies are being sought to mitigate hepatic damage, and there has been growing interest in the research on bioactive phytochemical agents (nutraceuticals) due to their versatility in health benefits reported in various epidemiological studies. Therefore, this review provides information and summarizes the juncture of antiviral, immunomodulatory, and hepatoprotective nutraceuticals that can be useful during the management of COVID-19.
1. COVID-19: From Outbreak to Pandemic
The Coronavirus Disease 19 (COVID-19) is a highly infectious disease caused by a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1] (Figure 1A). The COVID-19 disease, which has infected millions and shocked the planet, emerges from the animal kingdom. Since December 2019, the first case of coronavirus disease (COVID-19) resulted from zoonotic transmission from the seafood and live wild animal market in Wuhan, China. As of 12 December 2020, approximately 72 million infections and more than 1,610,684 deaths have been reported, and these statistics are likely to be higher due to inadequate monitoring in parts of the world and asymptomatic carriers [2,3,4] (Figure 1B). The SARS-CoV-2 pandemic can be regarded as the largest global public health disaster since the onset of the 1918 pandemic.
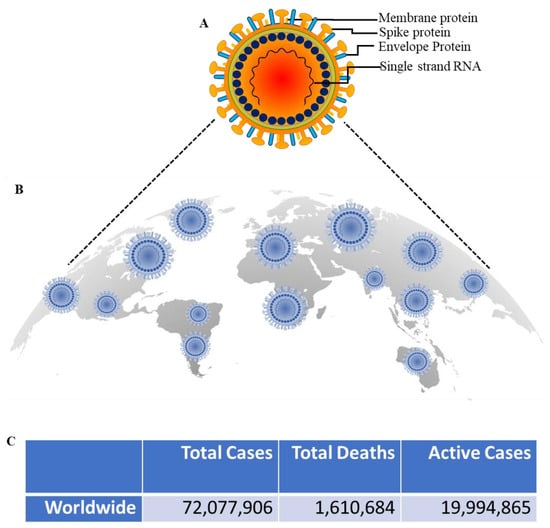
Figure 1.
Global COVID-19 pandemic. (A); SARS-CoV-2 structure (B); Schematic representation of putative worldwide distribution of total cases, total deaths, and the active cases of COVID-19. (C); The number of total COVID-19 cases and deaths associated to COVID-19 was derived from Worldometer. Information obtained from https://www.worldometers.info/coronavirus/ on 12 December 2020.
SARS-CoV-2 primarily causes respiratory symptoms, including flu-like symptoms and interstitial pneumonia, which may further progress into fatal Acute Respiratory Distress Syndrome (ARDS) condition [5,6]. However, other organs, particularly the liver, heart, and kidneys, are also affected, resulting in multi-organ failure and death in some patients [6]. Higher incidences of aminotransferases in and/or bilirubin were reported in COVID-19 patients to a varying degree. Moreover, several COVID-19 patients have experienced some type of liver injury, particularly those with serious or critical cases [7]. This prompted hepatologists to collaborate with other physicians, such as internal medicine and emergency departments, to protect the health status and avoid the adverse effects of COVID-19 in people with liver disease. Given the global burden of chronic liver disease, this pandemic could further worsen the treatment of patients at risk [8]. This review aims to gather information on medicinal plants and nutraceuticals with hepatoprotective activity, which can protect against the hepatic damage caused by COVID-19 and antiviral drugs.
2. Pathogenesis of COVID-19
Coronaviruses are single-stranded RNA viruses known to cause human respiratory tract or animal intestinal infection [9,10,11] (Figure 2A). Primarily, there are four main types, including α-coronavirus, β-coronavirus, δ-coronavirus, and γ-coronavirus [12]. Six coronaviruses, including SARS-CoV (Severe Acute Respiratory Syndrome CoV) and MERS-CoV (Middle East Respiratory Syndrome CoV), were reported to cause illness in humans prior to SARS-CoV-2 [13]. Similar to SARS-CoV and MERS-CoV, SARS-CoV-2 belongs to β-coronavirus. SARS-CoV-2 and SARS have a genome sequence homology of approximately 79% and is more closely related to SARS-like bat coronaviruses than SARS-CoV [14]. The spike protein recognizes and binds to the receptor and invades the host cell via clathrin-mediated endocytosis [15]. After internalization, the virus manipulates the cells’ reproductive machinery to produce more copies to infect other cells. SARS-CoV-2 also utilizes the cofactors, Furin and transmembrane proteases, serine 2 (TMPRSS2), protein cleaving enzymes, to cleave viral S-protein and further facilitate the virus-cell fusion [16,17]. As revealed in structure model analysis, SARS-CoV-2 binds to Angiotensin I Converting Enzyme 2 (ACE2), a host receptor, with more than a 10-fold higher affinity as compared to SARS-CoV [18] (Figure 2B). These findings explain the greater propagation capacity of SARS-CoV-2 in humans relative to SARS-CoV and the higher number of reported cases of COVID-19 relative to SARS-CoV infection [19]. The precise mechanism by which SARS-CoV-2 influences humans via S-protein binding to ACE2, the association intensity for the danger of human transmission, and how SARS-CoV-2 causes organ damage remains unclear, and further studies are required.
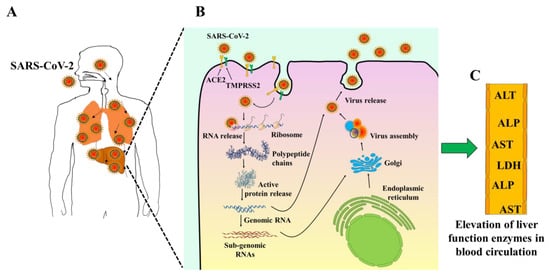
Figure 2.
Schematic diagram showing the potential mechanisms of liver injury and abnormality in liver. (A); COVID-19 infection through the respiratory tract. (B); SARS-CoV-2 binds via its spike proteins to target cells receptor angiotensin-converting enzyme II (ACE2). (C); Liver function impairment with a mild-to-moderate increase of liver function enzymes level in the bloodstream.
3. Evidence for the Involvement of Liver in COVID-19 Infections
Early observational studies have shown the elevation in hepatic enzymes, including aspartate transferase, alanine transferase, and total bilirubin in COVID-19 patients [20,21,22,23,24,25,26,27] (Figure 2C). A study conducted by Chen et al. (2019) showed that more than a third of patients with COVID-19 have some liver function test abnormalities [25]. It is uncertain if these laboratory test variations are linked with a poorer prognosis. In another study of 1099 patients from 552 hospitals, Guan and colleagues found elevated AST levels in 112 (18.2 percent) patients with non-serious disease and 56 (39.4 percent) patients with severe disease [21]. In comparison, the proportion of pathological ALTs in severe cases (28.1%) was higher than in moderate cases (19.8%). Correspondingly, Huang et al. recorded that the proportion of ICU patients with liver damage (61.5%) was higher than non-ICU patients (25.0%) (25.0%) [24]. Recent clinical trials of COVID-19 suggest that elevated transaminases, elevated bilirubin, prolonged prothrombin period, hypoproteinemia, and intensity of blood test abnormalities can predict a worse outcome [28].
