Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. HPLC Assay of Curcumin
2.2.2. Preliminary Screening Studies
2.2.3. Preparation of Curcumin-Loaded Proniosomes
2.2.4. Experimental Design
2.2.5. Preparation of Proniosome-Derived Niosomal Dispersions of Curcumin
2.2.6. In Vitro Characterization of Curcumin-Loaded Proniosomes
Determination of Drug Content and EE% of Curcumin-Loaded Proniosomes
In Vitro Release Study of Curcumin-Loaded Proniosomes
Statistical Optimization of Curcumin-Loaded Proniosomes
2.2.7. Comparative Study of the Optimized Curcumin-Loaded Proniosomal Formula and the Conventional Niosomes
Formulation of Curcumin-Loaded Niosomes
Evaluation of EE% and In Vitro Release of Curcumin-Loaded Niosomes
The Stability Test
2.2.8. Characterization of the Optimized Curcumin-Loaded Proniosomes
Scanning Electron Microscopy (SEM)
Vesicle size and Zeta Potential Determination
Determination of the Micromeritic Properties
2.2.9. Evaluation of the Antiviral Activity and Cytotoxicity
HS-1 Virus and the Cell Culture
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Screening Studies
3.2. Formulation of Curcumin-Loaded Proniosomes
3.3. Reconstitution of Curcumin-Loaded Proniosomes into Niosomes
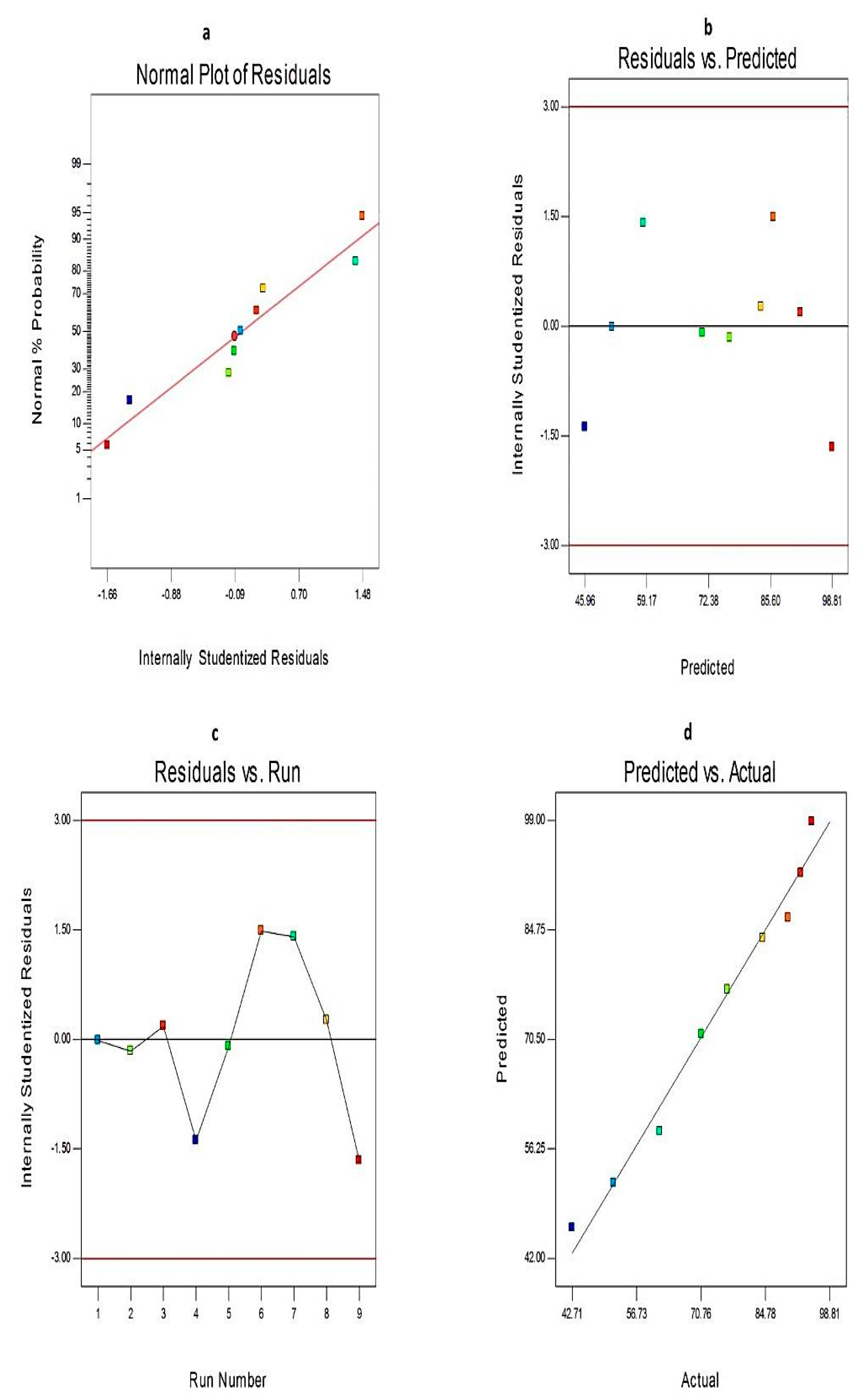
3.4. Analysis of the 32 Factorial Design
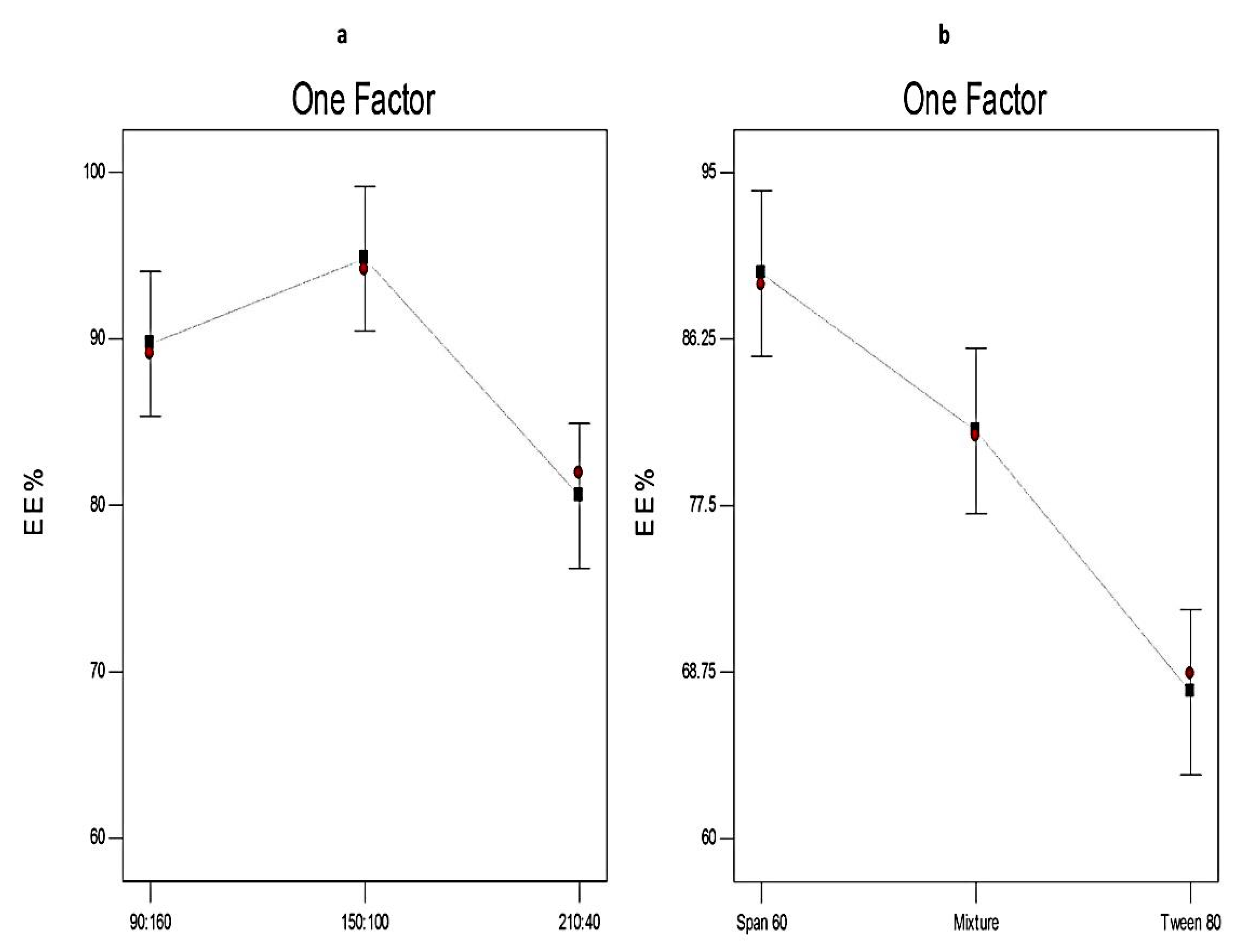
3.4.1. The Effect of Formulation Variables on EE% of Curcumin-Loaded Proniosomes
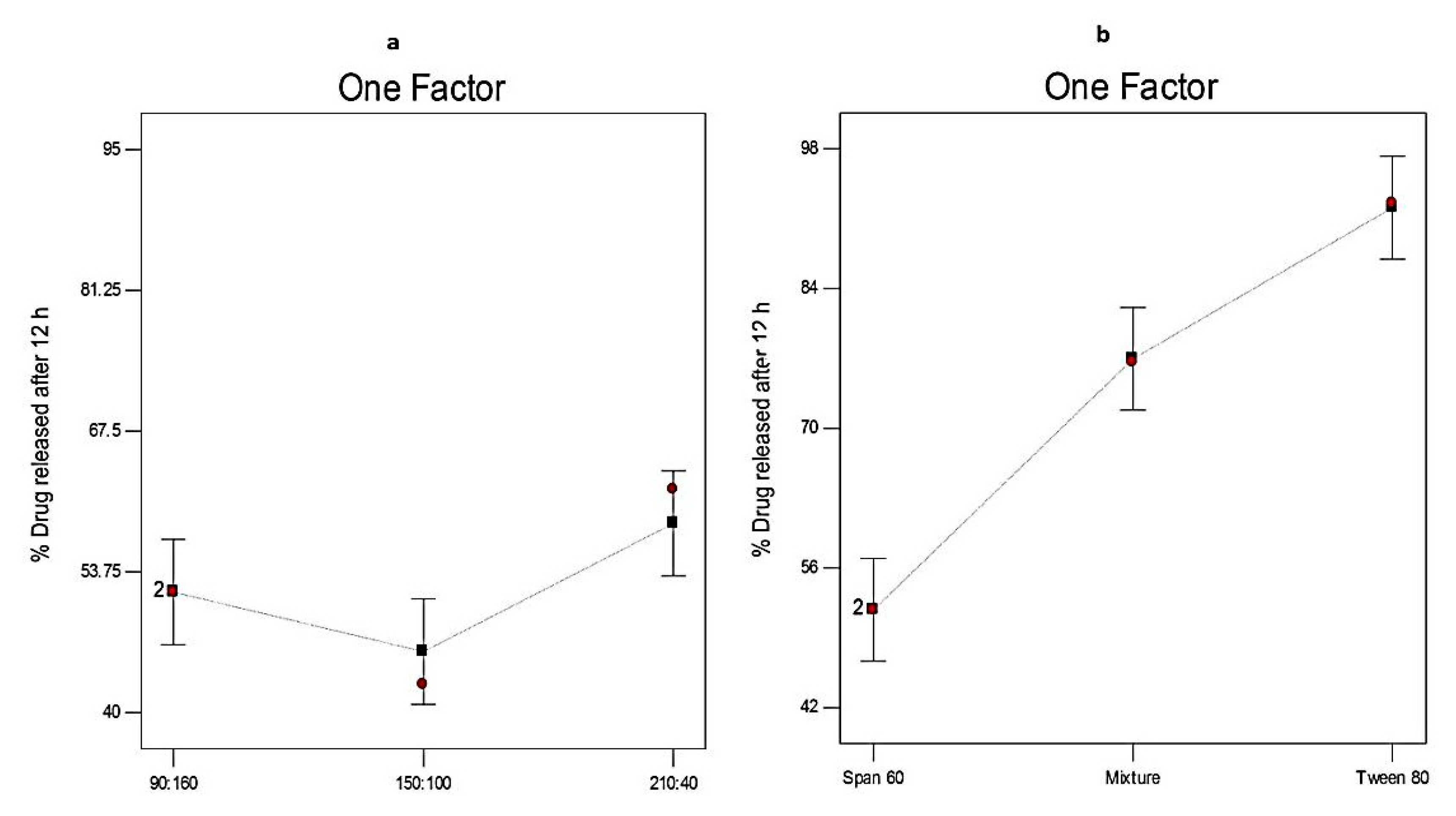
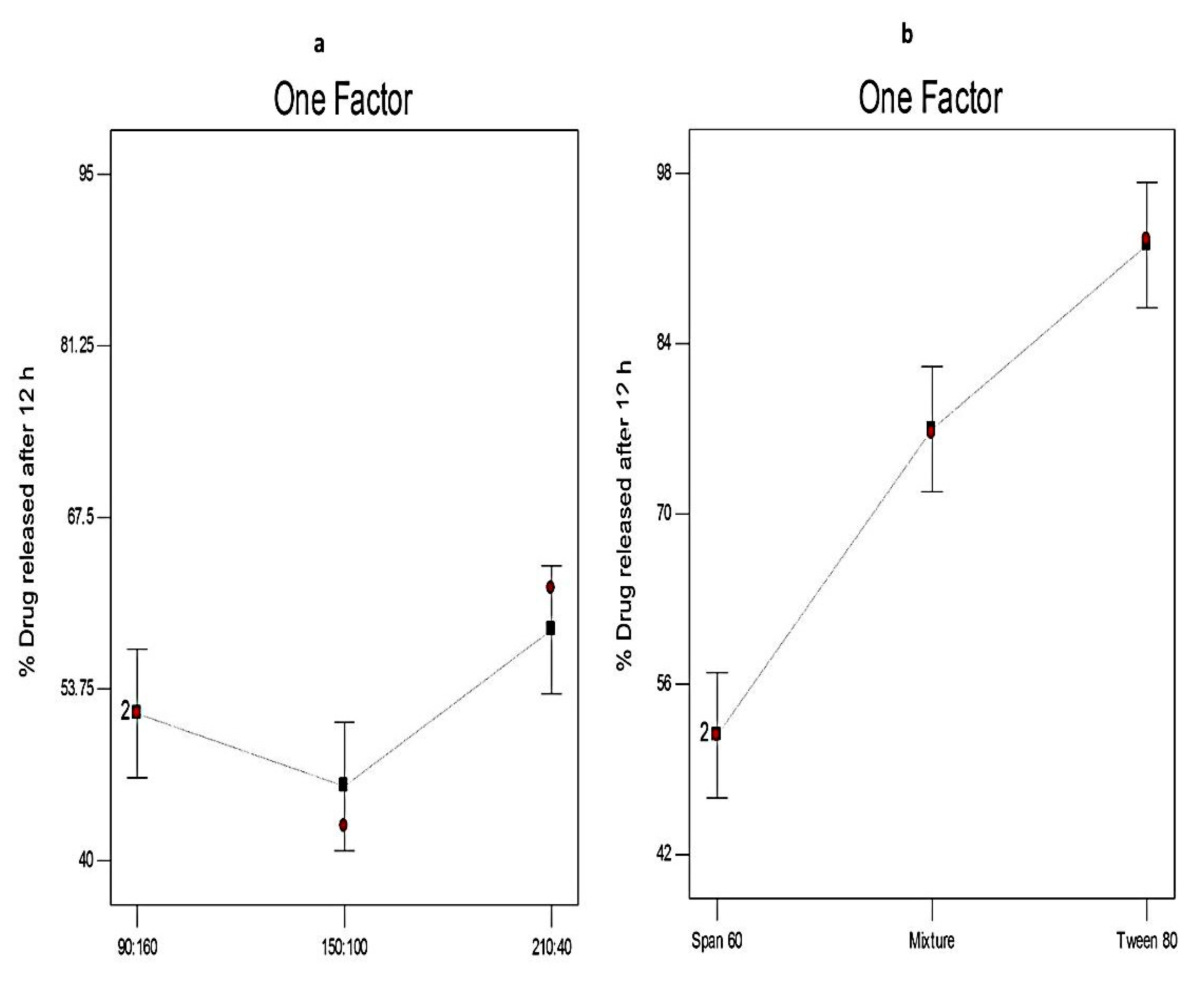
3.4.2. The Effect of Formulation Variables on Q12h of Curcumin-Loaded Proniosomes
3.4.3. The Optimization of Curcumin-Loaded Proniosomes
3.5. Comparative Study of the Optimized Curcumin-Loaded Proniosomal Formula and the Conventional Niosomes
3.5.1. Determination of EE%
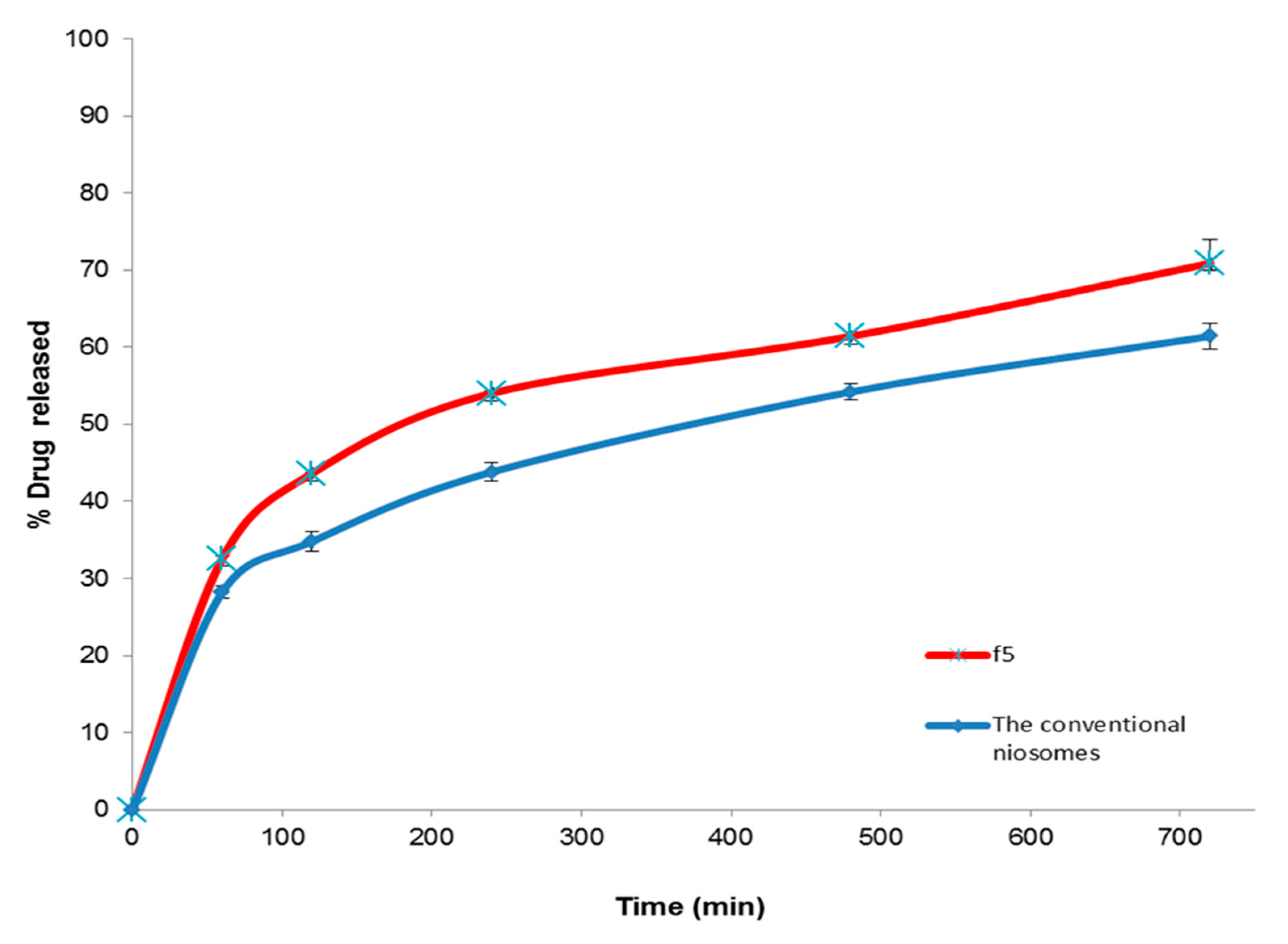
3.5.2. In Vitro Release Study
3.5.3. The Stability Study
3.6. Characterization of the Optimized Curcumin-Loaded Proniosomes
3.6.1. Morphological Characterization by SEM
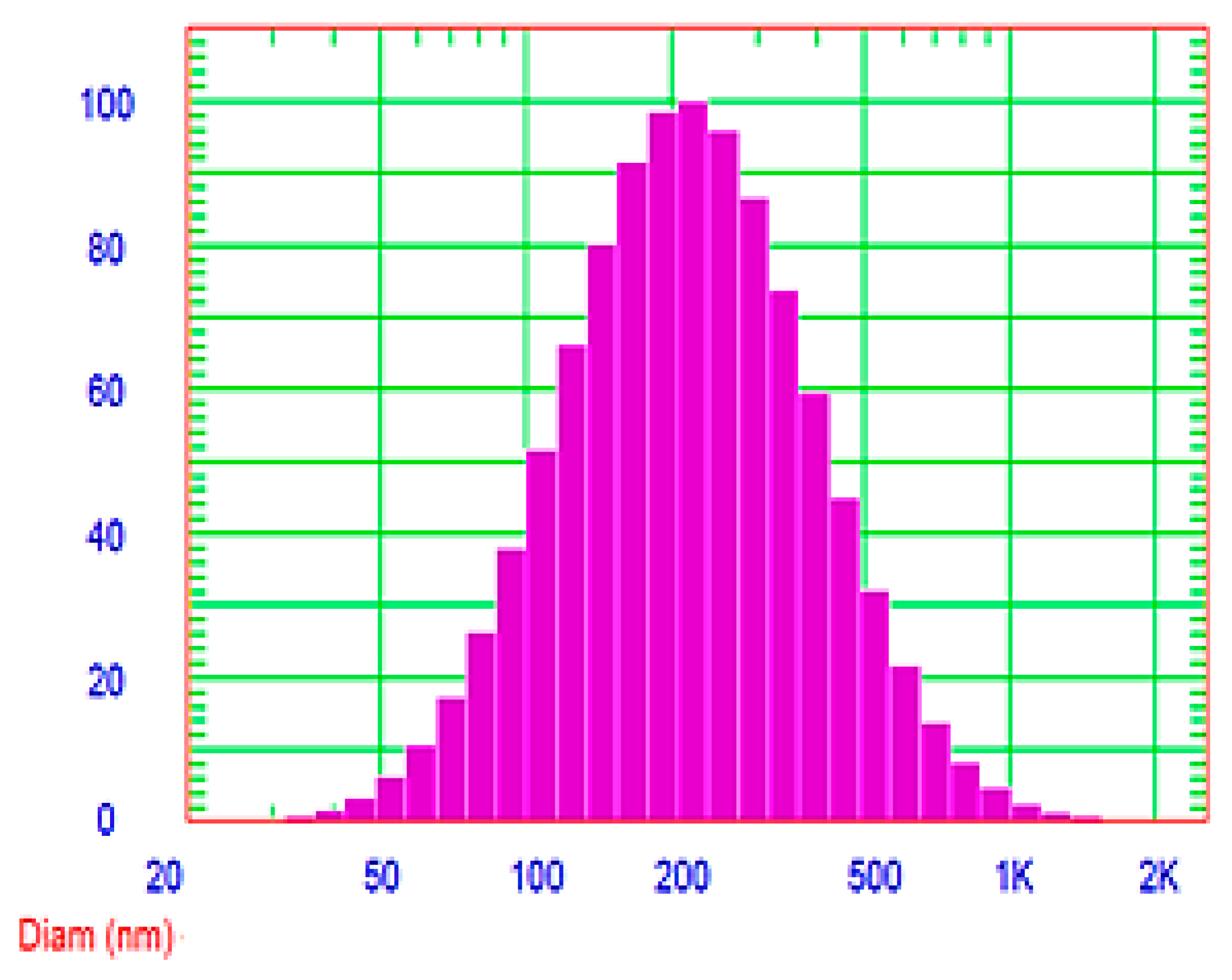
