Increasing γ-Aminobutyric Acid Content in Vegetable Soybeans via High-Pressure Processing and Efficacy of Their Antidepressant-Like Activity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. High-Performance Liquid Chromatography (HPLC) Determination of GABA and L-Glutamate Contents
2.2.2. Extraction and HPLC Analysis of GABA
2.2.3. Animal Experiments
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Optimal Conditions for HPP of Vegetable Soybeans
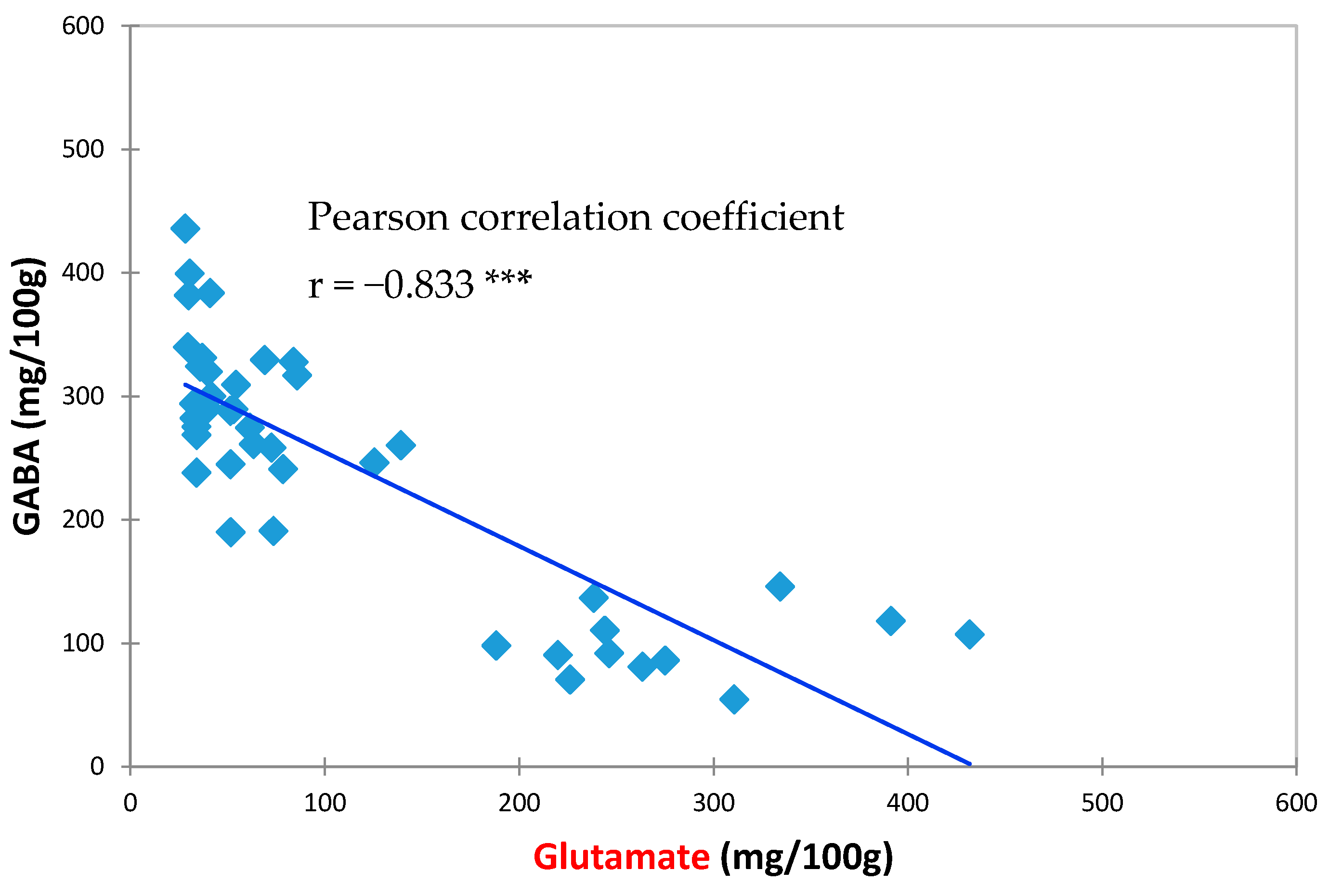
3.2. Effects of HPP Treatment on GABA and Glutamate Contents in Vegetable Soybeans
3.3. Animal Experiments
3.3.1. Weight Changes during the Feeding Period
