New Insights into Immunological Involvement in Congenital Disorders of Glycosylation (CDG) from a People-Centric Approach
Abstract
1. Introduction
Study Rationale and Aims
- (1)
- characterized the infection profile of PMM2-CDG patients, including infection types and frequency, age association, and treatment effectiveness;
- (2)
- studied allergies (types, prevalence, association with infection and age);
- (3)
- evaluated autoimmune diseases (types, prevalence, infection and age relation);
- (4)
- examined vaccinations (adherence, perceived (in)effectiveness and relevant adverse reactions (ADRs);
- (5)
- assessed a potential correlation between genotype and (immune) phenotype, as well as correlated overall phenotypic severity and other clinical manifestations with immune-related manifestations;
- (6)
- measured the impact of immune-related manifestation on the quality of life (QoL) of these patients.
2. Experimental Section
2.1. Development of the ImmunoCDGQ and ImmunoHealthyQ (Immunology Questionnaire for the General “Healthy” Population)
2.2. Piloting, Translations, and Refinement by Clinical and Family Experts
2.3. Ethical Approval
2.4. Survey Monkey—E-Questionnaire Platform
2.5. Information and Recruitment Strategy
2.6. Inclusion/Exclusion Criteria
2.7. Statistical Analysis
2.8. Genotype Characterization
- Human Genome Mutation Database (HGMD ®, http://www.hgmd.cf.ac.uk/) [31];
- Leiden Open Variation Database 3.0 (LOVD 3, https://databases.lovd.nl) [32];
- Expasy (https://www.expasy.org/) [33];
- ClinVar (NCBI, https://www.ncbi.nlm.nih.gov/clinvar/) [34];
