QCM Sensor Arrays, Electroanalytical Techniques and NIR Spectroscopy Coupled to Multivariate Analysis for Quality Assessment of Food Products, Raw Materials, Ingredients and Foodborne Pathogen Detection: Challenges and Breakthroughs †
Abstract
:1. Introduction and Overview
2. General Challenges and Breakthroughs in Quality Assessment of Food Products
3. Recent Technological Innovations and Advances in NIR, QCM, and Electroanalytical Spectroscopic Instrument Development
3.1. NIR Spectroscopy
3.2. Portable NIR Sensors
3.3. Quartz Crystal Microbalance (QCM)
4. NIR Spectroscopy in Processed Foods
4.1. Edible Oils
4.2. Dairy Products
4.3. Grains and Flours
4.4. Chocolate and Syrups
4.5. Herbs and Spices
4.6. Food Additives
5. NIR Spectroscopy in Agricultural Produce Analysis
5.1. Fruits
5.2. Grains (Rice, Cereal) and Potatoes
5.3. Cassava and Wheat
6. NIR Spectroscopy in Food Supplements, Beverages, and Drinks
6.1. Food Supplements
6.2. Beverages (Fruit Juice, Soda, Energy Drink)
6.3. Alcoholic Drinks (Wines, Beers, Spirit)
7. NIR in Food and Pharmaceutical Raw Materials
8. NIR Spectroscopy in Meat and Meat Products, Fish, and Seafood Products
9. Chemometrics Approach and Multivariate Analyses of Spectral Data Analysis
10. Multivariate Analyses of NIR Spectra for Selected Food Quality Assessment
10.1. Meat and Pork
10.2. Fish and Eggs
11. Multivariate Analyses of NIR Spectra for Selected Food Quality Assessment
11.1. Dairy Products
11.2. Edible Oils
11.3. Agricultural Products
12. Quartz Crystal Microbalance (QCM) Coupled with Multivariate Analyses for Food Quality Assessment
12.1. Quartz Crystal Microbalance
12.2. Liquid-Phase QCM Advances
12.3. Gas-Phase QCM Advances
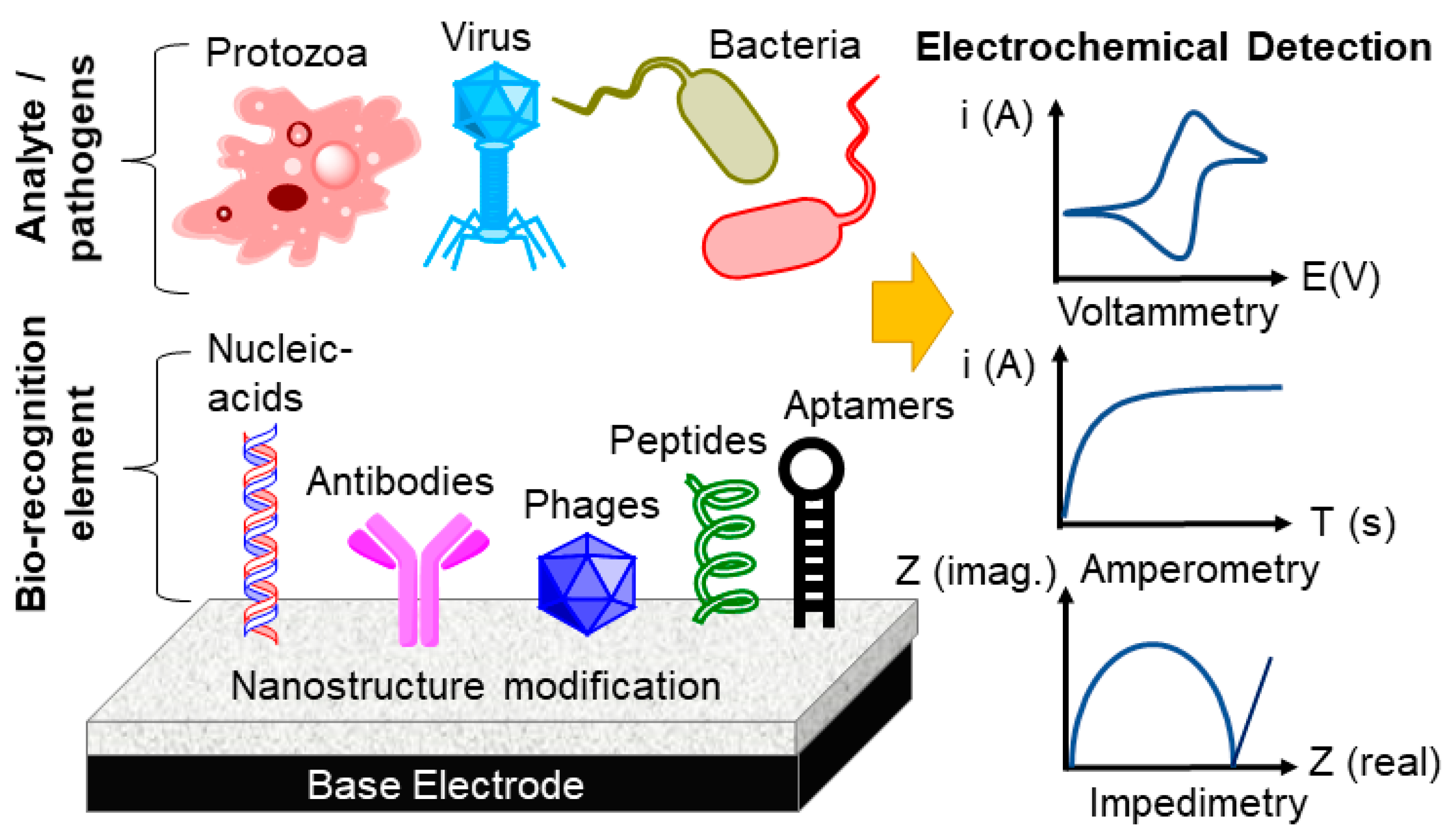
13. Electrochemical Biosensors for the Detection of Foodborne Pathogens
14. Conclusions and Future Trajectory
Funding
Conflicts of Interest
References
- European Commission. Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002/657/EC). Off. J. Eur. Commun. 2002, 221, 8–36. [Google Scholar]
- European Commission. Food Fraud 2016. Available online: https://ec.europa.eu/food/safety/food-fraud/ffn_en (accessed on 4 June 2018).
- European Food Safety Authority. Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Off. J. Eur. Commun. 2002, 31, 1–24. [Google Scholar]
- European Parliament–Committee on the Environment. Environment Committee Adopts Report on Fraud in the Food Supply Chain; Food Service Europe: Brussels, Belgium, 27 November 2013; Available online: https://issuu.com/office-epcas/docs/foodserviceeurope_newsletter_-_nove (accessed on 20 October 2020).
- Johnson, R. Food Fraud and ‘‘Economically Motivated Adulteration” of Food and Food Ingredients; Congressional Research Service: Washington, DC, USA, 2014. [Google Scholar]
- Wheatley, V.M.; Spink, J. Defining the Public Health Threat of Dietary Supplement Fraud. Compr. Rev. Food Sci. Food Saf. 2013, 12, 599–613. [Google Scholar] [CrossRef]
- U.S. Pharmacopeial Convention. USP’s Food Fraud Database. Available online: https://www.foodfraud.org/2016 (accessed on 16 October 2020).
- European Alliance for Access to Safe Medicines. Available online: http://www.eaasm.eu (accessed on 12 June 2020).
- World Health Organization. Sixty-Second World Health Assembly Counterfeit Medical Products. 2009. Available online: https://apps.who.int/gb/ebwha/pdf_files/A62/A62_13-en.pdf (accessed on 16 October 2020).
- World Health Organization. WHO Medicines Spurious Falsely Labeled Falsified Counterfeit (SFFC) Medicines. Available online: http://www.who.int/mediacentre/factsheets/fs275/en (accessed on 21 March 2013).
- Gostin, L.O.; Buckley, G.J. Committee on Understanding the Global Public Health Implications of Substandard, Falsified, and Counterfeit Medical Products; Countering the Problem of Falsified and Substandard Drugs; The National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- Anderson, T. Confusion over counterfeit drugs in Uganda. Lancet 2009, 373, 2097–2098. [Google Scholar] [CrossRef]
- Wertheimer, A.I.; Norris, J. Safeguarding against substandard/counterfeit drugs: Mitigating a macroeconomic pandemic. Res. Soc. Adm. Pharm. 2009, 5, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Panusa, A.; Multari, G.; Incarnato, G.; Gagliardi, L. High-performance liquid chromatography analysis of anti-inflammatory pharmaceuticals with ultraviolet and electrospray-mass spectrometry detection in suspected counterfeit homeopathic medicinal products. J. Pharm. Biomed. Anal. 2007, 43, 1221–1227. [Google Scholar] [CrossRef]
- Brant, J.; Malpani, R. Eye on the Ball: Medicine Regulation—Not IP Enforcement—Can Best Deliver Quality Medicine; Briefing Paper; Oxfam International: Oxford, UK, 2011. [Google Scholar]
- United States Pharmacopeia Drug Quality and Information Program. U. S. Pharmacopeia Report; United States Pharmacopeia Drug Quality and Information Program: Rockville, MD, USA, 2007. [Google Scholar]
- US Food and Drug Administration (FDA). Addressing challenges of Economically-Motivated Adulteration. In Proceedings of the Public Meeting on Economically Motivated Adulteration, College Park, MD, USA, 1 May 2009; Request for Comment. pp. 15497–15499. [Google Scholar]
- European Parliament-Committee on the Environment, Public Health and Food Safety, 2013/2091(INI). Available online: https://www.europarl.europa.eu/doceo/document/ENVI-PR-519759_EN.pdf?redirect (accessed on 6 December 2020).
