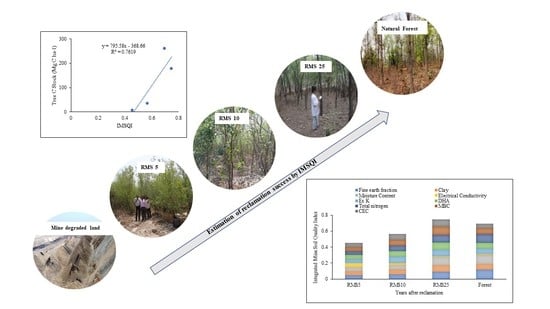
Assessment of Forest Ecosystem Development in Coal Mine Degraded Land by Using Integrated Mine Soil Quality Index (IMSQI): The Evidence from India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Study
2.3. Collection of Mine Soil Sample
2.4. Analysis of Soil Samples
2.5. Estimation of Carbon (SOC) and Nitrogen (N) Stock
2.6. Estimation of Carbon Sequestration
2.7. Integrated Soil Quality Index Based on PCA
2.8. Statistical Analysis
3. Results
3.1. Vegetation Biomass and Carbon Dynamics in Reclaimed Chronosequence
3.2. Litter Carbon Stock and CO2 Sequestration in Reclaimed Chronosequence
3.3. Soil Physicochemical Properties
3.4. Soil Organic Carbon (SOC) and Total Nitrogen (TN) Stock
3.5. Ecosystem Carbon Pool
3.6. Identification of Key Soil Parameters and Estimation of Integrated Mine Soil Quality Index (IMSQI)
4. Discussion
4.1. Effect of Revegetation in Recuperation of Soil Quality
4.2. Accretion of Carbon (C) and Total Nitrogen (TN) Stock
4.3. Ecosystem Carbon (C) Pool
4.4. Integrated Mine Soil Quality Index (IMSQI)–Tool to Evaluate Restoration Success
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Central Electricity Authority (CEA). Annual Report 2018–2019. Available online: http://www.cea.nic.in/reports/annual/annualreports/annual_report-2019.pdf (accessed on 5 October 2020).
- Rivas-Pérez, I.M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Monterroso, C.; Macías, F.; Álvarez-Rodríguez, E. Evolution of chemical characteristics of Technosols in an afforested coal mine dump over a 20-year period. Land Degrad. Dev. 2016, 27, 1640–1649. [Google Scholar] [CrossRef]
- Józefowska, A.; PietrzykowskiM, W.B.; Cajthaml, T.; Frouz, J. Theeffects of tree species and substrate on carbon sequestration andchemical and biological properties in reforested post-mining soils. Geoderma 2017, 292, 9–16. [Google Scholar] [CrossRef]
- Liu, X.; Bai, Z.; Zhou, W.; Cao, Y.; Zhang, G. Changes in soil properties in the soil profile after mining and reclamation in an opencast coalmine on the loess plateau, China. Ecol. Eng. 2017, 98, 228–239. [Google Scholar] [CrossRef]
- Ličina, V.; Akšić, M.F.; Tomić, Z.; Trajković, I.; Mladenović, S.A.; Marjanović, M.; Rinklebe, J. Bioassessment of heavy metals in the surface soil layer of an opencast mine aimed for its rehabilitation. J. Environ. Manag. 2017, 186, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Coal, Government of India. Report of the Working Group on Coal and Lignite for Formulation of 11th Five-Year Plan (2007–2012). Available online: https://niti.gov.in/planningcommission.gov.in/docs/aboutus/committee/wrkgrp11/wg11_coal.doc (accessed on 5 October 2020).
- IPCC Intergovernmental Panel on Climate Change (IPCC). IPCC Guidelines for National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change, NGGIP Publications, IGES: Japan, 2006; Available online: https://www.ipcc-nggip.iges.or.jp/meeting/pdfiles/Washington_Report.pdf (accessed on 11 October 2020).
