Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway
Abstract
:1. Introduction
2. The Kynurenine Pathway and Kynurenines
3. Kynurenines in the Brain, the Periphery, and the Gut-Brain Axis
3.1. Kynurenines in the Brain and the Periphery
3.2. Gut-Brain Axis
4. Neurodegenerative Diseases
4.1. Alzheimer’s Disease
4.2. Parkinson’s Disease
4.3. Amyotrophic Lateral Sclerosis
4.4. Huntington’s Disease
4.5. Multiple Sclerosis
5. Other Relevant Diseases
6. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α | alpha |
| α7nAchR | α-7 Nicotinic acetylcholine receptor |
| α-syn | alpha-Synuclein |
| AD | Alzheimer’s disease |
| AHRs | Aryl hydrocarbon receptors |
| ALS | Amyotrophic lateral sclerosis |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ATP | Adenosine triphosphate |
| BBB | Blood-brain barrier |
| CA | Cinnabarinic acid |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| DA | Dopamine |
| DOPAC | 3,4-Dihydroxyphenylacetic acid |
| EAE | Experimental autoimmune encephalitis |
| FTD | Frontotemporal dementia |
| GI | Gastrointestinal |
| GPR35 | G-protein receptor 35 |
| HD | Huntington’s disease |
| IDO-1 | Indolamine 2,3-dioxygenase 1 |
| IFNs | Interferons |
| KATs | Kynurenine aminotransferases |
| KMO | Kynurenine 3-monooxygenase |
| KP | Kynurenine pathway |
| KYN | Kynurenine |
| KYNA | Kynurenic acid |
| LBs | Lewy bodies |
| l-KYN | N-formyl-l-kynurenine |
| MPP(+) | 1-Methyl-4-phenylpyridinium |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MS | Multiple sclerosis |
| NAD+ | Nicotinamide adenine dinucleotide |
| NMDA | N-methyl-d-aspartate |
| PD | Parkinson’s disease |
| PIC | Picolinic acid |
| PPMS | Primary progressive MS |
| QUIN | Quinolinic acid |
| ROS | Reactive oxygen species |
| RRMS | Relapsing-remitting MS |
| SNP | Single nucleotide polymorphism |
| SNpc | Pars compacta of the substance nigra |
| TDO | Tryptophan 2,3-dioxygenase |
| TDP-43 | TAR DNA-binding protein 43 |
| TRP | Tryptophan |
| XA | Xanthurenic acid |
Appendix A
| Alzheimer’s Disease | |||
|---|---|---|---|
| In the CNS | Ref. | In the Periphery | Ref. |
| -Decreased KYNA in the CSF | [84] | -Increased KYN/TRP ratio (IDO activity) associated with reduced cognitive performance | [95] |
| -Increased KYNA in the putamen and caudate nucleus -Increased KAT I activities in both nuclei -Minor increased KAT II in the caudate nucleus -Marked increased KYNA in the caudate nucleus and putamen | [84] | -Decreased serum and red blood cell KYNA levels | [86] |
| -A β 1–42 induces production of QUIN by human macrophages and microglia | [143] | -Lower TRP and KYNA concentrations in plasma -Non-significant increase of KYN, 3-HK and AA levels, and a marked increase of QUIN -IncreaseKYN/TRp ratio which suggests increased IDO activity -Positive correlations between cognitive function tests and plasma KYNA levels, and inversely correlations between these tests and QUIN levels | [68] |
| -Enhanced IDO and QUIN immunoreactivity in the hippocampus in association with senile plaques | [56] | -Increased serum levels of 3-HK -No increases in other downstream KP metabolites -3-HK can be used as a biomarker (Schwarz et al., 2013) | [55] |
| - QUIN is co-localized with hyperphosphorylated tau within cortical neurons in AD brain -QUIN induces tau phosphorylation in human neurons | [57] | -Upregulation of serotonin pathway while downregulation of kynurenine pathway in AD transgenic mice urine | [144] |
| -Confirmed association of IDO-1 with senile plaques for the first time -IDO-1 specifically localized inconjunction with neurofibrillary tangles | [58] | -Decreased TRP, XA, 3-HAA and QUIN in plasma -KYN, AA, QUIN, and markers of immune activation increased with age, while XA decreased with age -Inflammation-related markers were associated with age, but not AD. -Elderly AD patients with high QUIN performed worse on the CamCog test | [61] |
| -Expression and cell distribution of TDO and QUIN, and their co-localization with neurofibrillary tangles and senile β amyloid deposition were also determined in hippocampal sections. -Higher TDO and IDO-1 immunoreactivity observed in the hippocampus -TDO co-localizes with QUIN, neurofibrillary tangles-tau and amyloid deposits in the hippocampus -TDO is highly expressed in the brains of AD mice and in AD patients, suggesting that TDO-mediated activation of the KP could be involved in neurofibrillary tangles formation and associated with senile plaque | [59] | -Elevated KYN, AA and 3-HK in serum in neocortical amyloid-β load (NAL+) versus NAL− females in preclinical AD -Observed positive correlation between NAL and the serum KP metabolite concentrations | [79] |
| -Higher KYNA and QUIN concentrations in CSF -This observation together with other TRP pathway intermediates were correlated with either CSF Amyloid β 1–42, or tau and phosphorylated Tau-181. | [60] | -Positive correlation between Neurofilament light chain (NFL) and IDO activity -Positive correlations between NFL and KYN, KYNA, 3-HK, AA and QUIN -Observed significant associations between plasma A β 40 and the KYN/TRP ratio, KYNA, KYNA, AA and QUIN -Significant associations between plasma A β 42 and the KYN/TRP ratio, kynurenic acid, anthranilic acid and quinolinic acid -On stratifying participants based on their NAL status, NFL correlated with KP metabolites irrespective of NAL status -But associations between plasma A β and KP metabolites were only pronounced in individuals with high NAL while associations in individuals with low NAL were nearly absent. | [145] |
| -Increased 3-HK/KYN ratio correlated with t-tau and p-tau in CSF | [98] | -Plasma concentrations of KYN, 3-HK, AA, PIC, and neopterin significantly correlated with their respective CSF levels -Plasma KYN and PIC inversely correlated with CSF p-tau and t-tau | [98] |