- European Parliament. Report—on the Food Crisis, Fraud in the Food Chain and the Control Thereof, Rapporteur (Chair): Esther de Lange, Committee on the Environment, Public Health and Food Safety; European Parliament: Brussels, Belgium, 2014. [Google Scholar]
- Everstine, K.; Spink, J.; Kennedy, S. Analysis of Food Fraud and Economically Motivated Adulteration incidents. J. Food Protect. 2013, 4, 560–735. [Google Scholar]
- Spink, J.; Moyer, D.C. Understanding and combating food fraud. Food Technol. 2013, 67, 30–35. [Google Scholar]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309–19336. [Google Scholar] [CrossRef]
- Patel, P.; Doddamani, A. Role of sensor in the food processing industries. Int. Arch. Appl. Sci. Technol. 2019, 10, 10–18. [Google Scholar]
- Viejo, C.G.; Torrico, D.D.; Dunshea, F.; Fuentes, S. Emerging Technologies Based on Artificial Intelligence to Assess the Quality and Consumer Preference of Beverages. Beverages 2019, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Kaya, A.; Keçeli, A.S.; Catal, C.; Tekinerdogan, B. Sensor Failure Tolerable Machine Learning-Based Food Quality Prediction Model. Sensors 2020, 20, 3173. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Calo-Mata, P.; Barros-Velázquez, J.; Ortea, I. Review of Recent DNA-Based Methods for Main Food-Authentication Topics. J. Agric. Food Chem. 2019, 67, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Tao, J.; Xi, B.; Xue, M.; Sun, W. A Multiplex PCR Assay Mediated by Universal Primers for the Detection of Adulterated Meat in Mutton. J. Food Prot. 2019, 82, 325–330. [Google Scholar] [CrossRef]
- Yin, R.; Sun, Y.; Wang, K.; Feng, N.; Zhang, H.; Xiao, M. Development of a PCR-based lateral flow strip assay for the simple, rapid, and accurate detection of pork in meat and meat products. Food Chem. 2020, 318, 126541. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, Y.; Chu, H.; Yin, R. Development of a polymerase chain reaction—Nucleic acid sensor assay for the rapid detection of chicken adulteration. LWT 2020, 131, 109679. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Yu, W.; Qiao, L.; Liu, R.; Li, S.; Zhao, Y.; Yang, S.; Chen, A. Determination of content of camel milk in adulterated milk samples by normalized real-time polymerase chain reaction system based on single-copy nuclear genes. J. Sci. Food Agric. 2020, 100, 3465–3470. [Google Scholar] [CrossRef]
- Vinayaka, A.C.; Ngo, T.A.; Kant, K.; Engelsmann, P.; Dave, V.P.; Shahbazi, M.-A.; Wolff, A.; Bang, D.D. Rapid detection of Salmonella enterica in food samples by a novel approach with combination of sample concentration and direct PCR. Biosens. Bioelectron. 2019, 129, 224–230. [Google Scholar] [CrossRef]
- Pakbin, B.; Basti, A.A.; Khanjari, A.; Azimi, L.; Karimi, A. Differentiation of stx1A gene for detection of Escherichia coli serotype O157: H7 and Shigella dysenteriae type 1 in food samples using high resolution melting curve analysis. Food Sci. Nutr. 2020, 8, 3665–3672. [Google Scholar] [CrossRef]
- Li, D.-D.; Hao, C.-B.; Liu, Z.-M.; Wang, S.-J.; Wang, Y.; Chao, Z.; Gao, S.-Y.; Chen, S. Development of a novel dual priming oligonucleotide system-based PCR assay for specific detection of Salmonella from food samples. J. Food Saf. 2020, 40, 12789. [Google Scholar] [CrossRef]
- Geng, Y.; Liu, G.; Liu, L.; Deng, Q.; Zhao, L.; Sun, X.X.; Wang, J.; Zhao, B.; Wang, J. Real-time recombinase polymerase amplification assay for the rapid and sensitive detection of Campylobacter jejuni in food samples. J. Microbiol. Methods 2019, 157, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Salihah, N.T.; Hossain, M.M.; Abdul Hamid, M.R.W.; Ahmed, M.U. A novel, rapid, and sensitive real-time PCR assay for cost-effective detection and quantification of Staphylococcus aureus in food samples with the ZEN doublequenched probe chemistry. Int. Food Res. J. 2019, 26, 193–201. [Google Scholar]
- Rani, S.; Pradhan, A.K. Evaluation and meta-analysis of test accuracy of direct PCR and bioassay methods for detecting Toxoplasma gondii in meat samples. LWT 2020, 131, 109666. [Google Scholar] [CrossRef]
- Köppel, R.; Sendic, A.; Waiblinger, H.-U. Two quantitative multiplex real-time PCR systems for the efficient GMO screening of food products. Eur. Food Res. Technol. 2014, 239, 653–659. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Baker, G.A.; Bwambok, D.K.; Bhawawet, N.; Elzey, B.; Siraj, N.; Macchi, S.; Pollard, D.A.; Perez, R.L.; Duncan, A.V.; et al. Molecular (Raman, NIR, and FTIR) spectroscopy and multivariate analysis in consumable products analysis1. Appl. Spectrosc. Rev. 2019, 55, 647–723. [Google Scholar] [CrossRef]
- Neves, M.D.G.; Poppi, R.J.; Siesler, H.W. Rapid Determination of Nutritional Parameters of Pasta/Sauce Blends by Handheld Near-Infrared Spectroscopy. Molecules 2019, 24, 2029. [Google Scholar] [CrossRef] [Green Version]
- Dzsenifer, N.; Gabor, B.; Hussein, G.D.; Zoltan, K.; Zsanett, B.; Zinia, Z.J.-L.; Victor, S.; Zoltan, V.K.; Noemi, K. Standard analytical methods, sensory evaluation, NIRS and electronic tongue for sensing taste attributes of different melon varieties. Sensors 2019, 19, 5010. [Google Scholar]
- Fernández, L.; Allende-Prieto, C.; Peón, J.; Recondo, C.; Rodríguez-Gonzálvez, P.; Gutiérrez, D.; Martínez, B.; García, P.; Rodríguez, A. Preliminary Assessment of Visible, Near-Infrared, and Short-Wavelength–Infrared Spectroscopy with a Portable Instrument for the Detection of Staphylococcus aureus Biofilms on Surfaces. J. Food Prot. 2019, 82, 1314–1319. [Google Scholar] [CrossRef]
- Horcada, A.; Valera, M.; Juárez, M.; Fernández-Cabanás, V.M. Authentication of Iberian pork official quality categories using a portable near infrared spectroscopy (NIRS) instrument. Food Chem. 2020, 318, 126471. [Google Scholar] [CrossRef]
- Sehlke, A.; Mirmalek, Z.; Burtt, D.; Haberle, C.W.; Santiago-Materese, D.; Nawotniak, S.E.K.; Hughes, S.S.; Garry, W.B.; Bramall, N.; Brown, A.J.; et al. Requirements for Portable Instrument Suites during Human Scientific Exploration of Mars. Astrobiology 2019, 19, 401–425. [Google Scholar] [CrossRef] [Green Version]
- Royer, C.; Poulet, F.; Reess, J.-M.; Pilorget, C.; Hamm, V.; Fouchet, T.; Maurice, S.; Forni, O.; Bernardi, P.; Montmessin, F.; et al. Pre-launch radiometric calibration of the infrared spectrometer onboard SuperCam for the Mars2020 rover. Rev. Sci. Instrum. 2020, 91, 063105. [Google Scholar] [CrossRef] [PubMed]
- Chia, K.S.; Jam, M.N.H.; Gan, Z.; Ismail, N. Pre-dispersive near-infrared light sensing in non-destructively classifying the brix of intact pineapples. J. Food Sci. Technol. 2020, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, C.; Sanden, K.W.; Wubshet, S.G.; Andersen, P.V.; Øyaas, J.; Wold, J.P. Estimating dry matter and fat content in blocks of Swiss cheese during production using on-line near infrared spectroscopy. J. Near Infrared Spectrosc. 2019, 27, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Wojtkiewicz, S.; Gerega, A.; Zanoletti, M.; Sudakou, A.; Contini, D.; Liebert, A.; Durduran, T.; Dehghani, H. Self-calibrating time-resolved near infrared spectroscopy. Biomed. Opt. Express 2019, 10, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Buttafava, M.; Behera, A.; Zanoletti, M.; Di Sieno, L.; Mora, A.D.; Contini, D.; Tosi, A. Eight-Wavelength, Dual Detection Channel Instrument for Near-Infrared Time-Resolved Diffuse Optical Spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fishell, A.K.; Arbeláez, A.M.; Valdés, C.P.; Burns-Yocum, T.M.; Sherafati, A.; Richter, E.J.; Torres, M.; Eggebrecht, A.T.; Smyser, C.D.; Culver, J.P. Portable, field-based neuroimaging using high-density diffuse optical tomography. NeuroImage 2020, 215, 116541. [Google Scholar] [CrossRef]
- Pal, S.; Das, A.; Nandy, S.; Kar, R.; Ghosh, J. Development of a near-infrared tunable diode laser absorption spectrometer for trace moisture measurements in helium gas. Rev. Sci. Instrum. 2019, 90, 103105. [Google Scholar] [CrossRef]
- Zhu, M.; Wen, B.; Wu, H.; Li, J.; Lin, H.; Li, Q.; Li, Y.; Huang, J.-A.; Liu, Z.-H. The Quality Control of Tea by Near-Infrared Reflectance (NIR) Spectroscopy and Chemometrics. J. Spectrosc. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Nock, J.F.; Al Shoffe, Y.; Watkins, C.B. Non-destructive prediction of soluble solids and dry matter contents in eight apple cultivars using near-infrared spectroscopy. Postharvest Biol. Tec. 2019, 151, 111–118. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Li, H.-D.; Deng, B.-C.; Cao, D.-S. An overview of variable selection methods in multivariate analysis of near-infrared spectra. TrAC Trends Anal. Chem. 2019, 113, 102–115. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, C.; Cui, X.; Cai, W.; Shao, X. A two-level strategy for standardization of near infrared spectra by multi-level simultaneous component analysis. Anal. Chim. Acta 2019, 1050, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Xiao, L.; Yao, H.; Ge, J.; Zhang, L.; Luan, S. Construction of global and robust near-infrared calibration models based on hybrid calibration sets using Partial Least Squares (PLS) regression. Anal. Lett. 2018, 52, 1177–1194. [Google Scholar] [CrossRef]
- Liao, Y.-Z.; Shi, H.; Chen, J.-H.; Lu, Y.; Du, M. Portable quartz crystal microbalance analyzer with adjustable reference frequency source. Fenxi Huaxue 2019, 47, 992–997. [Google Scholar]