- Ussiri, D.A.N.; Lal, R.; Jacinthe, P.A. Soil properties and carbon sequestrationof afforested pastures in reclaimed minesoils of Ohio. Soil Sci. Soc. Am. J. 2006, 70, 1797–1806. [Google Scholar] [CrossRef]
- Ahirwal, J.; Maiti, S.K.; Singh, A.K. Changes in ecosystem carbon pool and soil CO2flux following post-mine reclamation in dry tropical environment, India. Sci Total Environ. 2017, 583, 153–162. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, Y.; Gao, G.; Zhao, Z.; Niu, S. Intra-and Interspecific Interactions among Pioneer Trees Affect Forest-Biomass Carbon Accumulation in a Nutrient-Deficient Reclaimed Coal Mine Spoil. Forests 2020, 11, 819. [Google Scholar] [CrossRef]
- Ahirwal, J.; Maiti, S.K. Assessment of carbon sequestration potential of revegetated coal mine overburden dumps: A chronosequence study from dry tropical climate. J. Environ. Manag. 2017, 201, 369–377. [Google Scholar] [CrossRef]
- Karan, S.K.; Samadder, S.R.; Maiti, S.K. Assessment of the capability of remotesensing and GIS techniques for monitoring reclamation success in coal mine degraded lands. J. Environ. Manag. 2016, 182, 272–283. [Google Scholar] [CrossRef]
- Quinkenstein, A.; Jochheim, H. Assessing the carbon sequestration potential of poplar and black locust short rotation coppices on mine reclamation sites in Eastern Germanye Model development and application. J. Environ. Manag. 2016, 168, 53–66. [Google Scholar] [CrossRef]
- Pietrzykowski, M. Soil quality index as a tool for Scots pine (Pinus sylvestris) monoculture conversion planning on afforested, reclaimed mine land. J. For. Res. 2014, 25, 63–74. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of surface coal mining and land reclamation on soil properties: A review. Earth Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Sena, K.; Agouridis, C.; Miller, J.; Barton, C. Spoil type influences soil genesis and forest development on an Appalachian surface coal mine ten years after placement. Forests 2018, 9, 780. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, S.K.; Masto, R.E. Development of Mine Soil Quality Index (MSQI) for evaluation of reclamation success: A chronosequence study. Ecol. Eng. 2014, 71, 10–20. [Google Scholar] [CrossRef]
- Chodak, M.; Niklinska, M. Effect of texture and tree species on microbialproperties of mine soils. Appl. Soil Ecol. 2010, 46, 268–275. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Carbon and nitrogen pools in reclaimed landunder forest and pasture ecosystems in Ohio, USA. Geoderma 2010, 157, 196–205. [Google Scholar] [CrossRef]
- Ahirwal, J.; Kumar, A.; Pietrzykowski, M.; Maiti, S.K. Reclamation of coal mine spoil and its effect on Technosol quality and carbon sequestration: A case study from India. Environ. Sci. Pollut. Res. 2018, 25, 27992–28003. [Google Scholar] [CrossRef]
- Johnson, C.D.; Skousen, J.G. Minesoil properties of 15 abandoned mine land sites in West Virginia. J. Environ. Qual. 1995, 24, 635–643. [Google Scholar] [CrossRef]
- Indian Standards: 2720. In Grain Size Analysis; Part IV; Indian Standard Institute: New Delhi, India, 1985.