| -Higher KYNA concentration in CSF compared with healthy subjects or with frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and progressive supranuclear palsy (PSP) -No significant differences in KYNA levels in CSF between any other neurodegenerative groups (FTD, ALS, PSP) and controls. -Increased KYNA concentration in CSF specific to AD. | [67] | -Plasma KYN positive associations with plasma NF-L levels, both, before and after adjusting for potential confounding variables (age, sex, APOE ε4, BMI) -Plasma KYN correlated significantly with plasma NF-L in Aβ+ participants and a trend level significance were observed in Aβ- participants. | [146] |
| Parkinson’s Disease | |||
|---|---|---|---|
| In the CNS | Ref. | In the Periphery | Ref. |
| -Increased TRP/KYN and KYNA/TRP ratios in the frontal cortex, putamen and pars compacta of the SN (SNpc) -Higher 3-HK levels in the putamen and SNpc -Decreased KYNA level in the frontal cortex, putamen and SNpc | [76] | -Increased KYN/TRP ratio and neopterin levels in the serum | [146] |
| -MPP(+) and 3-NPA dose-dependently impaired the synthesis of KYNA in rat cortical slices -MPP(+) inhibited the activity of KAT II -3-NPA impaired the activity KAT I and KAT II | [147] | -Reduced activities of KAT-I and KAT-II parallel to lower KYNA levels tendency in plasma, but increased KYNA level and KAT II activity in the peripheral red blood cells | [93] |
| -Increased KYN/TRP ratio and neopterin levels in the CSF | [95] | -Altered KP were verified in urinary samples of PD patients | [131] |
| -Decreased KAT I expression in MPTP treated mice | [95] | -In plasma lower KYNA levels and KYNA/KYN ratio in PD patients compared to HD patients and controls -Higher QUIN level and QUIN/KYNA ratio in PD patients compared to HD patients and controls -PD patients at advanced stage (Hoehn-Yahr stage > 2) showed lower KYNA and KYNA/KYN ratio while higher QUIN and QUIN/KYNA ratio compared to PD patients at early stage (Hoehn-Yahr stage ≤ 2) and controls. -This metabolomic analyses demonstrate a number of plasma biomarker candidates for PD, suggesting a shift toward neurotoxic QUIN synthesis and away from neuroprotective KYNA production in KP. | [75] |
| -3-HK concentration was increased by one-third, and mean oxidized glutathione was decreased by 40% in CSF -The findings offer further support for a possible excitotoxic disease mechanism in PD as well as a biomarker for monitoring a therapeutic intervention against 3-HK formation. | [81] | -PD subjects had >100% higher 3-HK and 14% lower 3-HAA in plasma -3-HK in plasma was associated with both symptom severity and disease duration. | [74] |
| -23% lower KYNA in the CSF Higher QUIN levels in the CSF associated with more severe symptoms, -Lower levels of the KYNA linked to olfactory deficits. -An elevated QUIN/PIC ratio in the CSF correlated with higher R2*values in the substantia nigra -Plasma C-reactive protein and serum amyloid alpha were associated with signs of increased KP activity in the CSF | [74] | -Urine KYN level higher in the PD group -Urine KYN were significantly associated with PD severity and mild cognitive impairment. -Urine KYN may be a new biomarker for early-stage PD | [94] |
| Amyotrophic Lateral Sclerosis | |||
|---|---|---|---|
| In the CNS. | Ref. | In the Periphery | Ref. |
| -Higher CSF KYNA concentration in patients with bulbar onset compared to controls, and compared to patients with limb onset -higher CSF KYNA in patients with severe clinical status compared to controls. | [86] | -Lower serum KYNA in patients with severe clinical status compared to controls and patients with mild clinical status | [70] |
| -Significantly increased levels of CSF TRP, KYN and QUIN -Significant increase in activated microglia expressing HLA-DR -Increased neuronal and microglial expression of IDO and QUIN in ALS motor cortex and spinal cord | [69] | -Increased levels of serum TRP, KYN and QUIN -Decreased levels of serum PIC | [69] |
| -Reduced DOPAC concentrations in FTD and ALS in CSF -Increased in DA levels and decrease in DOPAC/DA ratios in FTD relative to CONTR | [148] | -Increased serum DA levels and decreased DOPAC concentrations and DOPAC/DA ratios in in FTD and ALS -Decreased HK/XA ratios in serum of ALS subjects compared to FTD -KP does not hold promise as a research/therapeutic target in FTD and ALS | [104] |
| Huntington’s disease | |||
|---|---|---|---|
| In the CNS | Ref. | In the periphery | Ref. |
| -Increased 3-HAO activity in the striatum, which is known to exhibit the most prominent nerve-cell loss in HD | [98] | -Lower TRP, higher KYN, neopterin levels and higher KYN/TRP ratios (increased IDO activity) in the serum | [64] |
| -Decreased KYNA concentrations in the putamen and CSF -Increased KYN/KYNA ratio in the putamen (decreased KAT activity) | [88] | -Greater KYN/TRP ratio (increased IDO activity) in plasma -Lower KYNA/KYN ratio (decreased KAT activity) in plasma -Decreased 3-HK and 3-HAA in plasma | [83] |
| -Reduced KYNA concentrations in the cortex | [89] | -Lower levels of TRP and a higher KYN/TRP ratios (enhanced IDO activation) in the most severely affected group -Marked correlations between AA and inflammatory status -TRP negatively correlated with symptom severity and number of CAG repeats -TRP metabolism along the KP is related to the degree of genetic abnormality, to clinical disease severity and to aspects of immunopathogenesis in HD | [71] |
| -Increased 3-HK concentration in the brain | [81] | - | - |
| -Decreased KAT activity in the neostriatum -Decreased KYNA level in neostriatum | [89] | - | - |
