Glycation Increases the Risk of Microbial Traversal through an Endothelial Model of the Human Blood-Brain Barrier after Use of Anesthetics
Abstract
1. Introduction
2. Experimental Section
2.1. Cells and Cell Culture
2.2. Treatment for Immunoblotting
2.3. Preparation of Cell Extracts
2.4. Immunoblotting
2.5. MTT-Assay
2.6. Measurement of Bacterial Traversal through the BBB
2.7. Treatment for Measurement of Bacterial Traversal through the BBB
2.8. RNA Extraction and Barrier Genes High-Throughput Multiplex qPCR
2.9. Statistical Analysis
3. Results
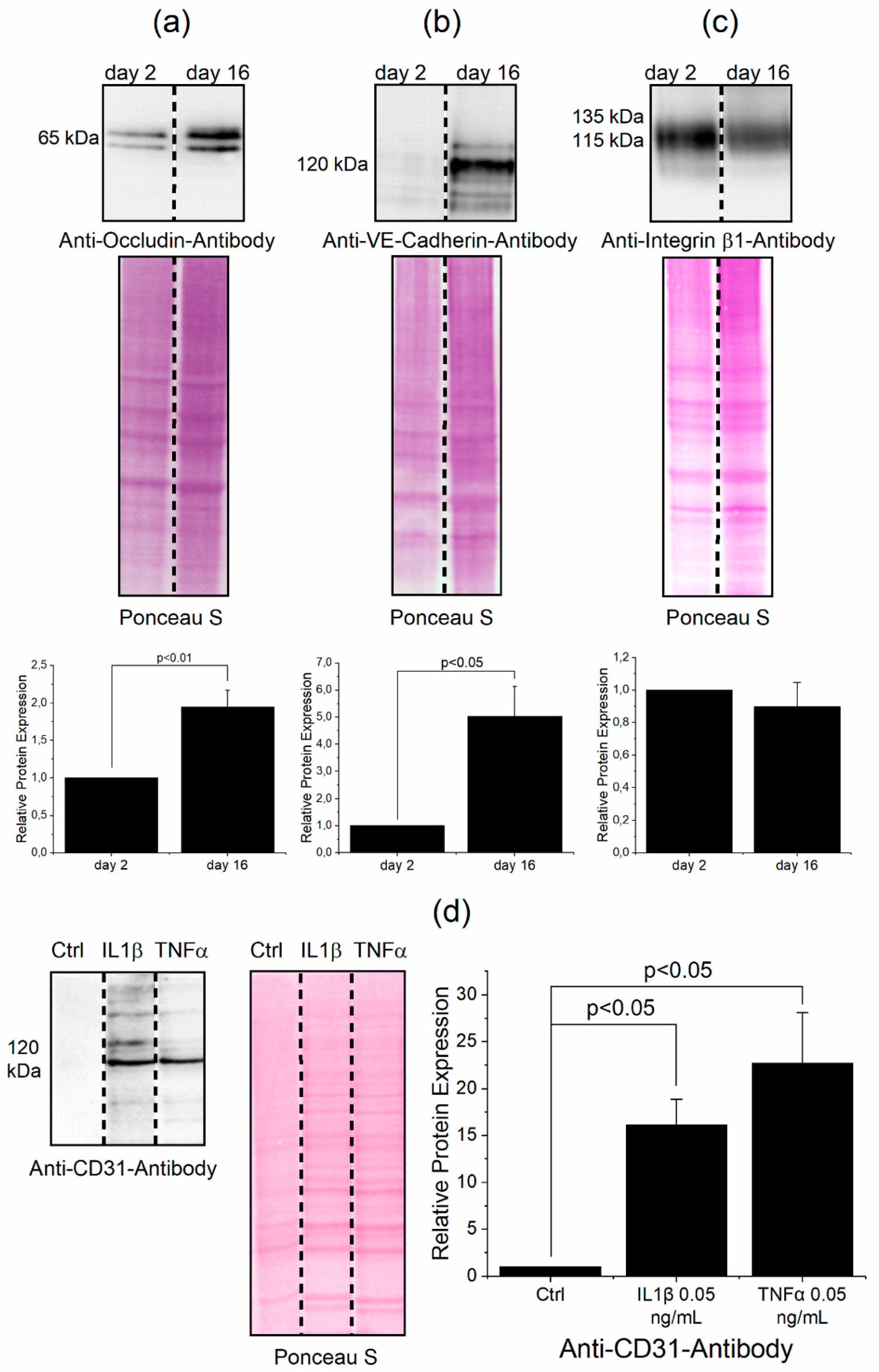
3.1. Determination of Protein Levels Building the Cell Junctions
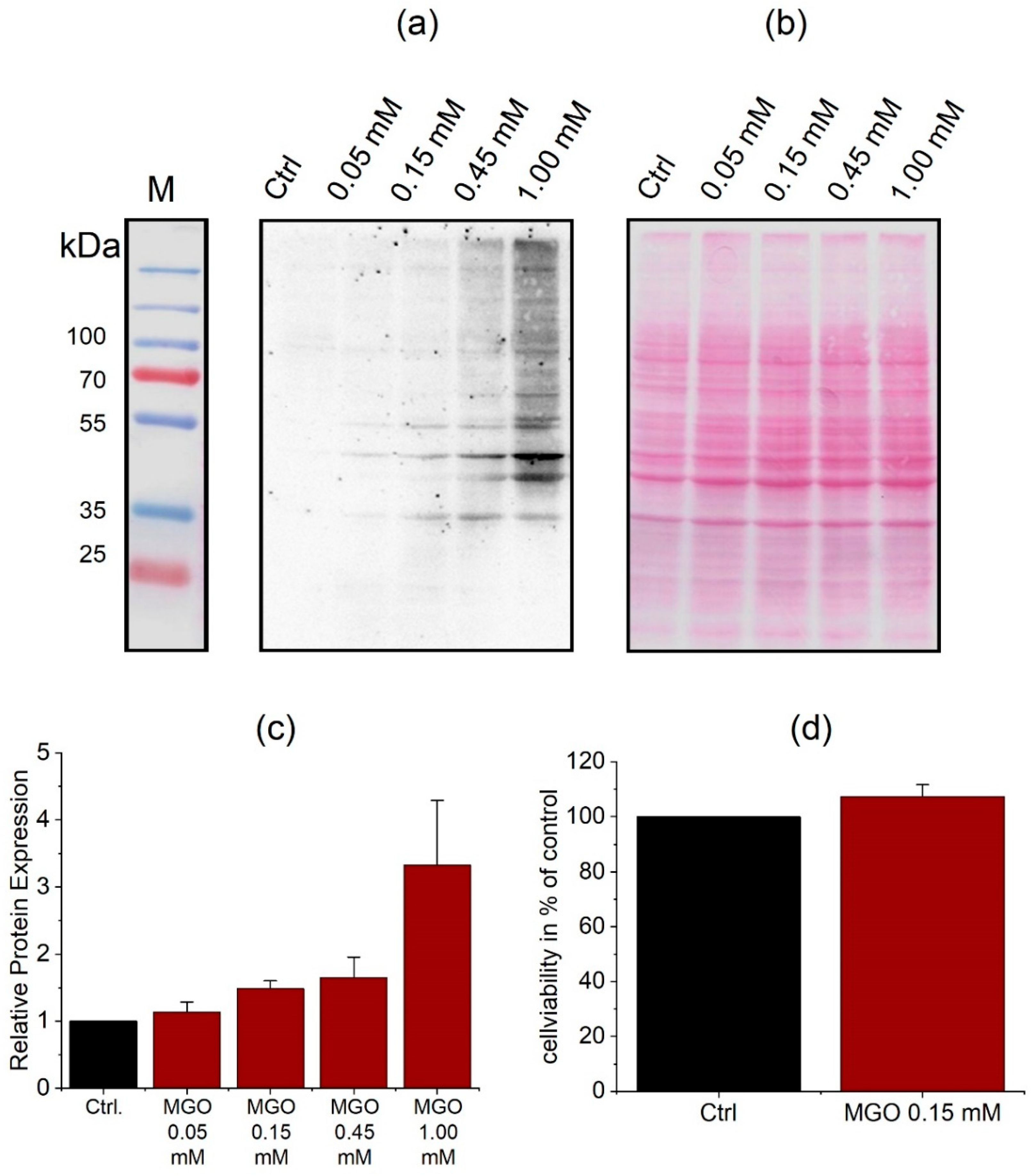
3.2. Protein Glycation Is Induced by MGO
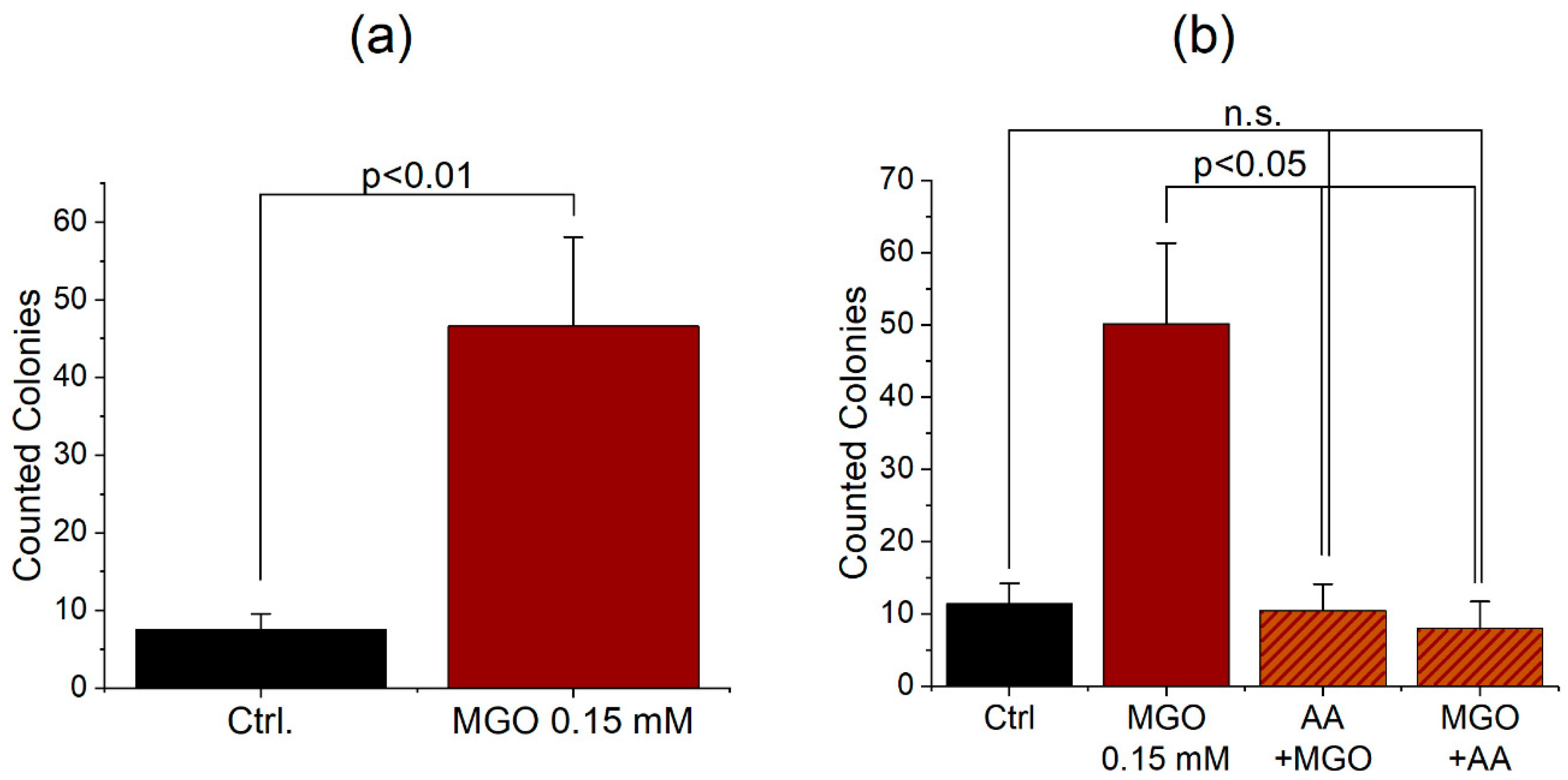
3.3. Protein Glycation Increases the Permeability of the BBB, Which Can Be Reverted by Antioxidant
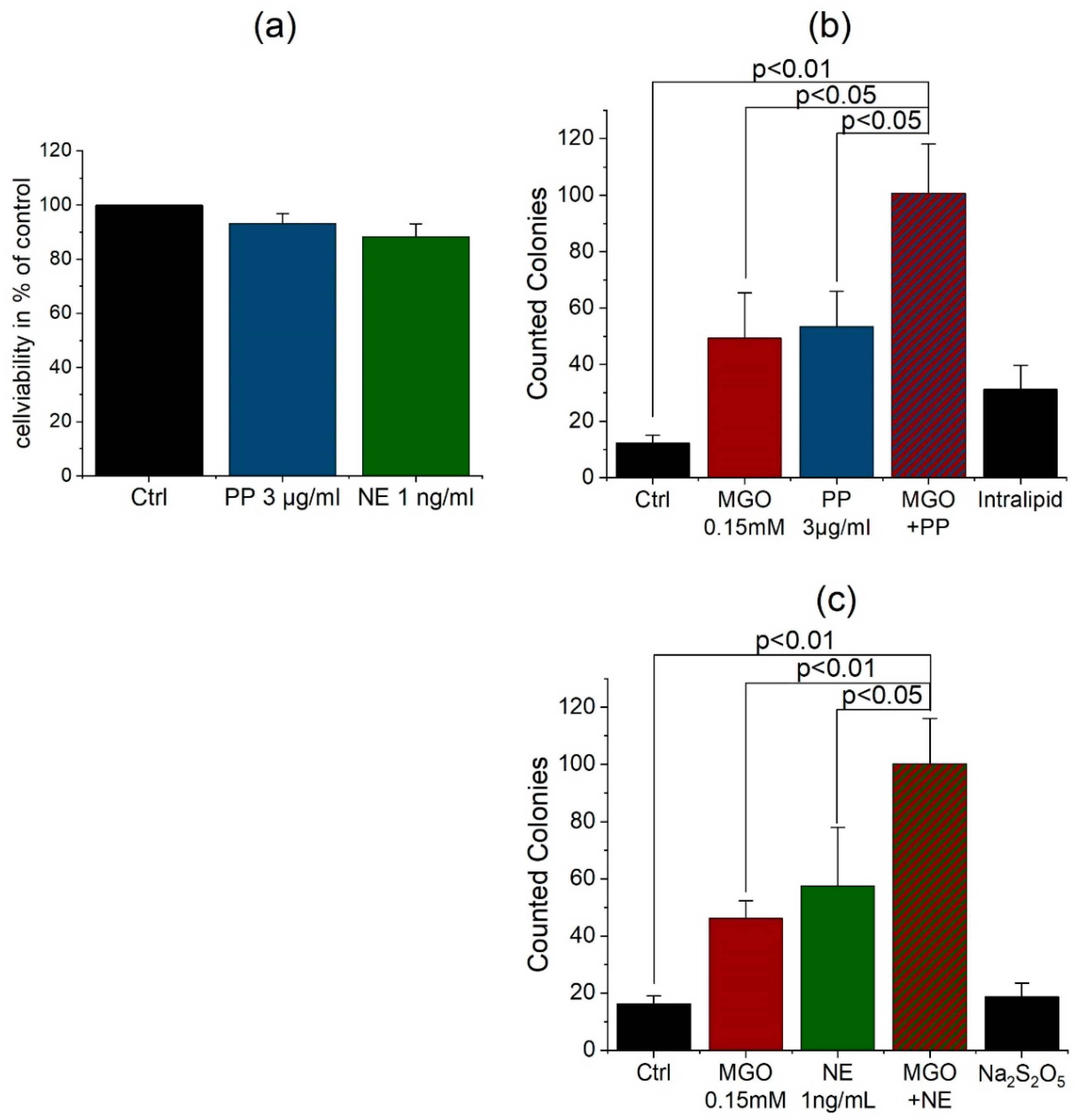
3.4. Anesthetics Increase the Permeability of the BBB and Bacterial Traversal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schalkwijk, C.G.; Miyata, T. Early- and advanced non-enzymatic glycation in diabetic vascular complications: The search for therapeutics. Amino Acids 2012, 42, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Bennmann, D.; Horstkorte, R.; Hofmann, B.; Jacobs, K.; Navarrete-Santos, A.; Simm, A.; Bork, K.; Gnanapragassam, V.S. Advanced glycation endproducts interfere with adhesion and neurite outgrowth. PLoS ONE 2014, 9, e112115. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Bohm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- John, W.G.; Lamb, E.J. The Maillard or browning reaction in diabetes. Eye 1993, 7, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Methylglyoxal and glucose metabolism: A historical perspective and future avenues for research. Drug Metabol. Drug Interact. 2008, 23, 69–91. [Google Scholar] [CrossRef]