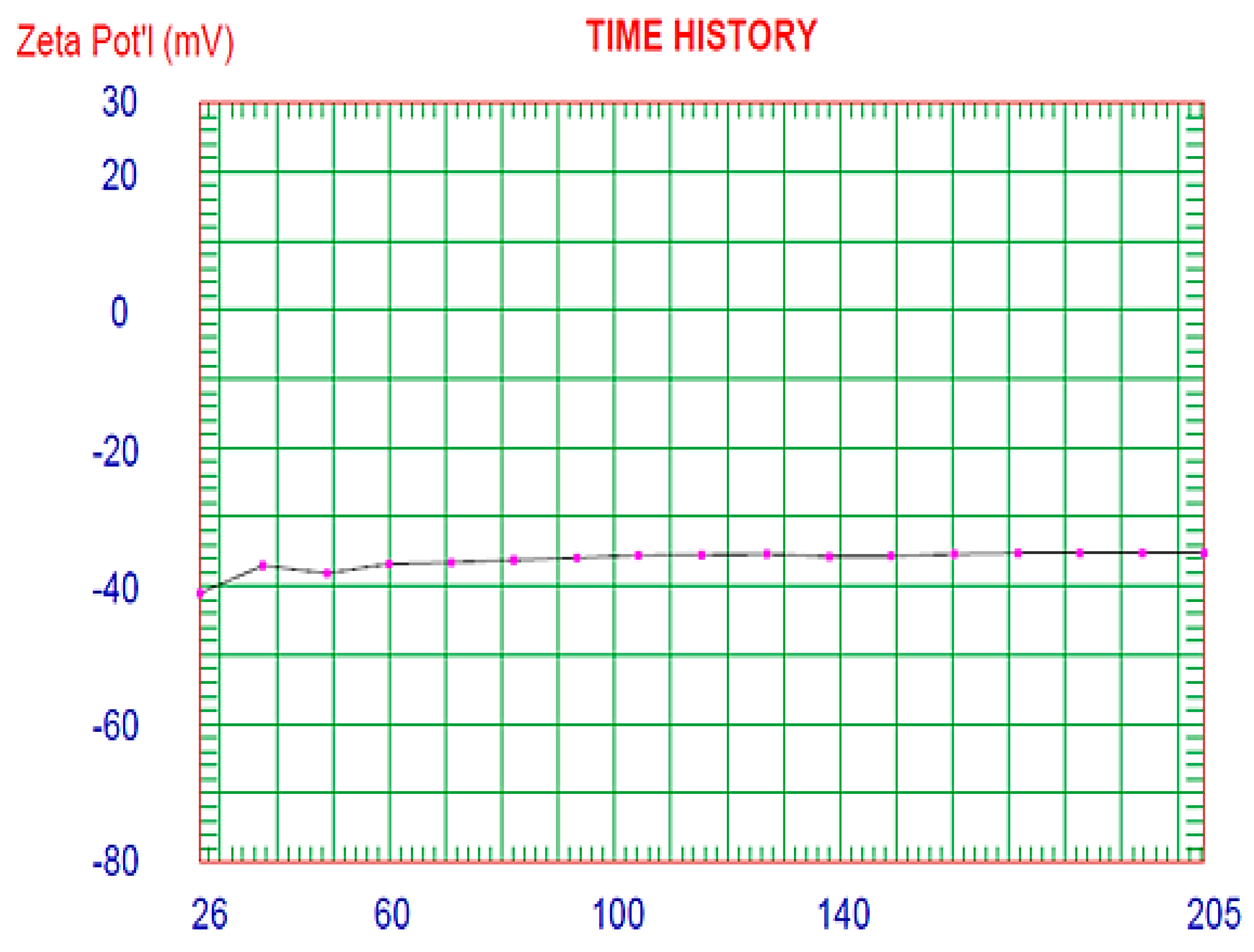
3.6.2. Determination of Vesicle Size and Zeta Potential
3.6.3. Determination of the Micromeritic Properties
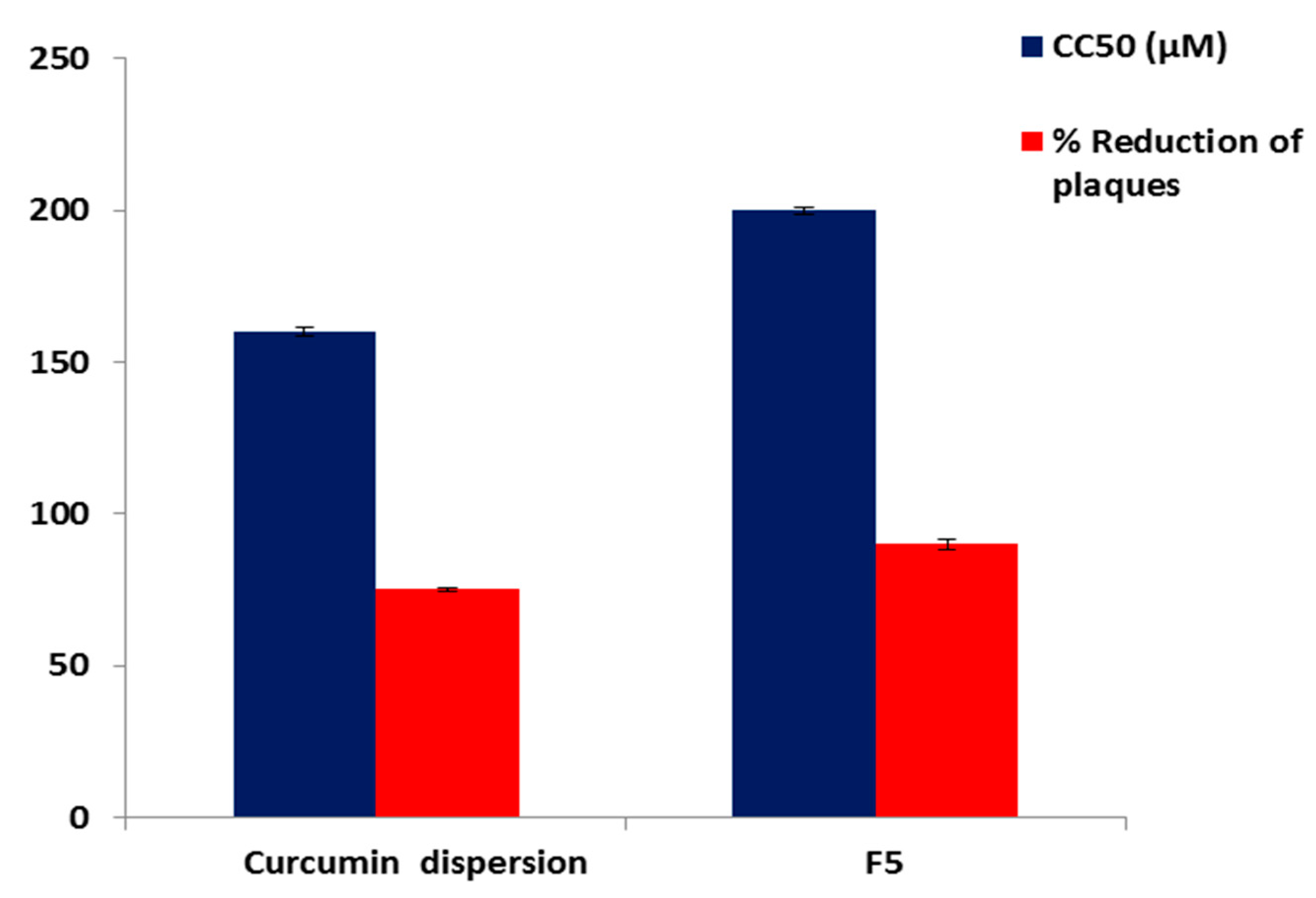
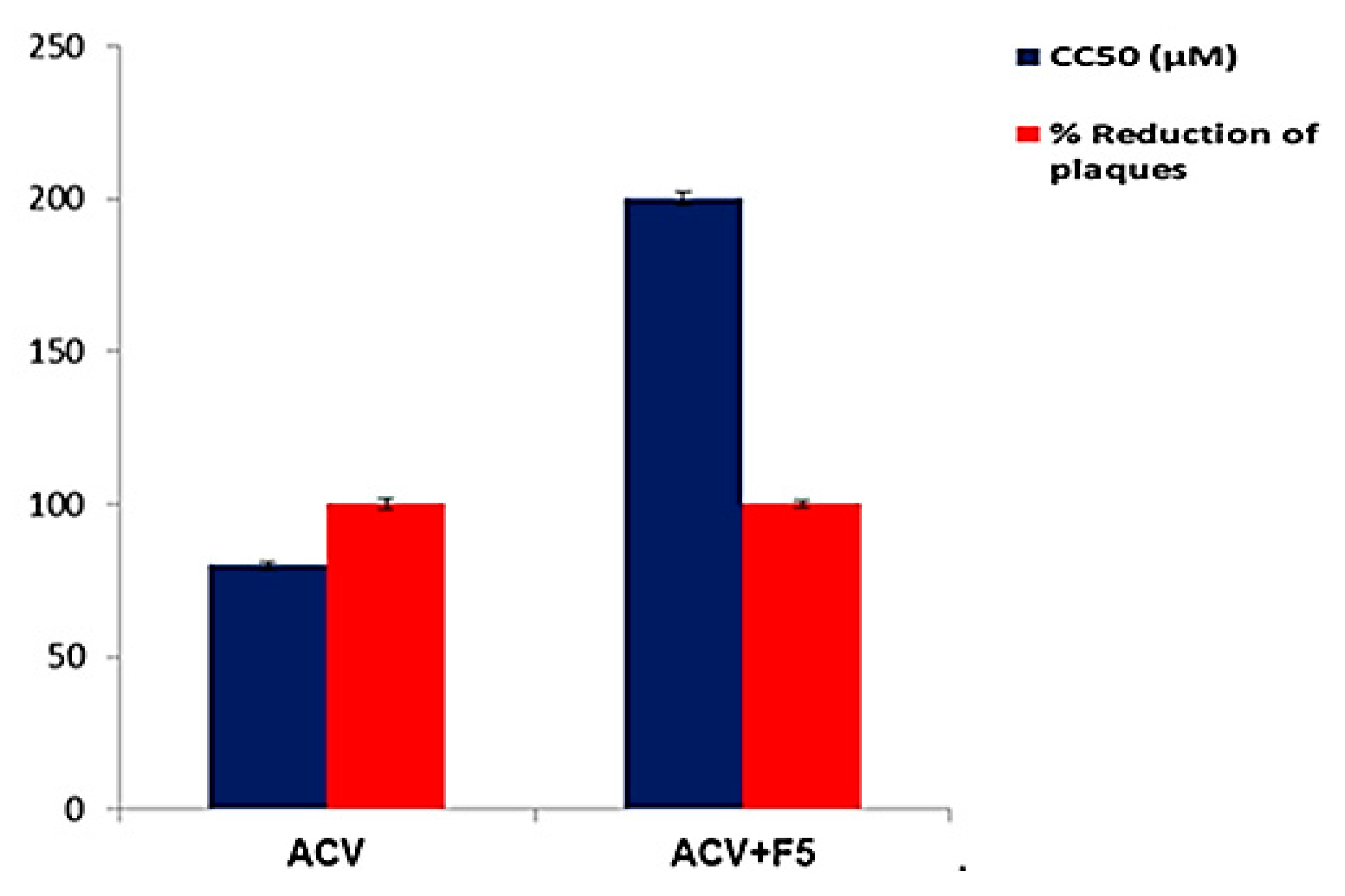
3.7. Evaluation of the Antiviral Activity and Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marchi, S.; Trombetta, C.M.; Gasparini, R.; Temperton, N.; Montomoli, E. Epidemiology of herpes simplex virus type 1 and 2 in Italy: A seroprevalence study from 2000 to 2014. J. Prev. Med. Hyg. 2017, 58, E27. [Google Scholar]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of curcumin-treated Herpes Simplex virus 1 and 2 in vero cells. Adv. Microbiol. 2016, 6, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Barton, S.; Celum, C.; Shacker, T. The role of anti-HSV therapeutics in the HIV-infected host and in controlling the HIV epidemic. Herpes J. IHMF 2005, 12, 15–22. [Google Scholar]
- Bag, P.; Chattopadhyay, D.; Mukherjee, H.; Ojha, D.; Mandal, N.; Sarkar, M.C.; Chatterjee, T.; Das, G.; Chakraborti, S. Anti-herpes virus activities of bioactive fraction and isolated pure constituent of Mallotus peltatus: An ethnomedicine from Andaman Islands. Virol. J. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, D.; Khan, M.T.H. Ethnomedicines and ethnomedicinal phytophores against herpesviruses. Biotechnol. Annu. Rev. 2008, 14, 297–348. [Google Scholar] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Bhawana, B.R.; Buttar, H.S.; Jain, V.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- El-Mahdy, M.M.; Hassan, A.S.; El-Badry, M.; El-Gindy, G.E.-D.A. Performance of Curcumin in Nanosized Carriers Niosomes and Ethosomes as Potential Anti-Inflammatory Delivery System for Topical Application. Bull. Pharm. Sci. Assiut. 2020, 43, 105–122. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Chen, W.-R.; Tsosie, J.K.; Xie, X.; Li, P.; Wan, J.-B.; He, C.-W.; Chen, M.-W. Niosome encapsulation of curcumin: Characterization and cytotoxic effect on ovarian cancer cells. J. Nanomater. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joe, B.; Vijaykumar, M.; Lokesh, B. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Hsu, W.-L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Manoharan, Y.; Haridas, V.; Vasanthakumar, K.; Muthu, S.; Thavoorullah, F.F.; Shetty, P. Curcumin: A Wonder Drug as a Preventive Measure for COVID19 Management. Indian J. Clin. Biochem. 2020, 35, 373–375. [Google Scholar] [CrossRef]
- Qin, Y.; Lin, L.; Chen, Y.; Wu, S.; Si, X.; Wu, H.; Zhai, X.; Wang, Y.; Tong, L.; Pan, B. Curcumin inhibits the replication of enterovirus 71 in vitro. Acta Pharm. Sin. B 2014, 4, 284–294. [Google Scholar] [CrossRef] [Green Version]
- El-Halim, S.M.A.; Mamdouh, M.A.; El-Haddad, A.E.; Soliman, S.M. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Sci. Pharm. 2020, 88, 9. [Google Scholar] [CrossRef] [Green Version]
- Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y. Ocular ketoconazole-loaded proniosomal gels: Formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017, 24, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Lembo, D.; Cavalli, R. Nanoparticulate delivery systems for antiviral drugs. Antivir. Chem. Chemother. 2010, 21, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Sharma Vijay, K.; Mishra, D.; Sharma, A.; Srivastava, B. Liposomes: Present prospective and future challenges. Int. J. Curr. Pharm. Rev. Res. 2010, 1, 6–16. [Google Scholar]
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal. Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Radha, G.; Rani, T.S.; Sarvani, B. A review on proniosomal drug delivery system for targeted drug action. J. Basic Clin. Pharm. 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Rai, A. Proniosomal formulation of curcumin having anti-inflammatory and anti-arthritic activity in different experimental animal models. Die Pharm. Int. J. Pharm. Sci. 2012, 67, 852–857. [Google Scholar]
