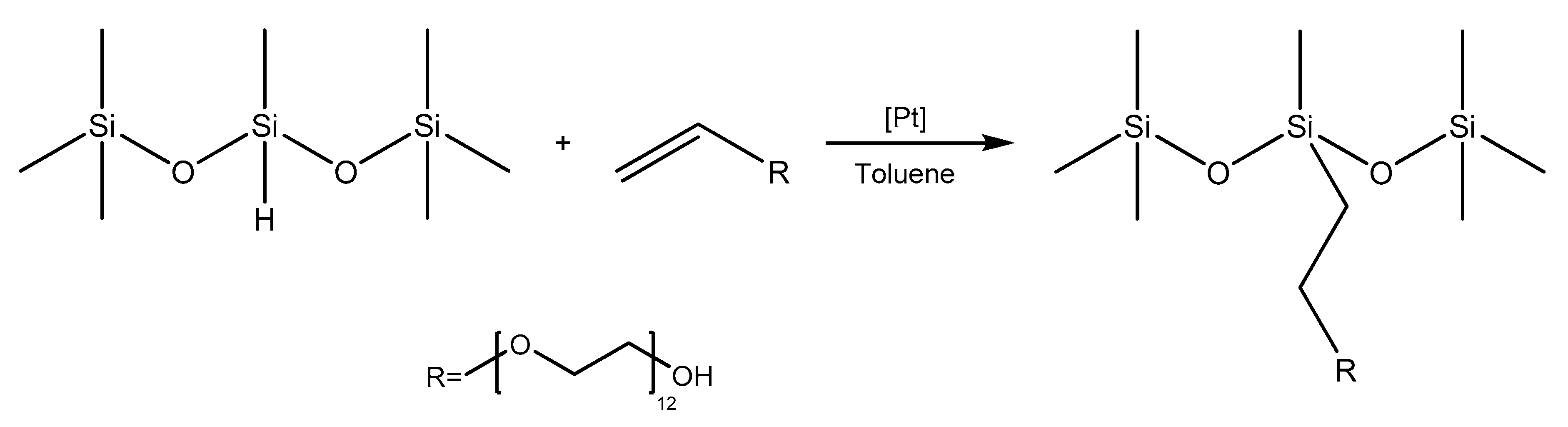
2.1. Synthesis Results
The siloxane polyether synthesis was performed using an allyl polyether containing 12 ethoxy groups with a terminal hydroxy group. Thus, two groups of contrasting surface properties, i.e., polyether groups showing hydrophilic properties and methyl groups in the siloxane showing hydrophobic properties, were linked in one compound. The reaction substrates, 1,1,1,3,5,5,5-heptamethyltrisiloxane and the olefin used are not sensitive to moisture, so the hydrosilylation reaction could be performed in an open system, which significantly facilitated the synthesis. All reactions were carried out in toluene as a solvent. The catalyst was the commercially available Karstedt one. The hydrosilylation reaction is shown in
Scheme 1.
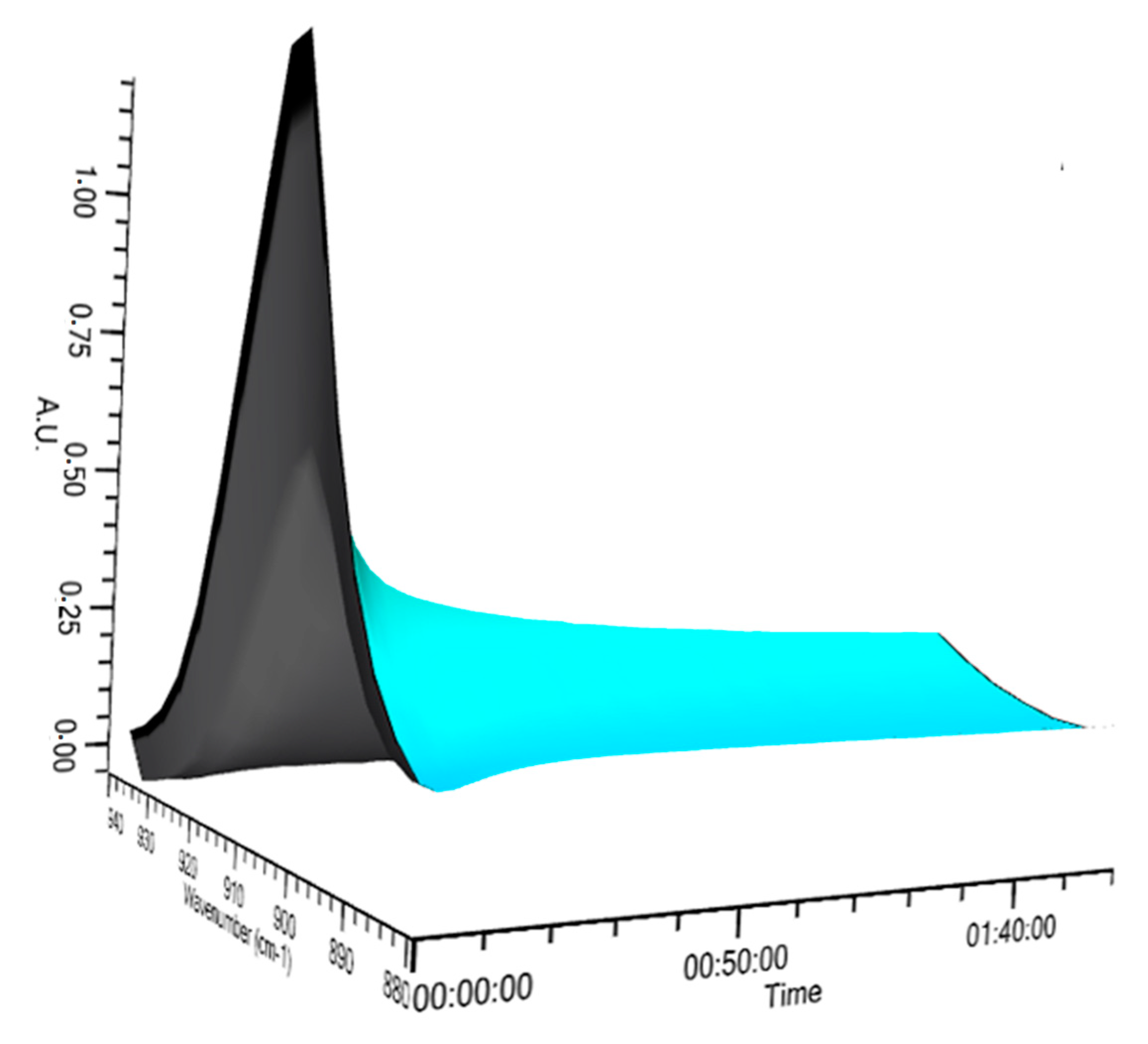
Hydrosilylation of allyl polyether with the terminal hydroxyl group with 1,1,1,3,5,5,5-heptamethyltrisiloxane was monitored using IR spectroscopy in real time. The reaction progress was quantified by checking the rate of changes in the area under the band with a maximum at a wavenumber (
) equal to 904 cm
−1 assigned to the stretching vibrations of the Si–H bond, illustrated in
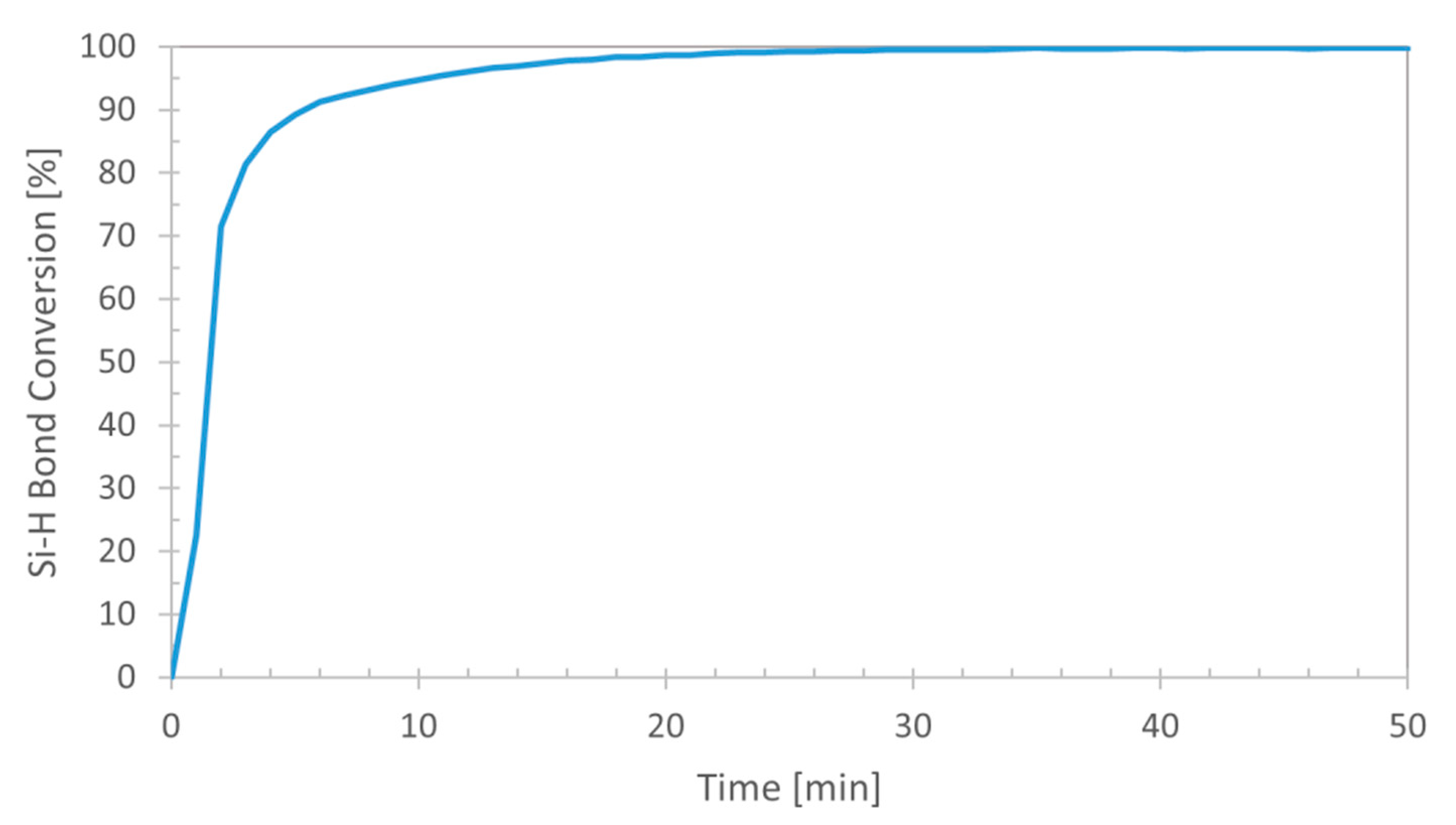
Figure 1. In order to analyze the kinetics of the process in more detail, the trend of changes in the area of the observed band at 904 cm
−1 in time is shown in
Figure 2, while