- Ahmed, N.; Battah, S.; Karachalias, N.; Babaei-Jadidi, R.; Horanyi, M.; Baroti, K.; Hollan, S.; Thornalley, P.J. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim. Biophys. Acta 2003, 1639, 121–132. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef]
- Meredith, M.E.; Qu, Z.C.; May, J.M. Ascorbate reverses high glucose- and RAGE-induced leak of the endothelial permeability barrier. Biochem. Biophys. Res. Commun. 2014, 445, 30–35. [Google Scholar] [CrossRef]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug. Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.W.; Sharp, M.M.; Albargothy, N.J.; Fernandes, R.; Hawkes, C.A.; Verma, A.; Weller, R.O.; Carare, R.O. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 2016, 131, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.S.; Nelson, W.J. VE-cadherin: At the front, center, and sides of endothelial cell organization and function. Curr. Opin. Cell Biol. 2010, 22, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Wass, C.A.; Cross, A.S. Blood-brain barrier permeability during the development of experimental bacterial meningitis in the rat. Exp. Neurol. 1997, 145, 253–257. [Google Scholar] [CrossRef]
- Kratz, T.; Heinrich, M.; Schlauß, E.; Diefenbacher, A. Preventing postoperative delirium. Dtsch. Arztebl. Int. 2015, 112, 289–296. [Google Scholar] [CrossRef]