- Varsome clinical (https://varsome.com/) [35].
3. Results
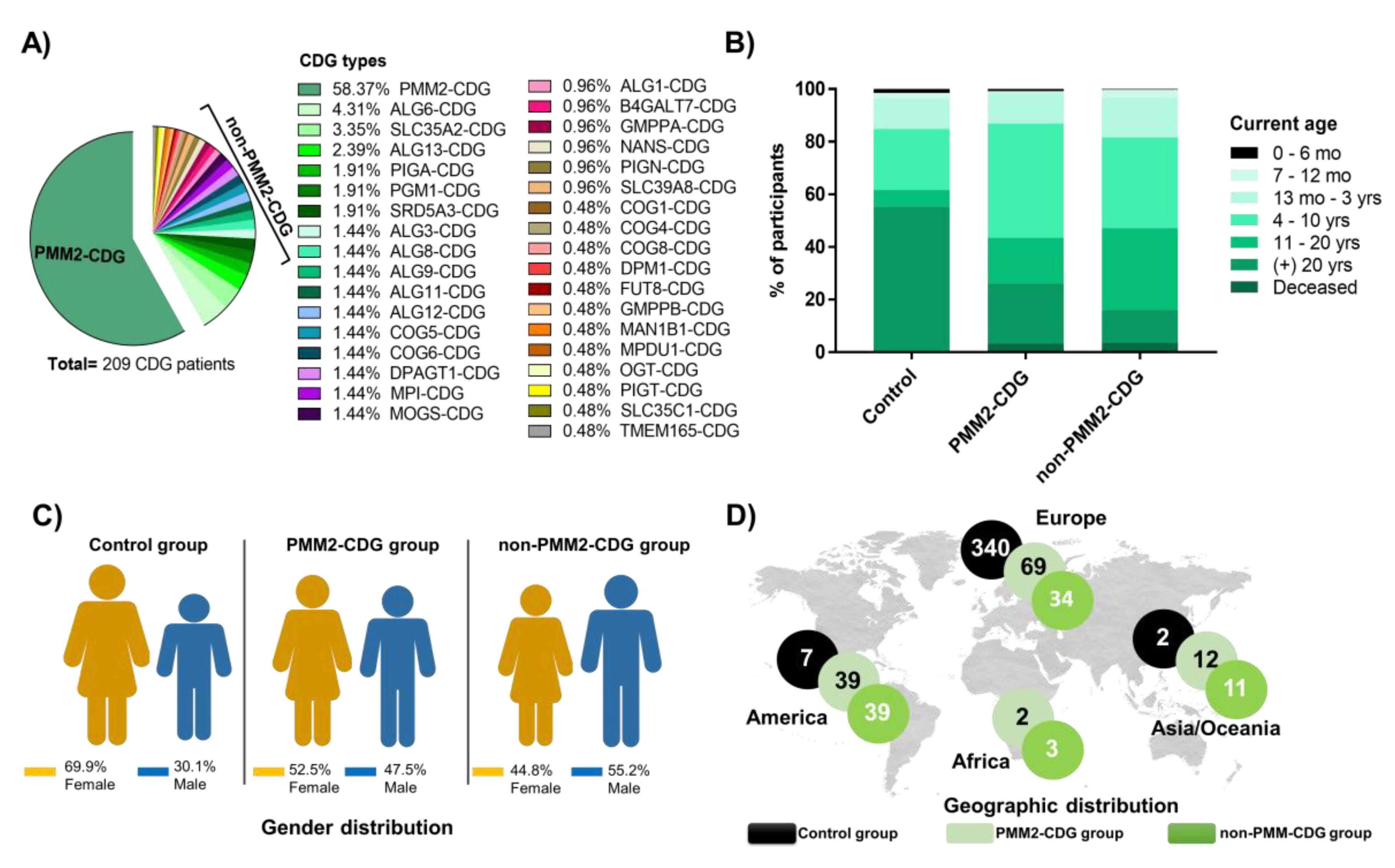
3.1. Participants Display Diversified Age, Gender and Worldwide Distribution
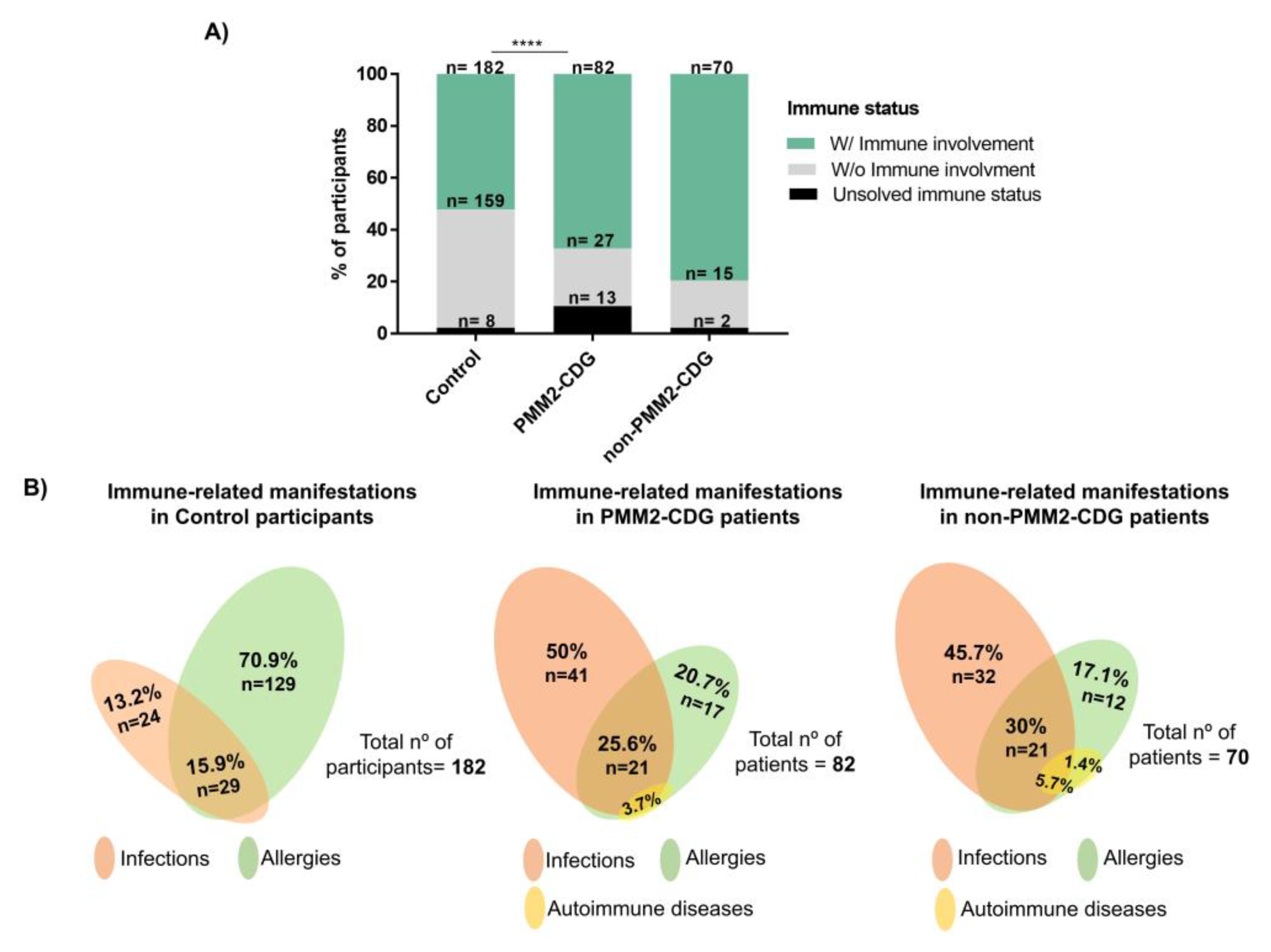
3.2. CDG Patients Show a Higher Prevalence of Immune-Related Manifestations
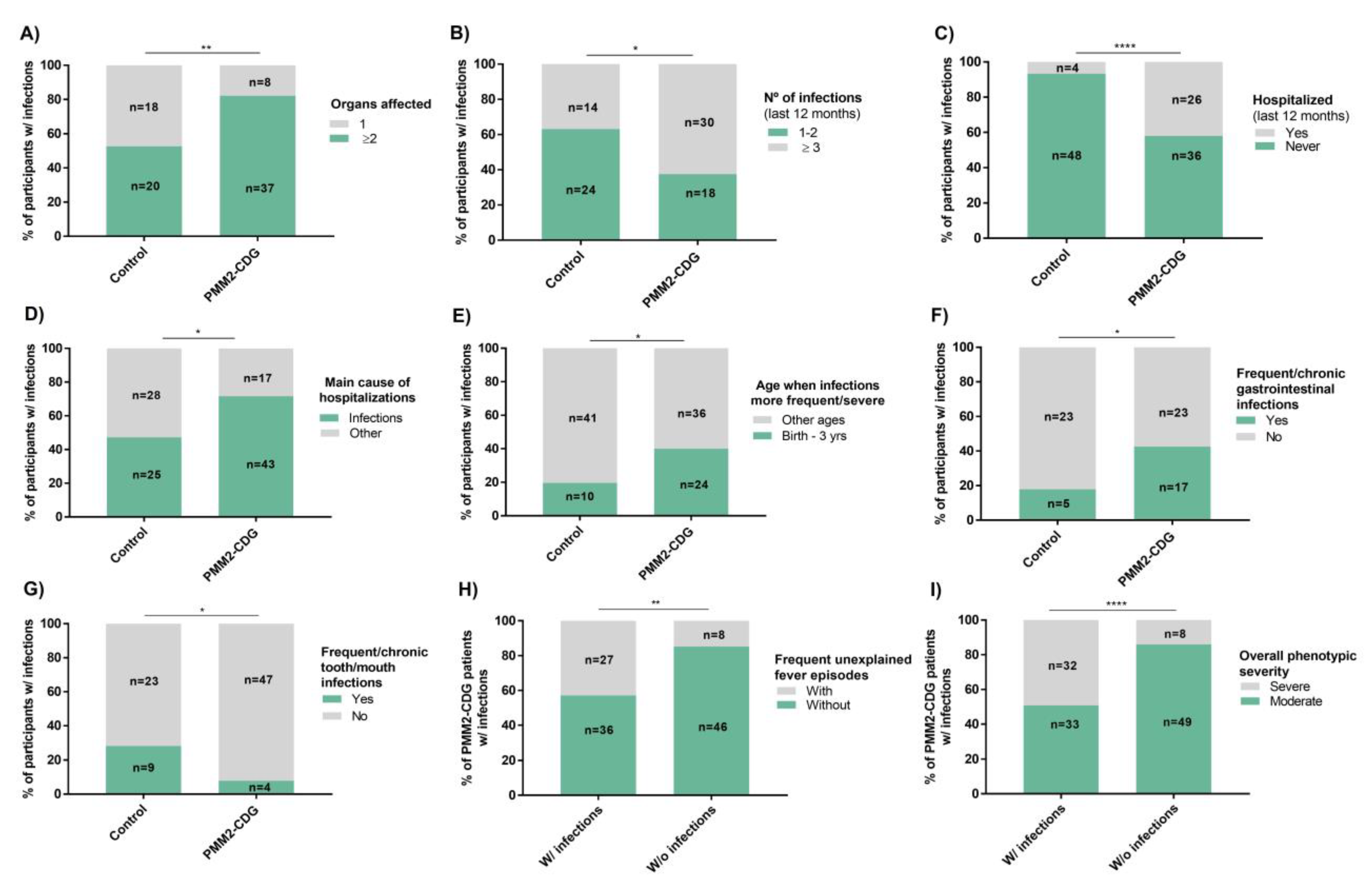
3.3. PMM2-CDG Patients Present Diverse and Severe Infection Patterns Which Are an Important Hospitalization Cause
3.4. Infections in PMM2-CDG Have Greater Clinical Relevance in Infancy and Significantly Associate with the GI Tract
3.5. PMM2-CDG Infections Do Not Relate to a Specific Recovery Time, Season, or Infectious Agent, but Significantly Correlate with Frequent Fever Episodes and Overall Phenotypic Severity
3.6. Infection Suspicion Is a Major Medical Care Trigger With Antibiotics Reported as the Most Common and Effective Infection Treatment in PMM2-CDG
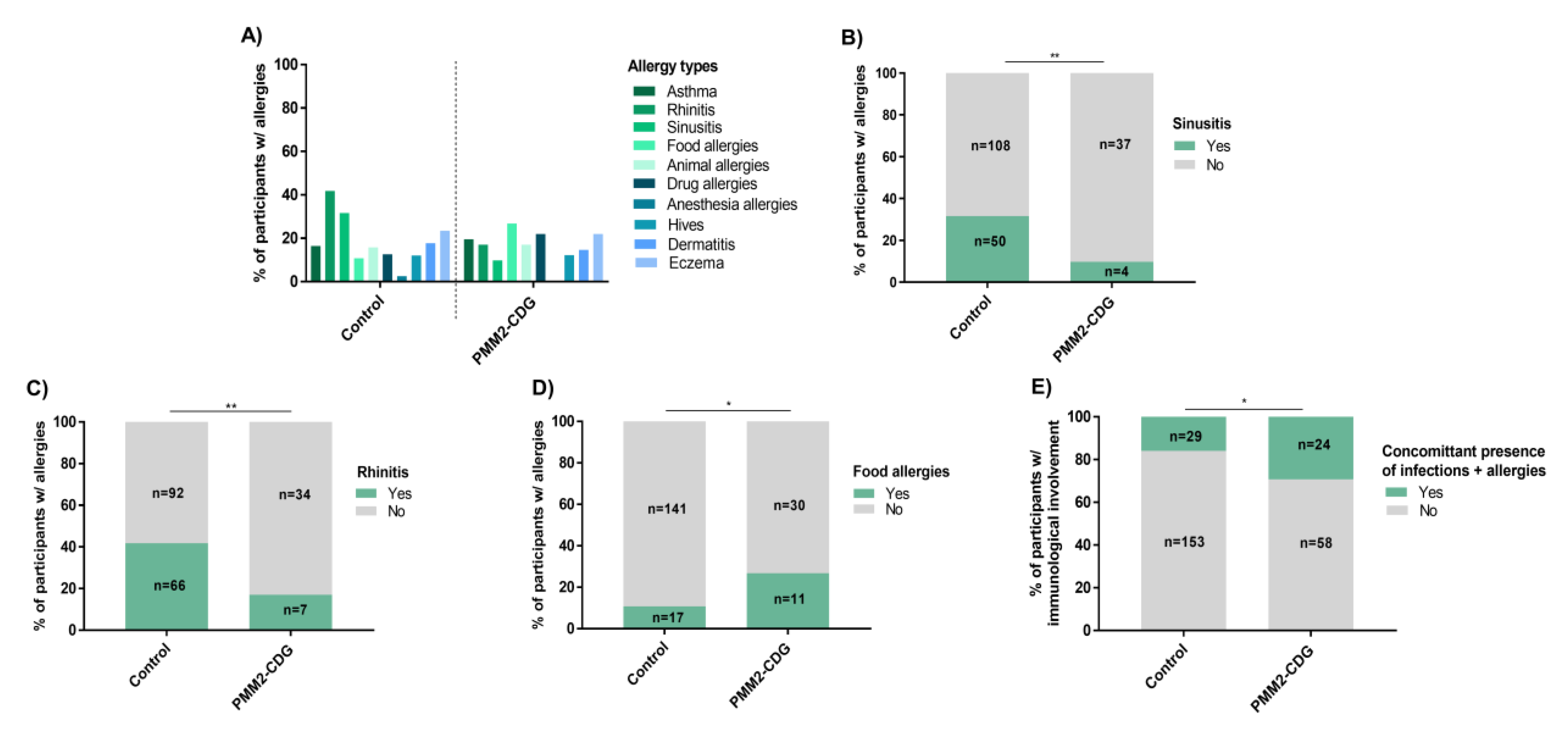
3.7. PMM2-CDG Have Low Overall Allergy Prevalence but a Significant Presence of Food Allergies
3.8. Infections and Allergies Associate with Relevant and Specific Non-Immune Phenotypic Features
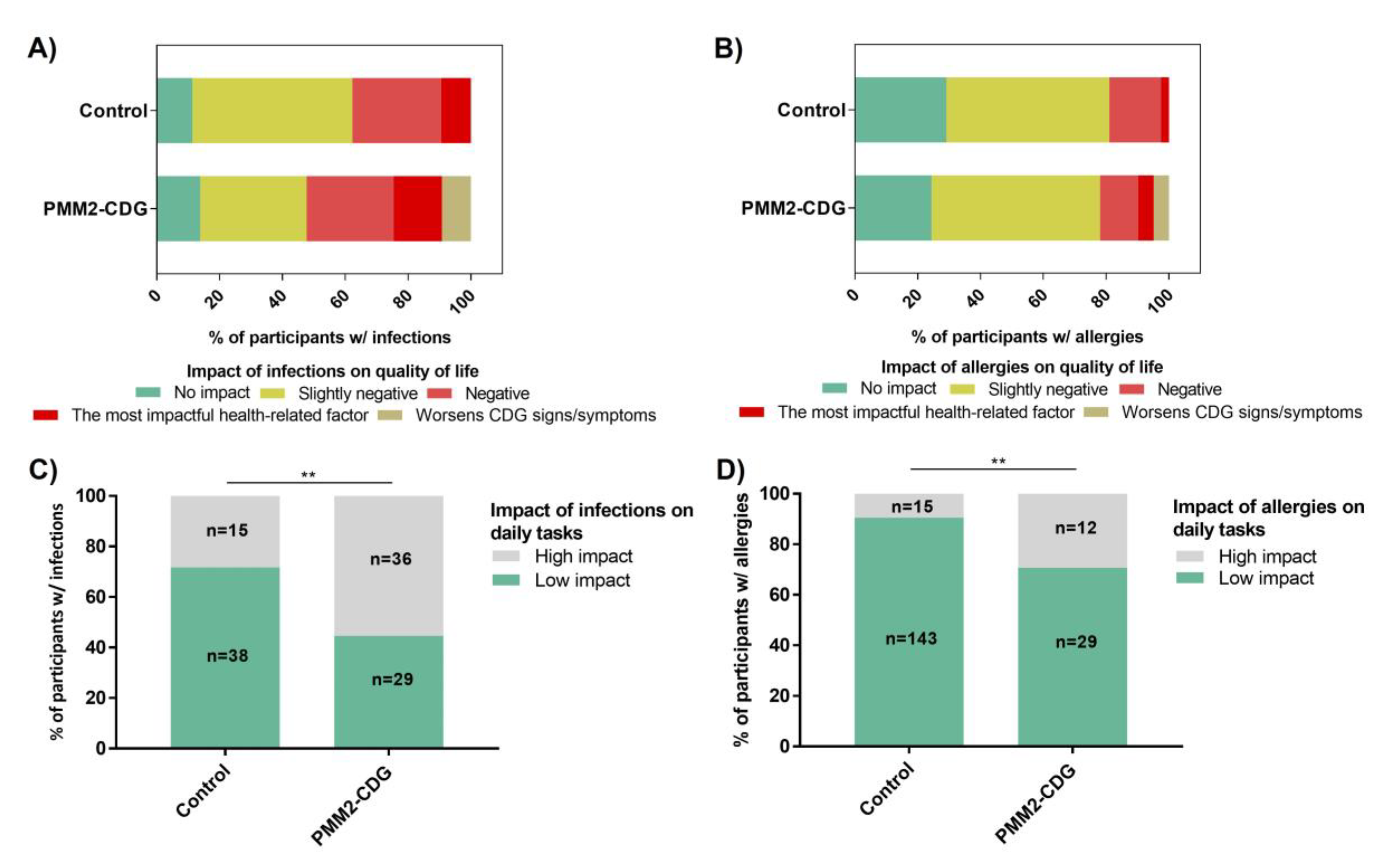
3.9. Infections and Allergies Negatively Impact the Daily Lives of PMM2-CDG Patients
3.10. No Clear Genotype-(Immune) Phenotype Correlation Identified in PMM2-CDG
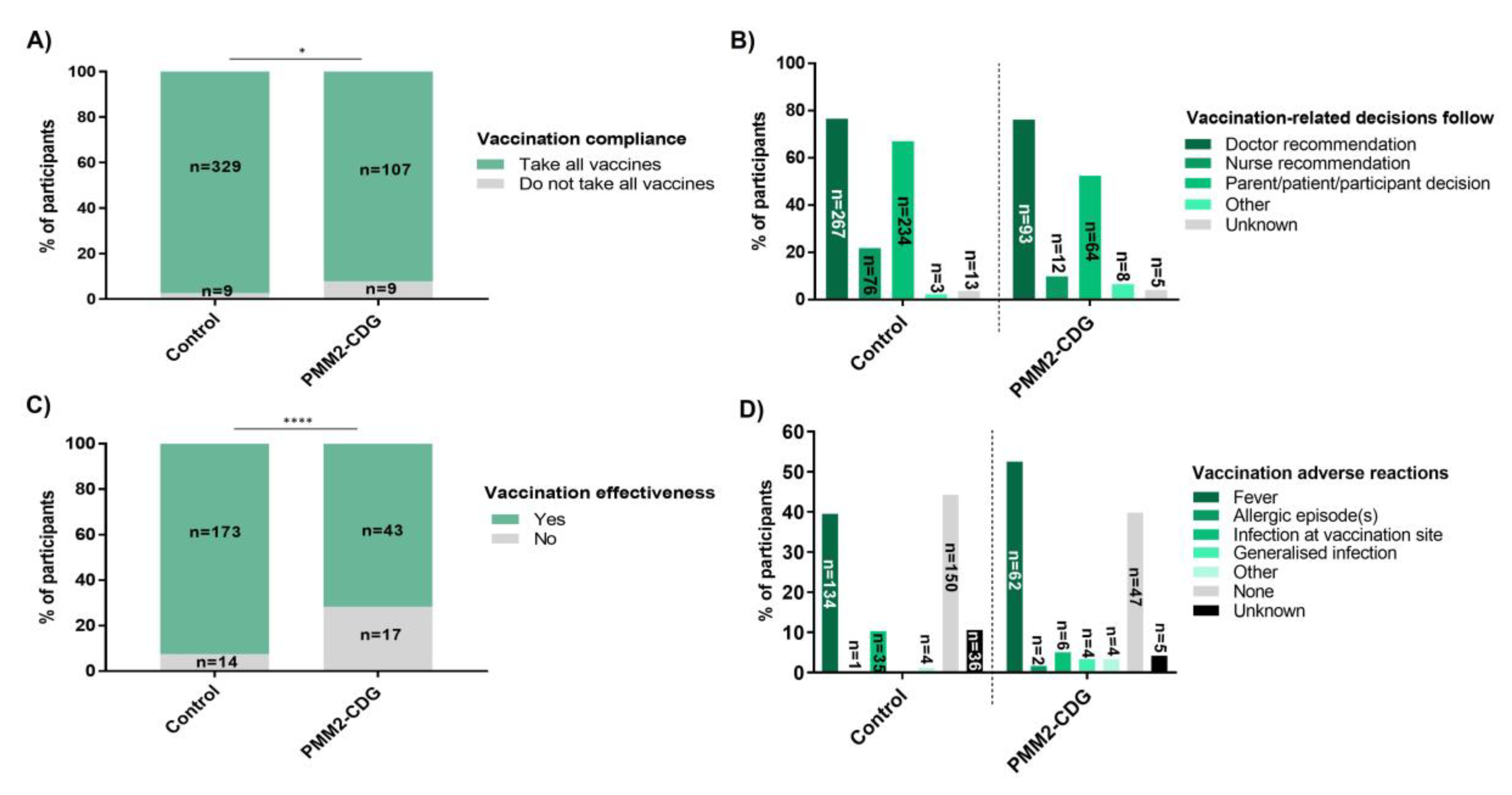
3.11. High Vaccination Compliance, with Greater Perceived Ineffectiveness and More Severe Adverse Reactions (ADRs) in PMM2-CDG
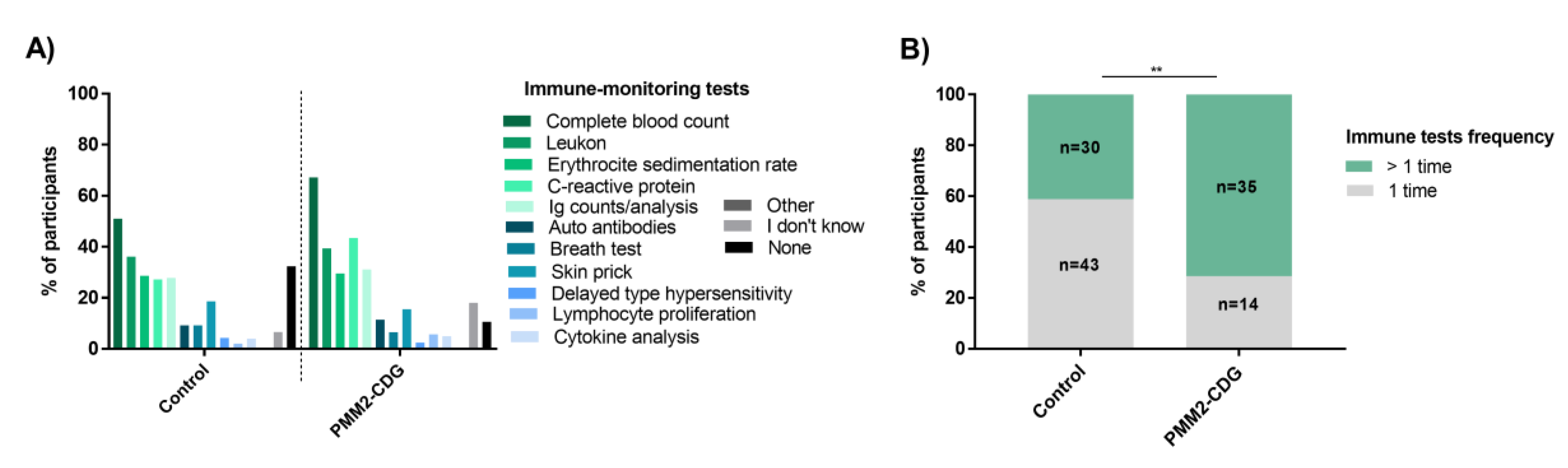