- Parkkila, P.; Viitala, T. Partitioning of Catechol Derivatives in Lipid Membranes: Implications for Substrate Specificity to Catechol-O-methyltransferase. ACS Chem. Neurosci. 2020, 11, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Dirri, F.; Palomba, E.; Longobardo, A.; Zampetti, E.; Saggin, B.; Scaccabarozzi, D. A review of quartz crystal microbalances for space applications. Sens. Actuators A Phys. 2019, 287, 48–75. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Sun, W.; Lu, N.; Yan, M.; Wu, Y.; Hua, Z.; Fan, S. Development of a Low-Cost Portable Electronic Nose for Cigarette Brands Identification. Sensors 2020, 20, 4239. [Google Scholar] [CrossRef]
- Garcia-Cabezon, C.; Teixeira, G.G.; Dias, L.G.; Salvo-Comino, C.; Garcia-Hernandez, C.; Rodriguez-Mendez, M.L.; Martin-Pedrosa, F. Analysis of Phenolic Content in Grape Seeds and Skins by Means of a Bio-Electronic Tongue. Sensors 2020, 20, 4176. [Google Scholar] [CrossRef]
- Kazi, A.P.; Routsi, A.M.; Kaur, B.; Christodouleas, D.C. Inexpensive, Three-Dimensional, Open-Cell, Fluid-Permeable, Noble-Metal Electrodes for Electroanalysis and Electrocatalysis. ACS Appl. Mater. Interfaces 2020, 12, 45582–45589. [Google Scholar] [CrossRef]
- Menon, S.; Mathew, M.R.; Sam, S.; Keerthi, K.; Kumar, K.G. Recent advances and challenges in electrochemical biosensors for emerging and re-emerging infectious diseases. J. Electroanal. Chem. 2020, 878, 114596. [Google Scholar] [CrossRef]
- Joshi, A.; Kim, K.-H. Recent advances in nanomaterial-based electrochemical detection of antibiotics: Challenges and future perspectives. Biosens. Bioelectron. 2020, 153, 112046. [Google Scholar] [CrossRef]
- Akshaya, K.B.; Nidhin, M.; Sudhakar, Y.N.; Louis, G.; Anitha, V. Electrochemical sensing of vitamin B12 deficiency marker methylmalonic acid using PdAu-PPy tailored carbon fiber paper electrode. Talanta 2020, 217, 121028. [Google Scholar] [CrossRef] [PubMed]
- Diniz, J.A.; Okumura, L.L.; Aleixo, H.; Gurgel, A.; Silva, Á.F.D. A voltammetric screening method to determine ronidazole in bovine meat. J. Environ. Sci. Health Part B 2020, 55, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Hannah, S.; Al-Hatmi, M.; Gray, L.; Corrigan, D.K. Low-cost, thin-film, mass-manufacturable carbon electrodes for detection of the neurotransmitter dopamine. Bioelectrochemistry 2020, 133, 107480. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.T.; Scarano, E.; Bergkamp, M.H.; Capria, A.M.; Sanger, K.; Nielsen, L.H.; Hwu, E.-T.; Zor, K.; Boisen, A.; Scarano, E. Modular, lightweight, wireless potentiostat-on-a-disc for electrochemical detection in centrifugal microfluidics. Anal. Chem. 2019, 91, 11620–11628. [Google Scholar] [CrossRef]
- Nantaphol, S.; Kava, A.A.; Channon, R.B.; Kondo, T.; Siangproh, W.; Chailapakul, O.; Henry, C.S. Janus electrochemistry: Simultaneous electrochemical detection at multiple working conditions in a paper-based analytical device. Anal. Chim. Acta 2019, 1056, 88–95. [Google Scholar] [CrossRef]
- Li, F.; Yu, Z.; Han, X.; Lai, R.Y. Electrochemical aptamer-based sensors for food and water analysis: A review. Anal. Chim. Acta 2019, 1051, 1–23. [Google Scholar] [CrossRef]
- Sohng, W.; Park, Y.; Jang, D.; Cha, K.; Jung, Y.M.; Chung, H. Incorporation of two-dimensional correlation analysis into discriminant analysis as a potential tool for improving discrimination accuracy: Near-infrared spectroscopic discrimination of adulterated olive oils. Talanta 2020, 212, 120748. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; Lozano, V.A.; Olivieri, A.C. Comparative chemometric analysis of fluorescence and near infrared spectroscopies for authenticity confirmation and geographical origin of Argentinean extra virgin olive oils. Food Control 2019, 96, 22–28. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Z.; Yao, L.; Wu, Y.; Zeng, S.; Li, H.; Li, Y. Discriminating geographic origin of sesame oils and determining lignans by near-infrared spectroscopy combined with chemometric methods. J. Food Compos. Anal. 2019, 84, 103327. [Google Scholar] [CrossRef]
- Willenberg, I.; Matthäus, B.; Gertz, C. A New Statistical Approach to Describe the Quality of Extra Virgin Olive Oils Using Near Infrared Spectroscopy (NIR) and Traditional Analytical Parameters. Eur. J. Lipid Sci. Technol. 2018, 121, 1800361. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Barrera, M.D.C.L.; García, M.T.; Zhang, Q.A.; Mateos, P.Á.; Martín, G.; Barrera, D.C.; Torres-García, M.; Álvarez-Mateos, P. Determination of the Acidity of Waste Cooking Oils by Near Infrared Spectroscopy. Processes 2019, 7, 304. [Google Scholar] [CrossRef] [Green Version]
- Pu, Y.-Y.; O’Donnell, C.; Tobin, J.T.; O’Shea, N. Review of near-infrared spectroscopy as a process analytical technology for real-time product monitoring in dairy processing. Int. Dairy J. 2020, 103, 104623. [Google Scholar] [CrossRef]
- Gastélum-Barrios, A.; Soto-Zarazúa, G.; Escamilla-García, A.; Toledano-Ayala, M.; Macias-Bobadilla, G.; Jauregui-Vazquez, D. Optical Methods Based on Ultraviolet, Visible, and Near-Infrared Spectra to Estimate Fat and Protein in Raw Milk: A Review. Sensors 2020, 20, 3356. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, M.I.; Vivar-Quintana, A.M.; Revilla, I.; Salvador-Esteban, J. The determination of fatty acids in cheeses of variable composition (cow, ewe’s, and goat) by means of near infrared spectroscopy. Microchem. J. 2020, 156, 104854. [Google Scholar] [CrossRef]
- Kamboj, U.; Kaushal, N.; Mishra, S.; Munjal, N. Application of Selective Near Infrared Spectroscopy for Qualitative and Quantitative Prediction of Water Adulteration in Milk. Mater. Today Proc. 2020, 24, 2449–2456. [Google Scholar] [CrossRef]
- Temizkan, R.; Can, A.; Dogan, M.A.; Mortas, M.; Ayvaz, H. Rapid detection of milk fat adulteration in yoghurts using near and mid-infrared spectroscopy. Int. Dairy J. 2020, 110, 104795. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Páscoa, R.N.; Castro, R.C.; Ribeiro, D.S.; Santos, J.L. Detection of melamine and sucrose as adulterants in milk powder using near-infrared spectroscopy with DD-SIMCA as one-class classifier and MCR-ALS as a means to provide pure profiles of milk and of both adulterants with forensic evidence: A short communication. Talanta 2020, 216, 120937. [Google Scholar] [CrossRef]
- Lapcharoensuk, R.; Chaiyanate, J.; Winichai, S.; Phetnak, A. Quantitative detection of buffalo milk adulteration with cow milk using Fourier transform near infrared spectroscopy. In Proceedings of the Seventh International Conference on Optical and Photonic Engineering (icOPEN 2019), Phuket, Thailand, 16–20 July 2019; p. 1120516. [Google Scholar] [CrossRef]
- Ejeahalaka, K.K.; On, S.L.W. Characterisation of the quality alterations in model fat-filled milk powders under inclement conditions and the prediction of the storage time using near infrared spectroscopy. Food Chem. 2020, 323, 126752. [Google Scholar] [CrossRef]
- Muñiz, R.; Cuevas-Valdés, M.; De La Roza-Delgado, B. Milk quality control requirement evaluation using a handheld near infrared reflectance spectrophotometer and a bespoke mobile application. J. Food Compos. Anal. 2020, 86, 103388. [Google Scholar] [CrossRef]
- Williams, P.C. Application of chemometrics to prediction of some wheat quality factors by near-infrared spectroscopy. Cereal Chem. 2020, 97, 958–966. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, T.; Chen, Q. Quantitative detection of fatty acid value during storage of wheat flour based on a portable near-infrared (NIR) spectroscopy system. Infrared Phys. Technol. 2020, 109, 103423. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Zhao, W.-Q.; Hu, X.-Z.; Li, X.-P.; Qiao, Y.-Y.; Ma, Z.; Zhang, Q.-A. Determination of polyphenols in oats by near-infrared spectroscopy (NIRS) and two-dimensional correlation spectroscopy. Anal. Lett. 2019, 52, 962–971. [Google Scholar] [CrossRef]
- Johnson, J.B. An overview of near-infrared spectroscopy (NIRS) for the detection of insect pests in stored grains. J. Stored Prod. Res. 2020, 86, 101558. [Google Scholar] [CrossRef]
- Kosmowski, F.; Worku, T. Evaluation of a miniaturized NIR spectrometer for cultivar identification: The case of barley, chickpea and sorghum in Ethiopia. PLoS ONE 2018, 13, e0193620/1–e0193620/17. [Google Scholar] [CrossRef]
- Blanch-Perez-del-Notario, C.; Saeys, W.; Lambrechts, A. Fast ingredient quantification in multigrain flour mixes using hyperspectral imaging. Food Control 2020, 118, 107366. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Peng, Y.; Yang, Y.; Wang, Q. Detection of fraud in high-quality rice by near-infrared spectroscopy. J. Food Sci. 2020, 85, 2773–2782. [Google Scholar] [CrossRef]
- Firmani, P.; La Piscopia, G.; Bucci, R.; Marini, F.; Biancolillo, A. Authentication of P.G.I. Gragnano pasta by near infrared (NIR) spectroscopy and chemometrics. Microchem. J. 2020, 152, 104339. [Google Scholar] [CrossRef]
- Assadzadeh, S.; Walker, C.K.; McDonald, L.S.; Maharjan, P.; Panozzo, J.F. Multi-task deep learning of near infrared spectra for improved grain quality trait predictions. J. Near Infrared Spec. 2020, 28, 275–286. [Google Scholar] [CrossRef]
- Sirisomboon, C.D.; Wongthip, P.; Sirisomboon, P. Potential of near infrared spectroscopy as a rapid method to detect aflatoxins in brown rice. J. Near Infrared Spectrosc. 2019, 27, 232–240. [Google Scholar] [CrossRef]
- Laborde, A.; Jaillais, B.; Roger, J.-M.; Metz, M.; Bouveresse, D.J.-R.; Eveleigh, L.; Cordella, C. Subpixel detection of peanut in wheat flour using a matched subspace detector algorithm and near-infrared hyperspectral imaging. Talanta 2020, 216, 120993. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Ni, X.; Chu, X.; Li, Y.-F.; Lu, C. Utilising near-infrared hyperspectral imaging to detect low-level peanut powder contamination of whole wheat flour. Biosyst. Eng. 2019, 184, 55–68. [Google Scholar] [CrossRef]