- Dubey, A.; Prabhu, P. Development and evaluation of lornoxicam loaded maltodextrin based proniosomes. Development 2013, 5, 865–872. [Google Scholar]
- Khatoon, M.; Shah, K.U.; Din, F.U.; Shah, S.U.; Rehman, A.U.; Dilawar, N.; Khan, A.N. Proniosomes derived niosomes: Recent advancements in drug delivery and targeting. Drug Deliv. 2017, 24, 56–69. [Google Scholar] [CrossRef] [Green Version]
- Peele, K.A.; Chandrasai, P.; Srihansa, T.; Krupanidhi, S.; Sai, A.V.; Babu, D.J.; Indira, M.; Reddy, A.R.; Venkateswarulu, T. Molecular docking and dynamic simulations for antiviral compounds against SARS-CoV-2: A computational study. Inform. Med. Unlocked 2020, 100345. [Google Scholar] [CrossRef]
- Shivanika, C.; Kumar, D.; Ragunathan, V.; Tiwari, P.; Sumitha, A. Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J. Biomol. Struct. Dyn. 2020, 3, 1–27. [Google Scholar]
- Dar, A.M.; Mir, S. Molecular docking: Approaches, types, applications and basic challenges. J. Anal. Bioanal. Tech. 2017, 8, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Sengodan, T.; Sunil, B.; Vaishali, R.; Chandra, R.J.; Nagar, S.; Nagar, O. Formulation and evaluation of maltodextrin based proniosomes loaded with indomethacin. Int. J. Pharmtech Res. 2009, 1, 517–523. [Google Scholar]
- Boddu, M.; Choppari, V.; Rapalli, V.K.; Badam, M. Formulation and Evaluation of Proniosomes of Felodipine. Drug Des. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Topical delivery of fenoprofen calcium via elastic nano-vesicular spanlastics: Optimization using experimental design and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2898–2909. [Google Scholar] [CrossRef]
- El Gamal, S.S.; Naggar, V.F.; Allam, A.N. Optimization of acyclovir oral tablets based on gastroretention technology: Factorial design analysis and physicochemical characterization studies. Drug Dev. Ind. Pharm. 2011, 37, 855–867. [Google Scholar] [CrossRef]
- Marwa, H.A.; Omaima, A.S.; Hanaa, A.E.-G.; Hanan, M.E.-N. Optimizing proniosomes for controlled release of ketoprofen using Box-Behnken experimental design. Int. J. Pharm. Sci. Res. 2011, 2, 2195. [Google Scholar]