3.12. PMM2-CDG Participants Describe Few Immune Cell and Protein Altered Counts but Have Higher Immune Monitoring and Information Needs
3.13. Participants Reported Good Content Understandability, Management, and Glossary Usefulness
4. Discussion
4.1. Limitations of the Study
- -
- Possible sampling bias created by (a) overrepresentation of social media users and/or of people involved in health/science-related associations, and (b) increased likelihood of people experiencing immune-related disturbances participating. Actions to minimize these possibilities include: (i) developing an informative and multi-platform recruitment strategy; (ii) adding logic to specific questions which result in a longer questionnaire if immune issues were present.
- -
- Gender and adult prevalence differences between the PMM2-CDG and controls. However, random subsampling using the sample () R function was attempted and no significant differences were found. Moreover, many tests were done between sub-groups (e.g., participants with relevant infections) and not through the comparison of the complete samples. Detailed descriptive characterization of these sub-groups is always provided.
- -
- Age ranges implementation, especially without the definition of a maximum age limit in adults, can lead to loss of information and non-optimal group characterization. However, within the subsections of the questionnaires, we added questions that allowed for an age/time framing to help overcome this limitation. Furthermore, in the open question format, participants often did not specify whether they were referring to age in months or years, resulting in significant data loss [21].
- -
- Although the ImmunoCDGQ was built with the intent to capture data from all CDG, it was mainly focused on PMM2-CDG, by far the most frequent CDG.
- -
- A more comprehensive listing of the types of medications taken by CDG patients is needed to properly establish their contribution to the onset, aggravation or improvement of clinical signs and symptoms, especially of those related to the immune system.
4.2. Strengths of the Study
- -
- -
- In a short period of time, our approach allowed the collection of data on a highly neglected medical and scientific topic in CDG, allowing the generation of new data and knowledge in a time- and cost-efficient manner.
- -
- This project paves the way for further progress and validation of people/patient-centered research. It also shows that citizens, particularly rare disease patients and caregivers, are willing to be involved in research and share their unique knowledge.
- -
- This study also provided new data on QoL, highlighting the negative impact of immune-related manifestations—particularly of infections—on the daily life of the patients. This information helps to better understand the full impact of immune signs and symptoms in CDG.
- -
- An informative and education campaign on immunology (and CDG) preceded data collection. Moreover, many support measures and resources were developed to both stimulate participation and improve the understanding of the content. Hence, this study also had an impact on health literacy.
