Abstract
Real-time detection of fatigue in the elderly during physical exercises can help identify the stability and thus falling risks which are commonly achieved by the investigation of kinematic parameters. In this study, we aimed to identify the change in gait variability parameters from inertial measurement units (IMU) during a course of 60 min brisk walking which could lay the foundation for the development of fatigue-detecting wearable sensors. Eighteen elderly people were invited to participate in the brisk walking trials for 60 min with a single IMU attached to the posterior heel region of the dominant side. Nine sets of signals, including the accelerations, angular velocities, and rotation angles of the heel in three anatomical axes, were measured and extracted at the three walking times (baseline, 30th min, and 60th min) of the trial for analysis. Sixteen of eighteen participants reported fatigue after walking, and there were significant differences in the median acceleration (p = 0.001), variability of angular velocity (p = 0.025), and range of angle rotation (p = 0.0011), in the medial–lateral direction. In addition, there were also significant differences in the heel pronation angle (p = 0.005) and variability and energy consumption of the angles in the anterior–posterior axis (p = 0.028, p = 0.028), medial–lateral axis (p = 0.014, p = 0.014), and vertical axis (p = 0.002, p < 0.001). Our study demonstrated that a single IMU on the posterior heel of the dominant side can address the variability of kinematics parameters for elderly performing prolonged brisk walking and could serve as an indicator for walking instability, and thus fatigue.
1. Introduction
Physical exercise is imperative for the elderly to maintain and promote a healthy lifestyle [1]. At least two and a half hours of moderate-intensity aerobic physical activity per week has significant health effects on the health of the elderly [2,3]. In addition, regular physical activity can reduce the risk of falls by 30% for the poor-mobility elderly [4]. It is recommended to integrate physical activity, such as walking, into daily routines [5] since it is convenient [6]. The elderly are recommended to walk 5000 steps per day to minimize the falling risk [7] and even encouraged to walk 7000 to 10,000 steps per day for health benefits [8]. However, the decline in muscle strength, coordination, and motor perception as aging impairs older people’s balance control ability increases the fear in older adults of falling [9]. As a result, high nervousness caused by the fear of falling and decreased muscle strength and tolerance make the elderly more prone to fatigue, which is associated with falling [10], and falling poses a serious threat to the elderly [11]. Therefore, timely identification of fatigue can reduce the risk of falls and enable the elderly to exercise more for better health.
Previous studies have found some indicators related to walking fatigue for the elderly. Fatigue attenuated the range of motion and muscle strength of the ankle joint of the dominant leg [12], in addition to the variability of step width and the minimum foot clearance (MFC) that were regarded as risks of tripping [10]. Gait asymmetry and variability measures are indirect indexes to evaluate fatigue [13]. Some researchers attempted to verify neuromuscular fatigue of lower limb and trunk muscles using electromyography [14,15]. The decreased coactivation of the ankle joint in the first half of the swing phase was found to be correlated with muscle fatigue in walking [16].
The application of an inertial measurement unit (IMU) is becoming popular recently due to its superiority in portability, cost, and accessibility compared to traditional gait labs with motion capture systems and force platforms [17]. IMUs were used to indirectly classify the fatigue of lower extremity muscles [18], to detect age-related gait deviation [19], and to direct balance improvement programs [20].
While there is an abundance of research on the walking fatigue of the elderly, the measurement (including the baseline and fatigue state) was often standalone from the fatigue-inducing task (e.g., long-distance walking [13]). This may or may not reliably acquire the state of fatigue from participants. Protocols in existing studies may allow the participants to recover when they switch from the fatiguing tasks to the test for measurement.
In this light, we propose to collect the kinematic data throughout the whole fatigue-inducing tasks for analysis without a standalone testing trial for measurement. This approach could ensure that the participants will not recover halfway, and the fatigue-inducing effect could be continuous. In addition, the advantage of this study lies in its potential to predict instability or fatigue using a single sensor measuring the orientation, angular velocity, and linear acceleration of the posterior heel. The main purpose of this study is to study the influence of fatigue induced by brisk walking on gait parameters by using an IMU and identify fatigue indicators. It is hypothesized that lower-limb muscle fatigue will manifest the deterioration of stability and increase energy consumption. This study is not only helpful for the elderly’s fast walking exercise but also the development of wearable fatigue recognition equipment, the elderly’s fall prevention, and rehabilitation training.