- Chauhan, M.K.; Sahoo, P.K.; Rawat, A.S.; Duggal, D.; Kandwal, M.; Sandal, N. Formulation, characterization and in vitro evaluation of tactically engineered proniosomes for successful oral delivery of ramipril. Der. Pharm. Lett. 2015, 7, 93–97. [Google Scholar]
- Sammour, R.M.; Taher, M.; Chatterjee, B.; Shahiwala, A.; Mahmood, S. Optimization of Aceclofenac Proniosomes by Using Different Carriers, Part 1: Development and Characterization. Pharmaceutics 2019, 11, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Menshawe, S.F.; Ali, A.A.; Halawa, A.A.; El-Din, A.S.S. A novel transdermal nanoethosomal gel of betahistine dihydrochloride for weight gain control: In-vitro and in-vivo characterization. Drug Des. Dev. Ther. 2017, 11, 3377. [Google Scholar] [CrossRef] [Green Version]
- Badria, F.; A Fayed, H.; K Ibraheem, A.; Mazyed, E.A. Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia. Pharmaceutics 2020, 12, 866. [Google Scholar] [CrossRef]
- Sahu, A.K.; Mishra, J.; Mishra, A.K. Introducing Tween-curcumin niosomes: Preparation, characterization and microenvironment study. Soft Matter 2020, 16, 1779–1791. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Zakaria, S. Enhancement of dissolution characteristics of clopidogrel bisulphate by proniosomes. Int. J. Appl. Pharm. 2019, 11, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-Vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [Green Version]
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics 2020, 12, 465. [Google Scholar] [CrossRef]
- Ammar, H.; Haider, M.; Ibrahim, M.; El Hoffy, N. In vitro and in vivo investigation for optimization of niosomal ability for sustainment and bioavailability enhancement of diltiazem after nasal administration. Drug Deliv. 2017, 24, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambhakar, S.; Paliwal, S.; Sharma, S.; Singh, B. Formulation of risperidone loaded proniosomes for effective transdermal delivery: An in-vitro and in-vivo study. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 239–247. [Google Scholar] [CrossRef]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of curves with an emphasis on in Vitro Dissolution Profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Bansal, S.; Aggarwal, G.; Chandel, P.; Harikumar, S. Design and development of cefdinir niosomes for oral delivery. J. Pharm. Bioall. Sci. 2013, 5, 318. [Google Scholar]
- Nasr, M. In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. AAPS PharmSciTech 2010, 11, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyol, S.; Gupta, M.M. Immediate drug release dosage form: A review. J. Drug Deliv. Ther. 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Badria, F.A.; Abu-Karam, M.; Mikhaeil, B.R.; Maatooq, G.T.; Amer, M. Anti-herpes activity of isolated compounds from frankincense. Biosci. Biotechnol. Res. Asia 2016, 1, 1–10. [Google Scholar]
- Henen, M.A.; El Bialy, S.A.; Goda, F.E.; Nasr, M.N.; Eisa, H.M. [1,2,4]Triazolo[4,3-a]quinoxaline: Synthesis, antiviral, and antimicrobial activities. Med. Chem. Res. 2012, 21, 2368–2378. [Google Scholar] [CrossRef]
- Abou-Karam, M.; Shier, W.T. A simplified plaque reduction assay for antiviral agents from plants. Demonstration of frequent occurrence of antiviral activity in higher plants. J. Nat. Prod. 1990, 53, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.A.; Attarde, V.B.; Parag Subhash, G. Optimization of Bifonazole-Loaded Nisomal Formulation Using Plackett-Burman Design and 23 Factorial Design. Open Pharm. Sci. J. 2016, 3, 31–48. [Google Scholar] [CrossRef] [Green Version]
- Ruckmani, K.; Sankar, V. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech 2010, 11, 1119–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food, U.; Administration, D. Inactive Ingredient Search for Approved Drug Products; FDA Database: Silver Spring, MD, USA, 2017.
- Food, U.; Administration, D. Gras Substances (Scogs) Database; FDA Database: Silver Spring, MD, USA, 2012.