- Margiotta, A.; Bianchetti, A.; Ranieri, P.; Trabucchi, M. Clinical characteristics and risk factors of delirium in demented and not demented elderly medical inpatients. J. Nutr. Health Aging 2006, 10, 535–539. [Google Scholar]
- Smulter, N.; Lingehall, H.C.; Gustafson, Y.; Olofsson, B.; Engstrom, K.G. Delirium after cardiac surgery: Incidence and risk factors. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 790–796. [Google Scholar] [CrossRef]
- van Veen, K.E.; Brouwer, M.C.; van der Ende, A.; van de Beek, D. Bacterial meningitis in diabetes patients: A population-based prospective study. Sci. Rep. 2016, 6, 36996. [Google Scholar] [CrossRef]
- Kalra, S.; Zargar, A.H.; Jain, S.M.; Sethi, B.; Chowdhury, S.; Singh, A.K.; Thomas, N.; Unnikrishnan, A.G.; Thakkar, P.B.; Malve, H. Diabetes insipidus: The other diabetes. Indian J. Endocrinol. Metab. 2016, 20, 9–21. [Google Scholar] [CrossRef]
- Schut, E.S.; Westendorp, W.F.; de Gans, J.; Kruyt, N.D.; Spanjaard, L.; Reitsma, J.B.; van de Beek, D. Hyperglycemia in bacterial meningitis: A prospective cohort study. BMC Infect. Dis. 2009, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Sano, Y.; Tominaga, O.; Maeda, T.; Abe, M.A.; Kanda, T. Advanced glycation end-products disrupt the blood-brain barrier by stimulating the release of transforming growth factor-beta by pericytes and vascular endothelial growth factor and matrix metalloproteinase-2 by endothelial cells in vitro. Neurobiol. Aging 2013, 34, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Bork, K.; Gnanapragassam, V.S.; Bennmann, D.; Jacobs, K.; Navarette-Santos, A.; Hofmann, B.; Simm, A.; Danker, K.; Horstkorte, R. Novel insights in the dysfunction of human blood-brain barrier after glycation. Mech. Ageing