This paper includes five parts. The Introduction section covers the research background and significance of fatigue indicators. The Materials and Methods section provides the information of participants, experimental protocol, outcome measures, and statistical analysis. The Results section highlights the changes in outcome measures across the time of long-term brisk walking as well as the results of the statistical analysis. Subsequently, we address the implications of our key findings and the limitations of our study in the Discussion section. Lastly, we conclude the feasibility of our system on fatigue detection with a cautious note on the relationship between the variability of kinematics and fatigue.
2. Materials and Methods
2.1. Participants
Eighteen older adults, nine males and nine females, were recruited from the university campus by posters and leaflets. The average age, height, and weight of the participants were 60.4 ± 7.9 years, 161.0 ± 7.6 cm, and 62.7 ± 11.7 kg, respectively. All these participants were independent walkers with a heel strike walking style. The exclusion criteria included persistent knee pain, osteoarthritis, unstable ankle, severe flat foot, severe hallux valgus, toe deformities, and other chronic diseases that might lead to safety issues. The participants shall also have no history of injuries in the past six months. All participants signed the informed consent, and this study was approved by the Human Subjects Ethics Sub-committee of The Hong Kong Polytechnic University (Reference Number: HSEARS20190919001).
2.2. Equipment and Experimental Procedures
All participants wore the same type of cushioned running shoes, ARHQ025-4 (Li-Ning Inc., Beijing, China), with self-selected sizes, from 36 to 43 (European size). The insole, upper, and outsole are made of Ethylene-vinyl acetate copolymer (EVA), synthetic, and rubber, respectively.
The closure type is lace-up. Before the running task, the safety precautions in using the treadmill (Unisen Inc., Tustin, CA, USA) and the general procedures of the experiment were explained to the participants. By increasing the treadmill speed by 0.5 km/h every 30 s [16], participants reached their self-preferred comfortable brisk walking speed. After 5 min of familiar walking on the treadmill, participants brisk walked for 1 h at this speed. The mean preferred comfortable fast walking speed was 3.9 ± 0.6 km/h. After the brisk walking trial, the participants were asked whether they experienced no fatigue or a mild or severe level of fatigue.
The acceleration, angular velocity, and angles of the posterior heel region of the dominant foot in the anterior–posterior axis, medial–lateral axis, and vertical axis were measured using a single IMU (BWT901BLE5.0, Wit motion Inc., Shenzhen, China). The hardware of the IMU utilized an MPU 9250 (InvenSense Inc., CA, USA). The software of the IMU integrated dynamics calculation and the Kalman filter algorithm and provided a mobile application that can collect data in real time. The IMU was attached on the middle posterior heel of the dominant foot as shown in Figure 1, sampling at 50Hz. The specifications of BWT901BLE5.0 are shown in Table 1 and the full-scale ranges of the accelerometer and gyroscope were ± 16 g and ± 2000 °/s. The x-axis, y-axis, and z-axis corresponded to the medial–lateral axis, anterior–posterior axis, and vertical axis, respectively, as shown in Figure 1. The dominant limb was determined by asking participants to kick a ball five meters away from the goal [21].

Figure 1.
The placement and orientation of the inertial measurement unit (IMU) on the posterior heel region.

Table 1.
The specifications of BWT901BLE5.0.
2.3. Data Processing
The average baseline orientation of the IMU was measured when the participants walked for the first 30 s of the walking trial. The calculated angles referred to the changes in orientation angle compared to the average baseline orientation.
We extracted the baseline (1st-min), 30th-min, and 60th-min data for analysis. The outcome measures included the acceleration, angular velocity, and angle in the anterior–posterior axis, medial–lateral axis, and vertical axis. Ten parameters were derived from these three time series’ primary data including the median absolute deviation (MAD), kurtosis, skewness, root mean square (RMS), variance, maximum absolute value, minimum absolute value, amplitude range, the median of absolute value, and the average of energy consumption (EC). In this study, signal power was regarded as EC, and the signal power of a real-valued signal is defined as follows [22]:
The process of data processing is summarized as follows.
- Extract the data of the baseline min, 30th min, and 60th min, and incomplete gait cycles are ignored.
- Calculate these 10 parameters of each gait cycle in these three time periods, then calculate the average value for each parameter.