| -Increased 3-HK and QUIN in the neocortex and in the neostriatum, but not in the cerebellum of the low grade HD brain -In contrast, the unchanged or decreased tendency was seen in 3-HK and QUIN levels in grade 2 and advanced grade (grades 3–4) HD brain -QUIN/KYNA and 3-HK/KYNA ratios indicated enhanced metabolism along the QUIN branch of the pathway in the neostriatum and the neocortex, but not in the cerebellum in the early stages of the disease -Results support a possible involvement of 3-HK and QUIN in the early phases of HD | [76] | - | - |
| pathophysiology and indicate novel therapeutic strategies against the disease. | |||
| -T. gondii infection resulted in elevation of cortical IDO activity in HD mice. HD-infected mice died significantly earlier than wild-type infected and HD control mice. | [108] | ||
| Multiple Sclerosis | |||
|---|---|---|---|
| In the CNS | Ref. | In the Periphery | Ref. |
| -Reduced TRP level in the CNS | [70] | -Reduced TRP level in the serum | [70] |
| -Neopterin and l-TRP correlated negatively in CSF of RRMS | [54] | -Higher KAT I and II activities in the red blood cells -Increased KYNA in plasma (compensatory protective mechanism) | [88] |
| -Expression and activity of KMO significantly increased in the spinal cord of rats with EAE -Increased formation of 3-HK and accumulated QUIN in the CNS of rats with EAE | [120] | -Increased IDO expression in serum IDO gene expression and activity in blood could be a useful marker to monitor the clinical course of RRMS -Therapeutic interventions modulating IDO activity may be beneficial in MS -IDO could contribute to remission of relapse in MS. -evaluation of IDO gene expression could be a useful predictive biomarker indicating the development of flares of disease | [149] |
| -IDO-1 inhibition exacerbated MS severity in EAE -IDO may contribute to the regulation of T cell activity associated with the different phases of this animal model of MS | [119] | -Lower glucose, 5-OH-TRP, and TRP in plasma (as a potential biomarker) | [116] |
| -Decreased KYNA in CSF during chronic remission | [117] | -Increased level KYNA and PIC in RRMS but not in SPMS or PPMS -Increased 3-HK and QUIN levels in both SPMS and PPMS -Moderately strong correlation between QUIN/KNYA ratio and MS severity -The first study using targeted KP metabolomics as a blood-based prognostic biomarker capable of distinguishing MS subtype. -TRP metabolism is more relevant to MS pathology than general inflammation -Serum KP profile is a suitably sensitive blood-based predictor of disease progression in MS -QUIN/KYNA levels, could be useful therapeutic approaches in slowing neurodegeneration in MS | [116] |
| -Elevated KYNA levels in CSF during acute relapse | [121] | - | - |
References
- Németh, G.; Jelinek, I. New directions in biomarker research, drug development and personalized medicine. Magy. Onkol. 2013, 57, 5–10. [Google Scholar] [PubMed]
- Aronson, J.K.; Ferner, R.E. Biomarkers-A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9.23.1–9.23.17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mándi, Y.; Vécsei, L. The kynurenine system and immunoregulation. J. Neural Transm. (Vienna) 2012, 119, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Nucci, N.; Menicali, E.; Morelli, S.; Bini, V.; Colella, R.; Mandarano, M.; Sidoni, A.; Puxeddu, E. The Aryl Hydrocarbon Receptor Is Expressed in Thyroid Carcinoma and Appears to Mediate Epithelial-Mesenchymal-Transition. Cancers 2020, 12, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panfili, E.; Gerli, R.; Grohmann, U.; Pallotta, M.T. Amino Acid Metabolism in Rheumatoid Arthritis: Friend or Foe? Biomolecules 2020, 10, 1280. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalardy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef]
- Jovanovic, F.; Candido, K.D.; Knezevic, N.N. The Role of the Kynurenine Signaling Pathway in Different Chronic Pain Conditions and Potential Use of Therapeutic Agents. Int. J. Mol. Sci. 2020, 21, 6045. [Google Scholar] [CrossRef]
- Anesi, A.; Rubert, J.; Oluwagbemigun, K.; Orozco-Ruiz, X.; Nothlings, U.; Breteler, M.M.B.; Mattivi, F. Metabolic Profiling of Human Plasma and Urine, Targeting Tryptophan, Tyrosine and Branched Chain Amino Acid Pathways. Metabolites 2019, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Grant, R.; Nguyen, S.; Guillemin, G. Kynurenine Pathway Metabolism is Involved in the Maintenance of the Intracellular NAD Concentration in Human Primary Astrocytes. Int. J. Tryptophan Res. 2010, 3, 151–156. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, H.; Toldi, J.; Vecsei, L. Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2005, 2, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.S.; Coggan, S.E.; Smythe, G.A. The physiological action of picolinic Acid in the human brain. Int. J. Tryptophan Res. 2009, 2, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Pol, J.A.; Klos, D.J.; Hamilton, P.D. Antiviral, cytotoxic and apoptotic activities of picolinic acid on human immunodeficiency virus-1 and human herpes simplex virus-2 infected cells. Anticancer Res. 2001, 21, 3773–3776. [Google Scholar] [PubMed]
- Cai, S.; Sato, K.; Shimizu, T.; Yamabe, S.; Hiraki, M.; Sano, C.; Tomioka, H. Antimicrobial activity of picolinic acid against extracellular and intracellular Mycobacterium avium complex and its combined activity with clarithromycin, rifampicin and fluoroquinolones. J. Antimicrob. Chemother. 2006, 57, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Varesio, L.; Neglia, R.; Colombari, B.; Pastorino, S.; Blasi, E. Antifungal activity of macrophages engineered to produce IFNgamma: Inducibility by picolinic acid. Med. Microbiol. Immunol. 2003, 192, 71–78. [Google Scholar] [CrossRef]
- Cuartero, M.I.; de la Parra, J.; Garcia-Culebras, A.; Ballesteros, I.; Lizasoain, I.; Moro, M.A. The Kynurenine Pathway in the Acute and Chronic Phases of Cerebral Ischemia. Curr. Pharm. Des. 2016, 22, 1060–1073. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohar, Z.; Martos, D.; Telegdy, G.; Vecsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Koola, M.M. Galantamine-Memantine combination in the treatment of Alzheimer’s disease and beyond. Psychiatry Res. 2020, 293, 113409. [Google Scholar] [CrossRef]