- -
- Our results point towards a new research avenue: the need for exploring GI (dys)function, more particularly the microbiome of PMM2-CDG patients since PMM2-CDG GI clinical signs were associated with all immune manifestations evaluated in this study. The role of the gut, most specifically of the microbiome, in the immune response and metabolism is just beginning to be uncovered, and our findings clearly indicate that PMM2-CDG pathophysiological comprehension could benefit from studies on this topic [92,93,94].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verheijen, J.; Wong, S.Y.; Rowe, J.H.; Raymond, K.; Stoddard, J.; Delmonte, O.M.; Bosticardo, M.; Dobbs, K.; Niemela, J.; Calzoni, E.; et al. Defining a new immune deficiency syndrome: MAN2B2-CDG. J. Allergy Clin. Immunol. 2020, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Francisco, R.; Marques-da-Silva, D.; Brasil, S.; Pascoal, C.; Dos Reis Ferreira, V.; Morava, E.; Jaeken, J. The challenge of CDG diagnosis. Mol. Genet. Metab. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Altassan, R.; Marques-Da-Silva, D.; Francisco, R.; Jaeken, J.; Morava, E. Recognizable phenotypes in CDG. J. Inherit. Metab. Dis. 2018, 41, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Bellai-Dussault, K.; Nguyen, T.T.M.; Baratang, N.V.; Jimenez-Cruz, D.A.; Campeau, P.M. Clinical variability in inherited glycosylphosphatidylinositol deficiency disorders. Clin. Genet. 2019, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.J.; He, M.; Lam, C.T. Congenital disorders of glycosylation. Ann. Transl. Med. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Péanne, R.; De Lonlay, P.; Foulquier, F.; Kornak, U.; Lefeber, D.J.; Morava, E.; Pérez, B.; Seta, N.; Thiel, C.; Van Schaftingen, E.; et al. Congenital disorders of glycosylation (CDG): Quo vadis? Eur. J. Med. Genet. 2018, 61, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Murthy, K.; Parton, Z.; Tsang, H.; Sam, F.S.; DiPrimio, N.; Lao, J.; Perlstein, E.O. Repurposing the aldose reductase inhibitor and diabetic neuropathy drug epalrestat for the congenital disorder of glycosylation PMM2-CDG. Dis. Models Mech. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Martínez-monseny, A.F.; Bolasell, M.; Callejón-póo, L.; Cuadras, D.; Freniche, V.; Itzep, D.C.; Gassiot, S.; Arango, P.; Casas-Alba, D.; La Morena, E.D.; et al. AZATAX: Acetazolamide safety and Ef fi cacy in cerebellar syndrome in PMM2 congenital disorder of glycosylation (PMM2-CDG). Ann. Neurol. 2019, 85, 740–751. [Google Scholar] [CrossRef]
- Brasil, S.; Pascoal, C.; Francisco, R.; Marques-da-Silva, D.; Andreotti, G.; Videira, P.A.; Morava, E.; Jaeken, J.; Dos Reis Ferreira, V. CDG therapies: From bench to bedside. Int. J. Mol. Sci. 2018, 19, 1304. [Google Scholar] [CrossRef]
- Yuste-Checa, P.; Brasil, S.; Gamez, A.; Underhaug, J.; Desviat, L.R.; Ugarte, M.; Perez-Cerda, C.; Martinez, A.; Perez, B. Pharmacological chaperoning: A potential treatment for PMM2-CDG. Hum. Mutat. 2017, 38, 160–168. [Google Scholar] [CrossRef]
- Jaeken, J.; Vanderschueren-Lodeweyckx, M.; Casaer, P. Familial psychomotor retardation with markedly fluctuating serum prolactin, FSH and GH levels, partial TBG deficiency, increased serum arylsulphatase A and increased CSF protein: A new syndrome? Pediatr. Res. 1980, 14, 179. [Google Scholar] [CrossRef]
- Vals, M.-A.; Pajusalu, S.; Kals, M.; Magi, R.; Ounap, K. The prevalence of PMM2-CDG in Estonia based on population carrier frequencies and diagnosed patients. JIMD Rep. 2018, 39, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Altassan, R.; Péanne, R.; Jaeken, J.; Barone, R.; Bidet, M.; Borgel, D.; Brasil, S.; Cassiman, D.; Cechova, A.; Coman, D.; et al. International clinical guidelines for the management of phosphomannomutase 2-congenital disorders of glycosylation: Diagnosis, treatment and follow up. J. Inherit. Metab. Dis. 2019, 42, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, C.; Francisco, R.; Ferro, T.; Dos Reis Ferreira, V.; Jaeken, J.; Videira, P.A. CDG and immune response: From bedside to bench and back. J. Inherit. Metab. Dis. 2019, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.; Roda, C.; Monin, M.-L.; Arion, A.; Barth, M.; Bednarek, N.; Bidet, M.; Bloch, C.; Boddaert, N.; Borgel, D.; et al. Clinical, laboratory and molecular findings and long-term follow-up data in 96 French patients with PMM2-CDG (Phosphomannomutase 2-congenital disorder of glycosylation) and review of the literature. J. Med. Genet. 2017, 54, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, M.; Ferro, T.; Dos Reis Ferreira, V.; Jaeken, J.; Videira, P.A. Immunological aspects of congenital disorders of glycosylation (CDG): A review. J. Inherit. Metab. Dis. 2016, 39, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Smith, L.; Hammer, D.; Fehrenbach, M.; DeLisser, H.; Perez, E.; Sullivan, K.E. Recurrent infections and immunological dysfunction in congenital disorder of glycosylation Ia (CDG Ia). J. Inherit. Metab. Dis. 2006, 29, 592. [Google Scholar] [CrossRef]
- Izquierdo-serra, M.; Martínez-Monseny, A.F.; López, L.; Carrillo-García, J.; Edo, A.; Ortigoza-Escobar, J.D.; García, Ó.; Cancho-Candela, R.; Carrasco-Marina, M.L.; Gutiérrez-Solana, L.G.; et al. Stroke-like episodes and cerebellar syndrome in phosphomannomutase deficiency (PMM2-CDG): Evidence for hypoglycosylation-driven channelopathy. Int. J. Mol. Sci. 2018, 19, 619. [Google Scholar] [CrossRef]
- De Haas, P.; Hendriks, W.J.A.J.; Lefeber, D.J.; Cambi, A. Biological and technical challenges in unraveling the role of N-Glycans in immune receptor regulation. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Robbins, D.A.; Curro, F.A.; Fox, C.H. Defining patient-centricity: Opportunities, challenges, and implications for clinical care and research. Ther. Innov. Regul. Sci. 2013, 47, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, D.; Francisco, R.; Dos Reis Ferreira, V.; Forbat, L.; Lagoa, R.; Videira, P.A.; Witters, P.; Jaeken, J.; Cassiman, D. An electronic questionnaire for liver assessment in congenital disorders of glycosylation (LeQCDG): A patient-centered study. JIMD Rep. 2018. [Google Scholar] [CrossRef]
- De Freitas, C.; Reis, V.; Silva, S.; Videira, P.A.; Morava, E. Public and patient involvement in needs assessment and social innovation: A people-centred approach to care and research for congenital disorders of glycosylation. BMC Health Serv. Res. 2017, 17, 682. [Google Scholar] [CrossRef]
- Tonsaker, T.; Bartlett, G.; Trpkov, C. Health information on the Internet: Gold mine or minefield? Can. Fam. Phys. 2014, 60, 407–408. [Google Scholar] [PubMed]
- Davies, W. Insights into rare diseases from social media surveys. Orphanet J. Rare Dis. 2016, 11, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, A.; Babajanyan, A.; Campbell, D.E.; Nanan, R. Validation of a comprehensive early childhood allergy questionnaire. Pediatr. Allergy Immunol. 2015, 26, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.-H.; Goldacker, S.; Haraldseide, J.; Großmann, K.; Gross, W.; Warnatz, K.; Grimbacher, B.; Rusch, S.; Nieters, A.; Vach, W. Construction and clinical validation of a questionnaire-based risk score to identify patients suffering from immunodeficiency or systemic autoimmunity. Br. J. Med. Med. Res. 2014, 4, 4751–4769. [Google Scholar] [CrossRef]
- Sievers, C.; Akmatov, M.K.; Kreienbrock, L.; Hille, K.; Ahrens, W.; Günther, K.; Flesch-Janys, D.; Obi, N.; Michels, K.B.; Fricke, J.; et al. Evaluation of a questionnaire to assess selected infectious diseases and their risk factors findings of a multicenter study. Bundesgesundheitsblatt Gesundh. Gesundh. 2014, 11, 1283–1291. [Google Scholar] [CrossRef]
- Aabenhus, R.; Thorsen, H.; Siersma, V.; Brodersen, J. The development and validation of a multidimensional sum-scaling questionnaire to measure patient-reported outcomes in acute respiratory tract infections in primary care: The acute respiratory tract infection questionnaire. Value Health 2013, 16, 987–992. [Google Scholar] [CrossRef]
- Loubet, P.; Kernéis, S.; Groh, M.; Loulergue, P.; Blanche, P.; Verger, P.; Launay, O. Attitude, knowledge and factors associated with influenza and pneumococcal vaccine uptake in a large cohort of patients with secondary immune deficiency. Vaccine 2015, 33, 3703–3708. [Google Scholar] [CrossRef]
- Champely, S.; Ekstrom, C.; Dalgaard, P.; Gill, J.; Weibelzahl, S.; Anandkumar, A.; Ford, C.; Volcic, R.; De Rosa, H. pwr: Basic Functions for Power Analysis. 2020. Available online: https://github.com/heliosdrm/pwr (accessed on 28 March 2020).