4. Mechanism of Liver Injury in COVID-19
The exact mechanism of COVID-19 mediated liver injury is not fully known. In the following sections, various putative mechanisms involved in underlying hepatic injury are presented (Figure 3).
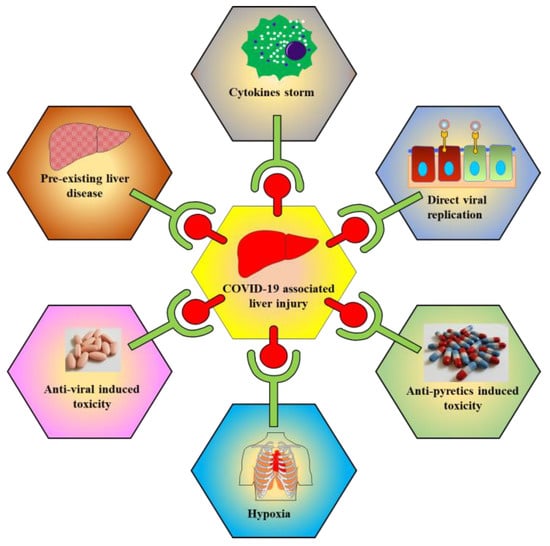
Figure 3.
Schematic representations show multiple factors are responsible for COVID-19 associated liver toxicity/injury. Factors include cytokine storm, direct viral replication, antipyretics induced toxicity, hypoxia, antiviral induced toxicity, and pre-existing liver disease.
4.1. Direct Effect of COVID-19 on Liver
As previously discussed, SARS-CoV-2 uses the ACE2 receptor for entry into the host cells, where the lung is the main target for infection. Reports from RNA-seq analysis have confirmed the expression of the ACE2 receptor in the liver. Studies have shown the expression of ACE2 receptor on liver tissues [29]. Furthermore, liver histology of patients infected with COVID-19 reveals microvascular steatosis, multinuclear syncytial hepatocytes, and moderate lobular and portal activity [30]. Moreover, mitochondrial swelling, endoplasmic reticulum dilatation, glycogen degradation, and damaged cell membranes are demonstrated by electron microscopy. These microscopic and ultrastructural characteristics co-occur with the SARS-CoV-2’s cytopathic influence on hepatocytes, suggesting its role for the viral replication within liver cells [31].
4.2. Cytokine Storm Mediated Hepatic Damage
It is observed that the host body activates an immune response against SARS-CoV-2 infection to facilitate virus clearance and induce a sustained adaptive immune response. Moreover, serologic analysis has shown elevated levels of Th17, CD8+ T-cells, IL-2, IL-6, IL-10, TNF-α, GM-CSF, MCP-1, and macrophage inflammatory protein 1 α in patients with severe COVID-19 infection, as compared to those in control [24,32,33,34]. Understandably, these SARS-CoV-2 associated cytokine storms can damage many organs, including the liver and gut. Many patients eventually died following organ failure [35]. In a recent meta-analysis, the prevalence of chronic liver disease (CLD) patients (73 studies, 24,299 patients) was 3% among all COVID-19 patients [36]. The prevalence of CLD patients was similar in COVID-19 positive and negative populations (pooled OR 0.79 [95% CI 0.60, 1.05], p = 0.10). The presence of CLD was significantly associated with more severe COVID-19 infection (pooled OR 1.48 [95% CI 1.17, 1.87], p = 0.001) and overall mortality (pooled OR 1.78 [95% CI 1.09, 2.93], p = 0.02) [36].
4.3. Hypoxia Associated Liver Damage
Extensive release of cytokines by the immune system in response to viral infection often results in sepsis symptoms, which caused mortality in 28% of COVID-19 cases [3]. Sepsis is generally referred to as the dysregulated immune response to an infection that results in multiple organ dysfunction [37]. The pathophysiology of sepsis-associated liver injury involves hypoxic liver injury due to several factors, including ischemia and shock, cholestasis, and overwhelming inflammation [38]. It is observed that sepsis is not uncommon in patients with existing liver cirrhosis [39], suggesting that pneumonia associated hypoxia is one of the most significant factors causing secondary liver injury in COVID-19 patients.
4.4. Antiviral Induced Hepatotoxicity
Microvascular steatosis and mild lobular and portal activities in postmortem biopsies reveal that liver injury is either caused by SARS-CoV-2 infection or drug-induced toxicity [30]. In another study, it was observed that antiviral therapies, including lopinavir/ritonavir used for the treatment of COVID-19, induces liver injury in patients [40]. Therefore, hepatic enzyme defects arose following the usage of a hepatotoxic medication; and antiviral induced damage may first be verified or removed.
4.5. Antipyretics Induced Hepatotoxicity
Many affected people were administered with antipyretic agents for fever relief during the COVID-19 outbreak. Many of these medicines include acetaminophen, a medication believed to inflict serious harm to the liver and/or trigger liver failure [30]. Acetaminophen is also known as APAP (acetyl-para-aminophenol) in the USA and paracetamol in Europe, is one of the most widely used antipyretics and analgesics medications in the world [41].
4.6. Pre-Existing Liver Disease Leads to Worst COVID-19 Outcome
Due to systemic immunodeficiency, patients with chronic liver disease and cirrhosis may have a higher risk of COVID-19 infections [42]. Moreover, a post-transplant patient is at higher risk due to immunosuppressive therapy [43,44]. However, the relationship between underlying liver disease and COVID-19 has not been fully studied. Patients with cirrhosis are at an increased risk of decompensation or development of acute-on-chronic liver failure when coupled with a bacterial, fungal, or viral infection [45,46]. Co-morbidities, including coronary artery disease, cerebrovascular disease, and chronic obstructive pulmonary disease, are more prevalent in hospitalized patients with severe/critical illnesses from COVID-19, and these patients are more likely to manifest abnormal liver chemistries [47]. Therefore, special attention should be paid to monitoring hepatic changes triggered by COVID-19 in patients with a pre-existing history of liver disease (especially older patients).
5. Hepatoprotective Agents
Most of the drugs are metabolized in the liver. As a result, liver injury can occur even though they are consumed for therapeutic purposes [48]. Since liver injury may result in fatty liver, hepatitis, fibrosis, cirrhosis, and cancer, this is considered as a serious health concern. Accumulated studies have reported that herbal compounds possess numerous medicinal properties. Natural products and nutraceuticals have shown potent therapeutic activity in liver injuries caused by several toxicants and drugs [49,50,51,52,53]. This section summarizes selective natural bioactive nutraceuticals exhibiting the hepatoprotective activity and pays specific attention to toxicants/antiviral induced liver injury (Figure 4). Few of these have shown potent antiviral activities and may be used in the future as possible options for the COVID-19 treatment
Figure 4.
Various medicinal plants possessing potential hepatoprotective activities.