Dev. 2016, 155, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, H.; Du, J.; Chen, B.; Li, Q.; Guo, X.; Huang, X.; Huang, Q. Advanced glycation end products induce moesin phosphorylation in murine brain endothelium. Brain Res. 2011, 1373, 1–10. [Google Scholar] [CrossRef]
- Kim, K.S.; Itabashi, H.; Gemski, P.; Sadoff, J.; Warren, R.L.; Cross, A.S. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Investig. 1992, 90, 897–905. [Google Scholar] [CrossRef]
- Doran, K.S.; Fulde, M.; Gratz, N.; Kim, B.J.; Nau, R.; Prasadarao, N.; Schubert-Unkmeir, A.; Tuomanen, E.I.; Valentin-Weigand, P. Host-pathogen interactions in bacterial meningitis. Acta Neuropathol. 2016, 131, 185–209. [Google Scholar] [CrossRef]
- Kim, K.S. Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef]
- Liu, W.-T.; Lv, Y.-J.; Yang, R.-C.; Fu, J.-Y.; Liu, L.; Wang, H.; Cao, Q.; Tan, C.; Chen, H.-C.; Wang, X.-R. New insights into meningitic Escherichia coli infection of brain microvascular endothelial cells from quantitative proteomics analysis. J. Neuroinflamm. 2018, 15, 291. [Google Scholar] [CrossRef]
- Sharma, H.S.; Pontén, E.; Gordh, T.; Eriksson, P.; Fredriksson, A.; Sharma, A. Propofol promotes blood-brain barrier breakdown and heat shock protein (HSP 72 kd) activation in the developing mouse brain. CNS Neurol. Disord. Drug Targets 2014, 13, 1595–1603. [Google Scholar] [CrossRef]
- Doronzio, A.; Lanni, F.; Ayrian, E.; Zhang, Y.P.; Bilotta, F.; Rosa, G. Postoperative delirium after anestesia with propofol, sevoflurane or desflurane: The Pinocchio trial. Interim analysis of safety and preliminary results: 7AP3-6. Eur. J. Anaesthesiol. 2013, 30, 108–109. [Google Scholar] [CrossRef]