- Normalize by dividing each participant’s baseline-min, 30th-min, and 60th-min data by their respective baseline-min data to get the proportion of change of each parameter relative to the baseline, thereby eliminating the difference in gait parameters between the experiments. The units of each parameter are 1 since each parameter uses a ratio.
Take the RMS of the 30th min as an example. The baseline min, the 30th min, and the 60th min consist of gait cycles, respectively. The calculation of the 30th min’s RMS is
2.4. Statistical Analysis
Statistical analysis was used to determine whether there were statistically significant differences in the outcome measures (detailed in Section 2.3) over the course of the brisk walking trial at the baseline, 30-min, and 60-min time points.
The data were not normally distributed as assessed by the Shapiro–Wilk test (p > 0.5). Therefore, the nonparametric test (Friedman test) was used. The significance level (α) was set at p = 0.05. If the findings of the Friedman test were statistically significant, a post hoc pairwise comparison, the Wilcoxon signed-rank test, was conducted with Bonferroni correction at p < 0.017.
3. Results
After the brisk walking trial, 12 out of the 18 participants reported severe fatigue and 4 participants reported a mild level of fatigue, while the remaining two participants reported non-fatigue. The outcome measures from the IMU after data processing are shown in Table 2. Twenty-five outcome variables demonstrated a statistically significant difference among the walking time conditions in the Friedman test. A post hoc pairwise comparison was conducted on the outcome variables with significance, as shown in Table 3.

Table 2.
The Friedman test results of three time periods for each parameter.

Table 3.
Post hoc analysis on outcome variables with significance in the Friedman test.
3.1. Influence of Walking Time (Fatigue) on Posterior Heel Acceleration
There were eight significant differences in statistical features in the three axes’ accelerations, as shown in Table 2, including the range (p < 0.03) and median (p < 0.002) of the x-axis acceleration, the RMS (p < 0.03), variance (p < 0.03), minimum (p < 0.002), and EC (p < 0.03) of the y-axis acceleration, and the skewness (p < 0.03) and median (p < 0.03) of the z-axis acceleration.
The post hoc pairwise comparison test (Table 3) revealed that, for , there were significant differences in the range between the baseline and 60th min, the median between the baseline and 60th min, and the 30th and 60 min, and the RMS between the baseline and 30 min. For there were significant differences in the variance between the baseline and 30th min, the minimum between the baseline and 60th min, and 30th and 60th mins, and the EC between the baseline and 30th min. For , there were significant differences in the skewness between the baseline and 60th min and the median between the 30th and 60th mins. Figure 2 shows that the RMS and EC of decreased after the first 30-min walking task, while the median of increased after the continued 30-min task (60-min).
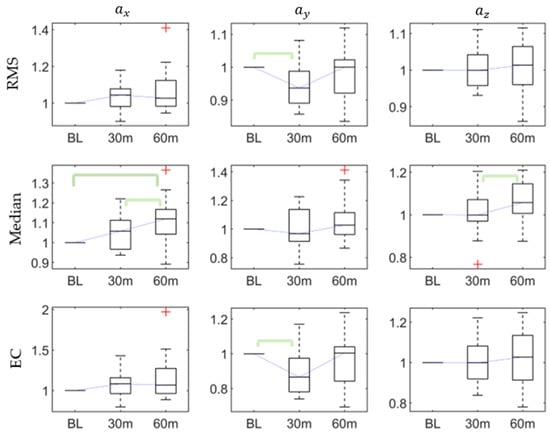
Figure 2.
The root mean square (RMS), median, and energy consumption (EC) of accelerations at baseline, 30th min, and 60th min. Bracket denotes statistical significance (p < 0.05) in the post hoc analysis with Bonferroni correction.
3.2. Influence of Walking Time (Fatigue) on Posterior Heel Angular Velocity
As Table 2 shows, there were significant differences in the RMS (p < 0.03), maximum (p < 0.03), and EC (p < 0.02) of the x-axis angular velocity, while there was no significant difference in the y-axis and z-axis angular velocities. The post hoc pairwise comparison test (Table 3) proved that the significant differences in the RMS, maximum, and EC exist at the baseline and 60th min, which all increased after 60 min. The post hoc results of the RMS, median, and EC characteristics of the three axes’ angular velocities are shown in Figure 3.