5.1. Silybum Marianum
Silybum marianum belongs to the Asteraceae family and is native to the Mediterranean region. It is commonly known as milk thistle. This plant has thorny branches and milky sap, with oval leaves of up to 30 cm. The flowers are light pink in colors and can be up to 8 cm in diameter [54]. This plant is grown in Hungary, China, and South America. In Mexico, milk thistle is consumed as a nutritional substitute [55]. Silymarin is a naturally occurring compound found in Silybum marianum and is most notably composed of numerous flavolignans, including silybin, silydianin, and silychristine. Silybin constitutes approximately 50% to 70% of silymarin extracts. This plant possesses several pharmacological activities and is used to treat disorders related to the liver, gall bladder, and spleen [56]. Notably, because of its antioxidant, anti-inflammatory, and anti-fibrotic activities, silymarin is probably the most commonly used natural compound for hepatic disease care worldwide [57]. Most importantly, its medicinal property has been explored as hepatoprotective for supportive therapy in case of hepatic dysfunctions such as hepatitis, cirrhosis, and fatty liver [58,59]. Moreover, studies have shown that Silybum marianum is very potent against stress induced by toxicants, including poisonous mushrooms, alcohol, and toxic chemicals [60]. Bioactive components isolated from Silybum spp., silymarin, showed therapeutic benefits in acute and chronic viral, alcohol, and chemically induced hepatitis [61]. Silymarin is the most frequently used natural compound for treating hepatic diseases worldwide due to its antioxidant, anti-inflammatory, and anti-fibrotic activities. Inhibition of cyclooxygenase cycle, leukotrienes, and free radical production contribute to its cytoprotective effects in liver [62].
Silymarin has also been known to increase protein synthesis in hepatocytes [57]. Owing to its phenolic existence, it can give electrons to stabilize free radicals and reactive oxygen molecules [63]. Silymarin also modulates intracellular glutathione, which inhibits membranes from lipid peroxidation [64]. Silymarin also has antiviral effects as it affects RNA and DNA synthesis [65]. Eurasil 85 is a strong oral bioavailable silymarin formulation with potent antioxidant activity are found in clinical studies, and co-administration with several antiretrovirals has been shown to be safe [66,67]. Although the use of silymarin in the management of hepatic dysfunctions remains a historical interest, its utility in the management of SAR-COV-2 induced liver dysfunction should be explored [68].
5.2. Solanum Nigrum
Solanum nigrum is commonly known as “Black nightshade” and is often cultivated in open, wild temperate climate regions [69,70,71]. This also constitutes food crops in several developing countries [72]. Solanum nigrum has numerous medicinal properties [73]. In traditional medicine, plant leaves have reportedly been used to treat many illnesses, including seizures, asthma, nausea, ulcers, vomiting, diarrhea, some eye infections, and jaundice [74,75]. The extracts contain many polyphenolic compounds, such as phenolic acids and flavones [76]. This herb is used as a potential hepatoprotective agent [69,70,71]. It exhibits several antioxidant activities and is known to inhibit lipid peroxidation as a means for their mechanism of action [77,78]. Aqueous extract of S. nigrum has been shown to reduce hepatic enzymes ALT, AST, and ALP significantly. Moreover, this inhibits bilirubin’s level and scavenge the free radicals production, as observed in CCL4 induced hepatic damage in rats [79]. The antioxidant activity may be attributable to the polyphenolic compounds’ existence in stems and leaves [80].
5.3. Cichorium Intybus
The plant Cichorium intybus, commonly known as “chicory”, is indigenous to Western Asia, Egypt, North America, and Europe [81]. This displays several therapeutic properties including anti-microbial [82,83], immunomodulatory [84], antihepatotoxic [85,86,87] and anti-hypertiglycemia activities [88]. Chichorium intybus extract showed remarkable antioxidative effects via inhibition of thiobarbituric acid reactive substances production [89]. Notably, it has been used in many liver tonics for the ailment of the liver and digestive disorders [90]. The root extract of C. intybus has shown anti-hepatotoxic activity against CCL4-induced hepatic damage as demonstrated by decreased levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin in treated groups as compared to control groups [91]. Interestingly, Zhang et al. (2014) studied the anti-hepatitis activity of chicoric acid isolated from Chichorium intybus leaves and observed that it can block the viral protein and DNA synthesis [92].
5.4. Allium Sativum
Allium sativum has been used for more than 5000 years and is commonly known as garlic. Allium sativum is used as a spice and has numerous medicinal properties. The active components present in this plant are diallyl thiosulfinate and diallyl disulfide. Studies have reported that pretreatment with extracts restored antioxidant enzyme levels in GalN/LPS-treated hepatitis in animal models [93]. Furthermore, it is known to attenuate the nitric oxide-induced oxidative stress, and lipid peroxidation in CCL4 treated mice [94]. Garlic oil has also reduced serum AST, ALT, alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) levels in CCL4-induced hepatotoxicity [95]. It has diverse pharmacological activities, including antibacterial, antiviral, antioxidant effects, anti-mutagenic, and immune-modulatory properties [96,97].
5.5. Glycyrrhiza Glabra
Glycyrrhiza glabra, commonly known as licorice, is native to the Mediterranean region, Western Asia and Europe. This plant is a very effective treatment for chest disease and asthma [56]. Studies have shown hepatoprotective activity of G. glabra in CCL4 induced hepatic damage. Following the oral administration, the level of hepatic iNOS, COX2, and TNF-alpha were significantly reduced in the treated group compared to the control group [98]. In another study, Kimura et al. showed that G. glabra is effective in attenuating the serum AST and ALT levels [99]. Glycyrrhizin, is a triterpenoid saponin, predominantly present in plant roots and possesses numerous pharmacological activities [100,101,102]. Interestingly, several studies have shown that glycyrrhizin exhibits potent activity against various human viruses [103,104,105,106,107,108,109,110,111,112,113,114,115,116]. Studies have shown the glycyrrhizin is used alone or in combination with other medications to combat coronavirus infections [117]. Recently, one study showed its ability to bind ACE2 that represses the SARS-CoV-2 receptor. Thus, targeting of ACE2 may also be very useful to prevent diffusion out of the virus from infected cells and invading new cells [118].
5.6. Phyllanthus Amarus
Phyllanthus amarus is a small plant and is commonly found in tropical regions and is used in many traditional medicines due to its diverse pharmacological activities [119]. Lignan phyllanthin, one of its essential bioactive constituents, has shown very potent activity as a hepatoprotective agent [120]. Histopathological analysis revealed that ethanolic extract of P. amarus attenuates the generation of intracellular ROS by enhancing the antioxidant levels against aflatoxin B1-induced hepatotoxicity [121]. Furthermore, an aqueous extract of P. amarus has been shown to inhibit HBV DNA polymerase activity as seen in in vitro experimental conditions, suggesting its potential use for viral infections in the liver [122]. Phytochemical screening of P. muellarianus, displayed the presence of several bioactive ingredients such as furosin, isoquercetin, phaselic acid, corilagin, nitidine, geranin, and gallic acid [123,124]. Aqueous extract of the P. muellarianus leaf has shown hepatoprotective activity against damage induced by p-acetaminophen in swiss albino mice [125]. It was observed that extract (p > 0.05) significantly attenuated acetaminophen-mediated alterations in ALT, alkaline phosphatase (ALP), AST, albumin (ALB), and total bilirubin (TB). Gallic acid, a well-known antioxidant agent, was documented to reverse AST, ALT, and ALP in acetaminophen-induced liver toxicity [126].