- Ishii, K.; Akiyama, D.; Hara, K.; Makita, T.; Sumikawa, K. Influence of general anesthetics on the incidence of postoperative delirium in the elderly. Masui 2011, 60, 856–858. [Google Scholar]
- Ishii, K.; Makita, T.; Yamashita, H.; Matsunaga, S.; Akiyama, D.; Toba, K.; Hara, K.; Sumikawa, K.; Hara, T. Total intravenous anesthesia with propofol is associated with a lower rate of postoperative delirium in comparison with sevoflurane anesthesia in elderly patients. J. Clin. Anesth. 2016, 33, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Weber, V.; Bork, K.; Horstkorte, R.; Olzscha, H. Analyzing the Permeability of the Blood-Brain Barrier by Microbial Traversal through Microvascular Endothelial Cells. J. Vis. Exp. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stins, M.F.; Badger, J.; Sik Kim, K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 2001, 30, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, K.; Irrgang, F.; Raju, R.; Harman, D.G.; Moran, C.; Srikanth, V.; Münch, G. Determination of glyoxal and methylglyoxal in serum by UHPLC coupled with fluorescence detection. Anal. Biochem. 2019, 573, 51–66. [Google Scholar] [CrossRef]
- Scheffler, J.; Bork, K.; Bezold, V.; Rosenstock, P.; Gnanapragassam, V.S.; Horstkorte, R. Ascorbic acid leads to glycation and interferes with neurite outgrowth. Exp. Gerontol. 2019, 117, 25–30. [Google Scholar] [CrossRef]
- Wessén, A.; Persson, P.M.; Nilsson, A.; Hartvig, P. Concentration-effect relationships of propofol after total intravenous anesthesia. Anesth. Analg. 1993, 77, 1000–1007. [Google Scholar]