- Stenson, P.D.; Mort, M.; Ball, E.V.; Evans, K.; Hayden, M.; Heywood, S.; Hussain, M.; Phillips, A.D.; Cooper, D.N. The human gene mutation database: Towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 2017, 136, 665–677. [Google Scholar] [CrossRef]
- Fokkema, I.F.A.C.; Taschner, P.E.M.; Schaafsma, G.C.P.; Celli, J.; Laros, J.F.J.; Den Dunnen, J.T. LOVD v.2.0: The next generation in gene variant databases. Hum. Mutat. 2011, 32, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; De Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Ng, B.G.; Sosicka, P.; Agadi, S.; Almannai, M.; Bacino, C.A.; Barone, R.; Botto, L.D.; Burton, J.E.; Carlston, C.; Chung, B.H.-Y.; et al. SLC35A2-CDG: Functional characterization, expanded molecular, clinical, and biochemical phenotypes of 30 unreported individuals. Hum. Mutat. 2019, 40, 908–925. [Google Scholar] [CrossRef] [PubMed]
- Monin, M.-L.; Mignot, C.; De Lonlay, P.; Heron, B.; Masurel, A.; Mathieu-Dramard, M.; Lenaerts, C.; Thauvin, C.; Gerard, M.; Roze, E.; et al. 29 French adult patients with PMM2-congenital disorder of glycosylation: Outcome of the classical pediatric phenotype and depiction of a late-onset phenotype. Orphanet J. Rare Dis. 2014, 9, 207. [Google Scholar] [CrossRef]
- Krasnewich, D.; Brien, K.O.; Sparks, S. Clinical features in adults with congenital disorders of glycosylation type Ia (CDG-Ia). Am. J. Med. Genet. Part C Semin. Med. Genet. 2007, 145 Pt C, 302–306. [Google Scholar] [CrossRef]
- Pearson, E.V.; Waite, J.; Oliver, C. Differences in the information needs of parents with a child with a genetic syndrome: A cross-syndrome comparison. J. Policy Pract. Intellect. Disabil. 2018, 15, 94–100. [Google Scholar] [CrossRef]
- Witters, P.; Tahata, S.; Barone, R.; Õunap, K.; Salvarinova, R.; Grønborg, S.; Hoganson, G.; Scaglia, F.; Lewis, A.M.; Mori, M.; et al. Clinical and biochemical improvement with galactose supplementation in SLC35A2-CDG. Genet. Med. 2020, 1–6. [Google Scholar] [CrossRef]
- Witters, P.; Cassiman, D.; Morava, E. Nutritional therapies in congenital disorders of glycosylation (CDG). Nutrients 2018, 9, 1222. [Google Scholar] [CrossRef]
- Witters, P.; Honzik, T.; Bauchart, E.; Altassan, R.; Pascreau, T.; Bruneel, A.; Vuillaumier, S.; Seta, N.; Borgel, D.; Matthijs, G.; et al. Long-term follow-up in PMM2-CDG: Are we ready to start treatment trials? Genet. Med. 2018, 21, 1181–1188. [Google Scholar] [CrossRef]
- De Lonlay, P.; Seta, N.; Barrot, S.; Chabrol, B.; Drouin, V.; Gabriel, B.M.; Journel, H.; Kretz, M.; Laurent, J.; Le Merrer, M.; et al. A broad spectrum of clinical presentations in congenital disorders of glycosylation I: A series of 26 cases. J. Med. Genet. 2001, 38, 14–19. [Google Scholar] [CrossRef]
- Wang, J. The sticky relationship between allergies and infections. Asia Pac. Allergy 2015, 5, 133–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Custovic, A.; Murray, C.; Simpson, A. Allergy and infection: Understanding their relationship. Allergy 2005, 60, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, C.; Brasil, S.; Francisco, R.; Marques-da-Silva, D.; Rafalko, A.; Jaeken, J.; Videira, P.A.; Barros, L.; Dos Reis Ferreira, V. Patient and observer reported outcome measures to evaluate health-related quality of life in inherited metabolic diseases: A scoping review. Orphanet J. Rare Dis. 2018, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Gluvic, Z.; Petrovic, N.; Obradovic, M.; Tomasevic, R.; Dugalic, P.; Isenovic, E.R. A quality of life assessment and the correlation between generic and disease-specific questionnaires scores in outpatients with chronic liver disease-pilot study. Rom. J. Intern. Med. 2017, 55, 129–137. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. Global Vaccine Safety. 2019. Available online: https://www.who.int/vaccine_safety/initiative/tools/vaccinfosheets/en/ (accessed on 27 March 2020).