- Seckler, J.M.; Lewis, S.J. Advances in d-Amino Acids in Neurological Research. Int. J. Mol. Sci. 2020, 21, 7325. [Google Scholar] [CrossRef]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorumski, C.F.; Izumi, Y. NMDA receptors and metaplasticity: Mechanisms and possible roles in neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2012, 36, 989–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Morrison, G.L.; Fontaine, C.J.; Hou, Q.; Harley, C.W.; Yuan, Q. Unlearning: NMDA receptor-mediated metaplasticity in the anterior piriform cortex following early odor preference training in rats. J. Neurosci. 2014, 34, 5143–5151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006, 402, 108–112. [Google Scholar] [CrossRef]
- Rózsa, E.; Robotka, H.; Vécsei, L.; Toldi, J. The Janus-face kynurenic acid. J. Neural Transm. (Vienna) 2008, 115, 1087–1091. [Google Scholar] [CrossRef]
- Tuboly, G.; Tar, L.; Bohar, Z.; Safrany-Fark, A.; Petrovszki, Z.; Kekesi, G.; Vecsei, L.; Pardutz, A.; Horvath, G. The inimitable kynurenic acid: The roles of different ionotropic receptors in the action of kynurenic acid at a spinal level. Brain Res. Bull. 2015, 112, 52–60. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Dobelis, P.; Staley, K.J.; Cooper, D.C. Lack of modulation of nicotinic acetylcholine alpha-7 receptor currents by kynurenic acid in adult hippocampal interneurons. PLoS ONE 2012, 7, e41108. [Google Scholar] [CrossRef]
- Watkins, L.R.; Orlandi, C. Orphan G Protein Coupled Receptors in Affective Disorders. Genes 2020, 11, 694. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Kuc, D.; Dobrowolski, P.; Lamorski, K.; Smolińska, K.; Donaldson, J.; Świetlicka, I.; Mielnik-Błaszczak, M.; Paluszkiewicz, P.; et al. Chronic dietary supplementation with kynurenic acid, a neuroactive metabolite of tryptophan, decreased body weight without negative influence on densitometry and mandibular bone biomechanical endurance in young rats. PLoS ONE 2019, 14, e0226205. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Tohyama, C. Embryonic and Postnatal Expression of Aryl Hydrocarbon Receptor mRNA in Mouse Brain. Front. Neuroanat. 2017, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, O.; Rochette, E.; Dore, E.; Maso, F.; Raoux, J.; Andrieux, F.; Fantini, M.L.; Merlin, E.; Pereira, B.; Walrand, S.; et al. Randomized Double-Blind Controlled Trial on the Effect of Proteins with Different Tryptophan/Large Neutral Amino Acid Ratios on Sleep in Adolescents: The PROTMORPHEUS Study. Nutrients 2020, 12, 1885. [Google Scholar] [CrossRef]
- Ramos-Chavez, L.A.; Lugo Huitron, R.; Gonzalez Esquivel, D.; Pineda, B.; Rios, C.; Silva-Adaya, D.; Sanchez-Chapul, L.; Roldan-Roldan, G.; Perez de la Cruz, V. Relevance of Alternative Routes of Kynurenic Acid Production in the Brain. Oxid. Med. Cell Longev. 2018, 2018, 5272741. [Google Scholar] [CrossRef] [Green Version]
- Di Biase, E.; Lunghi, G.; Maggioni, M.; Fazzari, M.; Pomè, D.Y.; Loberto, N.; Ciampa, M.G.; Fato, P.; Mauri, L.; Sevin, E.; et al. GM1 Oligosaccharide Crosses the Human Blood–Brain Barrier In Vitro by a Paracellular Route. Int. J. Mol. Sci. 2020, 21, 2858. [Google Scholar] [CrossRef] [Green Version]
- Diez-Iriepa, D.; Chamorro, B.; Talaván, M.; Chioua, M.; Iriepa, I.; Hadjipavlou-Litina, D.; López-Muñoz, F.; Marco-Contelles, J.; Oset-Gasque, M.J. Homo-Tris-Nitrones Derived from α-Phenyl-N-tert-butylnitrone: Synthesis, Neuroprotection and Antioxidant Properties. Int. J. Mol. Sci. 2020, 21, 7949. [Google Scholar] [CrossRef]
- Terai, M.; Londin, E.; Rochani, A.; Link, E.; Lam, B.; Kaushal, G.; Bhushan, A.; Orloff, M.; Sato, T. Expression of Tryptophan 2,3-Dioxygenase in Metastatic Uveal Melanoma. Cancers 2020, 12, 405. [Google Scholar] [CrossRef] [Green Version]
- Mbongue, J.C.; Nicholas, D.A.; Torrez, T.W.; Kim, N.S.; Firek, A.F.; Langridge, W.H. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines 2015, 3, 703–729. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jia, A.; Bi, Y.; Wang, Y.; Yang, Q.; Cao, Y.; Li, Y.; Liu, G. Targeting Myeloid-Derived Suppressor Cells in Cancer Immunotherapy. Cancers 2020, 12, 2626. [Google Scholar] [CrossRef]
- Hunt, C.; Macedo e Cordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Vilariño-García, T.; Sánchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Jin, T.; Kumar Sahu, S.; Xu, J.; Shi, Q.; Liu, H.; Wang, Y. The Distribution of Tryptophan-Dependent Indole-3-Acetic Acid Synthesis Pathways in Bacteria Unraveled by Large-Scale Genomic Analysis. Molecules 2019, 24, 1411. [Google Scholar] [CrossRef] [Green Version]
- Waclawiková, B.; El Aidy, S. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Croft, T.; Venkatakrishnan, P.; Lin, S.-J. NAD+ Metabolism and Regulation: Lessons from Yeast. Biomolecules 2020, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Stone, T.W.; Darlington, L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013, 169, 1211–1227. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.F.; Tan, L.; Wang, H.F.; Jiang, T.; Tan, M.S.; Tan, L.; Xu, W.; Li, J.Q.; Wang, J.; Lai, T.J.; et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord. 2016, 190, 264–271. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Ambar Akkaoui, M.; Geoffroy, P.A.; Roze, E.; Degos, B.; Garcin, B. Functional Motor Symptoms in Parkinson’s Disease and Functional Parkinsonism: A Systematic Review. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 4–13. [Google Scholar] [CrossRef] [PubMed]
- O’Day, D.H. Calmodulin Binding Proteins and Alzheimer’s Disease: Biomarkers, Regulatory Enzymes and Receptors That Are Regulated by Calmodulin. Int. J. Mol. Sci. 2020, 21, 7344. [Google Scholar] [CrossRef]