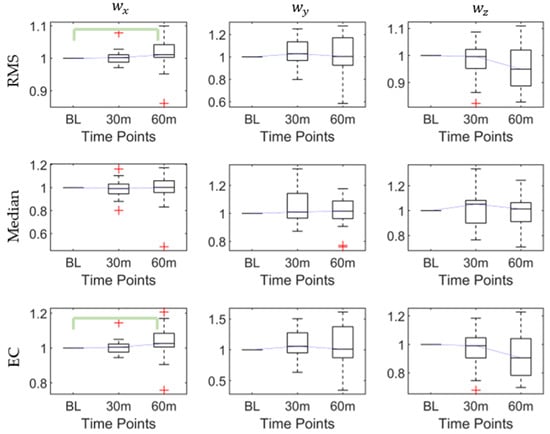
Figure 3.
The root mean square (RMS), median, and energy consumption (EC) of angular velocities at baseline, 30th min, and 60th min. Bracket denotes statistical significance (p < 0.05) in the post hoc analysis with Bonferroni correction.
3.3. Influence of Walking Time (Fatigue) on Posterior Heel Orientation (Rotation Angle)
There were significant differences in the RMS (p < 0.03), maximum (p < 0.002), range (p < 0.002), and EC (p < 0.03) of the x-axis angular; the RMS (p < 0.02) and EC (p < 0.02) of the y-axis angular; and in the kurtosis (p < 0.02), skewness (p < 0.05), RMS (p < 0.003), maximum (p < 0.03), minimum (p < 0.006), range (p < 0. 006), median (p < 0. 006), and EC (p < 0.001) of the z-axis angular, as shown in Table 2.
The post hoc pairwise comparison test (Table 3) demonstrated that for , there were significant differences in the RMS between the baseline and 60th min, the maximum between the baseline and 30th min, and the baseline and 60th min, and the range and EC between the baseline and 60th min; for , there were significant differences in the RMS and EC between the baseline and 60th min; and for , there were significant differences in the kurtosis and skewness between the baseline and 60th min and the RMS, maximum, minimum, range, median, and EC between the baseline and 30th min, and the baseline and 60th min. Figure 4 shows that the RMS, median, and EC of increased after the first 30-min walking session. After the continued 30-min task (60-min), the RMS and EC of , the RMS and EC of , and the RMS, median, and EC of all increased.
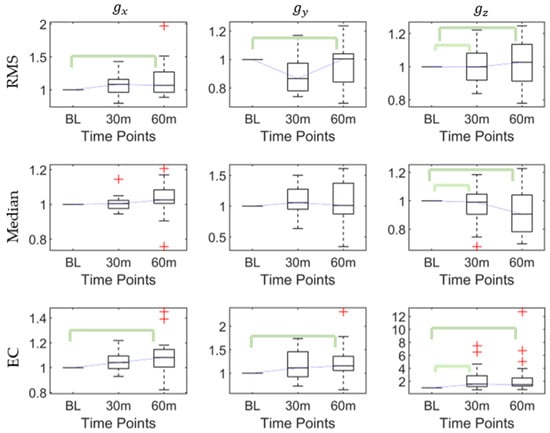
Figure 4.
The root mean square (RMS), median, and energy consumption (EC) of angles at baseline, 30th min, and 60th min. Bracket denotes statistical significance (p < 0.05) in the post hoc analysis with Bonferroni correction.
4. Discussion
This study explored the changes in kinematics and some derived kinematic variables after 60 min of brisk walking using an IMU. Our result showed that long-distance brisk walking may induce fatigue since most of the participants (16/18) responded that their subjective fatigue felt significantly increased, which imposed an apparent effect on the kinematic parameters of the individuals’ posterior heel of the dominant foot. After long-distance brisk walking, the participants showed a significant increase in (1) the median of acceleration in the medial–lateral axis (), (2) maximum, variability, and energy consumption of angular velocity in the medial–lateral axis (), (3) maximum, range, variability, and energy consumption of angle in the medial–lateral axis (), (4) variability of angle in the anterior–posterior axis (), and (5) median, maximum, range, variability, and energy consumption of angle in the vertical axis ().
The increment in the medial–lateral acceleration indicates greater vibration of the dominant lower limb in the medial–lateral axis. This signal was likely manifested by a muscle fatigue condition since the acceleration signal reflects the mechanical activity of the muscle [23], which was advocated by previous studies [24]. The greater variation in medial–lateral acceleration could also be a sign of instability, imbalance, and falling risks [10,25,26]. Variation in step width could be related to age and falling [27] and distinguished between young and old adults [28,29,30] or between fallers and non-fallers [31,32].