- Smith, M.J.; Reiter, M.J.; Crist, B.D.; Schultz, L.G.; Choma, T.J. Improving patient satisfaction through computer-based questionnaires. Orthopedics 2016, 39, 31–35. [Google Scholar] [CrossRef]
- He, P.; Srikrishna, G.; Freeze, H.H. N-glycosylation deficiency reduces ICAM-1 induction and impairs inflammatory response. Glycobiology 2014, 24, 392–398. [Google Scholar] [CrossRef]
- Van Dijk, W.; Koeleman, C.; Van het Hof, B.; Poland, D.; Jakobs, C.; Jaeken, J. Increased K 3-fucosylation of K 1-acid glycoprotein in patients with congenital disorder of glycosylation type IA (CDG-Ia). FEBS Lett. 2001, 494, 232–235. [Google Scholar] [CrossRef]
- Garcia-Lopez, R.; De la Morena-Barrio, M.E.; Alsina, L.; Perez-Duenas, B.; Jaeken, J.; Serrano, M.; Casado, M.; Hernandez-Caselles, T. Natural killer cell receptors and cytotoxic activity in phosphomannomutase 2 deficiency (PMM2-CDG). PLoS ONE 2016, 11, e0158863. [Google Scholar] [CrossRef][Green Version]
- Wu, R.; Li, D.; Tang, W.; Qiu, K.; Li, Y.; Liao, X.; Tang, D.; Qin, L.; Deng, B.; Luo, X. Atrial septal defect in a patient with congenital disorder of glycosylation type 1a: A case report. J. Med. Case Rep. 2018, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Korf, C.M.; Dale, R.C. The immune system in pediatric seizures and epilepsies. Pediatrics 2017, 140, e20163534. [Google Scholar] [CrossRef]
- Vezzani, A.; Lang, B.; Aronica, E. Immunity and inflammation in epilepsy. Cold Spring Harb Perspect. Med. 2016, 6, a022699. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.D.; Buijs, R.M.; Sitges, M. The anti-seizure drugs vinpocetine and carbamazepina, but not valproic acid, reduce inflammatory IL-1B and TNF-a expression in rat hippocampus. J. Neurochem. 2014, 130, 770–779. [Google Scholar] [CrossRef]
- Ximenes, J.C.M.; Gonçalves, D.D.O.; Siqueira, R.M.P.; Neves, K.R.T.; Cerqueira, G.S.; Correia, A.O.; Félix, F.H.C.; Leal, L.K.A.M.; De Castro Brito, G.A.; da Graca Naffah-Mazzacorati, M.; et al. Valproic acid: An anticonvulsant drug with potent antinociceptive and anti-inflammatory properties. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 575–587. [Google Scholar] [CrossRef]
- Stienen, M.N.; Haghikia, A.; Dambach, H.; Thöne, J.; Wiemann, M.; Gold, R.; Chan, A.; Dermietzel, R.; Faustmann, P.M.; Hinkerohe, D.; et al. Anti-inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGFb1 regulation. Br. J. Pharmacol. 2011, 162, 491–507. [Google Scholar] [CrossRef]
- Truin, G.; Guillard, M.; Lefeber, D.J.; Sykut-Cegielska, J.; Adamowicz, M.; Hoppenreijs, E.; Sengers, R.C.A.; Wevers, R.A.; Morava, E. Pericardial and abdominal fluid accumulation in Congenital Disorder of Glycosylation type Ia. Mol. Genet. Metab. 2008, 94, 481–484. [Google Scholar] [CrossRef]
- Noelle, V.; Knuepfer, M.; Pulzer, F.; Schuster, V.; Siekmeyer, W.; Matthijs, G.; Vogtmann, C. Unusual presentation of congenital disorder of glycosylation type 1a: Congenital persistent thrombocytopenia, hypertrophic cardiomyopathy and hydrops-like aspect due to marked peripheral oedema. Eur. J. Pediatr. 2005, 164, 223–226. [Google Scholar] [CrossRef]
- Kjaergaard, S.; Schwartz, M.; Skovby, F. Congenital disorder of glycosylation type Ia (CDG-Ia): Phenotypic spectrum of the R141H/F119L genotype. Arch. Dis. Child. 2001, 85, 236–239. [Google Scholar] [CrossRef]
- Altassan, R.; Witters, P.; Saifudeen, Z.; Quelhas, D.; Jaeken, J.; Levtchenko, E.; Cassiman, D.; Morava, E. Renal involvement in PMM2-CDG, a mini-review. Mol. Genet. Metab. 2018, 123, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Carrozzi, M.; Parini, R.; Battini, R.; Martinelli, D.; Elia, M.; Spada, M.; Lilliu, F.; Ciana, G.; Burlina, A.; et al. A nationwide survey of PMM2-CDG in Italy: High frequency of a mild neurological variant associated with the L32R mutation. J. Neurol. 2015, 262, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Citro, V.; Cimmaruta, C.; Monticelli, M.; Riccio, G.; Mele, B.H.; Cubellis, M.V.; Andreotti, G. The analysis of variants in the general population reveals that PMM2 is extremely tolerant to missense mutations and that diagnosis of PMM2-CDG can benefit from the identification of modifiers. Int. J. Mol. Sci. 2018, 19, 2218. [Google Scholar] [CrossRef] [PubMed]
- Westphal, V.; Peterson, S.; Patterson, M.; Tournay, A.; Blumenthal, A.; Treacy, E.P.; Freeze, H.H. Functional significance of PMM2 mutations in mildly affected patients with congenital disorders of glycosylation Ia. Genet. Med. 2001, 3, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Shang, J.; Huynh, D.; Manthati, V.L.; Arias, C.; Harding, H.P.; Kaufman, R.J.; Mohr, I.; Ron, D.; Falck, J.R.; et al. Mannose-6-phosphate regulates destruction of lipid-linked oligosaccharides. Mol. Biol. Cell 2011, 22, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Block, T.M.; Guo, J.-T. Viral resistance of MOGS-CDG patients implies a broad-spectrum strategy against acute virus infections. Antivir. Ther. 2015, 20, 257–259. [Google Scholar] [CrossRef]
- Dauber, A.; Ercan, A.; Lee, J.; James, P.; Jacobs, P.P.; Ashline, D.J.; Wang, S.R.; Miller, T.; Hirschhorn, J.N.; Nigrovic, P.A.; et al. Congenital disorder of fucosylation type 2c (LADII) presenting with short stature and developmental delay with minimal adhesion defect. Hum. Mol. Genet. 2014, 23, 2880–2887. [Google Scholar] [CrossRef]
- Aghamohammadi, A.; Abolhassani, H.; Mohammadinejad, P.; Rezaei, N. The approach to children with recurrent infections. Iran. J. Allergy Asthma Immunol. 2012, 11, 89–109. [Google Scholar]
- Stray-Pedersen, A.; Backe, P.H.; Sorte, H.S.; Morkrid, L.; Chokshi, N.Y.; Erichsen, H.C.; Gambin, T.; Elgstoen, K.B.P.; Bjoras, M.; Wlodarski, M.W.; et al. PGM3 mutations cause a congenital disorder of glycosylation with severe immunodeficiency and skeletal dysplasia. Am. J. Hum. Genet. 2014, 95, 1–12. [Google Scholar] [CrossRef]
- Al-Ghouleh, A.; Johal, R.; Sharquie, I.K.; Emara, M.; Harrington, H.; Shakib, F.; Ghaemmaghami, A.M. The glycosylation pattern of common allergens: The recognition and uptake of Der p 1 by epithelial and dendritic cells is carbohydrate dependent. PLoS ONE 2012, 7, e33929. [Google Scholar] [CrossRef]
- Shade, K.T.C.; Conroy, M.E.; Washburn, N.; Kitaoka, M.; Huynh, D.J.; Laprise, E.; Patil, S.U.; Shreffler, W.G.; Anthony, R.M. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature 2020, 582, 265–270. [Google Scholar] [CrossRef]
- Cartledge, N.; Chan, S. Atopic dermatitis and food allergy: A paediatric approach. Curr. Pediatr. Rev. 2018, 14, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Charles, J.F. Gut microbiome and bone: To build, destroy or both? Curr. Osteoporos. Rep. 2017, 15, 376–384. [Google Scholar] [CrossRef]