- Schwarz, M.J.; Guillemin, G.J.; Teipel, S.J.; Buerger, K.; Hampel, H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 26, 345–352. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Brew, B.J.; Noonan, C.E.; Takikawa, O.; Cullen, K.M. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol. Appl. Neurobiol. 2005, 31, 395–404. [Google Scholar] [CrossRef]
- Rahman, A.; Ting, K.; Cullen, K.M.; Braidy, N.; Brew, B.J.; Guillemin, G.J. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS ONE 2009, 4, e6344. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Mailankot, M.; Stone, J.G.; Garrett, M.R.; Staniszewska, M.; Castellani, R.J.; Siedlak, S.L.; Zhu, X.; Lee, H.G.; Perry, G.; et al. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer’s disease. Redox Rep. 2010, 15, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Nicolazzo, J.A.; Wen, L.; Chung, R.; Stankovic, R.; Bao, S.S.; Lim, C.K.; Brew, B.J.; Cullen, K.M.; Guillemin, G.J. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS ONE 2013, 8, e59749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Velpen, V.; Teav, T.; Gallart-Ayala, H.; Mehl, F.; Konz, I.; Clark, C.; Oikonomidi, A.; Peyratout, G.; Henry, H.; Delorenzi, M.; et al. Systemic and central nervous system metabolic alterations in Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Giil, L.M.; Midttun, O.; Refsum, H.; Ulvik, A.; Advani, R.; Smith, A.D.; Ueland, P.M. Kynurenine Pathway Metabolites in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Catanesi, M.; d’Angelo, M.; Tupone, M.G.; Benedetti, E.; Giordano, A.; Castelli, V.; Cimini, A. MicroRNAs Dysregulation and Mitochondrial Dysfunction in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 5986. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.M.; Ribeiro-dos-Santos, Â.; Vidal, A.F.; de Araújo, G.S. on behalf of the Alzheimer’s Disease Neuroimaging Initiative. Differential Expression and miRNA–Gene Interactions in Early and Late Mild Cognitive Impairment. Biology 2020, 9, 251. [Google Scholar] [CrossRef]
- Widner, B.; Leblhuber, F.; Walli, J.; Tilz, G.P.; Demel, U.; Fuchs, D. Tryptophan degradation and immune activation in Alzheimer’s disease. J. Neural Transm. (Vienna) 2000, 107, 343–353. [Google Scholar] [CrossRef]
- Yu, D.; Tao, B.B.; Yang, Y.Y.; Du, L.S.; Yang, S.S.; He, X.J.; Zhu, Y.W.; Yan, J.K.; Yang, Q. The IDO inhibitor coptisine ameliorates cognitive impairment in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, D.; Huang, S.Y.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Guidetti, P.; Wu, H.Q.; Lee, J.; Truong, J.; Andrews-Zwilling, Y.; Hsieh, E.; et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell 2011, 145, 863–874. [Google Scholar] [CrossRef] [Green Version]
- González-Sánchez, M.; Jiménez, J.; Narváez, A.; Antequera, D.; Llamas-Velasco, S.; Martín, A.-S.; Molina Arjona, J.A.; López de Munain, A.; Lleó Bisa, A.; Marco, M.-P.; et al. Kynurenic Acid Levels are Increased in the CSF of Alzheimer’s Disease Patients. Biomolecules 2020, 10, 571. [Google Scholar] [CrossRef] [Green Version]
- Gulaj, E.; Pawlak, K.; Bien, B.; Pawlak, D. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv. Med. Sci. 2010, 55, 204–211. [Google Scholar] [CrossRef]
- Chen, Y.; Stankovic, R.; Cullen, K.M.; Meininger, V.; Garner, B.; Coggan, S.; Grant, R.; Brew, B.J.; Guillemin, G.J. The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox. Res. 2010, 18, 132–142. [Google Scholar] [CrossRef]
- Widner, B.; Leblhuber, F.; Walli, J.; Tilz, G.P.; Demel, U.; Fuchs, D. Degradation of tryptophan in neurodegenerative disorders. Adv. Exp. Med. Biol. 1999, 467, 133–138. [Google Scholar]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Spiden, S.L.; Taylor, R.; Stone, T.W.; Darlington, L.G. Blood levels of kynurenines, interleukin-23 and soluble human leucocyte antigen-G at different stages of Huntington’s disease. J. Neurochem. 2010, 112, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Monaco, F.; Fumero, S.; Mondino, A.; Mutani, R. Plasma and cerebrospinal fluid tryptophan in multiple sclerosis and degenerative diseases. J. Neurol. Neurosurg. Psychiatry 1979, 42, 640–641. [Google Scholar] [CrossRef]
- Cocco, E.; Murgia, F.; Lorefice, L.; Barberini, L.; Poddighe, S.; Frau, J.; Fenu, G.; Coghe, G.; Murru, M.R.; Murru, R.; et al. (1)H-NMR analysis provides a metabolomic profile of patients with multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heilman, P.L.; Wang, E.W.; Lewis, M.M.; Krzyzanowski, S.; Capan, C.D.; Burmeister, A.R.; Du, G.; Escobar Galvis, M.L.; Brundin, P.; Huang, X.; et al. Tryptophan Metabolites Are Associated With Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Cheng, M.L.; Tang, H.Y.; Huang, C.Y.; Wu, Y.R.; Chen, C.M. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 6319–6328. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Luthi-Carter, R.E.; Augood, S.J.; Schwarcz, R. Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiol. Dis. 2004, 17, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Rejdak, K.; Bartosik-Psujek, H.; Dobosz, B.; Kocki, T.; Grieb, P.; Giovannoni, G.; Turski, W.A.; Stelmasiak, Z. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci. Lett. 2002, 331, 63–65. [Google Scholar] [CrossRef]
- Rejdak, K.; Petzold, A.; Kocki, T.; Kurzepa, J.; Grieb, P.; Turski, W.A.; Stelmasiak, Z. Astrocytic activation in relation to inflammatory markers during clinical exacerbation of relapsing-remitting multiple sclerosis. J. Neural Transm. (Vienna) 2007, 114, 1011–1015. [Google Scholar] [CrossRef]
- Chatterjee, P.; Goozee, K.; Lim, C.K.; James, I.; Shen, K.; Jacobs, K.R.; Sohrabi, H.R.; Shah, T.; Asih, P.R.; Dave, P.; et al. Alterations in serum kynurenine pathway metabolites in individuals with high neocortical amyloid-beta load: A pilot study. Sci. Rep. 2018, 8, 8008. [Google Scholar] [CrossRef]