- Quelal-Vásconez, M.A.; Lerma-García, M.J.; Perez-Esteve, É.; Arnau-Bonachera, A.; Barat, J.M.; Talens, P. Fast detection of cocoa shell in cocoa powders by near infrared spectroscopy and multivariate analysis. Food Control 2019, 99, 68–72. [Google Scholar] [CrossRef]
- Laborde, A.; Puig-Castellví, F.; Bouveresse, D.J.-R.; Eveleigh, L.; Cordella, C.; Jaillais, B. Detection of chocolate powder adulteration with peanut using near-infrared hyperspectral imaging and Multivariate Curve Resolution. Food Control 2020, 119, 107454. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Palma, M.; Barbero, G.F. A screening method based on Visible-NIR spectroscopy for the identification and quantification of different adulterants in high-quality honey. Talanta 2019, 203, 235–241. [Google Scholar] [CrossRef]
- Bisutti, V.; Merlanti, R.; Serva, L.; Lucatello, L.; Mirisola, M.; Balzan, S.; Tenti, S.; Fontana, F.; Trevisan, G.; Montanucci, L.; et al. Multivariate and machine learning approaches for honey botanical origin authentication using near infrared spectroscopy. J. Near Infrared Spectrosc. 2019, 27, 65–74. [Google Scholar] [CrossRef]
- De Oliveira, M.M.; Cruz-Tirado, J.; Barbin, D.F. Nontargeted Analytical Methods as a Powerful Tool for the Authentication of Spices and Herbs: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 670–689. [Google Scholar] [CrossRef] [Green Version]
- Wilde, A.S.; Haughey, S.A.; Galvin-King, P.; Elliott, C.T. The feasibility of applying NIR and FT-IR fingerprinting to detect adulteration in black pepper. Food Control 2019, 100, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.M.; Cruz-Tirado, J.P.; Roque, J.V.; Teófilo, R.F.; Barbin, D.F. Portable near-infrared spectroscopy for rapid authentication of adulterated paprika powder. J. Food Compos. Anal. 2020, 87, 103403. [Google Scholar] [CrossRef]
- Cantarelli, M.Á.; Moldes, C.A.; Marchevsky, E.J.; Azcarate, S.M.; Camiña, J.M. Low-cost analytic method for the identification of Cinnamon adulteration. Microchem. J. 2020, 159, 105513. [Google Scholar] [CrossRef]
- Li, S.; Xing, B.; Lin, D.; Yi, H.; Shao, Q. Rapid detection of saffron (Crocus sativus L.) Adulterated with lotus stamens and corn stigmas by near-infrared spectroscopy and chemometrics. Ind. Crop. Prod. 2020, 152, 112539. [Google Scholar] [CrossRef]
- Santana, M.C.D.; Ferreira, M.M.C.; Pallone, J.A.L. Control of ascorbic acid in fortified powdered soft drinks using near-infrared spectroscopy (NIRS) and multivariate analysis. J. Food Sci. Technol. 2020, 57, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Borba, K.R.; Spricigo, P.C.; Aykas, D.P.; Mitsuyuki, M.C.; Colnago, L.A.; Ferreira, M.D. Non-invasive quantification of vitamin C, citric acid, and sugar in ‘Valência’ oranges using infrared spectroscopies. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Wiedemair, V.; Ramoner, R.; Huck, C.W. Investigations into the total antioxidant capacities of cultivars of gluten-free grains using near-infrared spectroscopy. Food Control 2019, 95, 189–195. [Google Scholar] [CrossRef]
- Rosa, L.N.; Gonçalves, T.R.; Gomes, S.T.M.; Matsushita, M.; Gonçalves, R.P.; Março, P.H.; Valderrama, P. N-Way NIR data treatment through PARAFAC in the evaluation of protective effect of antioxidants in soybean oil. Molecules 2020, 25, 4366. [Google Scholar] [CrossRef] [PubMed]
- Georgouli, K.; Carrasco, B.; Vincke, D.; Del Rincon, J.M.; Koidis, A.; Baeten, V.; Fernández Pierna, J.A. Continuous statistical modeling in characterisation of complex hydrocolloid mixtures using near infrared spectroscopy. Chemometr. Intell. Lab. 2020, 196, 103910. [Google Scholar] [CrossRef]
- Mishra, P.; Roger, J.M.; Rutledge, D.N.; Woltering, E. SPORT pre-processing can improve near-infrared quality prediction models for fresh fruits and agro-materials. Postharvest Biol. Technol. 2020, 168, 111271. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Agyekum, A.A.; Wu, J.; Chen, Q.; Zuo, M.; El-Seedi, H.R.; Tao, F.; Shi, J.; Ouyang, Q.; et al. Quantitative detection of apple watercore and soluble solids content by near infrared transmittance spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, S.; Li, J.; Tian, X.; Huang, W.; Chen, L. Optimization and comparison of models for prediction of soluble solids content in apple by online Vis/NIR transmission coupled with diameter correction method. Chemom. Intell. Lab. Syst. 2020, 201, 104017. [Google Scholar] [CrossRef]
- Song, J.; Li, G.; Yang, X.; Liu, X.; Xie, L. Rapid analysis of soluble solid content in navel orange based on visible-near infrared spectroscopy combined with a swarm intelligence optimization method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117815. [Google Scholar] [CrossRef]
- Malegori, C.; Buratti, S.; Benedetti, S.; Oliveri, P.; Ratti, S.; Cappa, C.; Lucisano, M. A modified mid-level data fusion approach on electronic nose and FT-NIR data for evaluating the effect of different storage conditions on rice germ shelf life. Talanta 2020, 206, 120208. [Google Scholar] [CrossRef]
- Le, B.T. Application of deep learning and near infrared spectroscopy in cereal analysis. Vib. Spectrosc. 2020, 106, 103009. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, T.; Chen, Q. Dynamic monitoring of fatty acid value in rice storage based on a portable near-infrared spectroscopy system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118620. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, P.N.S.; Castanho, A.; Almeida, A.S.; Oliveira, J.; Brites, C. Identification of rice flour types with near-infrared spectroscopy associated with PLS-DA and SVM methods. Eur. Food Res. Technol. 2019, 246, 527–537. [Google Scholar] [CrossRef]
- Barnaby, J.Y.; Huggins, T.D.; Lee, H.; McClung, A.M.; Pinson, S.R.M.; Oh, M.; Bauchan, G.R.; Tarpley, L.; Lee, K.; Kim, M.S.; et al. Vis/NIR hyperspectral imaging distinguishes sub-population, production environment, and physicochemical grain properties in rice. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Bantadjan, Y.; Rittiron, R.; Malithong, K.; Narongwongwattana, S. Rapid Starch Evaluation in Fresh Cassava Root Using a Developed Portable Visible and Near-Infrared Spectrometer. ACS Omega 2020, 5, 11210–11216. [Google Scholar] [CrossRef]
- Bantadjan, Y.; Rittiron, R.; Malithong, K.; Narongwongwattana, S. Establishment of an Accurate Starch Content Analysis System for Fresh Cassava Roots Using Short-Wavelength Near Infrared Spectroscopy. ACS Omega 2020, 5, 15468–15475. [Google Scholar] [CrossRef]
- do Carmo, C.D.; Sousa, M.B.E.; Santos Pereira, J.d.S.; Ceballos, H.; Oliveira, E.J. Identification of waxy cassava genotypes using fourier-transform near-infrared spectroscopy. Crop Sci. 2020, 60, 883–895. [Google Scholar] [CrossRef]
- Firmani, P.; Nardecchia, A.; Nocente, F.; Gazza, L.; Marini, F.; Biancolillo, A. Multi-block classification of Italian semolina based on Near Infrared Spectroscopy (NIR) analysis and alveographic indices. Food Chem. 2020, 309, 125677. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, S.; Wu, T. Individual wheat kernels vigor assessment based on NIR spectroscopy coupled with machine learning methodologies. Infrared Phys. Technol. 2020, 105, 103213. [Google Scholar] [CrossRef]
- De Girolamo, A.; Arroyo, M.C.; Lippolis, V.; Cervellieri, S.; Cortese, M.; Pascale, M.; Logrieco, A.F.; Von Holst, C. A simple design for the validation of a FT-NIR screening method: Application to the detection of durum wheat pasta adulteration. Food Chem. 2020, 333, 127449. [Google Scholar] [CrossRef]
- Zaukuu, J.-L.Z.; Aouadi, B.; Lukács, M.; Bodor, Z.; Vitalis, F.; Gillay, B.; Gillay, Z.; Friedrich, L.; Kovacs, Z. Detecting Low Concentrations of Nitrogen-Based Adulterants in Whey Protein Powder Using Benchtop and Handheld NIR Spectrometers and the Feasibility of Scanning through Plastic Bag. Molecules 2020, 25, 2522. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tan, C.; Li, H. Untargeted identification of adulterated Sanqi powder by near-infrared spectroscopy and one-class model. J. Food Compos. Anal. 2020, 88, 103450. [Google Scholar] [CrossRef]
- Karunathilaka, S.R.; Choi, S.H.; Mossoba, M.M.; Yakes, B.J.; Brückner, L.; Ellsworth, Z.; Srigley, C.T. Rapid classification and quantification of marine oil omega-3 supplements using ATR-FTIR, FT-NIR and chemometrics. J. Food Compos. Anal. 2019, 77, 9–19. [Google Scholar] [CrossRef]
- Hespanhol, M.C.; Souza, J.C.; Pasquini, C. Feasibility of a portable, low-cost near-infrared spectrophotometer for the quality screening of omega-3 dietary supplements. J. Pharm. Biomed. Anal. 2020, 189, 113436. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, G.; Aleixandre-Tudo, J.-L.; Buica, A. Viability of IR spectroscopy for the accurate measurement of yeast assimilable nitrogen content of grape juice. Talanta 2020, 206, 120241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Marini, G.; Van Berkel, N.; Sarsenbayeva, Z.; Tan, Z.; Luo, C.; He, X.; Dingler, T.; Goncalves, J.; Kawahara, Y.; et al. Probing Sucrose Contents in Everyday Drinks Using Miniaturized Near-Infrared Spectroscopy Scanners. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2019, 3, 1–25. [Google Scholar] [CrossRef]
- Włodarska, K.; Szulc, J.; Khmelinskii, I.; Sikorska, E. Non-destructive determination of strawberry fruit and juice quality parameters using ultraviolet, visible, and near-infrared spectroscopy. J. Sci. Food Agric. 2019, 99, 5953–5961. [Google Scholar] [CrossRef]
- Ren, G.; Ning, J.; Zhang, Z. Intelligent assessment of tea quality employing visible-near infrared spectra combined with a hybrid variable selection strategy. Microchem. J. 2020, 157, 105085. [Google Scholar] [CrossRef]