- Takizawa, E.; Hiraoka, H.; Takizawa, D.; Goto, F. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. BJA Br. J. Anaesth. 2005, 96, 179–185. [Google Scholar] [CrossRef]
- Minami, K.; Körner, M.M.; Vyska, K.; Kleesiek, K.; Knobl, H.; Körfer, R. Effects of pulsatile perfusion on plasma catecholamine levels and hemodynamics during and after cardiac operations with cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1990, 99, 82–91. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Brannan, T.; Martinez-Tica, J.; Weinberger, J. Ischemia in the dorsal hippocampus is associated with acute extracellular release of dopamine and norepinephrine. J. Neural. Transm. Gen. Sect. 1990, 80, 195–201. [Google Scholar] [CrossRef]
- Yasuda, Y.; Nishikimi, M.; Nishida, K.; Takahashi, K.; Numaguchi, A.; Higashi, M.; Matsui, S.; Matsuda, N. Relationship Between Serum Norepinephrine Levels at ICU Admission and the Risk of ICU-Acquired Delirium: Secondary Analysis of the Melatonin Evaluation of Lowered Inflammation of ICU Trial. Crit. Care Explor. 2020, 2, e0082. [Google Scholar] [CrossRef] [PubMed]
- Windmann, V.; Spies, C.; Knaak, C.; Wollersheim, T.; Piper, S.; Vorderwülbecke, G.; Kurpanik, M.; Kuenz, S.; Lachmann, G. Intraoperative hyperglycemia increases the incidence of postoperative delirium. Minerva Anestesiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.G.; Rao, V.N. Ascorbic acid as a reducing agent in quantitative analysis. Fresenius Z. Anal. Chem. 1955, 147, 338–347. [Google Scholar] [CrossRef]
- Krone, C.A.; Ely, J.T. Ascorbic acid, glycation, glycohemoglobin and aging. Med. Hypotheses 2004, 62, 275–279. [Google Scholar] [CrossRef]