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Bachhav, A.A. Proniosome: A novel non-ionic provesicules as potential drug carrier. Asian J. Pharm. 2016, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Blazek-Welsh, A.I.; Rhodes, D.G. Maltodextrin-based proniosomes. AAPS Pharmsci. 2001, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Shehata, T.M.; Abdallah, M.H.; Ibrahim, M.M. Proniosomal oral tablets for controlled delivery and enhanced pharmacokinetic properties of acemetacin. AAPS PharmSciTech 2015, 16, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Prajapati, S.K.; Yadav, R.; Senyschyn, D.; Shea, P.R.; Trevaskis, N.L. Single intravenous dose of novel Flurbiprofen-loaded Proniosome formulations provides prolonged systemic exposure and anti-inflammatory effect. Mol. Pharm. 2016, 13, 3688–3699. [Google Scholar] [CrossRef]
- Hu, C.; Rhodes, D.G. Proniosomes: A novel drug carrier preparation. Int. J. Pharm. 1999, 185, 23–35. [Google Scholar] [CrossRef]
- Yuksel, N.; Bayindir, Z.S.; Aksakal, E.; Ozcelikay, A.T. In situ niosome forming maltodextrin proniosomes of candesartan cilexetil: In vitro and in vivo evaluations. Int. J. Biol. Macromol. 2016, 82, 453–463. [Google Scholar] [CrossRef]
- Naggar, V.F.; El Gamal, S.S.; Allam, A.N. Proniosomes as a stable carrier for oral acyclovir: Formulation and physicochemical characterization. J. Am. Sci. 2012, 8, 417–428. [Google Scholar]
- Abd-Elbary, A.; El-Laithy, H.; Tadros, M. Sucrose stearate-based proniosome-derived niosomes for the nebulisable delivery of cromolyn sodium. Int. J. Pharm. 2008, 357, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Gonzalez-Mira, E.; Egea, M.; Garcia, M.; Souto, E. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- El-Laithy, H.M.; Shoukry, O.; Mahran, L.G. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: Preclinical and clinical studies. Eur. J. Pharm. Biopharm. 2011, 77, 43–55. [Google Scholar] [CrossRef]
- Khoee, S.; Yaghoobian, M. Niosomes: A novel approach in modern drug delivery systems. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Eldeeb, E.A.; Salah, S.; Ghorab, M. Proniosomal gel-derived niosomes: An approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019, 26, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafa, M.G.; Ayoub, B.M. DOE optimization of nano-based carrier of pregabalin as hydrogel: New therapeutic & chemometric approaches for controlled drug delivery systems. Sci. Rep. 2017, 7, 41503. [Google Scholar] [PubMed] [Green Version]
- Al-mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: In-Vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int. J. Pharm. 2014, 472, 304–314. [Google Scholar] [CrossRef] [PubMed]
- John, B. Application of desirability function for optimizing the performance characteristics of carbonitrided bushes. Int. J. Ind. Eng. Comput. 2013, 4, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Akhilesh, D.; Faishal, G.; Prabhu, P.; Kamath, J. Development and Optimization of Proniosomes for Oral delivery of Glipizide. Int J. Pharm. Pharm. Sci. 2012, 4, 307–314. [Google Scholar]
- Khudair, N.; Agouni, A.; Elrayess, M.A.; Najlah, M.; Younes, H.M.; Elhissi, A. Letrozole-loaded nonionic surfactant vesicles prepared via a slurry-based proniosome technology: Formulation development and characterization. J. Drug Deliv. Sci. Technol. 2020, 58, 101721. [Google Scholar] [CrossRef]
- Bhama, S. Development and evaluation of letrozole loaded proniosomes as drug delivery system. Int. J. Res. Pharm. Sci. 2015, 6, 363–368. [Google Scholar]
- Alemi, A.; Reza, J.Z.; Haghiralsadat, F.; Jaliani, H.Z.; Karamallah, M.H.; Hosseini, S.A.; Karamallah, S.H. Paclitaxel and curcumin coadministration in novel cationic PEGylated niosomal formulations exhibit enhanced synergistic antitumor efficacy. J. Nanobiotechnology 2018, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Ismail, E.; Sabry, D.; Mahdy, H.; Khalil, M. Synthesis and Characterization of some Ternary Metal Complexes of Curcumin with 1, 10-phenanthroline and their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Tian, G.; Jian, S.; Gu, Z.; Zhou, L.; Yan, L.; Jin, S.; Yin, W.; Zhao, Y. TWEEN coated NaYF 4: Yb, Er/NaYF4 core/shell upconversion nanoparticles for bioimaging and drug delivery. Rsc. Adv. 2012, 2, 7037–7041. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Hussein, A.K.; Sarhan, H.A.; Mansour, H.F. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Drug Dev. Ind. Pharm. 2017, 43, 902–910. [Google Scholar] [CrossRef]
- Sun, P.; Yang, H.-J.; Wang, Y.-Q.; Liu, K.-Z.; Xu, Y.-W. Lipase-catalyzed synthesis and characterization of stearic acid dextrin ester. Res. Health Nutr. 2013, 1, 7–11. [Google Scholar]
- Sadeghi Ghadi, Z.; Ebrahimnejad, P. Curcumin entrapped hyaluronan containing niosomes: Preparation, characterisation and in vitro/in vivo evaluation. J. Microencapsul. 2019, 36, 169–179. [Google Scholar] [CrossRef]
- Chopra, M.; Jain, R.; Dewangan, A.K.; Varkey, S.; Mazumder, S. Design of curcumin loaded polymeric nanoparticles-optimization, formulation and characterization. J. Nanosci. Nanotechnol. 2016, 16, 9432–9442. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Fang, C.-L.; Liu, C.-H.; Su, Y.-H. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; Ramly, A.E. Formulation of tretinoin-loaded topical proniosomes for treatment of acne: In-vitro characterization, skin irritation test and comparative clinical study. Drug deliv. 2015, 22, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Das, S. Study of Decomposition Behaviour of Binders and the Effect of Binder Type on Strength and Density of Alumina Samples. Ph.D. Thesis, National Institute of Technology Rourkela, Rourkela, India, May 2011. [Google Scholar]
- Patil, H.N.; Hardikar, S.R.; Bhosale, A.V. Formulation development and evaluation of proniosomal gel of carvedilol. Int. J. Pharm. Pharm. Sci. 2012, 4, 191–197. [Google Scholar]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374. [Google Scholar] [PubMed]
- Das, M.K.; Palei, N.N. Sorbitan ester niosomes for topical delivery of rofecoxib. Indian J. Exp. Biol. 2011, 49, 438–445. [Google Scholar] [PubMed]
- Caracciolo, G.; Pozzi, D.; Caminiti, R.; Marianecci, C.; Moglioni, S.; Carafa, M.; Amenitsch, H. Effect of hydration on the structure of solid-supported Niosomal membranes investigated by in situ energy dispersive X-ray diffraction. Chem. Phys. Lett. 2008, 462, 307–312. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Supaperm, T. Effect of charged and non-ionic membrane additives on physicochemical properties and stability of niosomes. AAPS PharmSciTech 2008, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Essa, E.A. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J. Pharm 2014, 4, 227–233. [Google Scholar] [CrossRef]
- Uchechi, O.; Ogbonna, J.D.; Attama, A.A. Nanoparticles for dermal and transdermal drug delivery. Appl. Nanotechnol. Drug Deliv. 2014, 4, 193–227. [Google Scholar]