- Dakshinamurthi, C.; Gupta, R.P. Practicals in Soil Physics; IARI: New Delhi, Indian, 1968. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Hans Publishers: Bombay, Mumbai, 1966. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part 1—Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; SSSA Book Series 5; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; PHI Pvt. Ltd.: New Delhi, India, 1973; pp. 183–197. [Google Scholar]
- Olsen, S.R.; Sommer, L.E. Phosphorus. In Methods of Soil Analysis, Part 2; Page, A.L., Ed.; SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3, Chemical Methods, 3rd ed.; Sparks, D.L., Ed.; SSSA Book Series 5; ASA and SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of availablenitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Chatterjee, A.; Lal, R.; Shrestha, R.K.; Ussiri, D.A.N. Soil carbon pools of reclaimed minesoils under grass and forest landuses. Land Degrad. Dev. 2009, 20, 300–307. [Google Scholar] [CrossRef]
- Brown, S.; Gillespie, A.; Lugo, A.E. Biomass estimation methodsfor tropical forests with applications to forest inventory data. For. Sci. 1989, 35, 881–902. [Google Scholar] [CrossRef]
- Lodhiyal, L.S.; Singh, R.P.; Singh, S.P. Structure and function of anage series of poplar plantations in central Himalaya: I dry matterdynamics. Ann. Bot. 1995, 76, 191–199. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Treeallometry and improved estimation of carbon stocks and balancein tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Socha, J.; Wezyk, P. Allometric equations for estimating the foliagebiomass of Scots pine. Eur. J. For. Res. 2007, 126, 263–270. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Socha, J. An estimation of Scots pine (Pinus sylvestris L.) ecosystem productivity on reclaimed post-miningsites in Poland (central Europe) using of allometric equations. EcolEng 2011, 37, 381–386. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the abovegroundbiomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Masto, R.E.; Yadav, A.; George, J.; Ram, L.C.; Shukla, S.P. Soil quality index for evaluation of reclaimed coal minespoil. Sci. Total Environ. 2016, 542, 540–550. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental panel on climate change 2000. In Land Use, Land Use Change and Forestry; Special Report; Cambridge Univ. Press: Cambridge, UK, 2000. [Google Scholar]
- FAO. Global Forest Resources Assessment 2005. Global Assessment of Growing Stock, Biomass and Carbon Stock. Available online: www.fao.org/docrep/013/i1757e/i1757e.pdf (accessed on 11 October 2020).
- Vasu, D.; Singh, S.K.; Ray, S.K.; Duraisami, V.P.; Tiwary, P.; Chandran, P.; Nimkar, A.M.; Anantwar, S.G. Soil quality index (SQI) as a toolto evaluate crop productivity in semi-arid Deccan plateau, India. Geoderma 2016, 282, 70–79. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhonkar, P.K.; Singh, D.; Patra, A.K. Alternative soil quality indicesfor evaluating the effect of intensive cropping, fertilization and manuring for 31years in the semi-arid soils of India. Environ. Monit. Assess. 2008, 136, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.S.; Carroll, C.R. Designing a soil quality assessment tool for sustainable agroecosystem management. Ecol. Appl. 2001, 11, 1573–1585. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Society for Ecological Restoration International Science & Policy Working Group. The SER International Primer on Ecological Restoration; Society for Ecological Restoration International: Tucson, AZ, USA, 2004; Available online: http://www.ser.org/pdf/primer3.pdf (accessed on 11 October 2020).