- Hu, X.-Z.; Liu, S.-Q.; Li, X.-H.; Wang, C.-X.; Ni, X.-L.; Liu, X.; Wang, Y.; Liu, Y.; Xu, C.-H. Geographical origin traceability of Cabernet Sauvignon wines based on Infrared fingerprint technology combined with chemometrics. Sci. Rep. 2019, 9, 8256. [Google Scholar] [CrossRef]
- Anjos, O.; Caldeira, I.; Roque, R.; Pedro, S.I.; Lourenço, S.; Canas, S. Screening of Different Ageing Technologies of Wine Spirit by Application of Near-Infrared (NIR) Spectroscopy and Volatile Quantification. Processes 2020, 8, 736. [Google Scholar] [CrossRef]
- Joshi, I.; Truong, V.K.; Chapman, J.; Cozzolino, D. The use of two-dimensional spectroscopy to interpret the effect of temperature on the near infrared spectra of whisky. J. Near Infrared Spectrosc. 2020, 28, 148–152. [Google Scholar] [CrossRef]
- Román-Ospino, A.D.; Tamrakar, A.; Igne, B.; Dimaso, E.T.; Airiau, C.; Clancy, D.J.; Pereira, G.; Muzzio, F.J.; Singh, R.; Ramachandran, R. Characterization of NIR interfaces for the feeding and in-line monitoring of a continuous granulation process. Int. J. Pharm. 2020, 574, 118848. [Google Scholar] [CrossRef] [PubMed]
- Razuc, M.; Grafia, A.; Gallo, L.; Ramírez-Rigo, M.V.; Romañach, R.J. Near-infrared spectroscopic applications in pharmaceutical particle technology. Drug Dev. Ind. Pharm. 2019, 45, 1565–1589. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chem. A Eur. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Keller, M.D.; Baughman, T.; Wilson, B.K.; Keller, M.D. Evaluating Low-Cost Optical Spectrometers for the Detection of Simulated Substandard and Falsified Medicines. Appl. Spectrosc. 2019, 74, 323–333. [Google Scholar] [CrossRef]
- Picouet, P.A.; Gou, P.; Hyypio, R.; Castellari, M. Implementation of NIR technology for at-line rapid detection of sunflower oil adulterated with mineral oil. J. Food Eng. 2018, 230, 18–27. [Google Scholar] [CrossRef]
- Chen, Y.; Van Berkel, N.; Luo, C.; Sarsenbayeva, Z.; Koastakos, V. Application of miniaturized near-infrared spectroscopy in pharmaceutical identification. Smart Health 2020, 18, 100126. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, H.; Zhou, L.; Ju, W.; Yang, Z.; Yan, K.; Yan, B.; Qu, H. Development and validation of in-line near-infrared spectroscopy based analytical method for commercial production of a botanical drug product. J. Pharm. Biomed. Anal. 2019, 174, 674–682. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, W.; Chen, Q. Comparison of algorithms for wavelength variables selection from near-infrared (NIR) spectra for quantitative monitoring of yeast (Saccharomyces cerevisiae) cultivations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 366–371. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Shi, Z.; Drennen, J.K.; Anderson, C.A. Prediction of Dissolution Profiles from Process Parameters, Formulation, and Spectroscopic Measurements. J. Pharm. Sci. 2019, 108, 2119–2127. [Google Scholar] [CrossRef]
- Pedersen, T.; Rantanen, J.; Naelapää, K.; Skibsted, E. Near infrared analysis of pharmaceutical powders with empirical target distribution optimization (ETDO). J. Pharm. Biomed. Anal. 2020, 181, 113059. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Tan, H.X.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Non-destructive dose verification of two drugs within 3D printed polyprintlets. Int. J. Pharm. 2020, 577, 119066. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Peter, A.; Wolfgang, M.; Khinast, J. How to measure coating thickness of tablets: Method comparison of optical coherence tomography, near-infrared spectroscopy and weight-, height- and diameter gain. Eur. J. Pharm. Biopharm. 2019, 142, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.S.; Colbert, M.-J.; Santangelo, M.; Bartlett, J.A.; Lapointe-Garant, P.-P.; Simard, J.-S.; Gosselin, R. Monitoring microsphere coating processes using PAT tools in a bench scale fluid bed. Eur. J. Pharm. Sci. 2019, 135, 12–21. [Google Scholar] [CrossRef]
- Yang, J.; Lou, X.; Yang, H.; Yang, H.; Liu, C.; Wu, J.; Bin, J. Improved calibration transfer between near-Infrared (NIR) spectrometers using canonical correlation analysis. Anal. Lett. 2019, 52, 2188–2202. [Google Scholar] [CrossRef]
- Skotare, T.; Nilsson, D.; Xiong, S.; Geladi, P.; Trygg, J. Joint and Unique Multiblock Analysis for Integration and Calibration Transfer of NIR Instruments. Anal. Chem. 2019, 91, 3516–3524. [Google Scholar] [CrossRef]
- Weeranantanaphan, J.; Downey, G.; Allen, P.; Sun, D.-W. A Review of near Infrared Spectroscopy in Muscle Food Analysis: 2005–2010. J. Near Infrared Spectrosc. 2011, 19, 61–104. [Google Scholar] [CrossRef]
- Simon, C.J.; Rodemann, T.; Carter, C.G. Near-Infrared Spectroscopy as a Novel Non-Invasive Tool to Assess Spiny Lobster Nutritional Condition. PLoS ONE 2016, 11, e0159671. [Google Scholar] [CrossRef]
- Pochanagone, S.; Rittiron, R. Preliminary Study on the Determination of ppm-Level Concentration of Histamine in Tuna Fish Using a Dry Extract System for Infrared Coupled with Near-Infrared Spectroscopy. ACS Omega 2019, 4, 19164–19171. [Google Scholar] [CrossRef] [Green Version]
- Peyvasteh, M.; Popov, A.; Bykov, A.; Meglinski, I. Meat freshness revealed by visible to near-infrared spectroscopy and principal component analysis. J. Phys. Comm. 2020, 4, 095011. [Google Scholar] [CrossRef]
- Kademi, H.I.; Ulusoy, B.H.; Hecer, C. Applications of miniaturized and portable near infrared spectroscopy (NIRS) for inspection and control of meat and meat products. Food Rev. Int. 2018, 35, 201–220. [Google Scholar] [CrossRef]
- Fowler, S.M.; Morris, S.; Hopkins, D.L. Preliminary investigation for the prediction of intramuscular fat content of lamb in-situ using a hand- held NIR spectroscopic device. Meat Sci. 2020, 166, 108153. [Google Scholar] [CrossRef] [PubMed]
- Pochanagone, S.; Rittiron, R. Rapid detection of infrared inactive sodium chloride content in frozen tuna fish for determining commercial value using short wavelengths. J. Near Infrared Spectrosc. 2019, 27, 424–431. [Google Scholar] [CrossRef]
- Shim, K.B.; Jeong, Y. Freshness Evaluation in Chub Mackerel (Scomber japonicus) Using Near-Infrared Spectroscopy Determination of the Cadaverine Content. J. Food Prot. 2019, 82, 768–774. [Google Scholar] [CrossRef]
- Miller, M.R.; Puddick, J.; Symonds, J.E.; Walker, S.P.; Tian, H. (Sabrina) Application of a Fourier transform—near infrared reflectance spectroscopy method for the rapid proximate analysis of the greenshell mussel (Perna canaliculus) and king (Chinook) salmon (Oncorhynchus tshawytscha). Aquac. Res. 2019, 50, 1668–1677. [Google Scholar] [CrossRef]
- Caballero, D.; Asensio, M.; Fernández, C.; Martín, N.; Silva, A. Determining Mandatory Nutritional Parameters for Iberian Meat Products Using a New Method Based on Near Infra-Red Reflectance Spectroscopy and Data Mining. Meat Technol. 2019, 60, 73–83. [Google Scholar] [CrossRef]
- Ortiz, A.; Parrini, S.; Tejerina, D.; Pinto de Araújo, J.P.; Čandek-Potokar, M.; Crovetti, A.; Garcia-Casco, J.M.; González, J.; Hernández-García, F.I.; Karolyi, D.; et al. Potential Use of Near-Infrared Spectroscopy to Predict Fatty Acid Profile of Meat from Different European Autochthonous Pig Breeds. Appl. Sci. 2020, 10, 5801. [Google Scholar] [CrossRef]
- Adams, M.J. Chemometrics in Analytical Spectroscopy, 2nd ed.; Royal Society: London, UK, 1995. [Google Scholar]
- Beebe, K.R.; Pell, R.J.; Seasholtz, M.B. Chemometrics: A Practical Guide; John Wiley Publishing: New York, NY, USA, 1998. [Google Scholar]
- Malinowski, E.R. Factor Analysis in Chemistry; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Martens, H.; Naes, T. Multivariate Calibration; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Otto, M. Pattern Recognition and Classification, in Chemometrics; John Wiley & Sons Publishing: New York, NY, USA, 2016. [Google Scholar]
- Chapman, J.; Elbourne, A.; Truong, V.K.; Cozzolino, D. Shining light into meat—A review on the recent advances in in vivo and carcass applications of near infrared spectroscopy. Int. J. Food Sci. Technol. 2019, 55, 935–941. [Google Scholar] [CrossRef]
- Kartakoullis, A.; Comaposada, J.; Cruz-Carrión, A.; Gou, P.; Gou, P. Feasibility study of smartphone-based Near Infrared Spectroscopy (NIRS) for salted minced meat composition diagnostics at different temperatures. Food Chem. 2019, 278, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.; Manley, M.; Hoffman, L.C.; Beganovic, A.; Kirchler, C.G.; Huck, C.W.; Williams, P.J. Differentiation of South African Game Meat Using Near-Infrared (NIR) Spectroscopy and Hierarchical Modelling. Molecules 2020, 25, 1845. [Google Scholar] [CrossRef]
- Weng, S.; Guo, B.; Tang, P.; Yin, X.; Pan, F.; Zhao, J.; Huang, L.; Zhang, D. Rapid detection of adulteration of minced beef using Vis/NIR reflectance spectroscopy with multivariate methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118005. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Li, F.; Xiong, L.; Xiong, Q.; Zhu, M.; Chen, Y. Quantitative detection of binary and ternary adulteration of minced beef meat with pork and duck meat by NIR combined with chemometrics. Food Control 2020, 113, 107203. [Google Scholar] [CrossRef]
- López-Maestresalas, A.; Insausti, K.; Jarén, C.; Pérez-Roncal, C.; Urrutia, O.; Beriain, M.J.; Arazuri, S. Detection of minced lamb and beef fraud using NIR spectroscopy. Food Control 2019, 98, 465–473. [Google Scholar] [CrossRef]
- Al-Sarayreh, M.; Reis, M.M.; Yan, W.Q.; Klette, R. Potential of deep learning and snapshot hyperspectral imaging for classification of species in meat. Food Control 2020, 117, 107332. [Google Scholar] [CrossRef]