- Roy, J.; Galano, J.M.; Durand, T.; Le Guennec, J.Y.; Lee, J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ Dysfunction in Cardiac Surgery Patients-Review and Pragmatic Approach. Nutrients 2018, 10, 974. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Vinson, J.; Howard, T. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996, 7, 659–663. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, V.; Olzscha, H.; Längrich, T.; Hartmann, C.; Jung, M.; Hofmann, B.; Horstkorte, R.; Bork, K. Glycation Increases the Risk of Microbial Traversal through an Endothelial Model of the Human Blood-Brain Barrier after Use of Anesthetics. J. Clin. Med. 2020, 9, 3672. https://doi.org/10.3390/jcm9113672
Weber V, Olzscha H, Längrich T, Hartmann C, Jung M, Hofmann B, Horstkorte R, Bork K. Glycation Increases the Risk of Microbial Traversal through an Endothelial Model of the Human Blood-Brain Barrier after Use of Anesthetics. Journal of Clinical Medicine. 2020; 9(11):3672. https://doi.org/10.3390/jcm9113672
Chicago/Turabian StyleWeber, Veronika, Heidi Olzscha, Timo Längrich, Carla Hartmann, Matthias Jung, Britt Hofmann, Rüdiger Horstkorte, and Kaya Bork. 2020. "Glycation Increases the Risk of Microbial Traversal through an Endothelial Model of the Human Blood-Brain Barrier after Use of Anesthetics" Journal of Clinical Medicine 9, no. 11: 3672. https://doi.org/10.3390/jcm9113672
APA StyleWeber, V., Olzscha, H., Längrich, T., Hartmann, C., Jung, M., Hofmann, B., Horstkorte, R., & Bork, K. (2020). Glycation Increases the Risk of Microbial Traversal through an Endothelial Model of the Human Blood-Brain Barrier after Use of Anesthetics. Journal of Clinical Medicine, 9(11), 3672. https://doi.org/10.3390/jcm9113672