- Macdonald, S.E.; Landhäusser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest restoration following surface mining disturbance:challenges and solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef]
- Qu, J.F.; Hou, Y.L.; Ge, M.Y.; Wang, K.; Liu, S.; Zhang, S.L.; Li, G.; Chen, F. Carbon dynamics of reclaimed coal mine soil under agriculturaluse: A chronosequence study in the dongtan mining area, Shandong Province, China. Sustainability 2017, 9, 629. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Masto, R.E. Carbon storage in coal mine spoil by DalbergiasissooRoxb. Geoderma 2016, 84, 204–213. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Changes in physical and chemical properties of soil after surface mining and reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Kumar, S.; Maiti, S.K.; Chaudhuri, S. Soil development in 2–21 years old coalmine reclaimed spoil with trees: A case study from Sonepur-Bazari opencast project, Raniganj Coalfield, India. Ecol. Eng. 2015, 84, 311–324. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, S.K. Trace metal accumulation and naturalmycorrhizal colonisation in an afforested coalmine overburden dump—Acase study from India. Int. J. Min. Reclam. Environ. 2011, 25, 187–207. [Google Scholar] [CrossRef]
- Ahirwal, J.; Maiti, S.K. Assessment of soil properties of different land uses generated due to surface coal mining activities in tropical Sal (Shorea robusta) forest, India. Catena 2016, 140, 155–163. [Google Scholar] [CrossRef]
- Lei, H.; Peng, Z.; Yigang, H.; Yang, Z. Vegetation and soil restoration in refuse dumps from open pit coal mines. Ecol. Eng. 2016, 94, 638–646. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.; Niu, S.; Li, X.; Wang, Y.; Bai, Z. Reclamation promotes the succession of the soil and vegetation in opencast coal mine: A case study from Robiniapseudoacacia reclaimed forests, Pingshuo mine, China. Catena 2018, 165, 72–79. [Google Scholar] [CrossRef]
- Ganjegunte, G.K.; Wick, A.F.; Stahl, P.D.; Vance, G.F. Accumulation and compositionof total organic carbon in reclaimed coal mine lands. Land Degrad. Dev. 2009, 20, 156–175. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Mehrotraa, K.L.; Nair, R.; Wanjari, T.; Singh, S.K.; Chakrabarti, T. Carbon sequestration in reclaimed manganese mine land at Gumgaon, India. Environ. Monit. Assess. 2010, 160, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ahirwal, J.; Kumar, A.; Maiti, S.K. Effect of Fast-Growing Trees on Soil Properties and Carbon Storage in an Afforested Coal Mine Land (India). Minerals 2020, 10, 840. [Google Scholar] [CrossRef]