Figure 3 presents the trend of temperature changes in time. The parameters of the process were chosen so that the catalyst in a proper stoichiometric amount would be dosed after temperature stabilization. Analysis of the trends presented in
Figure 2 and
Figure 3, shows that the loss of Si–H bond conversion takes place directly after addition of a catalyst portion. At that time a jump in temperature, characteristic of exothermal hydrosilylation reaction takes place (
Figure 3), leading to almost immediate conversion of the Si–H conversion reaching over 90% (
Figure 2). Further increase in the Si–H conversion is induced by the heating of the reaction mixture as indicated by the correlated in time temperature change. Total conversion of Si–H bonds in the system studied was reached after about 55 min of the reaction measured from the moment of the catalyst addition. To sum up, the possibility of carrying the reaction out in an open system, high conversion reached in a relatively short time and high conversion of the process, reaching 97% indicate that the system is optimal and highly effective.
The isolated product was subjected to spectroscopic analysis (
1H-NMR,
13C-NMR,
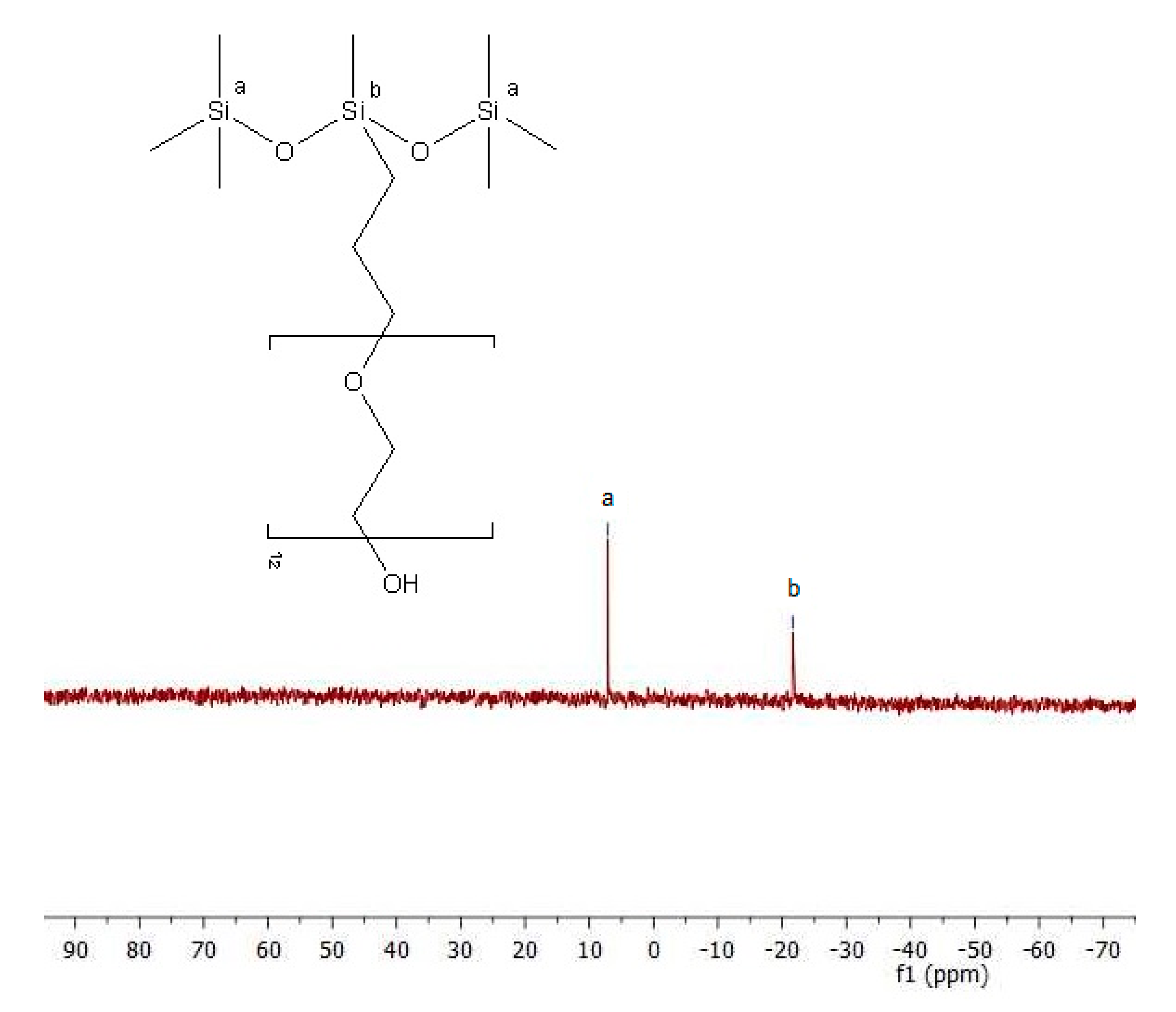
29Si-NMR) whose results are presented in
Figure 4,
Figure 5 and
Figure 6. In the
1H-NMR spectrum of the product, 3-[3-(hydroxy)(polyethoxy)propyl]-1,1,1,3,5,5,5-heptamethyltrisiloxane (
Figure 4), the characteristic signal at 5.15 ppm originating from the proton from Si–H bond disappeared. Also important is the lack of the characteristic bands assigned to the allyl groups, appearing at 5–6 ppm.