The maximum angular velocity in the medial–lateral axis tended to increase after long brisk walking, and we assumed that this is a strategy to shorten the time of dominant foot swing and increases the double support time. This assumption was consistent with one previous study that suggested, in order to adjust the microbalance before the swing phase of the dominant limb becomes more unstable after fatigue, a longer double support time and shorter support time are required [16]. The increase in the variability of medial–lateral angular velocity demonstrated that the elderly were less stable after prolong brisk walking (or fatigue), which is consistent with previous studies [13,33]. A similar finding that elderly participants increased the median absolute deviation (MAD) of the medial–lateral ankle angular velocity after long-distance walking was found in [13]. In addition, the energy consumption increase indicates that they need more energy to maintain stability, which is consistent with the result of increased angular velocity variability.
After prolonged brisk walking, the maximum rotation angle in the medial–lateral axis became larger and could result in a larger rotation range. This phenomenon may represent a longer double support time, agreeing with the results of previous studies [10,16,18,34,35]. The rotation angle in the vertical direction also increased, and this proved that the instability induced by long-distance walking, or fatigue, may induce heel pronation. The altered lower limb alignment may increase the risk of overuse injuries [36]. This could be the reason why anti-pronation shoes were developed for pronated feet runners [37].
Furthermore, both variation and energy consumption of rotation angles increased in the three axes of anterior–posterior, medial–lateral, and vertical directions after long-distance brisk walking. This result is similar to a previous study that found, when the muscles adjacent to the joint are fatigued, there could be a decrease in the synergy between the neuromuscular feedback of the joints and the proprioception of the joints [16,18,38,39], resulting in a decrease in human balance control [40], deterioration in gait stability, and increased variability, which needs more energy to consume [16].
There were some limitations to this study. Due to the ethical considerations, elderly participants recruited in this study were generally more physically active and fit, to minimize the falling risks by this challenging prolonged brisk walking task. The physical fitness of the elderly people could be the confounding or effect modifier to the identifiers. In the real case, some elderly people could be reluctant to exercise and could be less physically tolerable. Sampling elderly people with different levels of physical fitness or risk of falls may improve the external validity of our findings, which could be conducted using postural stability tests [41,42]. On the other hand, we assumed that “fatigue” was induced by the prolonged brisk walking task as supported by the subjective feedback of the participant. However, in real practice, “fatigue” was difficult to evaluate and could be categorized into mental, neuromuscular, and metabolic fatigue [43]. We heavily relied on the presupposition of the indirect relationship between fatigue and instability (variation in parameters) supported by some literature [44,45]. It would be interesting to evaluate the sensitivity and specificity of a fatigue-predicting algorithm [46], in addition to the use of a receiver-operating chart (ROC), for optimization [47]. However, extensive effort is required to develop a validated instrument for precise fatigue measurement that warrants future study. Furthermore, future studies may apply electromyography [14], a musculoskeletal model [48], or near-infrared spectroscopy [49] to address and correlate different classes and intensities of fatigue. In addition, the integration of a multi-unit synchronized system could further facilitate activity monitoring [50], as well as reducing the sensor node complexity [51].
While the IMU system in this research aims to simplify and minimize the number of IMUs for fatigue identification, it should be noted that a number of research works have endeavored to investigate the influence of fatigue on variability, stability, and gait asymmetry [13]. Incorporation of these features may help improve the accuracy of fatigue prediction. A biofeedback system [52] could also be developed to let the elderly be aware of their fatigue or instability conditions and minimize falling risks.
5. Conclusions
This study utilized a single IMU to measure the linear acceleration, angular velocity, and rotation at the posterior heel during a 60-min brisk walking trial among elderly participants. The prolonged brisk walking induced a significant change in the signal parameters in the medial–lateral directions, including the linear acceleration, angular velocity, and heel rotation range. In addition, higher variability and energy consumption were also found on the angular velocity in the medial–lateral direction, as well as on the heel rotation angles in all directions.
Our study demonstrates that the brisk walking trial induced fatigue in the elderly participants as demonstrated by the subjective feedback. It should be noted that fatigue is a complex symptom, being the result of the complex interaction of physiological systems and the brain [53]. Since the brisk walking trial also induced changes in our measured signal parameters, we can conclude that our IMU-based system can identify fatigue status based on signal parameters. The findings in this study will pave the way towards developing fatigue-detecting smart shoes for elderly walking exercises to minimize the risk of falls.