- Zhao, Z.; Shahrour, I.; Bai, Z.; Fan, W.; Feng, L.; Li, H. Soils development in opencast coal mine spoils reclaimed for 1–13 years in the West-Northern Loess Plateau of China. Eur. J. Soil Biol. 2013, 55, 40–46. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, R.S.; Chaulya, S.K. Dump stability and soil fertility of a coalmine spoil in Indian dry tropical environment: A long-term study. Environ. Manag. 2012, 50, 695–706. [Google Scholar] [CrossRef]
- Das, C.; Mondal, N.K. Litter fall, decomposition and nutrient release of Shorearobusta and Tectona grandis in a sub-tropical forest of West Bengal, Eastern India. J. For. Res. 2016, 27, 1055–1065. [Google Scholar] [CrossRef]
- Banning, N.C.; Grant, C.D.; Jones, D.L.; Murphy, D.V. Recovery of soil organic matter, organic matter turnover and nitrogen cycling in a post-mining forest rehabilitation chronosequence. Soil Biol. Biochem. 2008, 40, 2021–2031. [Google Scholar] [CrossRef]
- Akala, V.A.; Lal, R. Soil organic carbon pools and sequestration rates in reclaimed mine soils in Ohio. J. Environ. Qual. 2001, 30, 2098–2104. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, R.S.; Hills, C.D. Soil carbon development in rejuvenated Indiancoal mine spoil. Ecol. Eng. 2016, 90, 482–490. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, R.S.; Nathanail, C.P. Mine spoil acts as a sink of carbon dioxide in Indian dry tropical environment. Sci. Total Environ. 2014, 468, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, Z.; Zhang, P.; Chen, L.; Hu, T.; Niu, S.; Bai, Z. Soil organic carbon and nitrogen pools in reclaimed mine soils under forest and cropland ecosystems in the Loess Plateau, China. Ecol. Eng. 2017, 102, 137–144. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Krzaklewski, W. Potential for carbon sequestration in reclaimed mine soil on reforested surface mining areasin Poland. Nat. Sci. 2010, 2, 1015–1021. [Google Scholar] [CrossRef]
- Avera, B.N.; Strahm, B.D.; Burger, J.A.; Zipper, C.E. Development of ecosystem structure and function on reforested surface-mined lands in the Central Appalachian Coal Basin of the United States. New For. 2015, 46, 683–702. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Daniels, W.L. Estimation of carbon sequestration by pine (Pinus sylvestris L.) ecosystems developed on reforested post-mining sites in Poland on differing mine soil substrates. Ecol. Eng. 2014, 73, 209–218. [Google Scholar] [CrossRef]
- Frouz, J.; Jílková, V.; Cajthaml, T.; Pižl, V.; Tajovský, K.; Háněl, L.; Burešová, A.; Šimáčková, H.; Kolaříková, K.; Franklin, J.; et al. Soil biota in post-mining sites along a climatic gradient in the USA: Simple communities in shortgrass prairie recover faster than complex communities in tallgrass prairie and forest. Soil Biol. Biochem. 2013, 67, 212–225. [Google Scholar] [CrossRef]
- Vicentini, F.; Hendrychova, M.; Tajovský, K.; Pižl, V.; Frouz, J. The Effect of Topography on Long-Term Spontaneous Development of Soil and Woody Cover on Graded and Untreated Overburden. Forests 2020, 11, 602. [Google Scholar] [CrossRef]
- Baldrian, P.; Trögl, J.; Frouz, J.; Šnajdr, J.; Valášková, V.; Merhautová, V.; Cajthaml, T.; Herinková, J. Enzyme activities and microbial biomass in topsoil layer during spontaneous succession in spoil heaps after brown coal mining. Soil Biol. Biochem. 2008, 40, 2107–2115. [Google Scholar] [CrossRef]