- Ogawa, T.; Matson, W.R.; Beal, M.F.; Myers, R.H.; Bird, E.D.; Milbury, P.; Saso, S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 1992, 42, 1702–1706. [Google Scholar] [CrossRef]
- Lewitt, P.A.; Li, J.; Lu, M.; Beach, T.G.; Adler, C.H.; Guo, L.; Arizona Parkinson’s Disease Consortium. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov. Disord. 2013, 28, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Reynolds, G.P. Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington’s disease. Neurosci. Lett. 1992, 144, 199–201. [Google Scholar] [CrossRef]
- Stoy, N.; Mackay, G.M.; Forrest, C.M.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Heyes, M.P.; Saito, K.; Crowley, J.S.; Davis, L.E.; Demitrack, M.A.; Der, M.; Dilling, L.A.; Elia, J.; Kruesi, M.J.; Lackner, A.; et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992, 115 Pt 5, 1249–1273. [Google Scholar] [CrossRef]
- Baran, H.; Jellinger, K.; Deecke, L. Kynurenine metabolism in Alzheimer’s disease. J. Neural Transm. (Vienna) 1999, 106, 165–181. [Google Scholar] [CrossRef]
- Hartai, Z.; Juhasz, A.; Rimanoczy, A.; Janaky, T.; Donko, T.; Dux, L.; Penke, B.; Toth, G.K.; Janka, Z.; Kalman, J. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochem. Int. 2007, 50, 308–313. [Google Scholar] [CrossRef]
- Ilzecka, J.; Kocki, T.; Stelmasiak, Z.; Turski, W.A. Endogenous protectant kynurenic acid in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2003, 107, 412–418. [Google Scholar] [CrossRef]
- Beal, M.F.; Matson, W.R.; Swartz, K.J.; Gamache, P.H.; Bird, E.D. Kynurenine pathway measurements in Huntington’s disease striatum: Evidence for reduced formation of kynurenic acid. J. Neurochem. 1990, 55, 1327–1339. [Google Scholar] [CrossRef]
- Beal, M.F.; Matson, W.R.; Storey, E.; Milbury, P.; Ryan, E.A.; Ogawa, T.; Bird, E.D. Kynurenic acid concentrations are reduced in Huntington’s disease cerebral cortex. J. Neurol. Sci. 1992, 108, 80–87. [Google Scholar] [CrossRef]
- Jauch, D.; Urbanska, E.M.; Guidetti, P.; Bird, E.D.; Vonsattel, J.P.; Whetsell, W.O., Jr.; Schwarcz, R. Dysfunction of brain kynurenic acid metabolism in Huntington’s disease: Focus on kynurenine aminotransferases. J. Neurol. Sci. 1995, 130, 39–47. [Google Scholar] [CrossRef]
- Mancuso, R.; Hernis, A.; Agostini, S.; Rovaris, M.; Caputo, D.; Fuchs, D.; Clerici, M. Indoleamine 2,3 Dioxygenase (IDO) Expression and Activity in Relapsing-Remitting Multiple Sclerosis. PLoS ONE 2015, 10, e0130715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarugi, A.; Cozzi, A.; Ballerini, C.; Massacesi, L.; Moroni, F. Kynurenine 3-mono-oxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience 2001, 102, 687–695. [Google Scholar] [CrossRef]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vécsei, L. Kynurenine metabolism in plasma and in red blood cells in Parkinson’s disease. J. Neurol. Sci. 2005, 239, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.H.; Zheng, Y.L.; Yu, Y.P. Urinary kynurenine as a biomarker for Parkinson’s disease. Neurol. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Widner, B.; Leblhuber, F.; Fuchs, D. Increased neopterin production and tryptophan degradation in advanced Parkinson’s disease. J. Neural Transm. (Vienna) 2002, 109, 181–189. [Google Scholar] [CrossRef]
- Knyihar-Csillik, E.; Csillik, B.; Pakaski, M.; Krisztin-Peva, B.; Dobo, E.; Okuno, E.; Vecsei, L. Decreased expression of kynurenine aminotransferase-I (KAT-I) in the substantia nigra of mice after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment. Neuroscience 2004, 126, 899–914. [Google Scholar] [CrossRef]
- Hartai, Z.; Klivenyi, P.; Janaky, T.; Penke, B.; Dux, L.; Vecsei, L. Kynurenine metabolism in multiple sclerosis. Acta Neurol. Scand. 2005, 112, 93–96. [Google Scholar] [CrossRef]
- Jacobs, K.R.; Lim, C.K.; Blennow, K.; Zetterberg, H.; Chatterjee, P.; Martins, R.N.; Brew, B.J.; Guillemin, G.J.; Lovejoy, D.B. Correlation between plasma and CSF concentrations of kynurenine pathway metabolites in Alzheimer’s disease and relationship to amyloid-beta and tau. Neurobiol. Aging 2019, 80, 11–20. [Google Scholar] [CrossRef]
- Schwarcz, R.; Okuno, E.; White, R.J.; Bird, E.D.; Whetsell, W.O., Jr. 3-Hydroxyanthranilate oxygenase activity is increased in the brains of Huntington disease victims. Proc. Natl. Acad. Sci. USA 1988, 85, 4079–4081. [Google Scholar] [CrossRef] [Green Version]
- Papagno, C.; Trojano, L. Cognitive and behavioral disorders in Parkinson’s disease: An update. I: Cognitive impairments. Neurol. Sci. 2018, 39, 215–223. [Google Scholar] [CrossRef]
- Broen, M.P.; Narayen, N.E.; Kuijf, M.L.; Dissanayaka, N.N.; Leentjens, A.F. Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2016, 31, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Török, N.; Majlath, Z.; Szalardy, L.; Vécsei, L. Investigational alpha-synuclein aggregation inhibitors: Hope for Parkinson’s disease. Expert Opin. Investig. Drugs 2016, 25, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Mahul-Mellier, A.L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Török, N.; Török, R.; Szolnoki, Z.; Somogyvari, F.; Klivenyi, P.; Vécsei, L. The Genetic Link between Parkinson’s Disease and the Kynurenine Pathway Is Still Missing. Parkinsons Dis. 2015, 2015, 474135. [Google Scholar] [CrossRef]
- Dal Ben, M.; Bongiovanni, R.; Tuniz, S.; Fioriti, E.; Tiribelli, C.; Moretti, R.; Gazzin, S. Earliest Mechanisms of Dopaminergic Neurons Sufferance in a Novel Slow Progressing Ex Vivo Model of Parkinson Disease in Rat Organotypic Cultures of Substantia Nigra. Int. J. Mol. Sci. 2019, 20, 2224. [Google Scholar] [CrossRef] [Green Version]
- Török, N.; Maszlag-Török, R.; Molnár, K.; Szolnoki, Z.; Somogyvári, F.; Boda, K.; Tanaka, M.; Klivényi, P.; Vécsei, L. Single Nucleotide Polymorphisms of Indoleamine 2,3-Dioxygenase 1 Influenced the Age Onset of Parkinson’s Disease. Preprints 2020. [Google Scholar] [CrossRef]