The presence of the other bands confirms the complex structure of the product. In the
29Si-NMR spectrum (
Figure 6) the presence of a signal near 7 ppm testifies to the presence of Si(Me)
3O groups. The signal close to 21 ppm confirms that silicon occurs in different environment Si(CH
3)CH
2), which is characteristic for the compounds of this type. Formation of the product (
Figure 7) of the desired structure is also confirmed by analysis of its FT-IR spectrum (red line) juxtaposed to the spectra of the substrates used (yellow and blue lines). The spectrum of the product (red line) shows a band at 3500 cm
−1 characteristic for the stretching vibrations of hydroxyl groups (−OH).
The presence of this band in the spectrum, along with the disappearance of the bands at 2100 and 903 cm−1 assigned to the stretching vibrations of Si–H group present in the spectra of the substrate, testify to the formation of the product of hydrosilylation and not condensation of Si–H and −OH groups. The product structure is also confirmed by the presence of the overlapping symmetric and antisymmetric stretching vibrations, characteristic of C–H bonds of methyl and methylene groups in the range 2700–3000 cm−1 and the overlapping bands originating from the stretching vibrations of C–O–C bonds present in the polyether chains and asymmetric stretching vibrations of Si–O–Si bond in the range 1000–1200 cm−1.
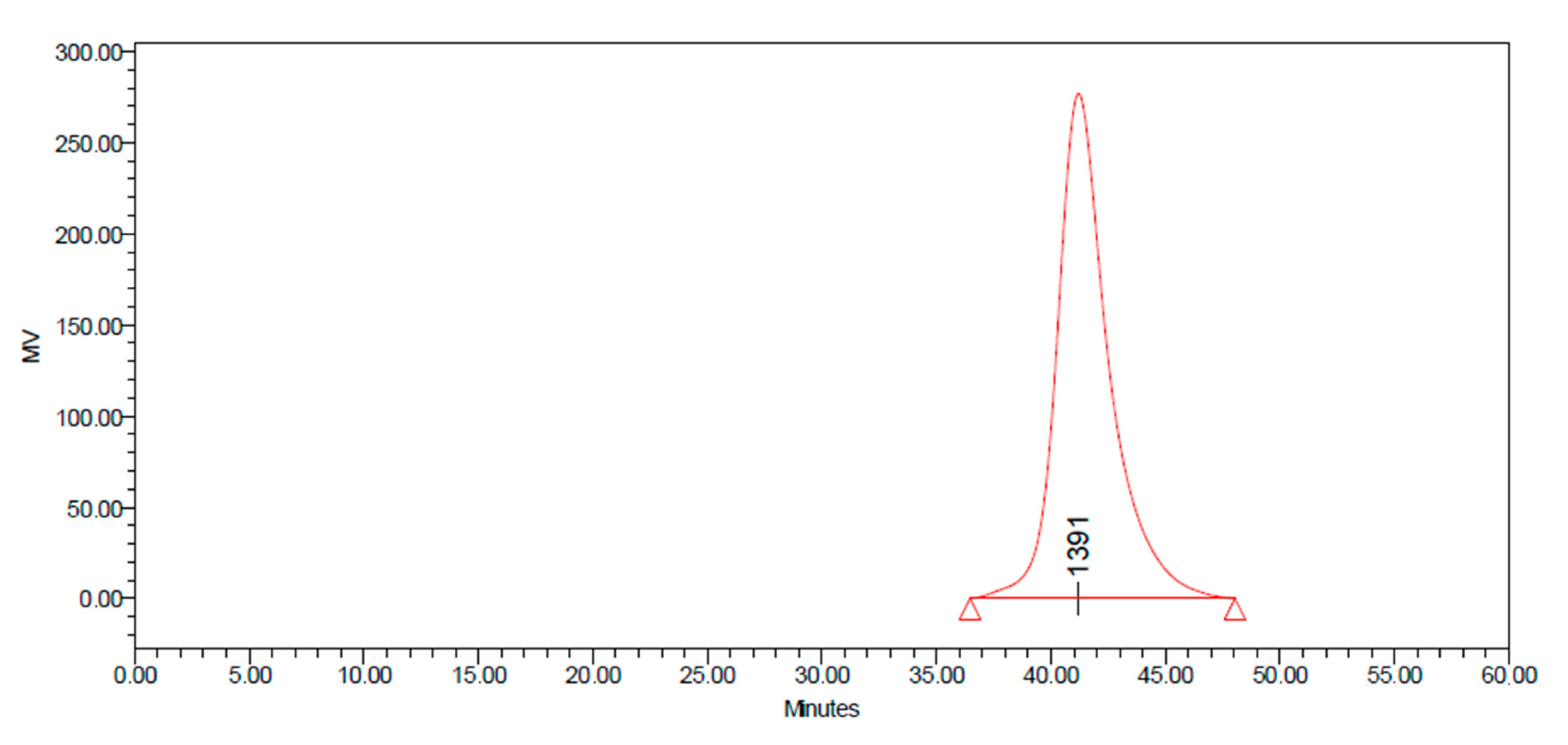
The molecular weight distributions of product was determined by GPC. The weight average molecular weight (MV) of functionalized siloxane was 1307 g/mol, with polymer dispersity index (PDI) of 1.353, indicating that the molecular weight distribution of product was relatively uniform (
Figure 8).
As shown in
Figure 9, the thermogravimetry of the product was also investigated. In
Figure 9 the TGA and DTG curves are compared. The polymer chains presented several decomposition stages. After an initial loss of solvents and absorbed water observed in sample, a decomposition step with complex shape was registered around 523.15 K and was assigned to cleavage of aliphatic fragments and ether bonds in the functional group and agree well with the molecular structure. Obviously, the siloxane backbone decomposes in higher temperature. The maximum weight loss appeared above 685.15 K.
There is only 1.37% of residue, which could be explained by the fact that siloxane backbone was decomposed into dimethylcyclosiloxane (DMC) at around 773.15 K, even under a nitrogen atmosphere, and pulled out along with the carrier gas. The decomposition mechanism seems to be affected by the chain structure and distribution. The decomposition temperature of a polymer is determined by the thermal stability of the molecules.
2.2. Equilibrium Surface Tension
From the practical point of view not only the knowledge of thermal stability of studied surfactant but also the temperature effect on the surface and aggregation properties are very desirable. They can be very helpful for understanding and explaining the large activity of TS-EO12 at the water-air interface under different conditions as well as low sensitivity of these surfactant properties with temperature.
In the case of siloxane surfactants the properties of the adsorbed monolayer at the water-air interface, which influences the changes in surface tension of their aqueous solutions, might depend on the structure and polymerization degree of the methylated siloxane hydrophobic moiety [
27] as well as on the type and length of a polyether polar chain of surfactant.
Determination of the properties of the adsorbed monolayer of 3-[3-(hydroxy)(polyethoxy) propyl]-1,1,1,3,5,5,5-heptamethyltrisiloxane) (TS-EO12) (
Scheme 1) as well as the thermodynamics of its adsorption at the water-air interface, requires the knowledge of the surface tension of aqueous solutions of the studied surfactant at different temperatures. The measured values of the equilibrium surface tension (
) of the studied surfactant aqueous solutions at 293 K, 303 K and 313 K are presented in
Figure 10.