- Mabood, F.; Boqué, R.; Alkindi, A.Y.; Al-Harrasi, A.; Al Amri, I.S.; Boukra, S.; Jabeen, F.; Hussain, J.; Abbas, G.; Naureen, Z.; et al. Fast detection and quantification of pork meat in other meats by reflectance FT-NIR spectroscopy and multivariate analysis. Meat Sci. 2020, 163, 108084. [Google Scholar] [CrossRef]
- Cáceres-Nevado, J.; Garrido-Varo, A.; De Pedro-Sanz, E.; Pérez-Marín, D. Fourier transform near-infrared spectroscopy coupled to a long fibre optic head for the quality control of IBERIAN pork loins: Intact versus minced. Meat Sci. 2019, 153, 86–93. [Google Scholar] [CrossRef]
- Piotrowski, C.; Garcia, R.; Garrido-Varo, A.; Pérez-Marín, D.; Riccioli CFearn, T. Short Communication: The potential of portable near infrared spectroscopy for assuring quality and authenticity in the food chain, using Iberian hams as an example. Animal 2019, 13, 3018–3021. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olio, S.; Aboagye, G.; Nanni, C.L.; Gallo, M.; Fontanesi, L. Effects of 17 performance, carcass and raw ham quality parameters on ham weight loss at first salting in heavy pigs, a meat quality indicator for the production of high quality dry-cured hams. Meat Sci. 2020, 162, 108012. [Google Scholar] [CrossRef]
- Perez-Palacios, T.; Caballero, D.; González-Mohino, A.; Mir-Bel, J.; Antequera, T. Near Infrared Reflectance spectroscopy to analyse texture related characteristics of sous vide pork loin. J. Food Eng. 2019, 263, 417–423. [Google Scholar] [CrossRef]
- Achata, E.M.; Inguglia, E.S.; Esquerre, C.A.; Tiwari, B.K.; O’Donnell, C.P. Evaluation of Vis-NIR hyperspectral imaging as a process analytical tool to classify brined pork samples and predict brining salt concentration. J. Food Eng. 2019, 246, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Q.; Wang, L.; Zareef, M.; Chen, Q.; Guo, Z.; Li, H. A feasibility of nondestructive rapid detection of total volatile basic nitrogen content in frozen pork based on portable near-infrared spectroscopy. Microchem. J. 2020, 157, 105020. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Yi, R.; Yu, S.; Tu, H. Comparison of variable selection algorithms on vis-NIR hyperspectral imaging spectra for quantitative monitoring and visualization of bacterial foodborne pathogens in fresh pork muscles. Infrared Phys. Technol. 2020, 107, 103327. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; You, J.; Xiong, S. Rapid determination of the textural properties of silver carp (Hypophthalmichthys molitrix) using near-infrared reflectance spectroscopy and chemometrics. LWT 2020, 129, 109545. [Google Scholar] [CrossRef]
- Wang, X.; Shan, J.; Han, S.; Zhao, J.; Zhang, Y. Optimization of Fish Quality by Evaluation of Total Volatile Basic Nitrogen (TVB-N) and Texture Profile Analysis (TPA) by Near-Infrared (NIR) Hyperspectral Imaging. Anal. Lett. 2019, 52, 1845–1859. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; Chen, Z.; You, J.; Xiong, S. Evaluation of freshness in freshwater fish based on near infrared reflectance spectroscopy and chemometrics. LWT 2019, 106, 145–150. [Google Scholar] [CrossRef]
- Uysal, R.S.; Boyaci, I.H. Authentication of liquid egg composition using ATR-FTIR and NIR spectroscopy in combination with PCA. J. Sci. Food Agric. 2020, 100, 855–862. [Google Scholar] [CrossRef]
- Puertas, G.; Vázquez, M. Fraud detection in hen housing system declared on the eggs’ label: An accuracy method based on UV-VIS-NIR spectroscopy and chemometrics. Food Chem. 2019, 288, 8–14. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Lin, Z. Non-destructive identification of native egg by near-infrared spectroscopy and data driven-based class-modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 484–490. [Google Scholar] [CrossRef]
- Puertas, G.; Vázquez, M. Cholesterol determination in egg yolk by UV-VIS-NIR spectroscopy. Food Control 2019, 100, 262–268. [Google Scholar] [CrossRef]
- Puertas, G.; Vázquez, M. UV-VIS-NIR spectroscopy and artificial neural networks for the cholesterol quantification in egg yolk. J. Food Compos. Anal. 2020, 86, 103350. [Google Scholar] [CrossRef]
- Visconti, L.G.; Rodríguez, M.S.; Di Anibal, C.V. Determination of grated hard cheeses adulteration by near infrared spectroscopy (NIR) and multivariate analysis. Int. Dairy J. 2020, 104, 104647. [Google Scholar] [CrossRef]
- Riu, J.; Gorla, G.; Chakif, D.; Boque, R.; Giussani, B. Rapid Analysis of Milk Using Low-Cost Pocket-Size NIR Spectrometers and Multivariate Analysis. Foods 2020, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, S.; Minaei, S. Combined data mining/NIR spectroscopy for purity assessment of lime juice. Infrared Phys. Technol. 2018, 91, 193–199. [Google Scholar] [CrossRef]
- Alamar, P.D.; Caramês, E.T.S.; Poppi, R.J.; Pallone, J.A.L. Detection of Fruit Pulp Adulteration Using Multivariate Analysis: Comparison of NIR, MIR and Data Fusion Performance. Food Anal. Methods 2020, 13, 1357–1365. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, M.; Liu, X.; Su, M.; Wang, L.; Zeng, Y.; Zang, H.; Nie, L. Comparative analysis of rapid quality evaluation of salvia miltiorrhiza (danshen) with fourier transform near-infrared spectrometer and portable near-infrared spectrometer. Microchem. J. 2020, 159, 105492. [Google Scholar] [CrossRef]
- Panesar, P.S. Fermented Dairy Products: Starter Cultures and Potential Nutritional Benefits. Food Nutr. Sci. 2011, 2, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Heaney, R.P. Dairy Intake, Dietary Adequacy, and Lactose Intolerance12. Adv. Nutr. 2013, 4, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.G.; Leite, A.I.N.; Andrade, J.; Bell, M.J.V.; Anjos, V. Evaluation of butter oil adulteration with soybean oil by FT-MIR and FT-NIR spectroscopies and multivariate analyses. LWT 2019, 107, 1–8. [Google Scholar] [CrossRef]
- Pereira, E.V.D.S.; Fernandes, D.D.D.S.; De Araújo, M.C.U.; Diniz, P.H.G.D.; Maciel, M.I.S. Simultaneous determination of goat milk adulteration with cow milk and their fat and protein contents using NIR spectroscopy and PLS algorithms. LWT 2020, 127, 109427. [Google Scholar] [CrossRef]
- Mabood, F.; Ali, L.; Boqué, R.; Abbas, G.; Jabeen, F.; Haq, Q.M.I.; Hussain, J.; Hamaed, A.M.; Naureen, Z.; Al-Nabhani, M.; et al. Robust Fourier transformed infrared spectroscopy coupled with multivariate methods for detection and quantification of urea adulteration in fresh milk samples. Food Sci. Nutr. 2020, 8, 5249–5258. [Google Scholar] [CrossRef]
- Teixeira, J.L.D.P.; Caramês, E.T.D.S.; Baptista, D.P.; Gigante, M.L.; Pallone, J.A.L. Vibrational spectroscopy and chemometrics tools for authenticity and improvement the safety control in goat milk. Food Control 2020, 112, 107105. [Google Scholar] [CrossRef]
- Grassi, S.; Strani, L.; Casiraghi, E.; Alamprese, C. Control and Monitoring of Milk Renneting Using FT-NIR Spectroscopy as a Process Analytical Technology Tool. Foods 2019, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balan, B.; Dhaulaniya, A.S.; Jamwal, R.; Yadav, A.; Kelly, S.; Cannavan, A.; Singh, D.K. Rapid detection and quantification of sucrose adulteration in cow milk using Attenuated total reflectance-Fourier transform infrared spectroscopy coupled with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118628. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.; Ahmed, S. Common milk adulteration and their detection techniques. Int. J. Food Contam. 2016, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, C.F.; Santos, P.M.; Pereira-Filho, E.R.; Rocha, F.R.P. Recent advances on determination of milk adulterants. Food Chem. 2017, 221, 1232–1244. [Google Scholar] [CrossRef]
- Strani, L.; Grassi, S.; Casiraghi, E.; Alamprese, C.; Marini, F. Milk Renneting: Study of Process Factor Influences by FT-NIR Spectroscopy and Chemometrics. Food Bioprocess Technol. 2019, 12, 954–963. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Cipolat-Gotet, C.; Cecchinato, A.; Schiavon, S.; Bittante, G. Chemometric authentication of farming systems of origin of food (milk and ripened cheese) using infrared spectra, fatty acid profiles, flavor fingerprints, and sensory descriptions. Food Chem. 2020, 305, 125480. [Google Scholar] [CrossRef]
- Bester, D.; Esterhuyse, A.J.; Truter, E.J.; Van Rooyen, J. Cardiovascular effects of edible oils: A comparison between four popular edible oils. Nutr. Res. Rev. 2010, 23, 334–348. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, L.; Zhang, Y.; Wang, D.; Wang, X.; Yu, L.; Zhang, W.; Li, P. Review of NIR spectroscopy methods for nondestructive quality analysis of oilseeds and edible oils. Trends Food Sci. Technol. 2020, 101, 172–181. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xia, Z.; Wang, Y.; Wu, Y.; Gong, Z. Rapid determination of phytosterols by NIRS and chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 336–341. [Google Scholar] [CrossRef]
- Kaufmann, K.C.; Favero, F.D.F.; De Vasconcelos, M.A.M.; Godoy, H.T.; Sampaio, K.A.; Barbin, D.F. Portable NIR Spectrometer for Prediction of Palm Oil Acidity. J. Food Sci. 2019, 84, 406–411. [Google Scholar] [CrossRef]
- Abu-Khalaf, N.; Hmidat, M. Visible/Near Infrared (VIS/NIR) spectroscopy as an optical sensor for evaluating olive oil quality. Comput. Electron. Agric. 2020, 173, 105445. [Google Scholar] [CrossRef]