- Anderson, J.D.; Ingram, L.J.; Stahl, P.D. Influence of reclamation management practices on microbial biomass carbon and soil organic carbon accumulation in semiarid mined lands of Wyoming. Appl. Soil Ecol. 2008, 40, 387–397. [Google Scholar] [CrossRef]





| Vegetation Characteristics | RMS 5 | RMS 10 | RMS 25 | Reference Forest |
|---|---|---|---|---|
| AGB (Mg ha−1) | 13.29 ± 2.06 | 57.89 ± 13.45 | 187.53 ± 52.73 | 445.40 ± 109.41 |
| BGB (Mg ha−1) | 3.41 ± 0.47 | 12.49 ± 2.56 | 35.26 ± 8.76 | 75.77 ± 16.68 |
| Total tree biomass (Mg ha−1) | 16.69 ± 2.52 | 70.38 ± 16.00 | 222.79 ± 61.49 | 521.17 ± 126.09 |
| Tree C stock (Mg C ha−1) | 8.35 ± 1.26 | 35.19 ± 8.00 | 111.40 ± 30.75 | 260.58 ± 63.05 |
| CO2 sequestered (Mg ha−1) | 30.61 ± 8.36 | 129.04 ± 32.71 | 408.49 ± 94.60 | 955.56 ± 279.63 |
| Tree Species | RMS5 | RMS10 | RMS25 | Reference Forest | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AGB | BGB | C Stock | CO2 Sequestered | AGB | BGB | C Stock | CO2 Sequestered | AGB | BGB | C Stock | CO2 Sequestered | AGB | BGB | C Stock | CO2 Sequestered | |
| Azadirachta indica | - | - | - | - | 36.34 | 8.30 | 22.32 | 81.84 | 261.6 | 47.5 | 154.5 | 566.74 | - | - | - | - |
| Terminalia arjuna | - | - | - | - | - | - | - | - | 208.91 | 38.92 | 123.91 | 454.38 | - | - | - | - |
| Dalbergia sissoo | 25.3 | 6.02 | 15.64 | 57.36 | - | - | - | - | 22.05 | 5.34 | 13.70 | 50.22 | - | - | - | - |
| Gmelina arborea | - | - | - | - | - | - | - | - | 19.03 | 4.68 | 11.86 | 43.48 | - | - | - | - |
| Eucalyptus spp. | 41.2 | 9.26 | 25.21 | 92.46 | 106.1 | 21.4 | 63.7 | 233.68 | 338.4 | 59.6 | 199.01 | 729.78 | - | - | - | - |
| Cassia siamea | - | - | - | - | - | - | - | - | 30.66 | 7.14 | 18.9 | 69.30 | - | - | - | - |
| Phyllanthus emblica | - | - | - | - | 51.2 | 11.2 | 31.2 | 114.43 | - | - | - | - | - | - | - | - |
| Terminalia bellirica | - | - | - | - | 63.7 | 13.6 | 38.7 | 141.82 | - | - | - | - | - | - | - | - |
| Terminalia chebula | - | - | - | - | 32.1 | 7.44 | 19.8 | 72.55 | 16.18 | 4.06 | 10.12 | 37.12 | - | - | - | - |
| Shorea robusta | - | - | - | - | - | - | - | - | - | - | - | - | 2151.2 | 305.46 | 1228.3 | 4504.19 |
| Diospyros melanoxylon | - | - | - | - | - | - | - | - | - | - | - | - | 14.98 | 3.79 | 9.38 | 34.41 |
| Butea monosperma | - | - | - | - | - | - | - | - | - | - | - | - | 60.9 | 13.1 | 36.9 | 135.65 |
| Tree Litter | RMS 5 | RMS 10 | RMS 25 | Reference Forest |
|---|---|---|---|---|
| Litter biomass (Mg ha−1) | 1.51 ± 0.03 | 2.10 ± 0.12 | 2.31 ± 0.22 | 2.50 ± 0.05 |
| Litter C stock (Mg C ha−1) | 0.71 ± 0.02 | 1.01 ± 0.06 | 1.13 ± 0.11 | 1.25 ± 0.03 |
| Equivalent CO2 sequestered (Mg ha−1) | 2.59 ± 0.30 | 3.72 ± 0.60 | 4.16 ± 0.75 | 4.58 ± 0.30 |
| Tree Species | RMS 5 | RMS 10 | RMS 25 | Reference Forest | ||||
|---|---|---|---|---|---|---|---|---|
| Litter Biomass | Litter Carbon | Litter Biomass | Litter Carbon | Litter Biomass | Litter Carbon | Litter Biomass | Litter Carbon | |
| Azadirachta indica | - | - | 2.1 ± 0.39 | 1.01 ± 0.19 | 2.24 ± 0.39 | 1.1 ± 0.19 | - | - |
| Terminalia arjuna | - | - | - | - | 2.1 ± 0.43 | 1.03 ± 0.21 | - | - |
| Dalbergia sissoo | 1.48 ± 0.18 | 0.7 ± 0.08 | - | - | 2.52 ± 0.33 | 1.24 ± 0.16 | - | - |