As follows from this figure, at all studied temperatures, the surface tension of the studied trisiloxane surfactant aqueous solutions decreases with increasing surfactant concentration (
CS). It is similar as in the case of classical hydrocarbon surfactants as well as some others. In addition the surface tension of aqueous solutions of TS-EO12 depends quite strongly on temperature and increases with decreasing temperature. These results are in a good agreement with the data presented earlier for other siloxane surfactants [
24,
31] as well as many classical hydrocarbon surfactants and biosurfactants [
32,
33,
34]. However, they are in contrast to those of some different siloxane surfactants [
35] whose surface tension increases with increasing temperature.
For TS-EO12 aqueous solutions the minimal surface tension decreases with increasing temperature and changes from 23.7 mN/m at 293 K to 22.5 mN/m in 313 K. It is worth noting that the changes in the surface tension of TS-OE12 aqueous solutions with temperature are similar to those of pure POE (wt. 3400 gmol
−1) aqueous solutions [
35]. This fact suggests that the orientation of TS-EO12 molecules at the water-air interface can change with temperature. Probably the OE chain in the surfactant molecule is bent or crooked at the water-air interface. As mentioned above the temperature influences the orientation of the TS-EO12 molecules at the water-air interface, which is also connected with the area occupied by a surfactant molecule at the interface or the surfactant surface excess concentration.
2.3. Surface Excess Concentration of TS-EO12 at the Water-Air Interface at Different Temperatures
In order to calculate the amount of the adsorbed studied surfactants per unit area at the air-water interface (the Gibbs surface excess concentration of surfactant at the water-air interface (Γ
S), the measured equilibrium surface tension (
) values (
Figure 10) were treated in terms of the Gibbs adsorption equation [
36]. This equation for dilute solutions (10
−2 M/dm
3 or less) of surfactants has the following form [
36]:
where
R is the gas constant,
T is the absolute temperature,
is the surfactant solution surface tension,
CS is the concentration of surfactant and
n is the constant which depends on the kind of surfactant (for studied nonionic trisiloxane surfactant it is equal to 1).
Γ
S of the studied surfactants corresponding to the unsaturated adsorption monolayer at the water-air interface was determined from Equation (1) and using the second order polynomial equations describing the relationship between
and
CS of a given surfactant at a given temperature. The maximal Γ
S values (
) corresponding to the saturated adsorption monolayer of TS-OE12 at the water-air interface (
Table 1) at a given temperature were determined from the linear dependence of
vs. log
CS and it is the parameter describing the effectiveness of a given surfactant adsorption at the water-air interface [
36]:
where
NA is Avogadro’s number.
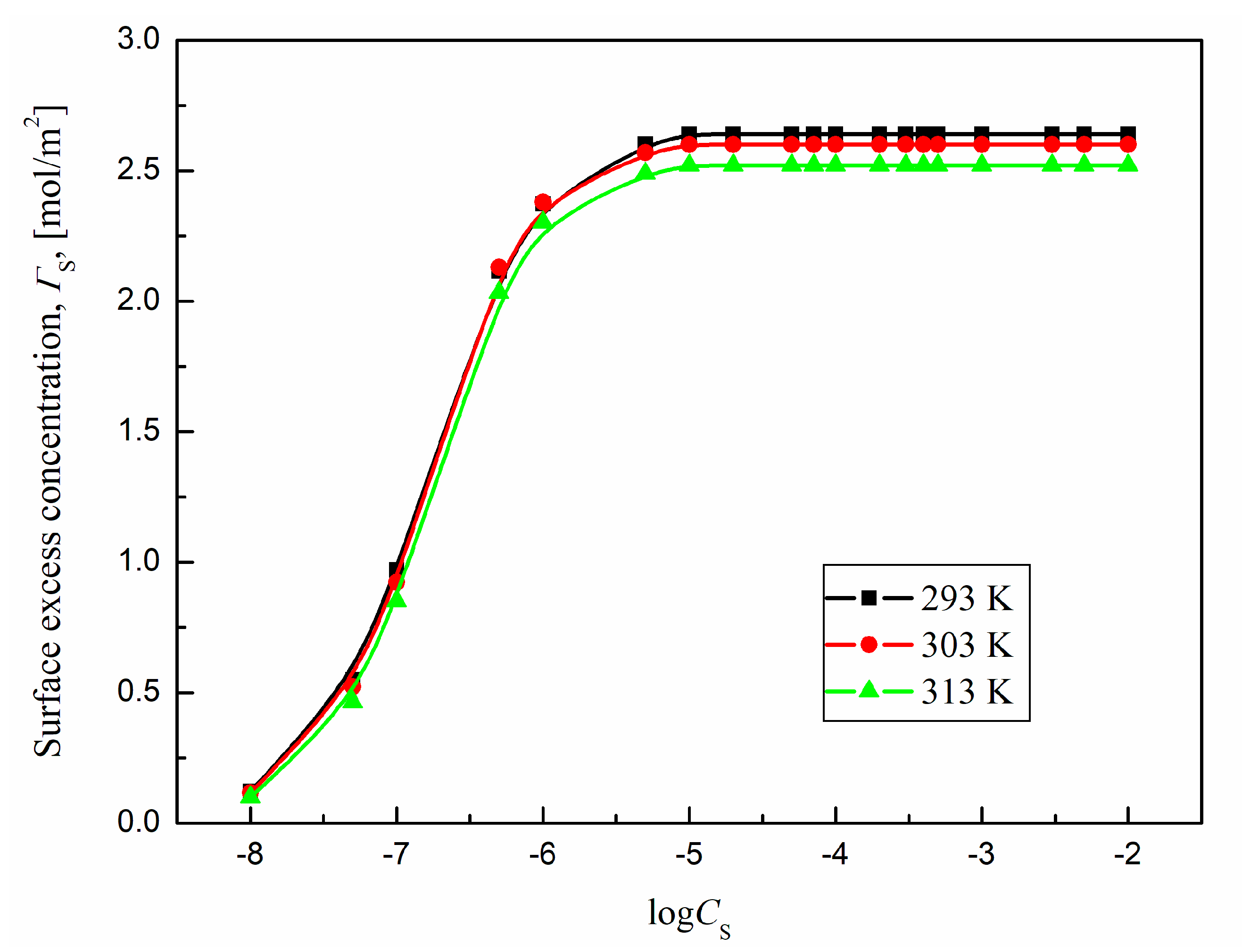
The changes in Γ
S values of TS-OE12 at 293 K, 303 K and 313 K with the surfactant concentration in the bulk phase are presented in
Figure 11. The
and
values calculated from Equations (1) and (2) for TS-OE12 at different temperatures are presented in
Table 1 and they are close to those reported in literature [
37]. It is difficult to compare the obtained
values at different temperatures with those reported in literature because of the lack of such data.
On the basis of our results it can be concluded that the minimal area per TS-OE12 molecule at the water-air interface rises with increasing temperature and according to Equation (2) it leads to a decrease in the maximal surface excess concentration of the studied surfactant at the interface with increasing temperature. However the changes in
and
with growing temperature are not significant (
Table 1).
In addition, using the values of Γ
S obtained from Equation (1) in a wide concentration range of TS-OE12 at different temperatures, the mole fraction of the area occupied by the molecules of the studied surfactant in the adsorption layer (
XS) at a given temperature was determined from the relation [
36]:
where Γ
W and Γ
S are the surface excess concentrations of water and surfactant at the water-air interface,
and
are the limiting areas of water or surfactant molecule at the water-air interface, respectively and:
For calculations of XS a proper value at a given temperature was used. AW values at different temperatures were calculated taking into account the increase of the distance between the water molecules in the surface region.
The value of
(
Table 1) can be determined from the Joos equation of state which for the aqueous solutions of surfactants can be written in the form [
38]:
where
and
are the limiting Gibbs surface excess concentrations of water and surfactant at the water-air interface, respectively,
π is the film pressure and
is the activity of a given surfactant at the water-air interface.