Author Contributions
Conceptualization, M.Z. and D.W.-C.W.; methodology, G.Z., and D.W.-C.W.; software, G.Z. and Y.P.; validation, T.T.-H.H., I.K.-K.W.; formal analysis, G.Z., and D.W.-C.W.; investigation, I.K.-K.W., and T.L.-W.C.; resources, I.K.-K.W. and T.T.-H.H.; data curation, G.Z., T.L.-W.C., and I.K.-K.W.; writing—original draft preparation, G.Z.; writing—review and editing, D.W., G.Z., and M.Z.; visualization, F.Y., Y.W., and Q.T.; supervision, M.Z.; project administration, M.Z.; funding acquisition, M.Z., and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Innovation and Technology Fund (ITF) of Hong Kong, China (ITS/262/18), and the National Natural Science Foundation of China (No. 11732015 and No. 11972315).
Acknowledgments
The authors are grateful to the experiment participants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98, 16218. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.H.; Warburton, D.E. Physical activity and functional limitations in older adults: A systematic review related to Canada’s Physical Activity Guidelines. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Committee, P.A.G.A. Physical Activity Guidelines Advisory Committee Report, 2008; US Department of Health and Human Services: Washington, DC, USA, 2008; pp. A1–H14.
- Paterson, D.H.; Jones, G.R.; Rice, C.L. Ageing and physical activity: Evidence to develop exercise recommendations for older adults. Appl. Physiol. Nutr. Metab. 2007, 32, S69–S108. [Google Scholar] [CrossRef]
- Global Recommendations on Physical Activity for Health. Available online: https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf (accessed on 6 December 2020).
- Chastin, S.; De Craemer, M.; De Cocker, K.; Powell, L.; Van Cauwenberg, J.; Dall, P.; Hamer, M.; Stamatakis, E. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br. J. Sports Med. 2019, 53, 370–376. [Google Scholar] [CrossRef]
- Aranyavalai, T.; Jalayondeja, C.; Jalayondeja, W.; Pichaiyongwongdee, S.; Kaewkungwal, J.; Laskin, J.J. Association between walking 5000 step/day and fall incidence over six months in urban community-dwelling older people. BMC Geriatr. 2020, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.L.; Aoyagi, Y.; Bell, R.C.; A Croteau, K.; De Bourdeaudhuij, I.; Ewald, B.; Gardner, A.; Hatano, Y.; Lutes, L.D.; et al. How many steps/day are enough? For older adults and special populations. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O.; Sparrow, W. Age and walking speed effects on muscle recruitment in gait termination. Gait Posture 2005, 21, 279–288. [Google Scholar] [CrossRef]
- Nagano, H.; James, L.; Sparrow, W.; Begg, R.K. Effects of walking-induced fatigue on gait function and tripping risks in older adults. J. Neuroeng. Rehabil. 2014, 11, 155. [Google Scholar] [CrossRef]
- Bruijn, S.M.; Meijer, O.G.; Beek, P.J.; Van Dieën, J.H. Assessing the stability of human locomotion: A review of current measures. J. R. Soc. Interface 2013, 10, 20120999. [Google Scholar] [CrossRef]
- Elhadi, M.M.; Ma, C.Z.-H.; Wong, D.W.-C.; Wan, A.H.; Lee, W.C.-C. Comprehensive Gait Analysis of Healthy Older Adults Who Have Undergone Long-Distance Walking. J. Aging Phys. Act. 2017, 25, 367–377. [Google Scholar] [CrossRef]
- Wong, D.W.-C.; Lam, W.-K.; Lee, W.C.-C. Gait asymmetry and variability in older adults during long-distance walking: Implications for gait instability. Clin. Biomech. 2020, 72, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lin, C.-B.; Chen, Y.; Chen, W.; Huang, T.-S.; Hsu, C.-Y. An EMG Patch for the Real-Time Monitoring of Muscle-Fatigue Conditions During Exercise. Sensors 2019, 19, 3108. [Google Scholar] [CrossRef] [PubMed]
- Moniri, A.; Terracina, D.; Rodriguez-Manzano, J.; Strutton, P.H.; Georgiou, P. Real-Time Forecasting of sEMG Features for Trunk Muscle Fatigue using Machine Learning. IEEE Trans. Biomed. Eng. 2020. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.F.; Soares, D.P.; Bertani, M.C.; Rodrigues, L.J.; Vila-boas, J.P. Effects of Fast-Walking on Muscle Activation in Young Adults and Elderly Persons. J. Novel Physiother. Rehabil. 2017, 1, 012–019. [Google Scholar]