| Gmelina arborea | - | - | - | - | 2.66 ± 0.23 | 1.31 ± 0.11 | - | - |
| Eucalyptus spp. | 1.57 ± 0.16 | 0.74 ± 0.07 | 2.11 ± 0.32 | 1.01 ± 0.15 | 2.24 ± 0.46 | 1.1 ± 0.23 | - | - |
| Cassia siamea | - | - | - | - | 2.37 ± 0.45 | 1.16 ± 0.22 | - | - |
| Phyllanthus emblica | - | - | 2.12 ± 0.32 | 1.02 ± 0.15 | - | - | - | - |
| Terminalia bellirica | - | - | 2.22 ± 0.35 | 1.06 ± 0.16 | - | - | - | - |
| Terminalia chebula | - | - | 1.94 ± 0.31 | 0.93 ± 0.15 | 2.2 ± 0.28 | 1.07 ± 0.14 | - | - |
| Shorea robusta | - | - | - | - | - | - | 2.5 ± 0.15 | 1.25 ± 0.08 |
| Diospyros melanoxylon | - | - | - | - | - | - | 2.54 ± 0.2 | 1.27 ± 0.1 |
| Butea monosperma | - | - | - | - | - | - | 2.38 ± 0.31 | 1.19 ± 0.15 |
| Soil Quality Parameter | Year after Reclamation | Reference Forest | ||
|---|---|---|---|---|
| RMS 5 | RMS 10 | RMS 25 | ||
| Fine earth fraction (%) | 46.5 ± 6.76 c | 65.04 ± 4.33 b | 76.45 ± 5.38 a | 79.03 ± 3.15 a |
| Water holding capacity (%) | 28.65 ± 1.60 c | 35.43 ± 1.37 b | 40.19 ± 1.07 a | 35.71 ± 1.05 b |
| Bulk density (Mg m−3) | 1.76 ± 0.05 a | 1.69 ± 0.08 a | 1.23 ± 0.08 b | 1.25 ± 0.01 b |
| Moisture content (%) | 3.97 ± 0.39 d | 6.45 ± 0.47 c | 13.73 ± 3.88 b | 18.57 ± 1.66 a |
| Sand (%) | 76.78 ± 2.49 b | 73.13 ± 1.49 c | 68.83 ± 2.12 d | 79.31 ± 2.22 a |
| Silt (%) | 14.39 ± 2.52 b | 18.73 ± 2.13 a | 19.12 ± 2.61 a | 10.79 ± 1.50 c |
| Clay (%) | 8.83 ± 0.28 c | 8.15 ± 0.84 c | 12.05 ± 2.72 a | 9.90 ± 1.71 b |
| pH (1:2.5, w/v) | 7.09 ± 0.06 b | 7.29 ± 0.10 a | 7.41 ± 0.11 a | 5.68 ± 0.23 c |
| EC (1:2.5, w/v) (µS/cm) | 198.29 ± 17.46 a | 150.27 ± 19.80 b | 113.20 ± 22.82 c | 80.1 ± 6.54 d |
| Soil organic carbon (%) | 0.99 ± 0.10 d | 1.54 ± 0.14 c | 2.71 ± 0.22 a | 2.01 ± 0.03 b |
| Available nitrogen (mg kg−1) | 65.93 ± 6.27 d | 78.96 ± 9.41 c | 139.65 ± 6.56 a | 99.97 ± 1.36 b |
| Total nitrogen (mg kg−1) | 1133.40 ± 98.72 c | 1635.09 ± 156.92 b | 2914.09 ± 179.45 a | 2983.10 ± 101.47 a |
| Available phosphorus (mg kg−1) | 0.39 ± 0.1 d | 0.68 ± 0.2 c | 1.53 ± 0.29 b | 5.11 ± 0.05 a |
| Available potassium (mg kg−1) | 45.35 ± 6.23 d | 91.71 ± 8.12 c | 149.23 ± 12.44 a | 140.3 ± 2.43 b |
| CEC (cmol+ kg−1) | 9.57 ± 0.84 c | 12.22 ± 0.95 b | 22.67 ± 1.98 a | 8.73 ± 0.07 c |
| DHA (µg TPF g−1 h−1) | 39.57 ± 5.30 d | 75.67 ± 6.46 b | 90.59 ± 7.31 a | 61.46 ± 3.99 c |
| MBC (Mg kg−1) | 97.41 ± 12.64 d | 178.90 ± 23.16 c | 456.73 ± 35.52 a | 322.65 ± 24.84 b |
| Stock Calculation | RMS 5 | RMS 10 | RMS 25 | Forest |
|---|---|---|---|---|
| SOC stock (Mg C ha−1) | 9.11 ± 1.23 | 19.89 ± 1.62 | 41.37 ± 3.76 | 31.70 ± 1.40 |
| TN stock (Mg N ha−1) | 1.06 ± 0.22 | 2.12 ± 0.17 | 4.45 ± 0.36 | 4.70 ± 0.21 |
| Carbon (C) Pool (Mg ha−1) | RMS 5 | RMS 10 | RMS 25 | Forest |
|---|---|---|---|---|
| Tree carbon stock | 8.35 | 35.19 | 111.40 | 260.58 |
| Litter carbon stock | 0.71 | 1.01 | 1.13 | 1.25 |
| Soil organic carbon stock | 9.11 | 19.89 | 41.37 | 31.70 |
| Ecosystem carbon pool | 18.17 | 56.09 | 153.9 | 293.53 |
| Equivalent CO2 sequestered | 66.68 | 205.85 | 564.81 | 1077.25 |
| Principal Component | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| Eigen value | 4.346 | 2.781 | 2.521 | 2.379 | 2.049 | 1.874 |