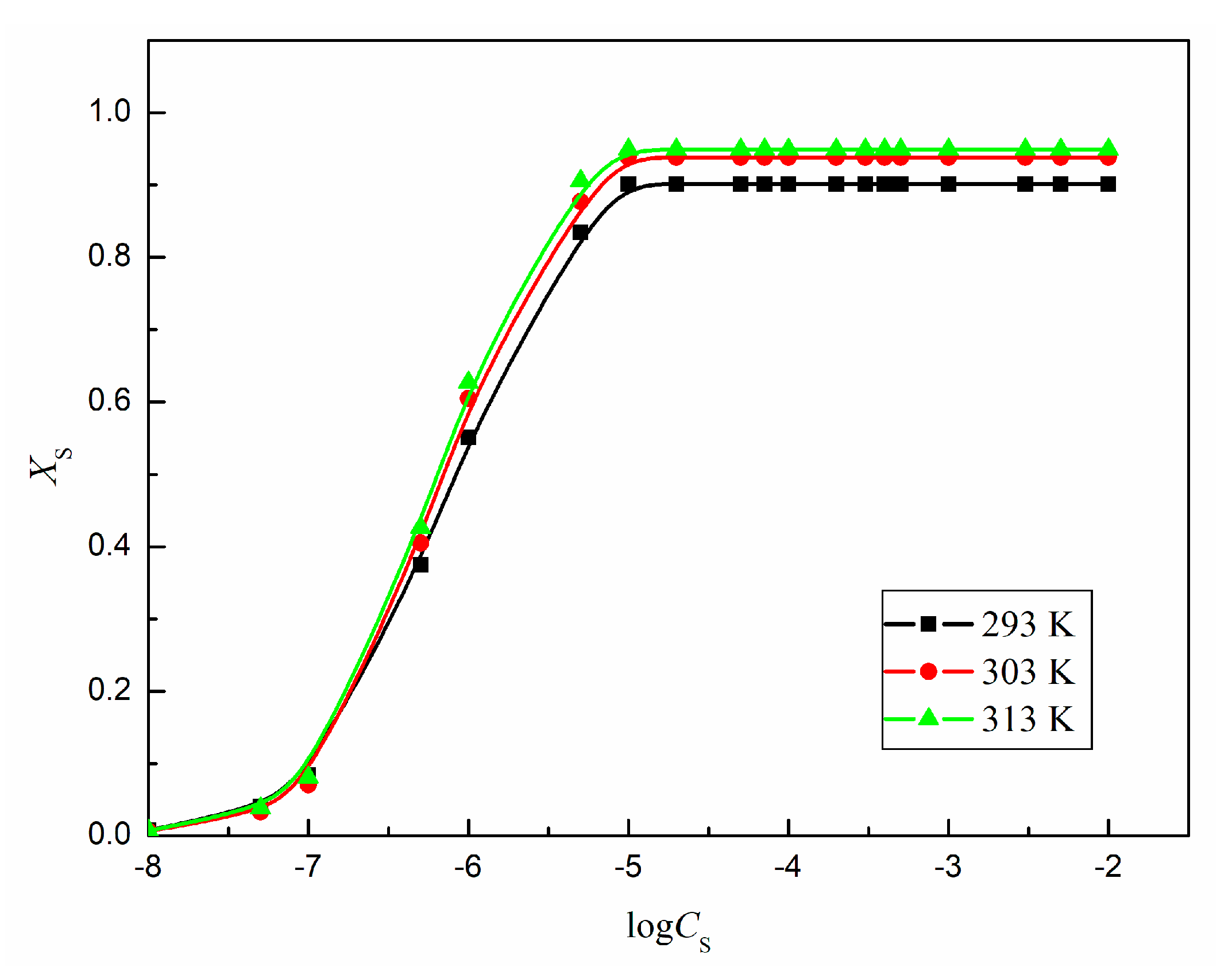
As follows from the obtained values,
XS only slightly increases with temperature but its maximal value is close to 1 and suggest that the adsorption layer of TS-OE12 at the water-air interface is more densely packed than, for example, that of hydrocarbon surfactants [
32,
33,
34].
On the other hand, as follows from
Table 1, the
values of the studied surfactant calculated from Equation (5) are somewhat higher than those of
in every case. Thus, on the basis of
, it is possible to determine the extent of coverage at the water-air interface by the surfactant molecules and the obtained values should be equal to those calculated from Equation (4) [
39]. The values of
XS of particular surfactants obtained from the ratio
are significantly higher than those calculated from Equation (4). As mentioned in earlier studies [
39] the discrepancies can result from different values of the surface area occupied by the water and surfactant molecules because one molecule of surfactant can replace more than one molecule of water. The number of water molecules which can be replaced by one surfactant molecule at the water-air interface can be equal to the ratio of
.
Assuming that
[
33,
36,
39], Equation (4) can be written as follows:
The values of
XS for TS-OE12 calculated from Equation (6) are practically the same as those obtained from the ratio
(
Figure 12 and
Figure S2). In addition, all isotherms of log
XS for TS-OE12 in the studied temperature range are of the Langmuir type, which indicates that the adsorbed monolayer is formed at the water-air interface (
Figure S3).
Analysis of the XS values of TS-OE12 and their changes with temperature (in the studied range) indicates that the dehydration process of polar heads probably proceeds to a smaller extent. Further consideration of the TS-OE12 adsorption at the water-air interface changes with temperature requires the knowledge of changes in the standard Gibbs free energy, enthalpy and entropy of adsorption.
It is also interesting from the practical point of view, whether it is possible to describe the changes in the surface tension of aqueous solutions of the studied surfactant as a function of its concentration on the basis of the solution components’ properties.
For this purpose the Szyszkowski equation [
36,
40] was used:
where
is the maximal surface excess of the studied surfactant concentration in the surface layer,
is the water surface tension and
b is a constant.
To solve Equation (7) the maximal and limiting Gibbs surface excess concentrations of TS-OE12 (
Table 1) were used.
Similarly as for the other quite different surfactants [
41,
42], the use of the limiting area of the surfactant at the water-air interface instead of the minimal one, gives better agreement of
values measured and those calculated from Equation (7) (
Figure S4).