5.7. Withania Somnifera
Withania somnifera belongs to the family of Solanaceae and is commonly known as ashwagandha, Indian ginseng, or Winter cherry. Multiple parts of this medicinal plant, such as leaves, fruits, and stems, have therapeutic effects [127]. The popular bioactive ingredients present in this are withaferin A and withanolides. Several studies have shown the hepatoprotective activity of this plant without toxicity [127,128]. Withaferin A performs a crucial function as antiviral agents against several viruses, including HIV-1 [129], HPV [130], HSV [131], and infectious bursal diseases virus (IBDV) [131]. In a recent study, it has been reported that withaferin-A and withanone could bind and stably interact with the catalytic site of TMPRSS2, suggesting their use in the blocking of COVID-19 viral entry into host cells [132].
5.8. Curcuma Longa
Curcuma longa is a very famous spice native to India and Southeast Asia. This plant has diverse pharmacological activity, including antibacterial, antifungal, antiviral, and anticancer activities [133]. Curcumin (diferuloylmethane), a polyphenol present in this plant’s rhizome, is responsible for its therapeutic activity [134,135]. The hepatoprotective function of curcumin depends primarily on its strong anti-inflammatory and antioxidant effects. It also exhibits immunomodulatory activity by suppressing the production of cytokines IFN-gamma, ILs, and TNF-alpha. Curcumin is a very effective blocker of NF-kappa B and inhibits the synthesis of iNOS [136,137,138]. Research has also shown that curcumin inhibits hepatic stellate cell activation and collagen expression. Moreover, it has shown hepatoprotective effects in thioacetamide-induced liver injury and fibrosis [139]. Curcumin has also been reported to conduct antiviral activities against a large variety of viruses, including HIV-1, HSV-2, HPV and hepatitis virus [140,141].
5.9. Other Hepatoprotective Agents
Capparis spinosa, generally found in west and Central Asia, is commonly used as a cooking flavoring agent [142]. Several traditional medicines utilize this plant for the treatment of liver diseases [143]. This possesses numerous bioactivities, including antioxidant, anticancer, and antibacterial properties. It has been demonstrated that polyphenol present in the plant is responsible for its therapeutic activity. Liv-52, an Indian herbal preparation for liver disorders, also contains Capparis spinosa, an essential constituent [144]. Studies suggest that Liv-52 showed significant therapeutic effects in cirrhotic patients [144]. Additionally, the chemical constituent p-methoxy benzoic acid, from the aqueous extract of C. spinosa, expresses potent hepatoprotective activity against paracetamol and CCL4-induced hepatotoxicity [145].
Aquilaria agallocha has several properties, including pharmacological effects, and shows anticancer, antioxidant, anti-inflammatory, antidiabetic, analgesic, antipyretic, laxative, antidiabetic, antimicrobial, antibacterial, and anticonvulsant protective activities [146]. The hepatoprotective effects of the ethanolic extract of A. agallocha leaves in PCM-induced hepatotoxicity in Sprague–Dawley (SD) rats show a substantial decrease in AST, ALP, ALT, lactate dehydrogenase (LDH), CHL, TB, and an increase in ALB, total protein concentration, and prevention of PCM-induced histopathological changes in the liver [147].
Dodonaea viscosa belongs to the soapberry family and is widely distributed in the subtropical, warm, tropical, temperate regions of Africa, the Americas, Australia, and Southern Asia. Aqueous: methanolic (70:30) leaves extract of D. viscosa has shown to exhibit the hepatoprotective activity by reducing the serum level of TAG, total cholesterol, LDL-C, HDL-CHL, ALT, and AST compared to experimental control sample [148]. Another plant Salix caprea, referred to as goat willow, is a predominant species of willow in Europe and western/central Asia [149]. The detailed knowledge of various natural compounds’ hepatoprotective activity has been summarized in the review [150].
Ethanolic extract of Salix subserrata has shown significant hepatoprotective activity in CCl4-induced liver toxicity [149] by decreasing serum enzymes levels and reversing hepatic tissue damage caused by CCl4. Studies have shown that the hepatoprotective activity of ethanolic Pandanus odoratissimus roots extracts in PCM-induced hepatotoxicity in rats resulted in a substantial decrease in the higher levels of serum marker enzymes [151]. Another plant named Alocasia indica, commonly cultivated especially in West Bengal, Assam, Maharashtra, and Southern India, has been assessed for hepatoprotective activity in CCL4-induced hepatic injury in male Albino Wistar rats [152]. It has been reported that the antioxidant activity of this plant has the potential for designing drugs for liver diseases. Opuntia ficus-indica, another plant, is a cactus species that are commonly distributed in the arid and semiarid parts of the world and is believed to have originated from Mexico. Aqueous extract (2 mL/kg) from the cactus leaves (cladodes) has shown potent hepatoprotective activity in CCl4-induced toxicity in Wistar male rats by decreasing AST and ALT levels [153]. The use of these natural phytoagents or nutraceuticals alone or in combination may protect the liver and modulates coronavirus infection, and provide recommendations for further study.
6. Conclusions
The COVID-19 pandemic has resulted in a global crisis in public health. As noted in COVID-19 patients, liver injury is very common, caused by either direct or indirect damage to organs, including the overshooting inflammatory response. Importantly, the drug-induced liver injury should not be overlooked during coronavirus infection treatment and should be carefully studied. Ultimately, it is imperative to find alternative methods for hepatoprotection. This review provides information on the medicinal plants used for various hepatic disorders over several decades. It also highlights the pathways that these plant-based medicines can seek to reduce the burden of disease. The potential efficacy of these bioactive nutraceuticals should be explored in COVID-19 patients and at-risk populations.
Author Contributions
Authors M.S. and S.M. worked on the conceptualization, information compilation, analysis, and manuscript writing equally. Review and editing, A.R., M.M.Y., A.S.N., S.K.S., V.D., S.C.C., and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support by the National Institutes of Health (R01 CA210192, R01 CA206069, R01 CA204552) and partial support from the Herb Kosten Foundation, and Faculty Start-up fund from UTRGV to S.C.C., M.J., and M.M.Y.