- Teye, E.; Elliott, C.; Sam-Amoah, L.K.; Mingle, C. Rapid and nondestructive fraud detection of palm oil adulteration with Sudan dyes using portable NIR spectroscopic techniques. Food Addit. Contam. Part A 2019, 36, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, L.; Wang, D.; Jiang, J.; Harrington, P.D.B.; Mao, J.; Zhang, Q.; Li, P. Detection of flaxseed oil multiple adulteration by near-infrared spectroscopy and nonlinear one class partial least squares discriminant analysis. LWT 2020, 125, 109247. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Palarea-Albaladejo, J.; García-Martín, J.F.; Pérez-Camino, M.D.C. Olive oil nutritional labeling by using Vis/NIR spectroscopy and compositional statistical methods. Innov. Food Sci. Emerg. Technol. 2019, 51, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Cortés, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Santos, P.M.; Simeone, M.L.F.; Pimentel, M.A.G.; Sena, M.M. Non-destructive screening method for detecting the presence of insects in sorghum grains using near infrared spectroscopy and discriminant analysis. Microchem. J. 2019, 149, 104057. [Google Scholar] [CrossRef]
- Biancolillo, A.; Firmani, P.; Bucci, R.; Magrì, A.; Marini, F. Determination of insect infestation on stored rice by near infrared (NIR) spectroscopy. Microchem. J. 2019, 145, 252–258. [Google Scholar] [CrossRef]
- Yazici, A.; Tiryaki, G.Y.; Ayvaz, H. Determination of pesticide residual levels in strawberry (Fragaria) by near-infrared spectroscopy. J. Sci. Food Agric. 2020, 100, 1980–1989. [Google Scholar] [CrossRef]
- Shan, R.; Chen, Y.; Meng, L.; Li, H.; Zhao, Z.; Gao, M.; Sun, X. Rapid prediction of atrazine sorption in soil using visible near-infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117455. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, L.; Zhang, D.; Ma, Y.; Ngadi, M. Discrimination of organic and conventional rice by chemometric analysis of NIR spectra: A pilot study. J. Food Meas. Charact. 2018, 13, 238–249. [Google Scholar] [CrossRef]
- Park, J.-Y.; Choi, J.-W. Review—Electronic Circuit Systems for Piezoelectric Resonance Sensors. J. Electrochem. Soc. 2020, 167, 037560. [Google Scholar] [CrossRef]
- McGinn, C.K.; Lamport, Z.A.; Kymissis, I. Review of Gravimetric Sensing of Volatile Organic Compounds. ACS Sens. 2020, 5, 1514–1534. [Google Scholar] [CrossRef] [PubMed]
- Länge, K. Bulk and Surface Acoustic Wave Sensor Arrays for Multi-Analyte Detection: A Review. Sensors 2019, 19, 5382. [Google Scholar] [CrossRef] [Green Version]
- Aghakhani, A.; Mohamadi, F.; Ghadimi, J. Novel alcohol vapour sensor based on the mixed-ligand modified MOF-199 coated quartz crystal microbalance. Int. J. Environ. Anal. Chem. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D.; Bassi, D.; Ferrini, F. Evaluation of Three Electronic Noses for Detecting Incipient Wood Decay. Sensors 2010, 10, 1062–1092. [Google Scholar] [CrossRef] [PubMed]
- Speller, N.C.; Siraj, N.; Regmi, B.P.; Marzoughi, H.; Neal, C.; Warner, I.M. Rational Design of QCM-D Virtual Sensor Arrays Based on Film Thickness, Viscoelasticity, and Harmonics for Vapor Discrimination. Anal. Chem. 2015, 87, 5156–5166. [Google Scholar] [CrossRef]
- Regmi, B.P.; Galpothdeniya, W.I.S.; Siraj, N.; Webb, M.H.; Speller, N.C.; Warner, I.M. Phthalocyanine- and porphyrin-based GUMBOS for rapid and sensitive detection of organic vapors. Sens. Actuators B Chem. 2015, 209, 172–179. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; Vaughan, S.; Warner, I.M. Assessment of QCM array schemes for mixture identification: Citrus scented odors. RSC Adv. 2016, 6, 95378–95386. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; McCarter, K.S.; Vaughan, S.; Warner, I.M. QCM virtual sensor array: Vapor identification and molecular weight approximation. Sens. Actuators B Chem. 2017, 246, 952–960. [Google Scholar] [CrossRef]
- Vaughan, S.R.; Speller, N.C.; Chhotaray, P.; McCarter, K.S.; Siraj, N.; Pérez, R.L.; Li, Y.; Warner, I.M. Class specific discrimination of volatile organic compounds using a quartz crystal microbalance based multisensor array. Talanta 2018, 188, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, M.; Nakamoto, T. Study of Room Temperature Ionic Liquids as Gas Sensing Materials in Quartz Crystal Microbalances. Sensors 2020, 20, 4026. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- Cervera-Chiner, L.; March, C.; Arnau, A.; Jiménez, Y.; Montoya, Á. Detection of DDT and carbaryl pesticides in honey by means of immunosensors based on high fundamental frequency quartz crystal microbalance (HFF-QCM). J. Sci. Food Agric. 2020, 100, 2468–2472. [Google Scholar] [CrossRef]
- Latif, U.; Can, S.; Sussitz, H.F.; Dickert, F.L. Molecular Imprinted Based Quartz Crystal Microbalance Sensors for Bacteria and Spores. Chemosensors 2020, 8, 64. [Google Scholar] [CrossRef]
- Emir Diltemiz, S.; Keçili, R.; Ersöz, A.; Say, R. Molecular Imprinting Technology in Quartz Crystal Microbalance (QCM) Sensors. Sensors 2017, 17, 454. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Wang, Y.; Wang, S.; Wang, J. Hollow molecularly imprinted polymer based quartz crystal microbalance sensor for rapid detection of methimazole in food samples. Food Chem. 2020, 309, 125787. [Google Scholar] [CrossRef]
- Shabani, H.; Mehdizadeh, M.; Mousavi, S.M.; Dezfouli, E.A.; Solgi, T.; Khodaverdi, M.; Rabiei, M.; Rastegar, H.; Alebouyeh, M. Halal authenticity of gelatin using species-specific PCR. Food Chem. 2015, 184, 203–206. [Google Scholar] [CrossRef]
- Baziwane, D.; He, Q. Gelatin: The Paramount Food Additive. Food Rev. Int. 2003, 19, 423–435. [Google Scholar] [CrossRef]
- Muharramah, A.; Permata, L.M.; Juwono, H.; Sugiarso, R.D.; Kurniawan, F. Detection of gelatin in ice cream using QCM sensor. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 493, p. 012028. [Google Scholar]
- Lim, S.A.; Ahmed, M.U. A label free electrochemical immunosensor for sensitive detection of porcine serum albumin as a marker for pork adulteration in raw meat. Food Chem. 2016, 206, 197–203. [Google Scholar] [CrossRef]
- Al-Jowder, O.; Kemsley, E.; Wilson, R. Mid-infrared spectroscopy and authenticity problems in selected meats: A feasibility study. Food Chem. 1997, 59, 195–201. [Google Scholar] [CrossRef]
- Cheubong, C.; Yoshida, A.; Mizukawa, Y.; Hayakawa, N.; Takai, M.; Morishita, T.; Kitayama, Y.; Sunayama, H.; Toshifumi, T. Molecularly Imprinted Nanogels Capable of Porcine Serum Albumin Detection in Raw Meat Extract for Halal Food Control. Anal. Chem. 2020, 92, 6401–6407. [Google Scholar] [CrossRef] [PubMed]
- Çakır, O.; Bakhshpour, M.; Yilmaz, F.; Baysal, Z. Novel QCM and SPR sensors based on molecular imprinting for highly sensitive and selective detection of 2,4-dichlorophenoxyacetic acid in apple samples. Mater. Sci. Eng. C 2019, 102, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Gobi, K.V.; Tanaka, H.; Shoyama, Y.; Miura, N. A simple and versatile self-assembled monolayer based surface plasmon resonance immunosensor for highly sensitive detection of 2,4-D from natural water resources. Sens. Actuators B Chem. 2008, 130, 281–289. [Google Scholar] [CrossRef]
- Sroysee, W.; Chunta, S.; Amatatongchai, M.; Lieberzeit, P.A. Molecularly imprinted polymers to detect profenofos and carbofuran selectively with QCM sensors. Phys. Med. 2019, 7, 100016. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Gu, S.; Wang, J.; Wang, Y. Discrimination of wood borers infested Platycladus orientalis trunks using quartz crystal microbalance gas sensor array. Sens. Actuators B Chem. 2020, 309, 127767. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C. Volatile organic compounds gas sensor based on quartz crystal microbalance for fruit freshness detection: A review. Food Chem. 2021, 334, 127615. [Google Scholar] [CrossRef]
- Toniolo, R.; Pizzariello, A.; Dossi, N.; Lorenzon, S.; Abollino, O.; Bontempelli, G. Room Temperature Ionic Liquids as Useful Overlayers for Estimating Food Quality from Their Odor Analysis by Quartz Crystal Microbalance Measurements. Anal. Chem. 2013, 85, 7241–7247. [Google Scholar] [CrossRef] [PubMed]
- Kuchmenko, T.A.; Lvova, L.B. A Perspective on Recent Advances in Piezoelectric Chemical Sensors for Environmental Monitoring and Foodstuffs Analysis. Chemosensors 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Del Pino, M.; Bienvenido, C.; Boyero, J.R.; Vela, J.M. Biology, ecology and integrated pest management of the white mango scale, Aulacaspis tubercularis Newstead, a new pest in southern Spain—A review. Crop. Prot. 2020, 133, 105160. [Google Scholar] [CrossRef]
- Li, L.; Ma, X.-W.; Zhan, R.-L.; Wu, H.-X.; Yao, Q.-S.; Xu, W.-T.; Luo, C.; Zhou, Y.-G.; Liang, Q.-Z.; Wang, S.-B. Profiling of volatile fragrant components in a mini-core collection of mango germplasms from seven countries. PLoS ONE 2017, 12, e0187487. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.S.; Chidley, H.G.; Kulkarni, R.S.; Pujari, K.H.; Giri, A.P.; Gupta, V.S. Cultivar relationships in mango based on fruit volatile profiles. Food Chem. 2009, 114, 363–372. [Google Scholar] [CrossRef]
- Ghatak, B.; Ali, S.B.; Tudu, B.; Pramanik, P.; Mukherji, S.; Bandhopadhyay, R. Detecting Ocimene in mango using mustard oil based quartz crystal microbalance sensor. Sens. Actuators B Chem. 2019, 284, 514–524. [Google Scholar] [CrossRef]
- Ali, S.B.; Ghatak, B.; Debabhuti, N.; Pal, S.; Sharma, P.; Tudu, B.; Bandyopadhyay, R. Sensitive Detection of β-Myrcene in Mango Using Ethyl Cellulose Modified Quartz Crystal Microbalance Sensor. Mater. Today Proc. 2019, 18, 1025–1032. [Google Scholar] [CrossRef]
- Ali, S.B.; Ghatak, B.; Debabhuti, N.; Sharma, P.; Ghosh, A.; Tudu, B.; Bhattacharya, N.; Bandhopadhyay, R. Detection of β-caryophyllene in mango using a quartz crystal microbalance sensor. Sens. Actuators B Chem. 2018, 255, 3064–3073. [Google Scholar] [CrossRef]