2.4. Critical Micelle Concentration (CMC) of TS-OE12
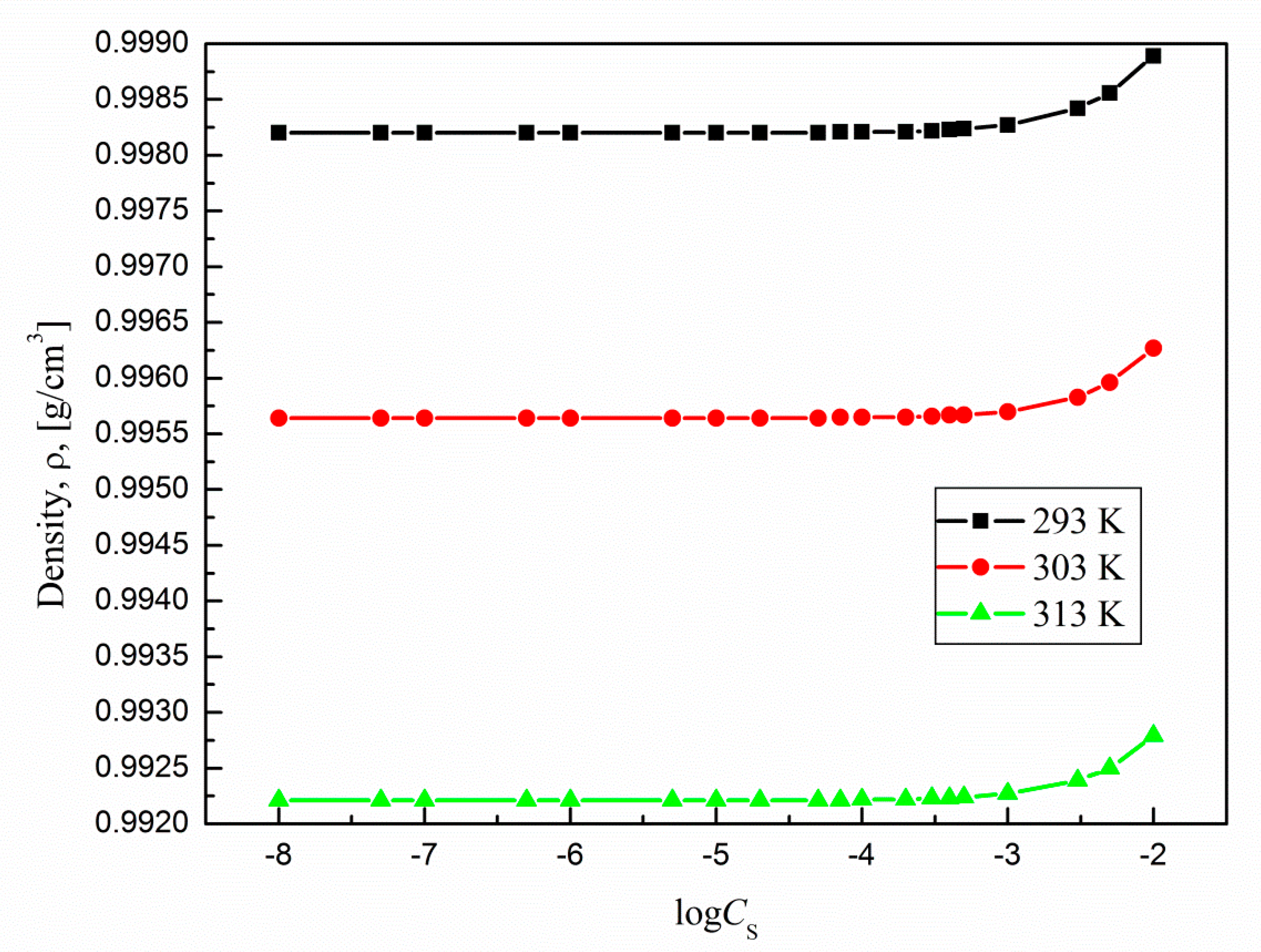
Based on the literature data it is difficult to find systematic informations about the mechanism of temperature effect on siloxane surfactants aggregation properties. Thus, at the beginning, the critical micelle concentration (CMC) of TS-OE12 in aqueous solutions were determined at different temperatures (293 K, 303 K and 313 K) by using different methods. Of all the methods that can be used for estimation of CMC the surface tension method is the most popular. In this method the CMC value can be determined from plots of surface tension versus log of surfactant concentration. In our studies the CMC of TS-OE12 at a given temperature was also determined on the basis of the density (
ρ) (
Figure 13) as well as pyrene (Py) (
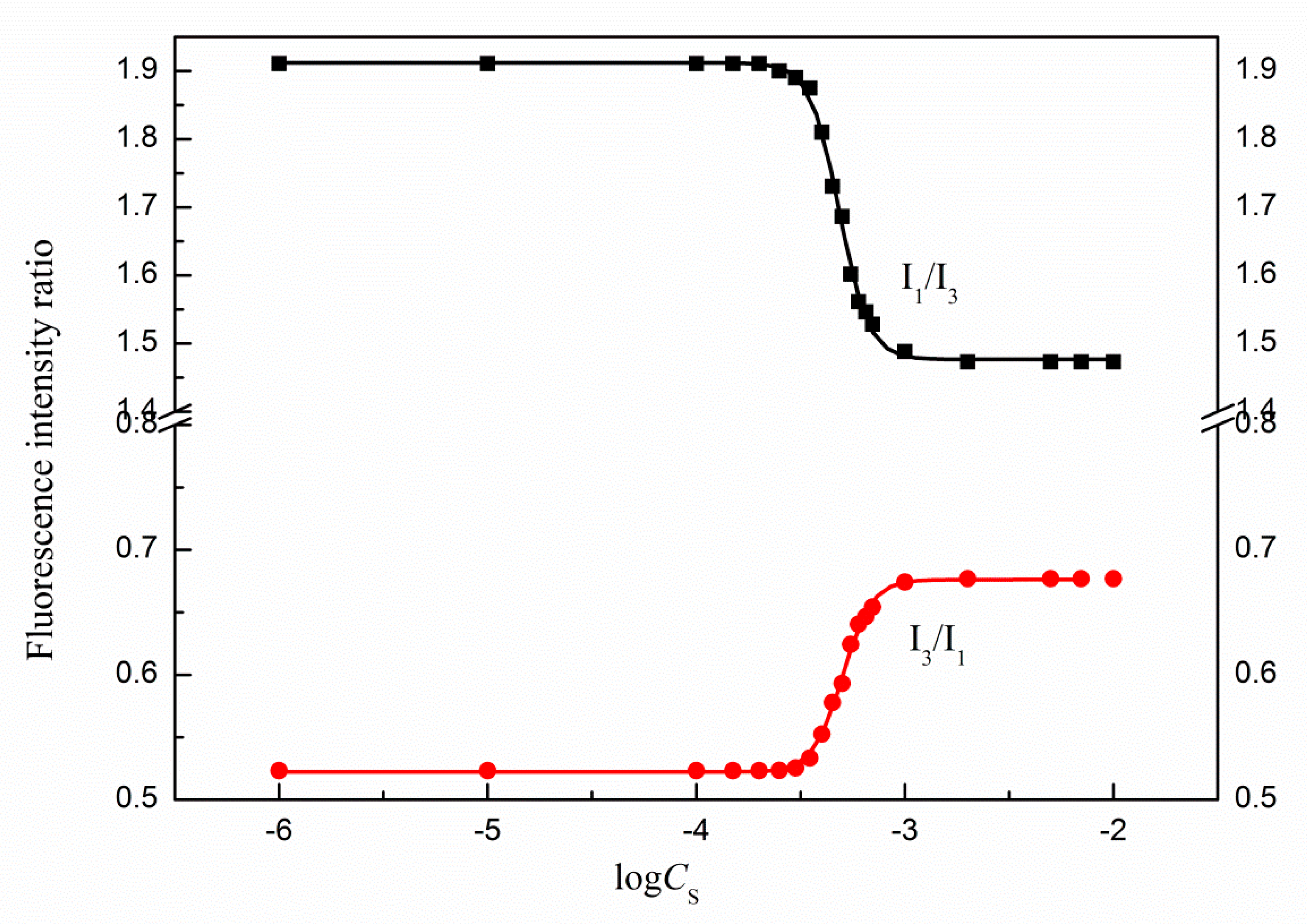
Figure 14) fluorescence intensity values changes as a function of the surfactant concentration in the solution. In the case of the Py fluorescence intensity measurements the CMC values of studied surfactants were determined from the changes in the first and third vibronic bands ratio (
I1/
I3) on the pyrene fluorescence spectra with the surfactant concentration in the solution.
The CMC values obtained in such a way for the studied surfactant are presented in
Table 1. According to
Table 1, TS-OE12 is not characterized by a single CMC value but can assume values from a certain range and depends on the method of its determination. The obtained CMC values for TS-OE12 are consistent with literature data [
24,
27]. However, it is difficult to compare all the obtained values of CMC in different temperatures because of the lack of such data.
The
Table 1 data also imply that CMC of TS-OE12 determined from the surface tension measurements decreases slightly with increasing temperature. This decrease can be connected with the dehydration process and the loss of hydration water. On the other hand the rise of temperature increases the flexibility of Si–O–Si linkage and extends the minimal area of surfactant at the water-air interface. This conclusion was also deduced from the spectroscopic measurement results as well as thermodynamic considerations.
The CMC values of TS-OE12 were also determined from the changes of the first and third vibronic bands ratio on the pyrene fluorescence spectra with the surfactant concentration in the solution (
Figure 14,
Figures S5 and S6). Pyrene is a strongly hydrophobic fluorescence probe and its solubility in water is in the range of 2–3 μM. Thus, in the presence of micelles, pyrene is preferentially solubilized in the interior hydrophobic regions of these micelles. This is the basis of successful use of pyrene as a fluorescence probe in the study of micellization process [
43]. For TS-OE12, like for the other types of surfactants, the changes in the ratio
I1/
I3 as a function of surfactant concentration can be described by the decreasing Boltzman sigmoid (
Figure 14,
Figures S5 and S6). As follows from the fluorescence measurements also for TS-OE12 its CMC decreases with temperature (
Table 1).