Acknowledgments
We want to thank Molly K. Vela for helping with manuscript proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perricone, C.; Triggianese, P.; Bartoloni, E.; Cafaro, G.; Bonifacio, A.F.; Bursi, R.; Perricone, R.; Gerli, R. The anti-viral facet of anti-rheumatic drugs: Lessons from COVID-19. J. Autoimmun. 2020, 111, 102468. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance 2020, 25, 2000180. [Google Scholar] [CrossRef] [PubMed]
- Laskar, P.; Yallapu, M.M.; Chauhan, S.C. “Tomorrow Never Dies”: Recent Advances in Diagnosis, Treatment, and Prevention Modalities against Coronavirus (COVID-19) amid Controversies. Diseases 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. Jama 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, S. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J. Med Virol. 2020, 92, 1491–1494. [Google Scholar] [CrossRef]
- Musa, S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab. J. Gastroenterol. 2020, 21, 3–8. [Google Scholar] [CrossRef]
- Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. COVID-19: Fighting the invisible enemy with microRNAs. Expert Rev. Anti-Infective Ther. 2020, 1–9. [Google Scholar] [CrossRef]
- Kashyap, V.K.; Dhasmana, A.; Massey, A.; Kotnala, S.; Zafar, N.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Smoking and COVID-19: Adding Fuel to the Flame. Int. J. Mol. Sci. 2020, 21, 6581. [Google Scholar] [CrossRef]
- Yang, P.; Wang, X. COVID-19: A new challenge for human beings. Cell Mol. Immunol. 2020, 17, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; To, K.K.-W.; Tse, H.; Jin, D.-Y.; Yuen, K.-Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Müller, M.A.; Allen, P.D.; Soilleux, E.J.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int J. Antimicrob Agents 2020, 55, 105948. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J. Infect Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, I.J.B.; Cacic, N.; Abdulazeem, H.M.; Von Groote, T.C.; Jayarajah, U.; Weerasekara, I.; Esfahani, M.A.; Civile, V.T.; Marusic, A.; Jerončić, A.; et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J. Clin. Med. 2020, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, L.; Zhao, M.; Xiao, J.; Zhao, Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020, 40, 2095–2103. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Mallapaty, S. Why does the coronavirus spread so easily between people? Nat. Cell Biol. 2020, 579, 183. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Huang, D.; Ou, P.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Ma, Z.; Zhang, Y.; Li, Z.; et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020, 75, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, J.-G. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J. Clin. Transl. Hepatol. 2020, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Chu, K.H.; Tsang, W.K.; Tang, C.S.; Lam, M.F.; Lai, F.M.; To, K.F.; Fung, K.S.; Tang, H.L.; Yan, W.W.; Chan, H.W.; et al. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005, 67, 698–705. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Satapathy, S.K.; Thuluvath, P.J. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Lelubre, C.; Vincent, J.-L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 2018, 14, 417–427. [Google Scholar] [CrossRef]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, P.S.F.T.A.K.C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef]
- Joung, J.-Y.; Cho, J.; Kim, Y.; Choi, S.; Son, C.-G. A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 2019, 9, e01235. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, L.; Li, J.; Cheng, X.; Yang, J.; Tian, C.; Zhang, Y.; Huang, S.; Liu, Z.; Cheng, J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin. Gastroenterol. Hepatol. 2020, 18, 1561–1566. [Google Scholar] [CrossRef]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Liang, J.; Shen, J.; Ghosh, S.; Zhu, L.-R.; Yang, H.; Wu, K.-C.; Chen, M. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol. Hepatol. 2020, 5, 425–427. [Google Scholar] [CrossRef]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K. “Fast, faster, and fastest: Science on the run during COVID-19 drama”—“Do not forget the liver”. Hepatol. Int. 2020, 14, 454–455. [Google Scholar] [CrossRef]
- Schütte, A.; Ciesek, S.; Wedemeyer, H.; Anastasiou, O. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J. Hepatol. 2019, 70, 797–799. [Google Scholar] [CrossRef]
- Qiu, H.; Wander, P.; Bernstein, D.; Satapathy, S.K. Acute on chronic liver failure from novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Liver International. 2020, 40, 1590–1593. [Google Scholar] [CrossRef]
- Kovalic, A.J.; Huang, G.; Thuluvath, P.J.; Satapathy, S.K. Elevated Liver Biochemistries in Hospitalized Chinese Patients with Severe COVID-19: Systematic Review and Meta-analysis. Hepatology 2020. [Google Scholar] [CrossRef]
- Allen, A.M.; Kim, W.R.; Moriarty, J.P.; Shah, N.D.; Larson, J.J.; Kamath, P.S. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology 2016, 64, 2165–2172. [Google Scholar] [CrossRef]
- Sikander, M.; Malik, S.; Yadav, D.; Biswas, S.; Katare, D.P.; Jain, S.K. Cytoprotective activity of a trans-chalcone against hydrogen peroxide induced toxicity in hepatocellular carcinoma (HepG2) cells. Asian Pac. J. Cancer Prev. 2011, 12, 2513–2516. [Google Scholar]
- Sikander, M.; Malik, S.; Parveen, K.; Ahmad, M.; Yadav, D.; Bin Hafeez, Z.; Bansal, M. Hepatoprotective effect of Origanum vulgare in Wistar rats against carbon tetrachloride-induced hepatotoxicity. Protoplasma 2012, 250, 483–493. [Google Scholar] [CrossRef]
- Deng, G.-F.; Xu, X.-R.; Zhang, Y.; Li, D.; Gan, R.-Y.; Li, H.-B. Phenolic Compounds and Bioactivities of Pigmented Rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kempson, S.A.; Vovor-Dassu, K.; Day, C.R. Betaine Transport in Kidney and Liver: Use of Betaine in Liver Injury. Cell. Physiol. Biochem. 2013, 32, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, S.; Guo, Y.; Jiao, Y.; Zhao, Y. Compositional characterisation of soluble apple polysaccharides, and their antioxidant and hepatoprotective effects on acute CCl4-caused liver damage in mice. Food Chem. 2013, 138, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Melzer, J.; Reichling, J.; Brignoli, R.; Meier, R. An Updated Systematic Review of the Pharmacology of Silymarin. Complement. Med. Res. 2007, 14, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Barzaghi, N.; Crema, F.; Gatti, G.; Pifferi, G.; Perucca, E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 1990, 15, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, T.; Fatima, K.; Ali, Q.U.A.; Ovais, M.; Khalil, A.T.; Ullah, I.; Raza, A.; Shinwari, Z.K.; Idrees, M. Selected hepatoprotective herbal medicines: Evidence from ethnomedicinal applications, animal models, and possible mechanism of actions. Phytotherapy Res. 2018, 32, 199–215. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H.-J. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Cacciapuoti, F.; Scognamiglio, A.; Palumbo, R.; Forte, R.; Cacciapuoti, F. Silymarin in non alcoholic fatty liver disease. World J. Hepatol. 2013, 5, 109–113. [Google Scholar] [CrossRef]