- Buckley, C.; O’Reilly, M.; Whelan, D.; Farrell, A.V.; Clark, L.; Longo, V.; Gilchrist, M.; Caulfield, B. Binary classification of running fatigue using a single inertial measurement unit. In Proceedings of the 2017 IEEE 14th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Eindhoven, The Netherlands, 9–12 May 2017; pp. 197–201. [Google Scholar]
- Zhang, J.; Lockhart, T.E.; Soangra, R. Classifying Lower Extremity Muscle Fatigue During Walking Using Machine Learning and Inertial Sensors. Ann. Biomed. Eng. 2014, 42, 600–612. [Google Scholar] [CrossRef]
- Hu, B.; Dixon, P.; Jacobs, J.; Dennerlein, J.; Schiffman, J. Machine learning algorithms based on signals from a single wearable inertial sensor can detect surface- and age-related differences in walking. J. Biomech. 2018, 71, 37–42. [Google Scholar] [CrossRef]
- Ma, C.Z.-H.; Wong, D.W.-C.; Lam, W.-K.; Wan, A.H.-P.; Lee, W.C.-C. Balance Improvement Effects of Biofeedback Systems with State-of-the-Art Wearable Sensors: A Systematic Review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef]
- Seeley, M.K.; Umberger, B.R.; Shapiro, R. A test of the functional asymmetry hypothesis in walking. Gait Posture 2008, 28, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Grami, A. Signals, Systems, and Spectral Analysis. In Introduction to Digital Communications; Springer: New York, NY, USA, 2007; pp. 41–150. [Google Scholar]
- Barry, D.T.; Hill, T.; Im, D. Muscle fatigue measured with evoked muscle vibrations. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1992, 15, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.; Gupta, A. A novel approach to detect localized muscle fatigue during isometric exercises. In Proceedings of the 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN), San Francisco, CA, USA, 14–17 June 2016. [Google Scholar]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Thies, B.S.; Richardson, J.K.; Ashton-Miller, J.A. Effects of surface irregularity and lighting on step variability during gait: A study in healthy young and older women. Gait Posture 2005, 22, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, D.; Singh, N.; Van Dieën, J.; Heller, M.; Taylor, W.R. Kinematic measures for assessing gait stability in elderly individuals: A systematic review. J. R. Soc. Interface 2011, 8, 1682–1698. [Google Scholar] [CrossRef] [PubMed]
- Woledge, R.C.; Birtles, D.; Newham, D.J. The Variable Component of Lateral Body Sway During Walking in Young And Older Humans. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2005, 60, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Owings, T.M.; Grabiner, M.D. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. J. Biomech. 2004, 37, 935–938. [Google Scholar] [CrossRef]
- Owings, T.M.; Grabiner, M.D. Variability of step kinematics in young and older adults. Gait Posture 2004, 20, 26–29. [Google Scholar] [CrossRef]
- Maki, B.E. Gait Changes in Older Adults: Predictors of Falls or Indicators of Fear? J. Am. Geriatr. Soc. 1997, 45, 313–320. [Google Scholar] [CrossRef]
- Brach, J.S.; Talkowski, J.B.; VanSwearingen, J.; Newman, A.B.; Studenski, S.A. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J. Neuroeng. Rehabil. 2005, 2, 21. [Google Scholar] [CrossRef]
- Chizewski, M.G.; Chiu, L.Z.F. Contribution of calcaneal and leg segment rotations to ankle joint dorsiflexion in a weight-bearing task. Gait Posture 2012, 36, 85–89. [Google Scholar] [CrossRef]
- Modarresi, S.; Divine, A.; Grahn, J.A.; Overend, T.J.; Hunter, S.W. Gait parameters and characteristics associated with increased risk of falls in people with dementia: A systematic review. Int. Psychogeriatr. 2018, 31, 1287–1303. [Google Scholar] [CrossRef]
- Morris, M.E.; Vermeulen, M.; Van Oers, M.H. Changes in gait and fatigue from morning to afternoon in people with multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2002, 72, 361–365. [Google Scholar] [CrossRef]