For pyrene, the ratio
I1/
I3 takes values of 1.04, 1.33 and 1.84 for toluene, methanol and water, respectively. The values of
I1/
I3 for TS-OE12 presented in
Figure 14 and
Figures S5 and S6 are in the range from 1.9 (no micelles) to about 1.5 (micellar solution) and decrease with increasing temperature. Above CMC it remains nearly constant and independent of the surfactant concentration, which confirms that the probe is located close to the micellar surface and the temperature increase induces changes in the
I1/
I3 values in the same direction as the CMC changes. In addition, it confirms high water penetration into the so-called palisade layer of the micelle and lose packing of the SF-OE12 micelles. As results from the changes in the ratio
I1/
I3 with the logarithm of the surfactant concentration at a given temperature, pyrene is a sensitive probe for determination of the TS-OE12 CMC. In addition, as follows from
Figure 12,
Figures S5 and S6, and from the changes in the third and first vibronic bands ratio, with increasing temperature the hydrophobicity of the interior of the studied micelles increases. However, this effect is not significant in the studied temperature range.
Additionally the morphology of TS-OE12 aggregates in the aqueous solution at the surfactant concentration well above its CMC (and at 293 K) was determined by using the confocal microscopy (
Figure 15a,b). From this analysis it results that aggregates of the surfactant could be considered as more or less spherical or ellipsoidal ones. It occurred that similar shape of aggregates were obtained by using both methods of glass surface modification that is sonication and immersing method. These findings demonstrate the need for further analysis of TS-OE12 aggregates structure.
Analysis of the changes in CMC and hydrophobicity of the interior of TS-OE12 micelles should be supplemented with thermodynamic considerations taking into account the standard Gibbs free energy, enthalpy and entropy of micellization as well as temperature changes in molar volume.
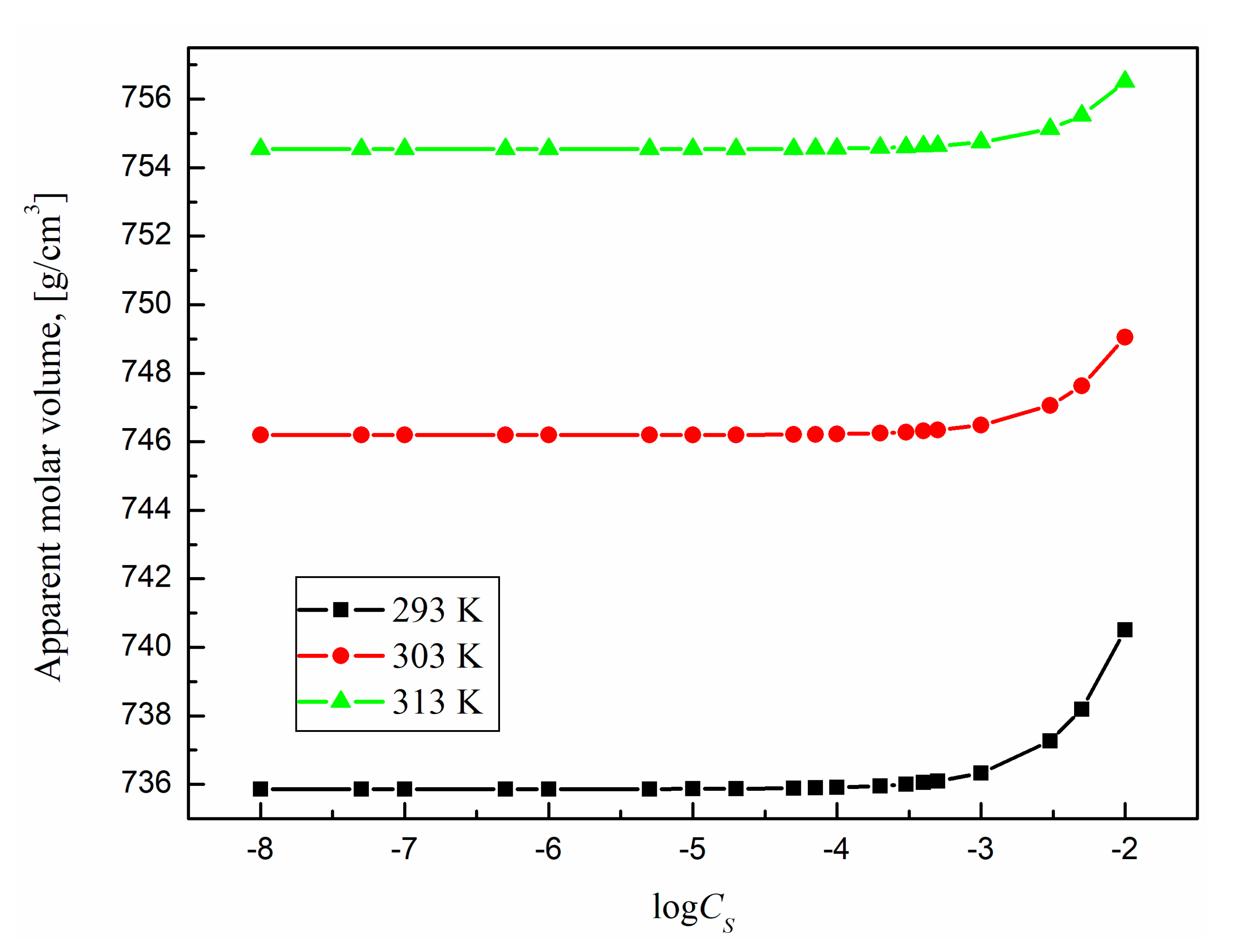
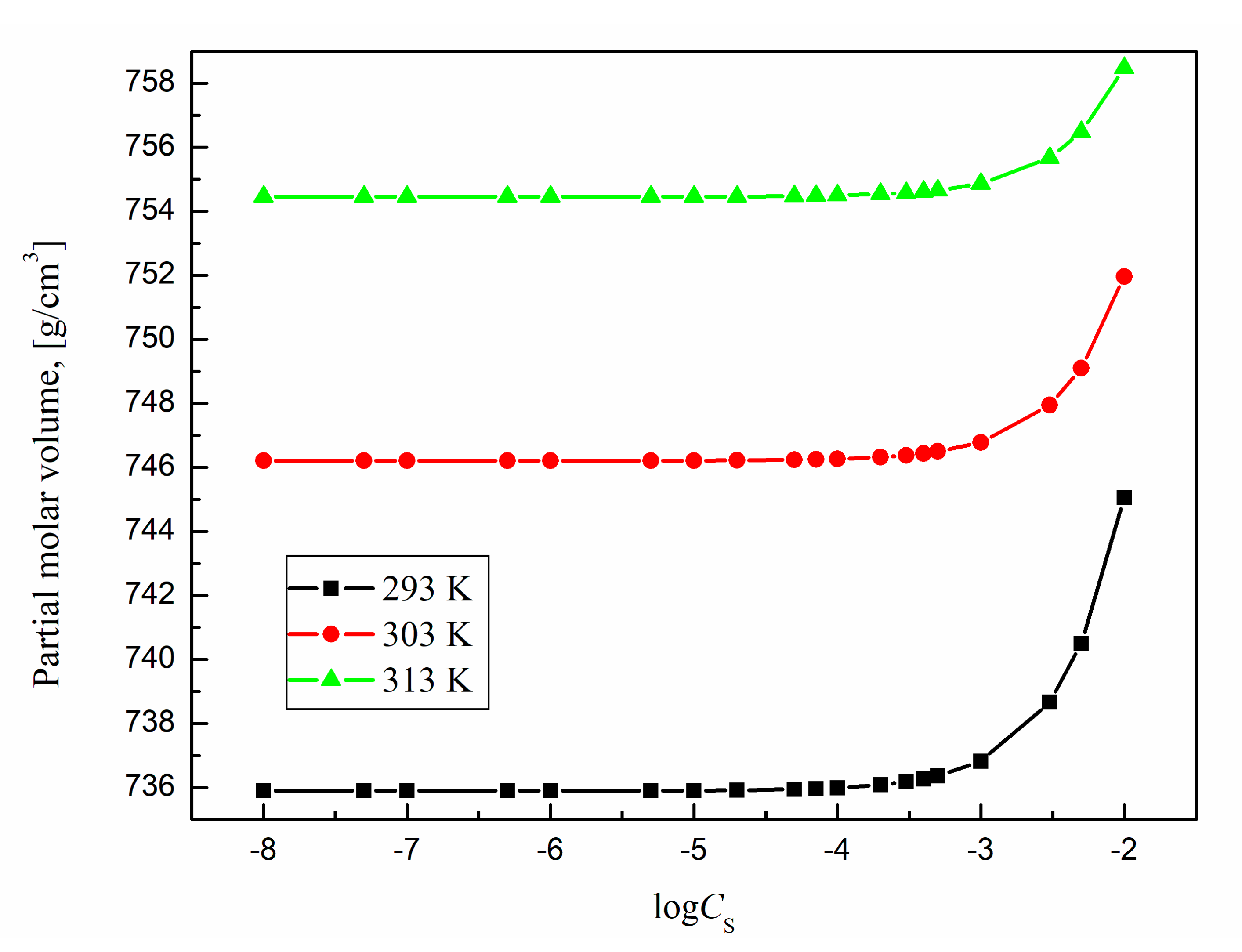
2.5. Apparent and Partial Molar Volume
The micellization process should be also reflected by changes in the surfactant aqueous solutions density (
Figure 13). As follows from this figure, the slopes of the plots of density vs concentration of surfactants before and after CMC are different, which allows determination of CMC values for a studied surfactant (
Table 1). However, the influence of surfactant concentration on the structure of the solution can be more visible from changes in the apparent (
) and partial (
) molar volumes with the surfactant concentration in the micellization process.