| Variance (%) | 25.567 | 16.360 | 14.831 | 13.996 | 12.056 | 11.025 |
| Cumulative variance (%) | 25.567 | 41.927 | 56.759 | 70.755 | 82.811 | 93.835 |
| Fine earth fraction | 0.152 | −0.174 | 0.196 | 0.909 | 0.041 | −0.114 |
| Sand | 0.797 | 0.296 | 0.122 | −0.092 | 0.425 | −0.084 |
| Silt | −0.620 | −0.273 | −0.097 | 0.119 | −0.698 | 0.003 |
| Clay | −0.065 | 0.088 | −0.005 | −0.115 | 0.920 | 0.171 |
| Bulk density | −0.175 | 0.203 | −0.122 | 0.906 | −0.293 | 0.044 |
| Moisture content | −0.019 | 0.086 | 0.034 | −0.161 | 0.073 | 0.966 |
| WHC | 0.332 | 0.506 | 0.090 | 0.263 | 0.182 | 0.718 |
| pH | 0.450 | 0.791 | 0.056 | 0.125 | −0.132 | 0.086 |
| EC | 0.241 | 0.833 | −0.380 | −0.089 | 0.245 | 0.164 |
| Organic carbon | 0.804 | 0.047 | 0.489 | −0.164 | 0.116 | 0.216 |
| Available nitrogen | 0.332 | −0.284 | 0.080 | −0.450 | 0.461 | −0.006 |
| Total nitrogen | 0.168 | −0.884 | −0.306 | −0.027 | −0.099 | −0.113 |
| Av P | 0.635 | −0.230 | 0.483 | −0.477 | 0.234 | 0.149 |
| Exchangeable K | 0.149 | 0.134 | 0.848 | −0.189 | 0.160 | 0.347 |
| CEC | 0.888 | 0.011 | −0.320 | 0.126 | −0.081 | 0.285 |
| DHA | −0.148 | −0.010 | −0.913 | −0.261 | 0.083 | 0.199 |
| MBC | 0.936 | 0.148 | 0.244 | 0.021 | −0.037 | −0.132 |
| FE | BD | Clay | MC | EC | SOC | TN | Ex K | CEC | DHA | MBC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FE | 1 | 0.718 * | −0.157 | −0.259 | −0.249 | 0.008 | 0.081 | −0.021 | 0.140 | −0.438 | 0.190 |
| BD | 1 | −0.345 | −0.098 | 0.027 | −0.367 | −0.182 | −0.316 | 0.037 | −0.124 | −0.143 | |
| Clay | 1 | 0.220 | 0.304 | 0.155 | −0.112 | 0.275 | −0.103 | 0.183 | −0.063 | ||
| MC | 1 | 0.247 | 0.231 | −0.239 | 0.383 | 0.225 | 0.192 | −0.150 | |||
| EC | 1 | 0.113 | −0.631 | −0.058 | 0.358 | 0.384 | 0.218 | ||||
| SOC | 1 | −0.055 | 0.676 * | 0.591 | −0.467 | 0.858 ** | |||||
| TN | 1 | −0.350 | 0.199 | 0.273 | 0.012 | ||||||
| Ex K | 1 | −0.089 | −0.625 | 0.342 | |||||||
| CEC | 1 | 0.167 | 0.715 * | ||||||||
| DHA | 1 | −0.376 | |||||||||
| MBC | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandyopadhyay, S.; Novo, L.A.B.; Pietrzykowski, M.; Maiti, S.K. Assessment of Forest Ecosystem Development in Coal Mine Degraded Land by Using Integrated Mine Soil Quality Index (IMSQI): The Evidence from India. Forests 2020, 11, 1310. https://doi.org/10.3390/f11121310
Bandyopadhyay S, Novo LAB, Pietrzykowski M, Maiti SK. Assessment of Forest Ecosystem Development in Coal Mine Degraded Land by Using Integrated Mine Soil Quality Index (IMSQI): The Evidence from India. Forests. 2020; 11(12):1310. https://doi.org/10.3390/f11121310
Chicago/Turabian StyleBandyopadhyay, Sneha, Luís A. B. Novo, Marcin Pietrzykowski, and Subodh Kumar Maiti. 2020. "Assessment of Forest Ecosystem Development in Coal Mine Degraded Land by Using Integrated Mine Soil Quality Index (IMSQI): The Evidence from India" Forests 11, no. 12: 1310. https://doi.org/10.3390/f11121310
APA StyleBandyopadhyay, S., Novo, L. A. B., Pietrzykowski, M., & Maiti, S. K. (2020). Assessment of Forest Ecosystem Development in Coal Mine Degraded Land by Using Integrated Mine Soil Quality Index (IMSQI): The Evidence from India. Forests, 11(12), 1310. https://doi.org/10.3390/f11121310