- Yang, D.-H.; Yang, M.-Y. The role of macrophage in the pathogenesis of osteoporosis. Int. J. Mol. Sci. 2019, 20, 2093. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.K.; Silverberg, J.I. Eczema is associated with osteoporosis and fractures in adults: A US population-based study. J. Allergy Clin. Immunol. 2014, 135, 2–6. [Google Scholar] [CrossRef]
- Ledford, D.; Apter, A.; Brenner, A.M.; Rubin, K.; Prestwood, K.; Frieri, M. Osteoporosis in the corticosteroid-treated patient with asthma. J. Allergy Clin. Immunol. 1998, 102, 353–362. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Takeda, J.; Tarutani, M.; Uchida, Y.; Holleran, W.M.; Endo, Y.; Elias, P.M.; Inoue, S. Epidermal-specific defect of GPI anchor in Pig-a null mice results in harlequin ichthyosis-like features. J. Invest. Dermatol. 2004, 123, 464–469. [Google Scholar] [CrossRef]
- Mclean, W.H.I. Filaggrin failure–From ichthyosis vulgaris to atopic eczema and beyond. Br. J. Dermatol. 2016, 175, 4–7. [Google Scholar] [CrossRef]
- Pereira, M.S.; Alves, I.; Vicente, M.; Campar, A. Glycans as key checkpoints of T cell activity and function. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and the altered glycan theory of autoimmunity: A critical review. J. Autoimmun. 2015, 1–13. [Google Scholar] [CrossRef]
- Wyrick-Glatzel, J.; MacDonald, J.K.; Chen, J.-J. Paroxysmal nocturnal hemoglobinemia:A molecular definition of the clinical biology of the disorder. Labmedicine 2006, 37, 237–243. [Google Scholar] [CrossRef]
- Ohya, T.; Nagai, T.; Araki, Y.; Yanagawa, T.; Tanabe, T.; Iyoda, K.; Kurihara, M.; Yamamoto, K.; Masunaga, K.; Iizuka, C.; et al. A pilot study on the changes in immunity after ACTH therapy in patients with West syndrome. Brain Dev. 2009, 31, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Grünert, S.C.; Marquardt, T.; Lausch, E.; Fuchs, H.; Thiel, C.; Sutter, M.; Schumann, A.; Hannibal, L.; Spiekerkoetter, U. Unsuccessful intravenous D-mannose treatment in PMM2-CDG. Orphanet J. Rare Dis. 2019, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Van Kempen, A.A.M.W.; Van der Heide, M.; Poll-The, B.T.; Van Slooten, H.J.; Troost, D.; Rozemuller-Kwakkel, J.M. Congenital disorder of glycosylation type Ia: A clinicopathological report of a newborn infant with cerebellar pathology. Acta Neuropathol. 2005, 109, 433–442. [Google Scholar] [CrossRef] [PubMed]
- De la Morena-Barrio, M.E.; Hernandez-Caselles, T.; Corral, J.; Garcia-Lopez, R.; Martinez-Martinez, I.; Perez-Duenas, B.; Altisent, C.; Sevivas, T.; Kristensen, S.R.; Guillen-Navarro, E.; et al. GPI-anchor and GPI-anchored protein expression in PMM2-CDG patients. Orphanet J. Rare Dis. 2013, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Kayser, L.; Karnoe, A.; Duminski, E.; Somekh, D.; Vera-Muñoz, C. A new understanding of health related empowerment in the context of an active and healthy ageing. BMC Health Serv. Res. 2019, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Chiauzzi, E.; Dasmahapatra, P.; Cochin, E.; Bunce, M. Factors in patient empowerment: A survey of an online patient research network. Patient Patient Cent. Outcomes Res. 2016, 9, 511–523. [Google Scholar] [CrossRef]
- Chatzimarkakis, J. Why patients should be more empowered: A european perspective on lessons learned in the management of diabetes. J. Diabetes Sci. Technol. 2010, 4, 1570–1573. [Google Scholar] [CrossRef]
- Pritchard, D.E.; Moeckel, F.; Villa, M.S.; Housman, L.T.; McCarty, C.A.; Mcleod, H.L. Strategies for integrating personalized medicine into healthcare practice. Per. Med. 2017, 14, 141–152. [Google Scholar] [CrossRef]
- Young, K.; Kaminstein, D.; Olivos, A.; Burroughs, C.; Castillo-Lee, C.; Kullman, J.; Mcalear, C.; Shaw, D.G.; Sreih, A.; Casey, G.; et al. Patient involvement in medical research: What patients and physicians learn from each other. Orphanet J. Rare Dis. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; De Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Mezouar, S.; Chantran, Y.; Michel, J.; Fabre, A.; Dubus, J.-C.; Leone, M.; Sereme, Y.; Mège, J.-L.; Ranque, S.; Desnues, B.; et al. Microbiome and the immune system: From a healthy steady-state to allergy associated disruption. Hum. Microbiome J. 2018, 10, 11–20. [Google Scholar] [CrossRef]

| Control | PMM2-CDG | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impact QoL | p-Value/OR | Impact Everyday Tasks | p-Value/OR | Impact QoL | p-Value/OR | Impact Everyday Tasks | p-Value/OR | ||||||
| High | Low | High | Low | High | Low | High | Low | ||||||
| Infection severity | Mild | 14.81% (n = 4) | 85.19% (n = 23) | 0.00048/9.69 | 11.11% (n = 3) | 88.89% (n = 24) | 0.0053/7.09 | 11.76% (n = 2) | 88.24% (n = 15) | 6.425 × 10−5/18.24 | 35.29% (n = 6) | 64.71% (n = 11) | 0.017/4.45 |
| Severe | 64% (n = 16) | 36% (n = 9) | 48% (n = 12) | 52% (n = 13) | 72.22% (n = 26) 1 | 27.78% (n = 10) | 71.43% (n = 30) | 28.57% (n = 12) | |||||
| Allergy severity | Mild | 9% (n = 9) | 91% (n = 91) | 1.99 × 10−5/6.75 | 2% (n = 2) | 98% (n = 98) | 2.05 × 10−5/16.44 | 5% (n = 1) | 95% (n = 19) | 0.012/13.09 | 15% (n = 3) | 75% (n = 17) | 0.014/6.84 |
| Severe | 40.43% (n = 19) | 59.57% (n = 28) | 25.53% (n = 12) | 74.47 (n = 35) | 42.86% (n = 6) 2 | 57.14% (n = 8) | 56.25% (n = 9) | 43.75% (n = 7) | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisco, R.; Pascoal, C.; Marques-da-Silva, D.; Brasil, S.; Pimentel-Santos, F.M.; Altassan, R.; Jaeken, J.; Grosso, A.R.; dos Reis Ferreira, V.; Videira, P.A. New Insights into Immunological Involvement in Congenital Disorders of Glycosylation (CDG) from a People-Centric Approach. J. Clin. Med. 2020, 9, 2092. https://doi.org/10.3390/jcm9072092
Francisco R, Pascoal C, Marques-da-Silva D, Brasil S, Pimentel-Santos FM, Altassan R, Jaeken J, Grosso AR, dos Reis Ferreira V, Videira PA. New Insights into Immunological Involvement in Congenital Disorders of Glycosylation (CDG) from a People-Centric Approach. Journal of Clinical Medicine. 2020; 9(7):2092. https://doi.org/10.3390/jcm9072092
Chicago/Turabian StyleFrancisco, Rita, Carlota Pascoal, Dorinda Marques-da-Silva, Sandra Brasil, Fernando M. Pimentel-Santos, Ruqaiah Altassan, Jaak Jaeken, Ana Rita Grosso, Vanessa dos Reis Ferreira, and Paula A. Videira. 2020. "New Insights into Immunological Involvement in Congenital Disorders of Glycosylation (CDG) from a People-Centric Approach" Journal of Clinical Medicine 9, no. 7: 2092. https://doi.org/10.3390/jcm9072092
APA StyleFrancisco, R., Pascoal, C., Marques-da-Silva, D., Brasil, S., Pimentel-Santos, F. M., Altassan, R., Jaeken, J., Grosso, A. R., dos Reis Ferreira, V., & Videira, P. A. (2020). New Insights into Immunological Involvement in Congenital Disorders of Glycosylation (CDG) from a People-Centric Approach. Journal of Clinical Medicine, 9(7), 2092. https://doi.org/10.3390/jcm9072092