The apparent molar volume can be determined from the following equation [
44,
45]:
where
MS is the molecular weight of the surface active agent,
C is the concentration of the surface active agent in mol/cm
3 and
ρ0 is the density of the solvent.
The partial molar volume
can be calculated from the equation [
45]:
It appeared that the
ρ data measured for TS-OE12 at different temperatures (
Figure 13) fit a polynomial of
Cp (the mass percentage concentration) given by:
where
a,
b and
d are constants.
On the basis of the above- mentioned relations between
ρ and
Cp, the apparent (
) and partial (
) molar volumes as well as their changes with the surfactant concentration and temperature were determined (
Figure 16 and
Figure 17).
From these figures it results that the partial and apparent molar volume values for TS-OE12 increase with surfactant concentration and there is no characteristic point corresponding to CMC. The growth of density with TS-OE12 concentration (
Figure 13) indicates also that the density of the micellar solution is higher than that of the surfactant in the monomeric form and that the size of micelles increases as a function of TS-OE12 concentration. It is possible to calculate the the size of aggregates as well as a mean aggregation number based on the length of the hydrophobic part of surfactant and its volume as well as on the basis of a diameter of the surfactant aggregate and molar volume of one molecule of surfactant determined from the density measurements. From
Figure 15a,b it is difficult to state one unequivocal diameter value of the studied surfactant micelles. Thus additional measurements should be done. In addition the siloxane chains in the core part are expected to be in a coiled form because of the high flexibility of the Si–O–Si bonds.
According to
Figure 16 and
Figure 17, the values of
similarly as those of
insignificantly increase with increasing temperature. This small increase probably results from the increase in the average distance between the surfactant and water molecules for the surfactant concentrations lower than CMC and between surfactant molecules in the micelles for the surfactant concentrations higher than CMC.
2.6. Thermodynamic Parameters of Adsorption and Micellization
Determination of standard thermodynamic functions (Gibbs free energy, enthalpy and entropy) of the studied surfactants adsorption at the water-air interface gives us more information about the mechanism of the process. Similarly, the standard enthalpy of micellization (), Gibbs free energy of micellization () and entropy of micellization () are important in understanding micelle formation in aqueous solution.
For these reasons, on the basis of the dependence of Γ
S on
CS for TS-OE12 at different temperatures, the standard Gibbs free energy (
) and next enthalpy (
) and entropy (
) of their adsorption at the water-air interface at a given temperature were determined. There are many approaches which can be used to determine
[
36,
40]. Among others, for dilute aqueous solutions whose concentration corresponds to the unsaturated monolayer at the water-air interface, the Langmuir equation can be used [
36,
40]. If mutual interactions between the adsorbed molecules are taken into account, the Langmuir equation is as follows [
36]:
where
AS is the area occupied by the surfactant molecule at the interface,
ω is the number of water moles in 1 dm
3 (at a given temperature). To calculate
from Equation (11), the values of
for a given surfactant must be known. These values were determined earlier from Equation (5) (
Table 2). The values of
AS at the water-air interface for studied surfactants at a given temperature were determined from the appropriate values of Γ
S (
Figure 11). The obtained
values of TS-OE12 are presented in
Table 2.
As follows from this table, the
values calculated from Equation (11) are constant only in the range of surfactants concentrations corresponding to the unsaturated monolayer at the water-air interface and the
values decrease with increasing temperature. According to the thermodynamic rule
fulfils the relationship [
36,
40]:
can be calculated from the relation:
if
is constant over the investigated temperature range. To calculate the standard enthalpy of adsorption of the studied surfactant, the standard entropy of adsorption determined from the
(from Equation (11)) linear changes with temperature in the studied temperature range was used. The obtained values of
and
for TS-OE12 are presented in
Table 2. The obtained values of standard Gibbs free energy (
), enthalpy (
) and entropy (
) of TS-OE12 adsorption at the water-air interface indicate the positive values of
. As the
values are influenced by bonds formation, thus positive values of standard enthalpy and entropy of TS-OE12 adsorption suggest that bond breaking predominates in the adsorption process. In addition, the
values suggest that in the studied temperature range, dehydration of the TS-OE12 molecules takes place (
Table 2).
There are also different approaches to determination of
. The phase separation model is the simplest one and treats micelles as a simple phase. In this model, micelle formation is treated as a phase separation phenomenon. In the mass action model, micelles and single surfactant molecules or ions are assumed to be in the association-dissociation equilibrium [
40].
According to the micellization process, for nonionic surfactants such as TS-OE12,
assumes the following form [
36]:
According to the thermodynamic rule, in the exothermic and isobaric conditions,
fulfills the relationship [
36,
40]:
Knowing the values of the standard Gibbs free energy of micellization at different temperatures it is possible to determine the standard enthalpy and entropy of micellization.
can be calculated from the relation:
if
is constant over the investigated temperature range.
Alternatively, when
is constant over the temperature range investigated, then it is possible to calculate
from the following equation:
As mentioned above, there are some differences in CMC for the same surfactant determined from the surface tension, density and spectrofluorimetric measurements (
Table 2). In the calculations of
of TS-OE12 only the CMC values obtained from the surface tension measurements were taken into account. The obtained
values are presented in
Table 2.
It occurs that the Gibbs free energy of micellization becomes more negative as the temperature increases, indicating that the formation of micelle becomes more spontaneous at higher temperatures. Next, taking into account the relation between
and
T, it was possible to determine
of micellization of TS-OE12 assuming that
of micellization in the studied range of temperatures is constant. It appeared that there is a linear dependence between
and temperature. The slope of this dependence is equal to
. Knowing the values of
,
were found from Equation (17). The obtained values of
and
are presented in
Table 2. According to this table, the standard enthalpy and entropy of TS-OE12 micellization take positive values and those of
are higher than zero. The values of
indicate that the micellization process is endothermic. This parameter shows a very strong dependence on temperature, indicating changes in the environment surrounding the hydrocarbon chain of the surfactant molecule with temperature changes. It is also known that
indicates whether during the micellization process more bonds are formed than broken.