- Tong, J.W.; Kong, P.W. Association Between Foot Type and Lower Extremity Injuries: Systematic Literature Review With Meta-analysis. J. Orthop. Sports Phys. Ther. 2013, 43, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Jafarnezhadgero, A.; Alavi-Mehr, S.M.; Granacher, U. Effects of anti-pronation shoes on lower limb kinematics and kinetics in female runners with pronated feet: The role of physical fatigue. PLoS ONE 2019, 14, e0216818. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Alexander, I.J.; Hayes, K.C. Nerve supply of the human knee and its functional importance. Am. J. Sports Med. 1982, 10, 329–335. [Google Scholar] [CrossRef]
- Baratta, R.; Solomonow, M.; Zhou, B.H.; Letson, D.; Chuinard, R.; D’ambrosia, R. Muscular coactivation: The role of the antagonist musculature in maintaining knee stability. Am. J. Sports Med. 1988, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R., 3rd; Howard, M.E.; Cawley, P.W.; Losse, G.M. Effect of lower extremity muscular fatigue on motor control performance. Med. Sci. Sports Exerc. 1998, 30, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Z.-H.; Wong, D.W.-C.; Wan, A.H.-P.; Lee, W.C.-C. Effects of orthopedic insoles on static balance of older adults wearing thick socks. Prosthet. Orthot. Int. 2018, 42, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chen, S.-H.; Jiang, B.C.; Sun, T.-L. Estimating Postural Stability Using Improved Permutation Entropy via TUG Accelerometer Data for Community-Dwelling Elderly People. Entropy 2020, 22, 1097. [Google Scholar] [CrossRef]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in Healthy and Diseased Individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Chen, T.L.-W.; Wong, D.W.-C.; Wang, Y.; Tan, Q.; Lam, W.-K.; Zhang, M. Changes in segment coordination variability and the impacts of the lower limb across running mileages in half marathons: Implications for running injuries. J. Sport Health Sci. 2020. [Google Scholar] [CrossRef]
- Wong DW, C.; Lam, W.K.; Yeung, L.F.; Lee, W.C. Does long-distance walking improve or deteriorate walking stability of transtibial amputees? Clin. Biomech. 2015, 30, 867–873. [Google Scholar] [CrossRef]
- Huang, C.L.-C.; Hsu, S.-Y.; Lin, E. A comparison of classification methods for predicting Chronic Fatigue Syndrome based on genetic data. J. Transl. Med. 2009, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.M.; Salavati, M.; Ebrahimi, I.; Mousavi, M.E. Sensitivity, specificity and predictive value of the clinical trunk muscle endurance tests in low back pain. Clin. Rehabil. 2007, 21, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wong, D.W.-C.; Wang, Y.; Chen, T.L.-W.; Tan, Q.; Chen, Z.; Jin, Z.-M.; Zhang, M. Immediate Effects of Medially Posted Insoles on Lower Limb Joint Contact Forces in Adult Acquired Flatfoot: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 2226. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Wang, Y.; Chen, T.L.-W.; Wong, D.W.-C.; Yan, F.; Li, Z.; Zhang, M. Exercise-Induced Hemodynamic Changes in Muscle Tissue: Implication of Muscle Fatigue. Appl. Sci. 2020, 10, 3512. [Google Scholar] [CrossRef]
- Coviello, G.; Avitabile, G. Multiple Synchronized Inertial Measurement Unit Sensor Boards Platform for Activity Monitoring. IEEE Sens. J. 2020, 20, 8771–8777. [Google Scholar] [CrossRef]
- Rucksana, S.; Babu, C.; Saranyabharathi, S. Efficient timing-sync protocol in wireless sensor network. In Proceedings of the 2015 International Conference on Innovations in Information, Embedded and Communication Systems (ICIIECS), Trivandrum, India, 16–19 December 2015. [Google Scholar]
- Ma, C.Z.-H.; Wan, A.H.-P.; Wong, D.W.-C.; Zheng, Y.-P.; Lee, W.C.-C. A Vibrotactile and Plantar Force Measurement-Based Biofeedback System: Paving the Way towards Wearable Balance-Improving Devices. Sensors 2015, 15, 31709–31722. [Google Scholar] [CrossRef]
- Lambert, E.; Gibson, A.S.C.; Noakes, T. Complex systems model of fatigue: Integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br. J. Sports Med. 2005, 39, 52–62. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).