- Al-Hashemi, H.M.B.; Al-Amoudi, O.S.B. A review on the angle of repose of granular materials. Powder Technol. 2018, 330, 397–417. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Soppimath, K.S.; Betageri, G.V. Controlled release application of multilamellar vesicles: A novel drug delivery approach. Drug Deliv. 2010, 17, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, K.; Piret, J.; Boivin, G. Herpesvirus DNA polymerases: Structures, functions and inhibitors. Virus Res. 2017, 234, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. In Chemical Biology; Springer: New York, NY, USA, 2015; pp. 243–250. [Google Scholar]
- Hu, L.; Zhang, Y.; Zhu, H.; Liu, J.; Li, H.; Li, X.-N.; Sun, W.; Zeng, J.; Xue, Y.; Zhang, Y. Filicinic Acid Based Meroterpenoids with Anti-Epstein–Barr Virus Activities from Hypericum japonicum. Org. Lett. 2016, 18, 2272–2275. [Google Scholar] [CrossRef] [PubMed]
- Sterling, T.; Irwin, J.J. ZINC 15–ligand discovery for everyone. J. Chem. Inf. Modeling 2015, 55, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery; ACS Publications: Washington, DC, USA, 2011. [Google Scholar]
- Fukunishi, Y.; Yamasaki, S.; Yasumatsu, I.; Takeuchi, K.; Kurosawa, T.; Nakamura, H. Quantitative Structure-activity Relationship (QSAR) Models for Docking Score Correction. Mol. Inform. 2017, 36, 1600013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgazar, A.A.; Knany, H.R.; Ali, M.S. Insights on the molecular mechanism of anti-inflammatory effect of formula from Islamic traditional medicine: An in-silico study. J. Tradit. Complementary Med. 2019, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Rollinger, J.M.; Stuppner, H.; Langer, T. Virtual screening for the discovery of bioactive natural products. In Natural Compounds as Drugs Volume I; Springer: New York, NY, USA, 2008; pp. 211–249. [Google Scholar]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Mujoriya, R.Z.; Bodla, R. Niosomes–challenge in preparation for pharmaceutical scientist. Int. J. App. Pharm. 2011, 3, 11–15. [Google Scholar]
- Monavari, S.H. The inhibitory effect of Acyclovir loaded nano-niosomes against herpes simplex virus type-1 in cell culture. Med. J. Islamic Repub. Iran 2014, 28, 99. [Google Scholar]
- Shah, J.; Nair, A.B.; Shah, H.; Jacob, S.; Shehata, T.M.; Morsy, M.A. Enhancement in antinociceptive and anti-inflammatory effects of tramadol by transdermal proniosome gel. AJPS 2019. [Google Scholar] [CrossRef]
- Aboelwafa, A.A.; El-Setouhy, D.A.; Elmeshad, A.N. Comparative study on the effects of some polyoxyethylene alkyl ether and sorbitan fatty acid ester surfactants on the performance of transdermal carvedilol proniosomal gel using experimental design. AAPS PharmSciTech 2010, 11, 1591–1602. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.Y.; Murakami, E.; Zorca, S.M.; Johnson, A.A.; Johnson, K.A.; Schinazi, R.F.; Furman, P.A.; Anderson, K.S. Relationship between antiviral activity and host toxicity: Comparison of the incorporation efficiencies of 2′, 3′-dideoxy-5-fluoro-3′-thiacytidine-triphosphate analogs by human immunodeficiency virus type 1 reverse transcriptase and human mitochondrial DNA polymerase. Antimicrob. Agents Chemother. 2004, 48, 1300–1306. [Google Scholar]
- Brezáni, V.; Leláková, V.; Hassan, S.T.; Berchová-Bímová, K.; Nový, P.; Klouček, P.; Maršík, P.; Dall’Acqua, S.; Hošek, J.; Šmejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Mata, É.C.G.; Mourão, C.B.F.; Rangel, M.; Schwartz, E.F. Antiviral activity of animal venom peptides and related compounds. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.; Bankwitz, D.; Steinmann, J.; Ott, M. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014, 63, 1137–1149. [Google Scholar]



| Formula | Time of Hydration (min) | Volume of Hydration (mL) | * EE% |
|---|---|---|---|
| P1 | 5 | 10 | 94.11 ± 1.27 |
| P2 | 5 | 20 | 82.13 ± 2.11 |
| P3 | 30 | 10 | 94.23 ± 1.27 |
| P4 | 30 | 20 | 82.30 ± 1.32 |
| Formula Code | Variables | ||||
|---|---|---|---|---|---|
| Independent | Dependent | ||||
| X1 | X2 | Y1 * | Y2 * | ||
| F1 | −1 | −1 | 89.06 ± 2.12 | 51.74 ± 1.23 | |
| F2 | −1 | 0 | 81.11 ± 1.36 | 76.56 ± 1.41 | |
| F3 | −1 | 1 | 68.61 ± 1.62 | 92.52 ± 2.69 | |
| F4 | 0 | −1 | 94.11 ± 1.27 | 42.71 ± 1.74 | |
| F5 # | 0 | 0 | 89.94 ± 2.31 | 70.89 ± 1.62 | |
| F6 | 0 | 1 | 70.11 ± 1.49 | 89.76 ± 1.44 | |
| F7 | 1 | −1 | 81.89 ± 1.25 | 61.78 ± 1.38 | |
| F8 | 1 | 0 | 69.17 ± 1.41 | 84.26 ± 1.34 | |
| F9 | 1 | 1 | 60.33 ± 1.61 | 94.91 ± 2.18 | |
| Independent variables | Low (−1) | Medium (0) | High (+1) | ||
| X1: Ratio of surfactant to CHOL | 90:160 | 150:100 | 210:40 | ||
| X2: Type of surfactant | Span 60 | Span 60 & Tween 80 | Tween 80 | ||
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision |
|---|---|---|---|---|
| EE% (Y1) | 0.9676 | 0.9352 | 0.8359 | 16.372 |
| Q12h (Y2) | 0.9822 | 0.9643 | 0.9097 | 20.165 |
| Depndent Variable | Source | SS | DF | MS | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Y1 | Model | 1053.93 | 4 | 263.48 | 29.84 | 0.0031 |
| X1 | 312.89 | 2 | 156.45 | 17.72 | 0.0103 | |
| X2 | 471.03 | 2 | 370.52 | 41.97 | 0.0021 | |
| Y2 | Model | 2724.45 | 4 | 681.11 | 55.09 | 0.0009 |
| X1 | 235.90 | 2 | 117.95 | 9.54 | 0.0300 | |
| X2 | 2488.55 | 2 | 1244.28 | 100.64 | 0.0004 |
| Formula | Zero Order | First Order | Higuchi Model | Hixson Crowell | Korsmeyer-Pappas |
|---|---|---|---|---|---|
| F1 | 0.9890 | −0.9958 | 0.9991 | 0.9940 | 0.9988 |
| F2 | 0.9887 | −0.9948 | 0.9978 | 0.9958 | 0.9924 |
| F3 | 0.9902 | −0.9839 | 0.9997 | 0.9964 | 0.9889 |
| F4 | 0.9852 | −0.9913 | 0.9957 | 0.9896 | 0.9952 |
| F5 | 0.9854 | −0.9952 | 0.9966 | 0.9939 | 0.9949 |
| F6 | 0.9927 | −0.9846 | 0.9993 | 0.9955 | 0.9860 |
| F7 | 0.9842 | −0.9948 | 0.9966 | 0.9925 | 0.9960 |
| F8 | 0.9928 | −0.9959 | 0.9998 | 0.9992 | 0.9948 |
| F9 | 0.9931 | −0.9663 | 0.9990 | 0.9890 | 0.9801 |
| Curcumin | 0.9903 | −0.9897 | 0.9989 | 0.9982 | 0.9927 |
| Parameter | Proniosomal Formula | Niosomal Formula | ||
|---|---|---|---|---|
| Fresh | Stored | Fresh | Stored | |
| Drug Content (%) | 97.44 ± 1.69 | 96.95 ± 1.32 | 95.94 ± 1.27 | 90.22 ± 1.44 |
| EE (%) | 89.94 ± 2.31 | 87.86 ± 1.82 | 90.78 ± 1.52 | 83.02 ± 2.11 |
| Q12h (%) | 70.89 ± 1.62 | 68.94 ± 0.90 | 61.42 ± 1.71 | 53.12 ± 0.97 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badria, F.A.; Abdelaziz, A.E.; Hassan, A.H.; Elgazar, A.A.; Mazyed, E.A. Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness. Molecules 2020, 25, 5668. https://doi.org/10.3390/molecules25235668
Badria FA, Abdelaziz AE, Hassan AH, Elgazar AA, Mazyed EA. Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness. Molecules. 2020; 25(23):5668. https://doi.org/10.3390/molecules25235668
Chicago/Turabian StyleBadria, Farid A., Abdelaziz E. Abdelaziz, Amira H. Hassan, Abdullah A. Elgazar, and Eman A. Mazyed. 2020. "Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness" Molecules 25, no. 23: 5668. https://doi.org/10.3390/molecules25235668
APA StyleBadria, F. A., Abdelaziz, A. E., Hassan, A. H., Elgazar, A. A., & Mazyed, E. A. (2020). Development of Provesicular Nanodelivery System of Curcumin as a Safe and Effective Antiviral Agent: Statistical Optimization, In Vitro Characterization, and Antiviral Effectiveness. Molecules, 25(23), 5668. https://doi.org/10.3390/molecules25235668