- Debabhuti, N.; Sharma, P.; Ali, S.B.; Tudu, B.; Bandyopadhyay, R.; Sarkar, M.P.; Bhattacharyya, N. Discrimination of the maturity stages of Indian mango using QCM based electronic nose. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–2. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Gu, S.; Wang, J.; Wang, Y.; Wei, Z. Detection of hexanal and 1-octen-3-ol in refrigerated grass carp fillets using a QCM gas sensor based on hydrophobic Cu(I)-Cys nanocomposite. Sens. Actuators B Chem. 2020, 305, 127476. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Gu, S.; Wang, J.; Wang, Y.; Wei, Z. Hydrophobic amino-functionalized graphene oxide nanocomposite for aldehydes detection in fish fillets. Sens. Actuators B Chem. 2020, 306, 127579. [Google Scholar] [CrossRef]
- Kalinichenko, A.; Arseniyeva, L. Electronic nose combined with chemometric approaches to assess authenticity and adulteration of sausages by soy protein. Sens. Actuators B Chem. 2020, 303, 127250. [Google Scholar] [CrossRef]
- Rajkovic, A.; Smigic, N.; Devlieghere, F. Contemporary strategies in combating microbial contamination in food chain. Int. J. Food Microbiol. 2010, 141, S29–S42. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Qu, Q.; Li, L.; Ran, X.; Zuo, L.; Huang, R.; Wang, Q. Ultrasensitive Electrochemical Detection of Clostridium perfringens DNA Based Morphology-Dependent DNA Adsorption Properties of CeO2 Nanorods in Dairy Products. Sensors 2018, 18, 1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhull, N.; Kaur, G.; Jain, P.; Mishra, P.; Singh, D.; Ganju, L.; Gupta, V.; Gupta, V. Label-free amperometric biosensor for Escherichia coli O157:H7 detection. Appl. Surf. Sci. 2019, 495, 143548. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, L.; Yang, H.; Cheng, C.; Deng, W.; Tan, Y.; Xie, Q.; Yao, S. An electrochemical immunobiosensor for ultrasensitive detection of Escherichia coli O157:H7 using CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags. Biosens. Bioelectron. 2019, 126, 493–500. [Google Scholar] [CrossRef]
- Wang, H.; Xiu, Y.; Chen, Y.; Sun, L.; Yang, L.; Chen, H.; Niu, X. Electrochemical immunosensor based on an antibody-hierarchical mesoporous SiO2 for the detection of Staphylococcus aureus. RSC Adv. 2019, 9, 16278–16287. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Kim, M.W.; Park, C.Y.; Choi, C.-S.; Kailasa, S.K.; Park, J.P.; Park, T.J. Development of a rapid and sensitive electrochemical biosensor for detection of human norovirus via novel specific binding peptides. Biosens. Bioelectron. 2019, 123, 223–229. [Google Scholar] [CrossRef]
- Wu, W.; Yu, C.; Wang, Q.; Zhao, F.; He, H.; Liu, C.; Yang, Q. Research advances of DNA aptasensors for foodborne pathogen detection. Crit. Rev. Food Sci. Nutr. 2020, 60, 2353–2368. [Google Scholar] [CrossRef]
- Muniandy, S.; Teh, S.J.; Appaturi, J.N.; Thong, K.L.; Lai, C.W.; Ibrahim, F.; Leo, B.F. A reduced graphene oxide-titanium dioxide nanocomposite based electrochemical aptasensor for rapid and sensitive detection of Salmonella enterica. Bioelectrochemistry 2019, 127, 136–144. [Google Scholar] [CrossRef]
- Peng, H.; Hui, Y.; Ren, R.; Wang, B.; Song, S.; He, Y.; Zhang, F. A sensitive electrochemical aptasensor based on MB-anchored GO for the rapid detection of Cronobacter sakazakii. J. Solid State Electrochem. 2019, 23, 3391–3398. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.-W.; Bie, S.; Suo, T.; Jia, G.-C.; Liu, B.; Ye, R.; Li, Z. Development of an Electrochemical Biosensor for Rapid and Effective Detection of Pathogenic Escherichia coli in Licorice Extract. Appl. Sci. 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Reta, N.; Saint, C.P.; Michelmore, A.; Prieto-Simon, B.; Voelcker, N.H. Nanostructured Electrochemical Biosensors for Label-Free Detection of Water- and Food-Borne Pathogens. ACS Appl. Mater. Interfaces 2018, 10, 6055–6072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Nanomaterial-based biosensors for sensing key foodborne pathogens: Advances from recent decades. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1465–1487. [Google Scholar] [CrossRef]
- Appaturi, J.N.; Pulingam, T.; Thong, K.L.; Muniandy, S.; Ahmad, N.; Leo, B.F. Rapid and sensitive detection of Salmonella with reduced graphene oxide-carbon nanotube based electrochemical aptasensor. Anal. Biochem. 2020, 589, 113489. [Google Scholar] [CrossRef]
- Sobhan, A.; Lee, J.; Park, M.-K.; Oh, J.-H. Rapid detection of Yersinia enterocolitica using a single–walled carbon nanotube-based biosensor for Kimchi product. LWT 2019, 108, 48–54. [Google Scholar] [CrossRef]
- Pandey, A.; Gurbuz, Y.; Ozguz, V.; Niazi, J.H.; Qureshi, A. Graphene-interfaced electrical biosensor for label-free and sensitive detection of foodborne pathogenic E. coli O157:H7. Biosens. Bioelectron. 2017, 91, 225–231. [Google Scholar] [CrossRef]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.F.; McLamore, E.S.; Hondred, J.A.; Gomes, C.L. Laser-Induced Graphene Electrochemical Immunosensors for Rapid and Label-Free Monitoring of Salmonella enterica in Chicken Broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef]
- Lin, D.; Pillai, R.G.; Lee, W.E.; Jemere, A.B. An impedimetric biosensor for E. coli O157:H7 based on the use of self-assembled gold nanoparticles and protein G. Microchim. Acta 2019, 186, 169. [Google Scholar] [CrossRef]
- Pissuwan, D.; Gazzana, C.; Mongkolsuk, S.; Cortie, M.B. Single and multiple detections of foodborne pathogens by gold nanoparticle assays. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1584. [Google Scholar] [CrossRef]
- Shoaie, N.; Moghadam, M.F.; Omidfar, K. Voltammetric determination of the Escherichia coli DNA using a screen-printed carbon electrode modified with polyaniline and gold nanoparticles. Microchim. Acta 2018, 185, 217. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.; Magalhães, J.M.; Barroso, M.F.; Oliva-Teles, T.; Freire, C.; Delerue-Matos, C. In situ formation of gold nanoparticles in polymer inclusion membrane: Application as platform in a label-free potentiometric immunosensor for Salmonella typhimurium detection. Talanta 2019, 194, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.; Neves, M.; Magalhães, J.M.S.; Freire, C.; Delerue-Matos, C. Emerging electrochemical biosensing approaches for detection of Listeria monocytogenes in food samples: An overview. Trends Food Sci. Technol. 2020, 99, 621–633. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Güner, A.; Çevik, E.; Şenel, M.; Alpsoy, L. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 by using chitosan, MWCNT, polypyrrole with gold nanoparticles hybrid sensing platform. Food Chem. 2017, 229, 358–365. [Google Scholar] [CrossRef]
- Singh, A.T.; Sinsinbar, G.; Choudhary, M.; Kumar, V.; Pasricha, R.; Verma, H.; Singh, S.P.; Arora, K. Graphene oxide-chitosan nanocomposite based electrochemical DNA biosensor for detection of typhoid. Sens. Actuators B Chem. 2013, 185, 675–684. [Google Scholar] [CrossRef]
- Hasan, R.; Pulingam, T.; Appaturi, J.N.; Zifruddin, A.N.; Teh, S.J.; Lim, T.W.; Ibrahim, F.; Leo, B.F.; Thong, K.L. Carbon nanotube-based aptasensor for sensitive electrochemical detection of whole-cell Salmonella. Anal. Biochem. 2018, 554, 34–43. [Google Scholar] [CrossRef]
- Villalonga, M.L.; Borisova, B.; Arenas, C.B.; Villalonga, A.; Arévalo-Villena, M.; Sánchez, A.; Pingarrón, J.M.; Briones-Pérez, A.; Villalonga, R. Disposable electrochemical biosensors for Brettanomyces bruxellensis and total yeast content in wine based on core-shell magnetic nanoparticles. Sens. Actuators B 2019, 279, 15–21. [Google Scholar] [CrossRef]
- He, S.; Yuan, Y.; Nag, A.; Feng, S.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C.; Organ, D.R. A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality. Int. J. Environ. Res. Public Health 2020, 17, 5220. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bwambok, D.K.; Siraj, N.; Macchi, S.; Larm, N.E.; Baker, G.A.; Pérez, R.L.; Ayala, C.E.; Walgama, C.; Pollard, D.; Rodriguez, J.D.; et al. QCM Sensor Arrays, Electroanalytical Techniques and NIR Spectroscopy Coupled to Multivariate Analysis for Quality Assessment of Food Products, Raw Materials, Ingredients and Foodborne Pathogen Detection: Challenges and Breakthroughs. Sensors 2020, 20, 6982. https://doi.org/10.3390/s20236982
Bwambok DK, Siraj N, Macchi S, Larm NE, Baker GA, Pérez RL, Ayala CE, Walgama C, Pollard D, Rodriguez JD, et al. QCM Sensor Arrays, Electroanalytical Techniques and NIR Spectroscopy Coupled to Multivariate Analysis for Quality Assessment of Food Products, Raw Materials, Ingredients and Foodborne Pathogen Detection: Challenges and Breakthroughs. Sensors. 2020; 20(23):6982. https://doi.org/10.3390/s20236982
Chicago/Turabian StyleBwambok, David K., Noureen Siraj, Samantha Macchi, Nathaniel E. Larm, Gary A. Baker, Rocío L. Pérez, Caitlan E. Ayala, Charuksha Walgama, David Pollard, Jason D. Rodriguez, and et al. 2020. "QCM Sensor Arrays, Electroanalytical Techniques and NIR Spectroscopy Coupled to Multivariate Analysis for Quality Assessment of Food Products, Raw Materials, Ingredients and Foodborne Pathogen Detection: Challenges and Breakthroughs" Sensors 20, no. 23: 6982. https://doi.org/10.3390/s20236982
APA StyleBwambok, D. K., Siraj, N., Macchi, S., Larm, N. E., Baker, G. A., Pérez, R. L., Ayala, C. E., Walgama, C., Pollard, D., Rodriguez, J. D., Banerjee, S., Elzey, B., Warner, I. M., & Fakayode, S. O. (2020). QCM Sensor Arrays, Electroanalytical Techniques and NIR Spectroscopy Coupled to Multivariate Analysis for Quality Assessment of Food Products, Raw Materials, Ingredients and Foodborne Pathogen Detection: Challenges and Breakthroughs. Sensors, 20(23), 6982. https://doi.org/10.3390/s20236982