- Freitag, A.F.; Cardia, G.F.E.; Da Rocha, B.A.; Aguiar, R.P.; Silva-Comar, F.M.D.S.; Spironello, R.A.; Grespan, R.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Hepatoprotective Effect of Silymarin (Silybum marianum) on Hepatotoxicity Induced by Acetaminophen in Spontaneously Hypertensive Rats. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Lu, C.; Lu, Y.; Chen, J.; Zhang, W.; Wu, W. Synchronized and sustained release of multiple components in silymarin from erodible glyceryl monostearate matrix system. Eur. J. Pharm. Biopharm. 2007, 66, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, A.; Guerra, R.; Garrido, A. Silybin Dihemisuccinate Protects Rat Erythrocytes Against Phenylhydrazine-Induced Lipid Peroxidation and Hemolysis. Planta Medica 2007, 53, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Nencini, C.; Giorgi, G.; Micheli, L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine 2007, 14, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Di Fabio, G.; Zarrelli, A.; Zappavigna, S.; Stiuso, P.; Tuccillo, C.; Caraglia, M.; Loguercio, C. Silybin-Phosphatidylcholine Complex Protects Human Gastric and Liver Cells from Oxidative Stress. Vivo 2015, 29, 569–575. [Google Scholar]
- Vargas-Mendoza, N.; Madrigal-Santillán, E.; Morales-González, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; García-Luna, Y.G.-R.M.; Gayosso-de-Lucio, J.A.; Morales-González, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef]
- Moltó, J.; Valle, M.; Miranda, C.; Cedeño, S.; Negredo, E.; Clotet, B. Effect of Milk Thistle on the Pharmacokinetics of Darunavir-Ritonavir in HIV-Infected Patients. Antimicrob. Agents Chemother. 2012, 56, 2837–2841. [Google Scholar] [CrossRef]
- Bosch-Barrera, J.; Martin-Castillo, B.; Buxó, M.; Brunet, J.; Encinar, J.A.; Menendez, J.A. Silibinin and SARS-CoV-2: Dual Targeting of Host Cytokine Storm and Virus Replication Machinery for Clinical Management of COVID-19 Patients. J. Clin. Med. 2020, 9, 1770. [Google Scholar] [CrossRef]
- Ferenci, P.; Scherzer, T.; Kerschner, H.; Rutter, K.; Beinhardt, S.; Hofer, H.; Schöniger–Hekele, M.; Holzmann, H.; Steindl-Munda, P. Silibinin Is a Potent Antiviral Agent in Patients With Chronic Hepatitis C Not Responding to Pegylated Interferon/Ribavirin Therapy. Gastroenterology 2008, 135, 1561–1567. [Google Scholar] [CrossRef]
- Jimoh, F.; Adedapo, A.A.; Afolayan, A. Comparison of the nutritional value and biological activities of the acetone, methanol and water extracts of the leaves of Solanum nigrum and Leonotis leonorus. Food Chem. Toxicol. 2010, 48, 964–971. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Feng, T.; Li, K. Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia 2008, 79, 548–556. [Google Scholar] [CrossRef]
- Liu, F.; Ma, X.; Li, M.-M.; Li, Z.; Han, Q.; Li, R.; Li, C.-W.; Chang, Y.-C.; Zhao, C.-W.; Lin, Y.-X. Hepatoprotective effects of Solanum nigrum against ethanol-induced injury in primary hepatocytes and mice with analysis of glutathione S-transferase A1. J. Chin. Med Assoc. 2016, 79, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Campisi, A.; Acquaviva, R.; Raciti, G.; Duro, A.; Rizzo, M.; Santagati, N.A. Antioxidant Activities of Solanum Nigrum L. Leaf Extracts Determined in in vitro Cellular Models. Foods 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Lucia, L.M.; Ghedira, K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomedicine 2009, 5, 31. [Google Scholar] [CrossRef]
- Jain, R.; Sharma, A.; Gupta, S.; Sarethy, I.P.; Gabrani, R. Solanum nigrum: Current perspectives on therapeutic properties. Altern. Med. Rev. 2011, 16, 78–85. [Google Scholar]
- Wang, Z.; Li, J.; Ji, Y.; An, P.; Zhang, S.; Li, Z. Traditional Herbal Medicine: A Review of Potential of Inhibitory Hepatocellular Carcinoma in Basic Research and Clinical Trial. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Huang, H.-C.; Syu, K.-Y.; Lin, J.-K. Chemical Composition of Solanum nigrum Linn Extract and Induction of Autophagy by Leaf Water Extract and Its Major Flavonoids in AU565 Breast Cancer Cells. J. Agric. Food Chem. 2010, 58, 8699–8708. [Google Scholar] [CrossRef]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cacciapuoti, C. Hepatitis C virus (HCV) genotypes distribution: An epidemiological up-date in Europe. Infect. Agents Cancer 2016, 11, 53. [Google Scholar] [CrossRef]
- Sultana, S.; Perwaiz, S.; Iqbal, M.; Athar, M. Crude extracts of hepatoprotective plants, Solanum nigrum and Cichorium intybus inhibit free radical-mediated DNA damage. J. Ethnopharmacol. 1995, 45, 189–192. [Google Scholar] [CrossRef]
- Lin, H.M.; Tseng, H.C.; Wang, C.J.; Lin, J.J.; Lo, C.W.; Chou, F.P. Hepatoprotective effects of Solanum nigrum Linn extract against CCl(4)-induced oxidative damage in rats. Chem Biol Interact 2008, 171, 283–293. [Google Scholar] [CrossRef]
- Upadhyay, P.; Ara, S.; Prakash, P. Antibacterial and Antioxidant Activity of Solanum nigrum Stem and Leaves. Chem. Sci. Trans. 2015, 4, 1013–1017. [Google Scholar] [CrossRef]
- Nørbaek, R.; Nielsen, K.; Kondo, T. Anthocyanins from flowers of Cichorium intybus. Phytochemistry 2002, 60, 357–359. [Google Scholar]
- Mares, D.; Romagnoli, C.; Tosi, B.; Andreotti, E.; Chillemi, G.; Poli, F. Chicory extracts from Cichorium intybus L. as potential antifungals. Mycopathologia 2005, 160, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Liu, Y.; Chen, G.; Cui, J.; Liu, H.; Wang, Q.; Liu, Y.; Chen, G.; Cui, J. Antimicrobial and Antioxidant Activities ofCichorium IntybusRoot Extract Using Orthogonal Matrix Design. J. Food Sci. 2013, 78, M258–M263. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mun, Y.-J.; Woo, W.-H.; Jeon, K.-S.; An, N.-H.; Park, J.-S. Effects of the ethanol extract of Cichorium intybus on the immunotoxicity by ethanol in mice. Int. Immunopharmacol. 2002, 2, 733–744. [Google Scholar] [CrossRef]
- Gilani, A.-H.; Janbaz, K. Evaluation of the liver protective potential of Cichorium intybus seed extract on Acetaminophen and CCl4-induced damage. Phytomedicine 1994, 1, 193–197. [Google Scholar] [CrossRef]
- Hassan, H.A.; Yousef, M.I. Ameliorating effect of chicory (Cichorium intybus L.)-supplemented diet against nitrosamine precursors-induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2010, 48, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Al-Howiriny, T.A.; Siddiqui, A.B. Antihepatotoxic activity of seeds of Cichorium intybus. J. Ethnopharmacol. 2003, 87, 237–240. [Google Scholar] [CrossRef]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): A comparative in vitro study with the effects of caffeic and ferulic acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef]