- Pfaff, A.L.; Bubb, V.J.; Quinn, J.P.; Koks, S. An Increased Burden of Highly Active Retrotransposition Competent L1s Is Associated with Parkinson’s Disease Risk and Progression in the PPMI Cohort. Int. J. Mol. Sci. 2020, 21, 6562. [Google Scholar] [CrossRef]
- Rojas, P.; Ramirez, A.I.; Fernandez-Albarral, J.A.; Lopez-Cuenca, I.; Salobrar-Garcia, E.; Cadena, M.; Elvira-Hurtado, L.; Salazar, J.J.; de Hoz, R.; Ramirez, J.M. Amyotrophic Lateral Sclerosis: A Neurodegenerative Motor Neuron Disease with Ocular Involvement. Front. Neurosci. 2020, 14, 566858. [Google Scholar] [CrossRef]
- Piccione, E.A.; Sletten, D.M.; Staff, N.P.; Low, P.A. Autonomic system and amyotrophic lateral sclerosis. Muscle Nerve 2015, 51, 676–979. [Google Scholar] [CrossRef] [Green Version]
- Fang, T.; Jozsa, F.; Al-Chalabi, A. Nonmotor Symptoms in Amyotrophic Lateral Sclerosis: A Systematic Review. Int. Rev. Neurobiol. 2017, 134, 1409–1441. [Google Scholar]
- Carvalho, T.L.; de Almeida, L.M.; Lorega, C.M.; Barata, M.F.; Ferreira, M.L.; de Brito-Marques, P.R.; Correia Cda, C. Depression and anxiety in individuals with amyotrophic lateral sclerosis: A systematic review. Trends Psychiatry Psychother. 2016, 38, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renton, A.E.; Majounie, E.; Waite, A.; Simon-Sanchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Z.Y.; Zhou, Z.R.; Che, C.H.; Liu, C.Y.; He, R.L.; Huang, H.P. Genetic epidemiology of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [Green Version]
- Tripolszki, K.; Csanyi, B.; Nagy, D.; Ratti, A.; Tiloca, C.; Silani, V.; Kereszty, E.; Török, N.; Vecsei, L.; Engelhardt, J.I.; et al. Genetic analysis of the SOD1 and C9ORF72 genes in Hungarian patients with amyotrophic lateral sclerosis. Neurobiol. Aging 2017, 53, 195.e1–195.e5. [Google Scholar] [CrossRef] [Green Version]
- Tripolszki, K.; Török, D.; Goudenege, D.; Farkas, K.; Sulak, A.; Török, N.; Engelhardt, J.I.; Klivényi, P.; Procaccio, V.; Nagy, N.; et al. High-throughput sequencing revealed a novel SETX mutation in a Hungarian patient with amyotrophic lateral sclerosis. Brain Behav. 2017, 7, e00669. [Google Scholar] [CrossRef] [Green Version]
- Török, N.; Török, R.; Klivényi, P.; Engelhardt, J.; Vécsei, L. Investigation of vitamin D receptor polymorphisms in amyotrophic lateral sclerosis. Acta Neurol. Scand. 2016, 133, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Dash, D.; Mestre, T.A. Therapeutic Update on Huntington’s Disease: Symptomatic Treatments and Emerging Disease-Modifying Therapies. Neurotherapeutics 2020. [Google Scholar] [CrossRef]
- Donley, D.W.; Olson, A.R.; Raisbeck, M.F.; Fox, J.H.; Gigley, J.P. Huntingtons Disease Mice Infected with Toxoplasma gondii Demonstrate Early Kynurenine Pathway Activation, Altered CD8+ T-Cell Responses, and Premature Mortality. PLoS ONE 2016, 11, e0162404. [Google Scholar] [CrossRef] [Green Version]
- Boeschoten, R.E.; Braamse, A.M.J.; Beekman, A.T.F.; Cuijpers, P.; van Oppen, P.; Dekker, J.; Uitdehaag, B.M.J. Prevalence of depression and anxiety in Multiple Sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 372, 331–341. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Ho, P.P.; Youssef, S.; Fontoura, P.; Garren, H.; Hur, E.M.; Gupta, R.; Lee, L.Y.; Kidd, B.A.; Robinson, W.H.; et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science 2005, 310, 850–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pemberton, L.A.; Kerr, S.J.; Smythe, G.; Brew, B.J. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J. Interferon Cytokine Res. 1997, 17, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Zou, J.P.; Tschetter, J.R.; Ward, J.M.; Shearer, G.M. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002, 129, 186–196. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef]
- Kwidzinski, E.; Bechmann, I. IDO expression in the brain: A double-edged sword. J. Mol. Med. (Berl) 2007, 85, 1351–1359. [Google Scholar] [CrossRef] [Green Version]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; et al. Serum Kynurenines Correlate with Depressive Symptoms and Disability in Poststroke Patients: A Cross-sectional Study. Neurorehabilit. Neural Repair 2020. [Google Scholar] [CrossRef]
- Ulivieri, M.; Wierońska, J.M.; Lionetto, L.; Martinello, K.; Cieslik, P.; Chocyk, A.; Curto, M.; Di Menna, L.; Iacovelli, L.; Traficante, A.; et al. The Trace Kynurenine, Cinnabarinic Acid, Displays Potent Antipsychotic-Like Activity in Mice and Its Levels Are Reduced in the Prefrontal Cortex of Individuals Affected by Schizophrenia. Schizophr. Bull. 2020. [Google Scholar] [CrossRef] [PubMed]
- Małgorzata, P.; Paweł, K.; Iwona, M.L.; Brzostek, T.; Andrzej, P. Glutamatergic dysregulation in mood disorders: Opportunities for the discovery of novel drug targets. Expert Opin. Ther. Targets 2020, 3, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Koola, M.M. Alpha7 nicotinic-N-methyl-d-aspartate hypothesis in the treatment of schizophrenia and beyond. Hum. Psychopharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Ahn, H.-S.; Lee, M.Y.; Yu, J.; Yeom, J.; Jeong, H.; Min, H.; Lee, H.J.; Kim, K.; Ahn, Y.M. An Exploratory Pilot Study with Plasma Protein Signatures Associated with Response of Patients with Depression to Antidepressant Treatment for 10 Weeks. Biomedicines 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Telegdy, G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav. Brain Res. 2014, 259, 196–199. [Google Scholar] [CrossRef]
- Tanaka, M.; Kádár, K.; Tóth, G.; Telegdy, G. Antidepressant-like effects of urocortin 3 fragments. Brain Res. Bull. 2011, 84, 414–418. [Google Scholar] [CrossRef]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the LHRH antagonist Cetrorelix on the brain function in mice. Neuropeptides 2009, 43, 229–234. [Google Scholar] [CrossRef]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–391. [Google Scholar] [CrossRef]