- Kim, T.-W.; Yang, K.-S. Antioxidative effects of cichorium intybus root extract on LDL (low density lipoprotein) oxidation. Arch. Pharmacal Res. 2001, 24, 431–436. [Google Scholar] [CrossRef]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Elgengaihi, S.; Mossa, A.-T.H.; Refaie, A.A.E.-R.; Aboubaker, D. Hepatoprotective Efficacy ofCichorium intybusL. Extract Against Carbon Tetrachloride-induced Liver Damage in Rats. J. Diet. Suppl. 2016, 13, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Dai, L.-H.; Wu, Y.-H.; Yu, X.-P.; Zhang, Y.-Y.; Guan, R.-F.; Liu, T.; Zhao, J. Evaluation of Hepatocyteprotective and Anti-hepatitis B Virus Properties of Cichoric Acid from Cichorium intybus Leaves in Cell Culture. Biol. Pharm. Bull. 2014, 37, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Vimal, V.; Devaki, T. Hepatoprotective effect of allicin on tissue defense system in galactosamine/endotoxin challenged rats. J. Ethnopharmacol. 2004, 90, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liong, E.C.; Ling, M.-T.; Ching, Y.-P.; Fung, M.-L.; Tipoe, G.L. S-allylmercaptocysteine reduces carbon tetrachloride-induced hepatic oxidative stress and necroinflammation via nuclear factor kappa B-dependent pathways in mice. Eur. J. Nutr. 2012, 51, 323–333. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, C.W.; Oh, S.J.; Yun, J.; Kang, M.R.; Han, S.-B.; Park, H.; Jung, J.C.; Chung, Y.H.; Kang, J.S. Hepatoprotective Effect of Aged Black Garlic Extract in Rodents. Toxicol. Res. 2014, 30, 49–54. [Google Scholar] [CrossRef]
- Meghwal, M. A Review on the Functional Properties, Nutritional Content, Medicinal Utilization and Potential Application of Fenugreek. J. Food Process. Technol. 2012, 3, 1–10. [Google Scholar] [CrossRef]
- Bongiorno, P.B.; Fratellone, P.M.; Logiudice, P. Potential Health Benefits of Garlic (Allium Sativum): A Narrative Review. J. Complement. Integr. Med. 2008, 5, 5. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, S.-W.; Kim, Y.S.; Kang, S.S.; Kim, J.A.; Lee, S.H.; Lee, S.-M. Protective Mechanism of Glycyrrhizin on Acute Liver Injury Induced by Carbon Tetrachloride in Mice. Biol. Pharm. Bull. 2007, 30, 1898–1904. [Google Scholar] [CrossRef]
- Kimura, M.; Moro, T.; Motegi, H.; Maruyama, H.; Sekine, M.; Okamoto, H.; Inoue, H.; Sato, T.; Ogihara, M. In vivo glycyrrhizin accelerates liver regeneration and rapidly lowers serum transaminase activities in 70% partially hepatectomized rats. Eur. J. Pharmacol. 2008, 579, 357–364. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytotherapy Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Xu, C.; Shi, J.; Chen, S.; Tan, M.; Chen, J.; Zou, L.; Chen, C.; Liu, Z.; et al. A Comprehensive Review for Phytochemical, Pharmacological, and Biosynthesis Studies on Glycyrrhiza spp. Am. J. Chin. Med. 2020, 48, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza spp. and Its Bioactive Constituents: Update and Review. Phytotherapy Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, U.A.; Masoud, M.S.; Nawaz, Z.; Riazuddin, S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. J. Transl. Med. 2011, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Shigeta, S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antivir. Res. 1987, 7, 99–107. [Google Scholar] [CrossRef]
- Baltina, L.A.; Tasi, Y.-T.; Huang, S.-H.; Lai, H.-C.; Petrova, S.F.; Yunusov, M.S.; Lin, C.-W. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 126645. [Google Scholar] [CrossRef] [PubMed]
- Bentz, G.L.; Lowrey, A.J.; Horne, D.C.; Nguyen, V.; Satterfield, A.R.; Ross, T.D.; Harrod, A.E.; Uchakina, O.N.; McKallip, R.J. Using glycyrrhizic acid to target sumoylation processes during Epstein-Barr virus latency. PLoS ONE 2019, 14, e0217578. [Google Scholar] [CrossRef]
- Briolant, S.; Garin, D.; Scaramozzino, N.; Jouan, A.; Crance, J. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: Synergistic effect of interferon-α and ribavirin combination. Antivir. Res. 2004, 61, 111–117. [Google Scholar] [CrossRef]
- Crance, J.-M.; Lévèque, F.; Biziagos, E.; Van Cuyck-Gandré, H.; Jouan, A.; Deloince, R. Studies on mechanism of action of glycyrrhizin against hepatitis a virus replication in vitro. Antivir. Res. 1994, 23, 63–76. [Google Scholar] [CrossRef]
- Crance, J.M.; Biziagos, E.; Passagot, J.; Van Cuyck-Gandré, H.; Deloince, R. Inhibition of hepatitis A virus replication in vitro by antiviral compounds. J. Med Virol. 1990, 31, 155–160. [Google Scholar] [CrossRef]
- Lin, J.-C. Mechanism of action of glycyrrhizic acid in inhibition of Epstein-Barr virus replication in vitro. Antivir. Res. 2003, 59, 41–47. [Google Scholar] [CrossRef]
- Lin, J.-C.; Cherng, J.-M.; Hung, M.-S.; Baltina, L.A.; Baltina, L.; Kondratenko, R. Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: Structure–activity relationships. Antivir. Res. 2008, 79, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Matsuura, T.; Aoyagi, H.; Matsuda, M.; Hmwe, S.S.; Date, T.; Watanabe, N.; Watashi, K.; Suzuki, R.; Ichinose, S.; et al. Antiviral Activity of Glycyrrhizin against Hepatitis C Virus In Vitro. PLoS ONE 2013, 8, e68992. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Ogbomo, H.; Altenbrandt, B.; Leutz, A.; Doerr, H.W.; Cinatl, J.; Cinatl, J. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med Microbiol. Immunol. 2010, 199, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Geiler, J.; Naczk, P.; Sithisarn, P.; Leutz, A.; Doerr, H.W.; Cinatl, J. Glycyrrhizin Exerts Antioxidative Effects in H5N1 Influenza A Virus-Infected Cells and Inhibits Virus Replication and Pro-Inflammatory Gene Expression. PLoS ONE 2011, 6, e19705. [Google Scholar] [CrossRef]
- Sakai-Sugino, K.; Uematsu, J.; Kamada, M.; Taniguchi, H.; Suzuki, S.; Yoshimi, Y.; Kihira, S.; Yamamoto, H.; Kawano, M.; Tsurudome, M.; et al. Glycyrrhizin inhibits human parainfluenza virus type 2 replication by the inhibition of genome RNA, mRNA and protein syntheses. Drug Discov. Ther. 2017, 11, 246–252. [Google Scholar] [CrossRef]
- Wolkerstorfer, A.; Kurz, H.; Bachhofner, N.; Szolar, O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antivir. Res. 2009, 83, 171–178. [Google Scholar] [CrossRef]
- Chen, L.; Hu, C.; Hood, M.; Zhang, X.; Zhang, L.; Kan, J.; Du, J. A Novel Combination of Vitamin C, Curcumin and Glycyrrhizic Acid Potentially Regulates Immune and Inflammatory Response Associated with Coronavirus Infections: A Perspective from System Biology Analysis. Nutrients 2020, 12, 1193. [Google Scholar] [CrossRef]
- Luo, P.; Liu, D.; Li, J. Pharmacological perspective: Glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105995. [Google Scholar] [CrossRef]
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmacol. 2011, 138, 286–313. [Google Scholar] [CrossRef]
- Hanh, N.D.; Sinchaipanid, N.; Mitrevej, A. Physicochemical characterization of phyllanthin from Phyllanthus amarus Schum. et Thonn. Drug Dev. Ind. Pharm. 2013, 40, 793–802. [Google Scholar] [CrossRef]