- Rog, J.; Błażewicz, A.; Juchnowicz, D.; Ludwiczuk, A.; Stelmach, E.; Kozioł, M.; Karakula, M.; Niziński, P.; Karakula-Juchnowicz, H. The Role of GPR120 Receptor in Essential Fatty Acids Metabolism in Schizophrenia. Biomedicines 2020, 8, 243. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Rodríguez de Fonseca, F. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef]
- Cantón-Habas, V.; Rich-Ruiz, M.; Romero-Saldaña, M.; Carrera-González, M.P. Depression as a Risk Factor for Dementia and Alzheimer’s Disease. Biomedicines 2020, 8, 45. [Google Scholar] [CrossRef]
- Kowalska, K.; Krzywoszański, Ł.; Droś, J.; Pasińska, P.; Wilk, A.; Klimkowicz-Mrowiec, A. Early Depression Independently of Other Neuropsychiatric Conditions, Influences Disability and Mortality after Stroke (Research Study—Part of PROPOLIS Study). Biomedicines 2020, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.S.; Luís, Â.; Barroso, M.; Gallardo, E.; Pereira, L. Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases—A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines 2020, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bak, A.; Kim, S.; Nam, Y.; Kim, H.; Yoo, D.-H.; Moon, M. Animal-Assisted and Pet-Robot Interventions for Ameliorating Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. Biomedicines 2020, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, L.; Li, Y.; Dong, J.; Li, M.; Huang, J.; Lin, S.; Cai, Z. Urinary Metabolomics Reveals Alterations of Aromatic Amino Acid Metabolism of Alzheimer’s Disease in the Transgenic CRND8 Mice. Curr. Alzheimer Res. 2016, 13, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Zetterberg, H.; Goozee, K.; Lim, C.K.; Jacobs, K.R.; Ashton, N.J.; Hye, A.; Pedrini, S.; Sohrabi, H.R.; Shah, T.; et al. Plasma neurofilament light chain and amyloid-beta are associated with the kynurenine pathway metabolites in preclinical Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 186. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Cheong, Y.J.; Bhatnagar, A.; Goozee, K.; Wu, Y.; McKay, M.; Martins, I.J.; Lim, W.L.F.; Pedrini, S.; Tegg, M.; et al. Plasma metabolites associated with biomarker evidence of neurodegeneration in cognitively normal older adults. J. Neurochem. 2020. [Google Scholar] [CrossRef]
- Luchowski, P.; Luchowska, E.; Turski, W.A.; Urbanska, E.M. 1-Methyl-4-phenylpyridinium and 3-nitropropionic acid diminish cortical synthesis of kynurenic acid via interference with kynurenine aminotransferases in rats. Neurosci. Lett. 2002, 330, 49–52. [Google Scholar] [CrossRef]
- Janssens, J.; Vermeiren, Y.; van Faassen, M.; van der Ley, C.; Kema, I.; De Deyn, P.P. Monoaminergic and Kynurenergic Characterization of Frontotemporal Dementia and Amyotrophic Lateral Sclerosis in Cerebrospinal Fluid and Serum. Neurochem. Res. 2020, 45, 1191–1201. [Google Scholar] [CrossRef] [Green Version]
- Obal, I.; Engelhardt, J.I.; Siklos, L. Axotomy induces contrasting changes in calcium and calcium-binding proteins in oculomotor and hypoglossal nuclei of Balb/c mice. J. Comp. Neurol. 2006, 499, 17–32. [Google Scholar] [CrossRef]
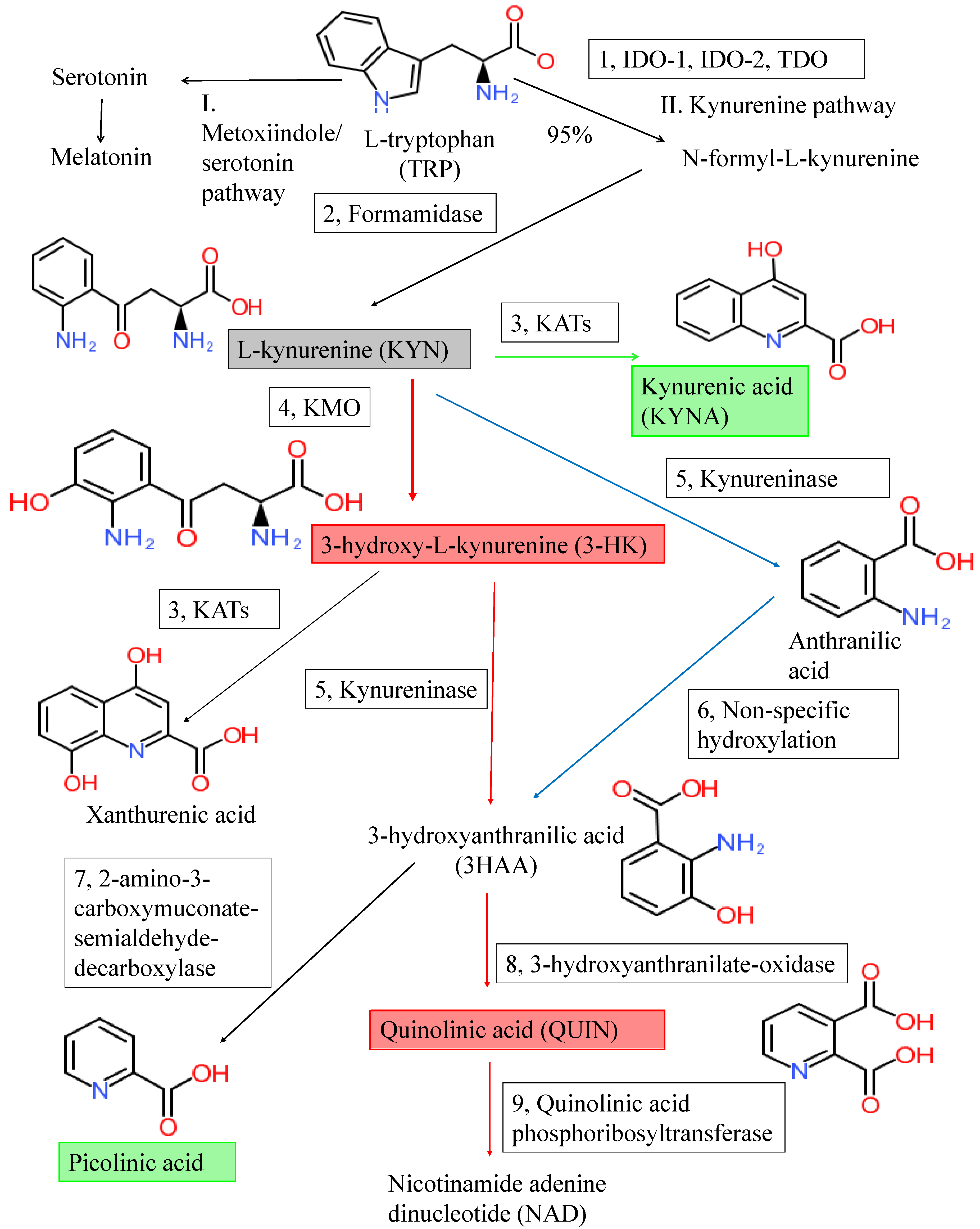
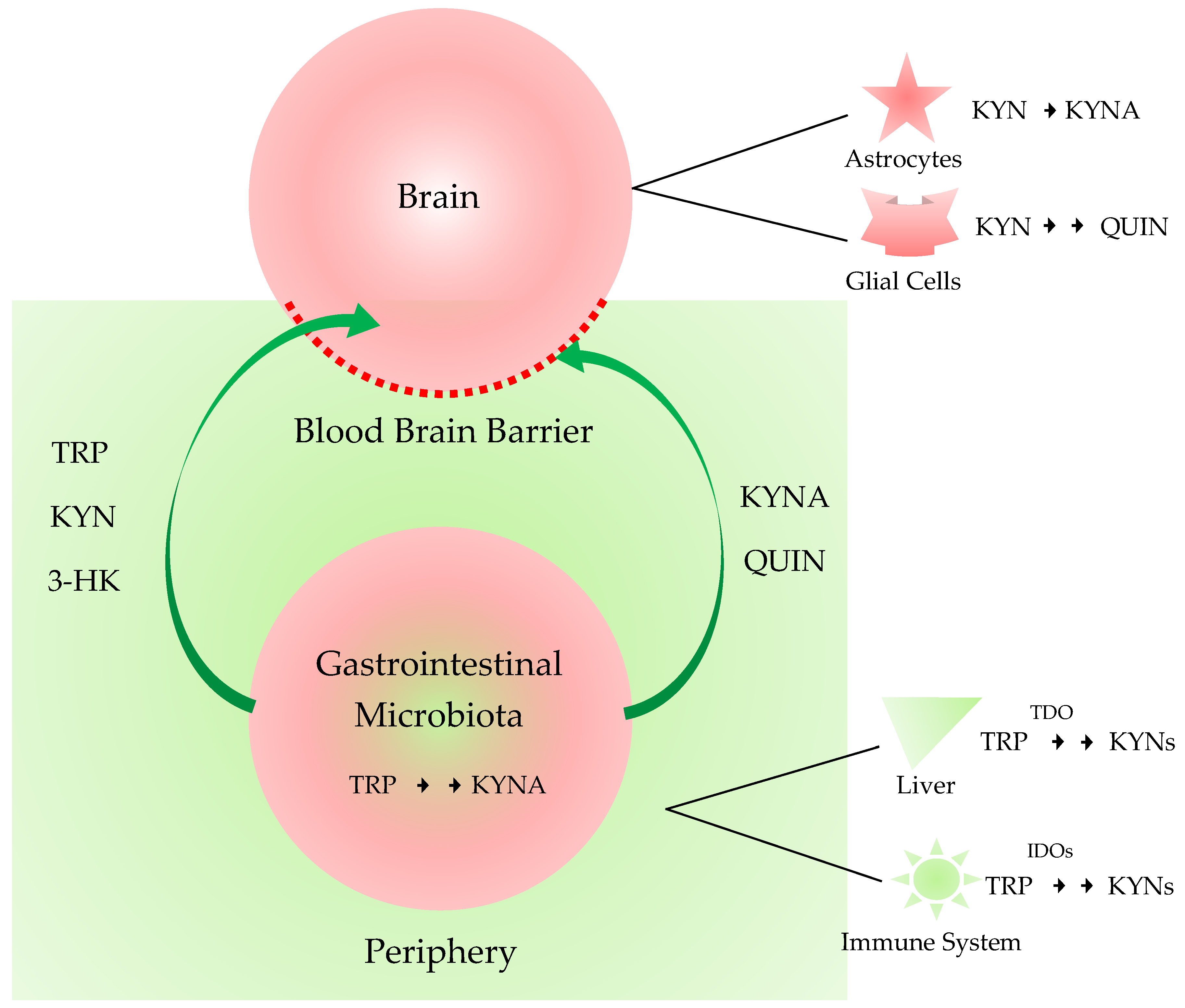
| Metabolites | Alzheimer’s Disease | Parkinson’s Disease | Amyotrophic Lateral Sclerosis | Huntington’s Disease | Multiple Sclerosis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CNS | Peripheral | CNS | Peripheral | CNS | Peripheral | CNS | Peripheral | CNS | Peripheral | |
| TRP | - | ↓ [61] ↓ [68] | - | - | ↑ [69] ↑ [69] | ↑ [69] | - | ↓ [70] ↓ [71] | ↓ [72] | ↓ [72] ↓ [73] |
| QUIN | ↑ [56] ↑ [59] ↑ [60] | ↓ [61] ↑ [68] | ↑ [74] | ↑ [75] | ↑ [69] | ↑ [69] | ↑ [76] | - | ↑ [77] | ↑ [78] |
| 3-HK | - | ↑ [55] ↑* [68] ↑ [79] | ↑ [80] ↑ [81] | ↑ [74] | - | - | ↑ [76] ↑ [82] | ↓ [83] | ↑ [77] | ↑ [78] |
| KYNA | ↑ [60] ↑ [67] ↓ [84] ↑ [85] | ↓ [68] ↓ [79] | ↓ [74] ↓ [80] | ↓ [75] ↓ [86] | ↑ [87] | - | ↓ [88] ↓ [89] ↓ [90] | - | ↓ [91] ↑ [92] | ↑ [78] ↑ [93] |
| AA | - | ↑ [66] ↑* [68] | - | - | - | - | - | - | - | - |
| KYN | - | ↑* [68] ↑ [79] | - | ↑ [94] | ↑ [85] | ↑ [85] | - | ↑ [67] | - | - |
| XA | - | ↓ [61] | - | - | - | - | - | - | - | - |
| 3-HAA | - | ↓ [61] | - | ↓ [74] | - | - | - | ↓ [83] | - | - |
| PIC | - | - | - | - | - | ↓ [69] | - | - | - | ↑ [78] |
| Enzyme Activity | Alzheimer’s Disease | Parkinson’s Disease | Amyotrophic Lateral Sclerosis | Huntington’s Disease | Multiple Sclerosis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CNS | Peripheral | CNS | Peripheral | CNS | Peripheral | CNS | Peripheral | CNS | Peripheral | |
| IDO | ↑ [56] ↑ [59] | ↑ [64] ↑ [68] | ↑ [80] ↑ [95] | ↓ [56] ↑ [86] | ↑ [69] | - | - | ↑ [70] ↑ [71] ↑ [83] | - | ↑ [91] |
| TDO | ↑ [59] | - | - | - | - | - | - | - | - | - |
| KAT I | ↑ [85] | - | ↓ [96] ↓ [97] | ↓ [97] | - | - | ↓ [88] ↓ [90] | ↓ [83] | - | ↑ [93] |
| KAT II | ↑ [98] | - | ↓ [97] ↑ [97] | - | - | - | ↓ [88] ↓ [90] | ↓ [83] | - | ↑ [93] |
| KMO | - | - | - | - | - | - | - | - | ↑ [92] | - |
| 3-HAO | - | - | - | - | - | - | ↑ [99] | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. https://doi.org/10.3390/ijms21249338
Török N, Tanaka M, Vécsei L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. International Journal of Molecular Sciences. 2020; 21(24):9338. https://doi.org/10.3390/ijms21249338
Chicago/Turabian StyleTörök, Nóra, Masaru Tanaka, and László Vécsei. 2020. "Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway" International Journal of Molecular Sciences 21, no. 24: 9338. https://doi.org/10.3390/ijms21249338
APA StyleTörök, N., Tanaka, M., & Vécsei, L. (2020). Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. International Journal of Molecular Sciences, 21(24), 9338. https://doi.org/10.3390/ijms21249338