- Naaz, F.; Javed, S.; Abdin, M. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. et Thonn. on aflatoxin B1-induced liver damage in mice. J. Ethnopharmacol. 2007, 113, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, P.S.; Millman, I.; Blumberg, B.S. Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: In vitro and in vivo studies. Proc. Natl. Acad. Sci. USA 1987, 84, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Lechtenberg, M.; Deters, A.; Petereit, F.; Hensel, A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 2011, 18, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Cesari, I.; Grisoli, P.; Paolillo, M.; Milanese, C.; Massolini, G.; Brusotti, G. Isolation and characterization of the alkaloid Nitidine responsible for the traditional use of Phyllanthus muellerianus (Kuntze) Excell stem bark against bacterial infections. J. Pharm. Biomed. Anal. 2015, 105, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Ahmad, F.M.; Daisi, A.O.; Yahaya, A.A.; Ibitoye, O.B.; Muritala, H.F.; Sunmonu, T.O. Hepatoprotective potential of Phyllanthus muellarianus leaf extract: Studies on hepatic, oxidative stress and inflammatory biomarkers. Pharm. Biol. 2017, 55, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Sabina, E.P.; Ramya, S.R.; Preety, P.; Patel, S.; Mandal, N.; Mishra, P.P.; Samuel, J. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 2010, 62, 638–643. [Google Scholar] [CrossRef]
- Widodo, N.; Kaur, K.; Shrestha, B.G.; Takagi, Y.; Ishii, T.; Wadhwa, R.; Kaul, S.C. Selective Killing of Cancer Cells by Leaf Extract of Ashwagandha: Identification of a Tumor-Inhibitory Factor and the First Molecular Insights to Its Effect. Clin. Cancer Res. 2007, 13, 2298–2306. [Google Scholar] [CrossRef]
- Elberry, A.A.; Harraz, F.M.; Ghareib, S.A.; Nagy, A.A.; Gabr, S.A.; Suliaman, M.I.; Abdel-Sattar, E. Antihepatotoxic Effect of Marrubium Vulgare and Withania Somnifera Extracts on Carbon Tetrachloride-Induced Hepatotoxicity in Rats. J. Basic Clin. Pharm. 2010, 1, 247–254. [Google Scholar]
- Mofed, D.; Ahmed, W.; Zekri, A.-R.; Said, O.; Rahouma, M.; Faraag, A.H.I. The Antiviral Efficacy of Withania somnifera (Ashwagandha) against Hepatitis C Virus Activity: In Vitro and in Silico Study. Adv. Microbiol. 2020, 10, 463–477. [Google Scholar] [CrossRef]
- Munagala, R.; Kausar, H.; Munjal, C.; Gupta, R. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis 2011, 32, 1697–1705. [Google Scholar] [CrossRef]
- Pant, M.; Ambwani, T.; Umapathi, V. Antiviral Activity of Ashwagandha Extract on Infectious Bursal Disease Virus Replication. Indian J. Sci. Technol. 2012, 5, 2. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Bhargava, P.; Kaul, A.; Wang, J.; Zhang, H.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct Dyn 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In Advances in Experimental Medicine and Biology; Aggarwal, B.B., Surh, Y.J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; Volume 595, pp. 105–125. [Google Scholar]
- Dhasmana, A.; Kashyap, V.K.; Dhasmana, S.; Kotnala, S.; Haque, S.; Ashraf, G.M.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Neutralization of SARS-CoV-2 Spike Protein via Natural Compounds: A Multi Layered High Throughput Virtual Screening Approach. Curr. Pharm. Des. 2020. [CrossRef] [PubMed]
- Dhasmana, A.; Uniyal, S.; Anukriti; Kashyap, V.K.; Somvanshi, P.; Gupta, M.; Bhardwaj, U.; Jaggi, M.; Yallapu, M.M.; Haque, S.; et al. Topological and system-level protein interaction network (PIN) analyses to deduce molecular mechanism of curcumin. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V. REGULATION OF COX AND LOX BY CURCUMIN. Results Probl. Cell Differ. 2007, 595, 213–226. [Google Scholar] [CrossRef]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Chun, K.S. Cancer chemopreventive effects of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Boston, MA, USA, 2007; pp. 149–172. [Google Scholar]
- Bruck, R.; Ashkenazi, M.; Weiss, S.; Goldiner, I.; Shapiro, H.; Aeed, H.; Genina, O.; Helpern, Z.; Pines, M. Prevention of liver cirrhosis in rats by curcumin. Liver Int 2007, 27, 373–383. [Google Scholar] [CrossRef]
- Das, S.; Sarmah, S.; Lyndem, S.; Singha Roy, A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct Dyn. 2020, 1–11. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef]
- Panico, A.; Cardile, V.; Garufi, F.; Puglia, C.; Bonina, F.; Ronsisvalle, G. Protective effect of Capparis spinosa on chondrocytes. Life Sci. 2005, 77, 2479–2488. [Google Scholar] [CrossRef]
- Ahmed, B.; Alam, T.; Varshney, M.; Alam Khan, S. Hepatoprotective activity of two plants belonging to the Apiaceae and the Euphorbiaceae family. J. Ethnopharmacol. 2002, 79, 313–316. [Google Scholar] [CrossRef]
- Huseini, H.F.; Alavian, S.; Heshmat, R.; Heydari, M.; Abolmaali, K. The efficacy of Liv-52 on liver cirrhotic patients: A randomized, double-blind, placebo-controlled first approach. Phytomedicine 2005, 12, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Gadgoli, C.; Mishra, S.H. Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J. Ethnopharmacol. 1999, 66, 187–192. [Google Scholar] [CrossRef]
- Alam, J.; Mujahid, M.; Badr, B.; Rahman; Akhtar, J.; Khalid, M.; Jahan, Y.; Basit, A.; Khan, A.; Shawwal, M.; et al. An insight of pharmacognostic study and phytopharmacology of Aquilaria agallocha. J. Appl. Pharm. Sci. 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Alam, J.; Mujahid; Badruddeen; Jahan, Y.; Bagga, P.; Rahman, A. Hepatoprotective potential of ethanolic extract of Aquilaria agallocha leaves against paracetamol induced hepatotoxicity in SD rats. J. Tradit. Complement. Med. 2017, 7, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Kabir, N.; Muhammad, A.; Shah, M.R.; Musharraf, S.G.; Iqbal, N.; Nadeem, S. Hautriwaic acid as one of the hepatoprotective constituent of Dodonaea viscosa. Phytomedicine 2014, 21, 131–140. [Google Scholar] [CrossRef]
- Wahid, A.; Hamed, A.N.; Eltahir, H.M.; Abouzied, M.M. Hepatoprotective activity of ethanolic extract of Salix subserrata against CCl4-induced chronic hepatotoxicity in rats. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Domitrović, R.; Potočnjak, I. A comprehensive overview of hepatoprotective natural compounds: Mechanism of action and clinical perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef]
- Mishra, G.; Khosa, R.L.; Singh, P.; Jha, K.K. Hepatoprotective potential of ethanolic extract of Pandanus odoratissimus root against paracetamol-induced hepatotoxicity in rats. J. Pharm. Bioallied Sci. 2015, 7, 45–48. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharjee, A.; Mukherjee, S.; Bhattacharya, K.; Khowala, S. Antioxidant and Hepatoprotective Activity of Ethanolic Extract of Alocasia indica Tuber. Am. J. Phytomedicine Clin. Ther. 2014, 2, 191–208. [Google Scholar]
- González-Ponce, H.A.; Martínez-Saldaña, M.C.; Rincón-Sánchez, A.R.; Sumaya-Martínez, T.; Buist-Homan, M.; Faber, K.N.; Moshage, H.; Jaramillo-Juárez, F. Hepatoprotective Effect of Opuntia robusta and Opuntia streptacantha Fruits against Acetaminophen-Induced Acute Liver Damage. Nutrients 2016, 8, 607. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).