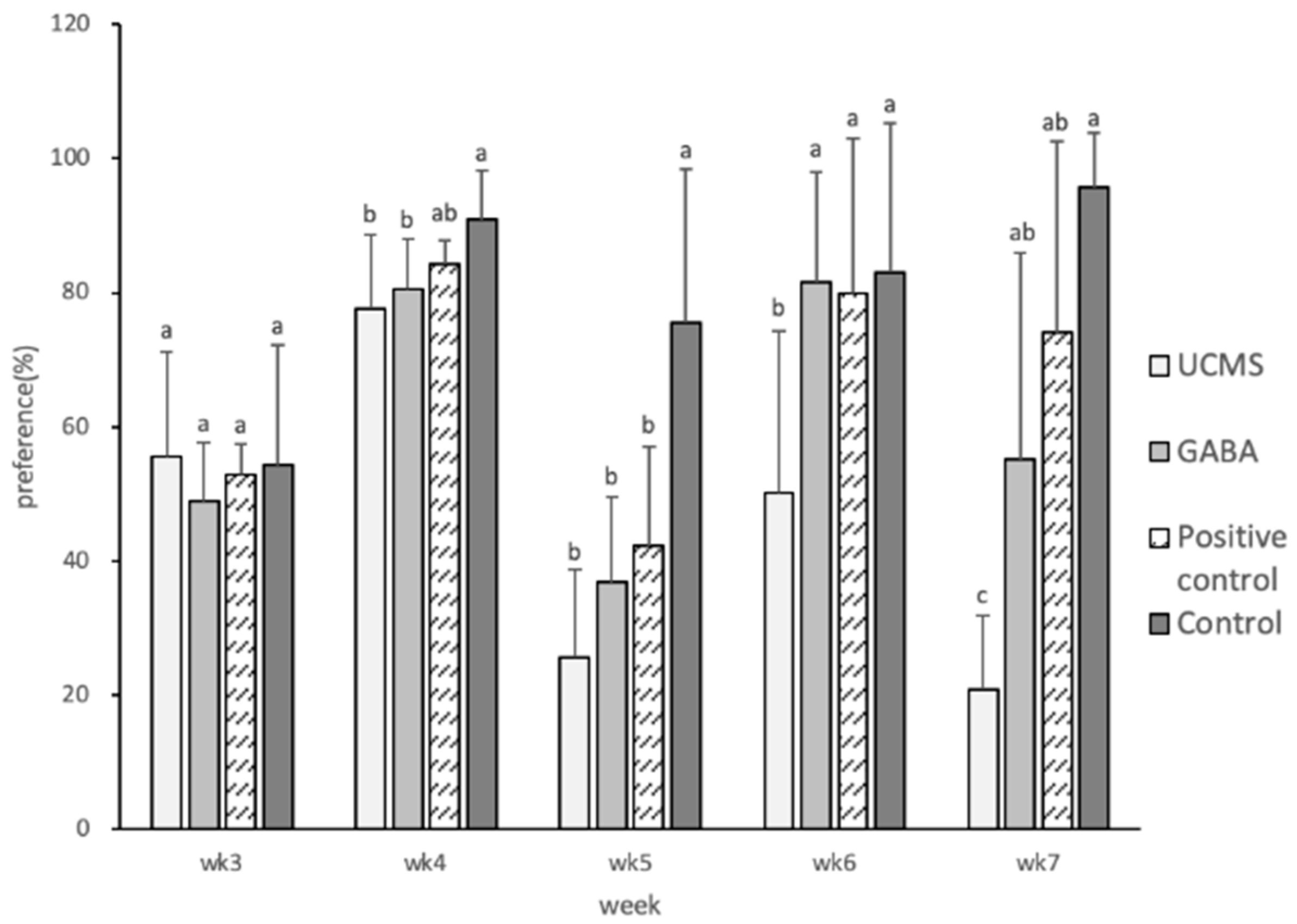
3.3.2. Degree of Sucrose Preference
3.3.3. Tail Suspension Test
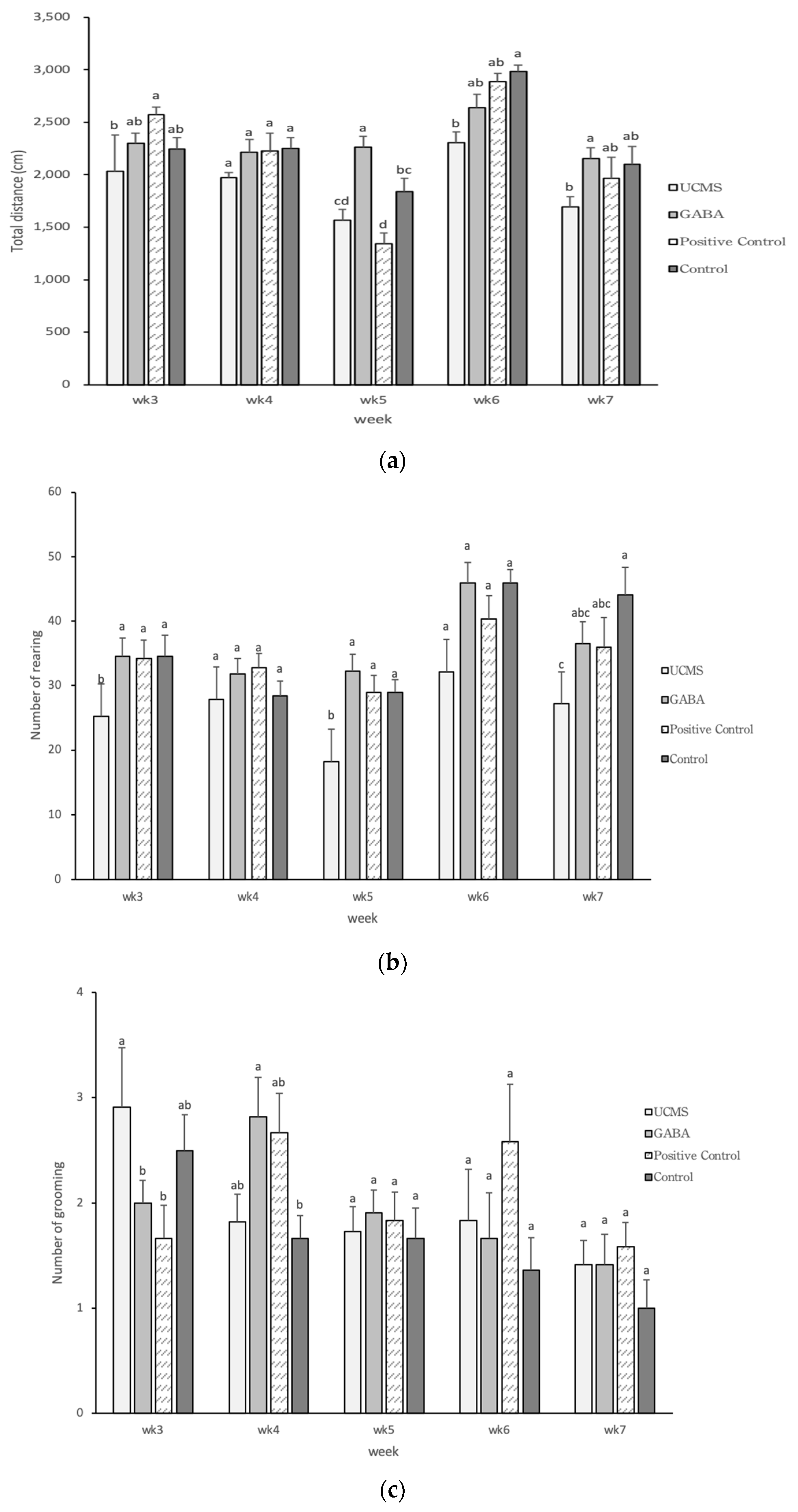
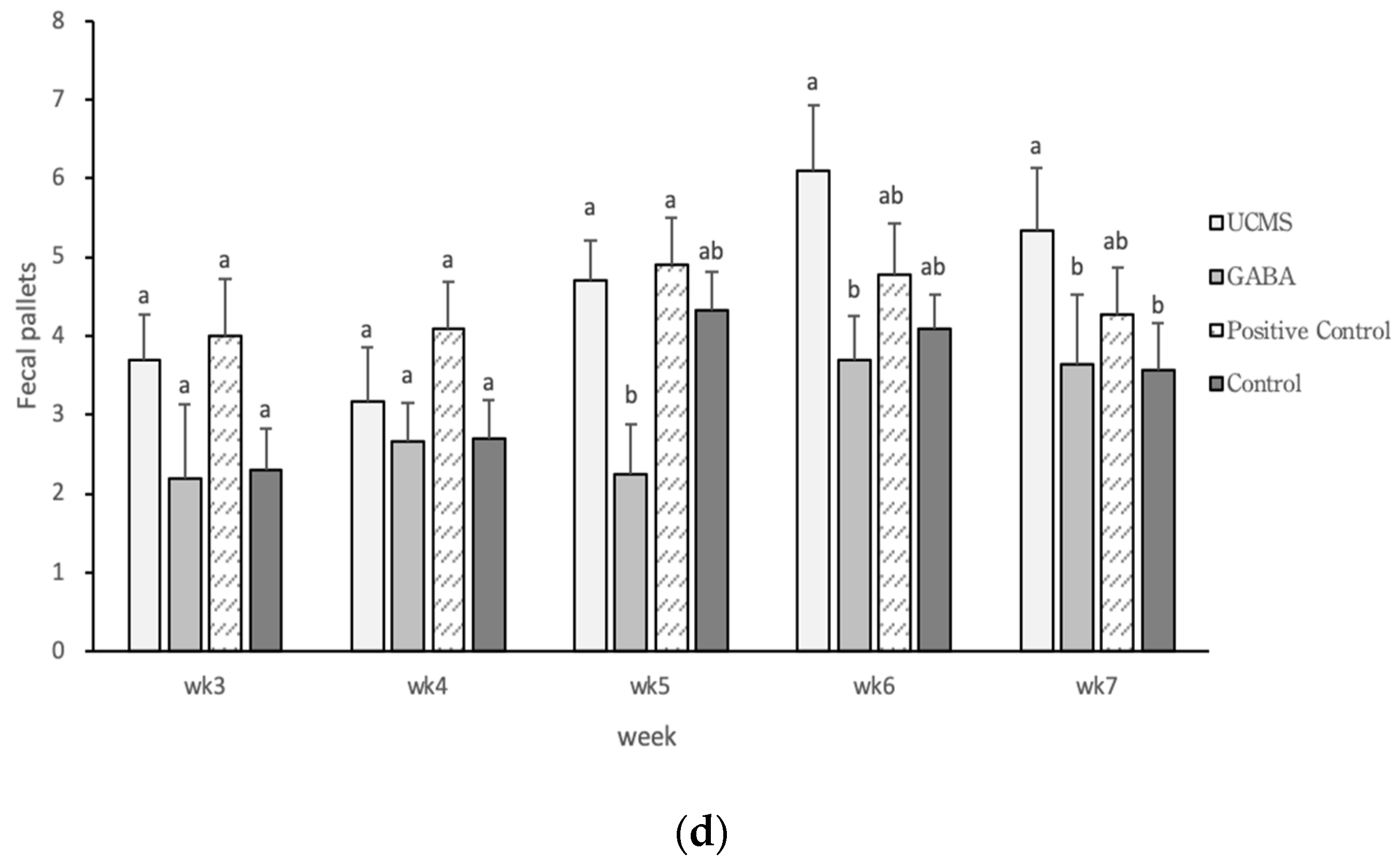
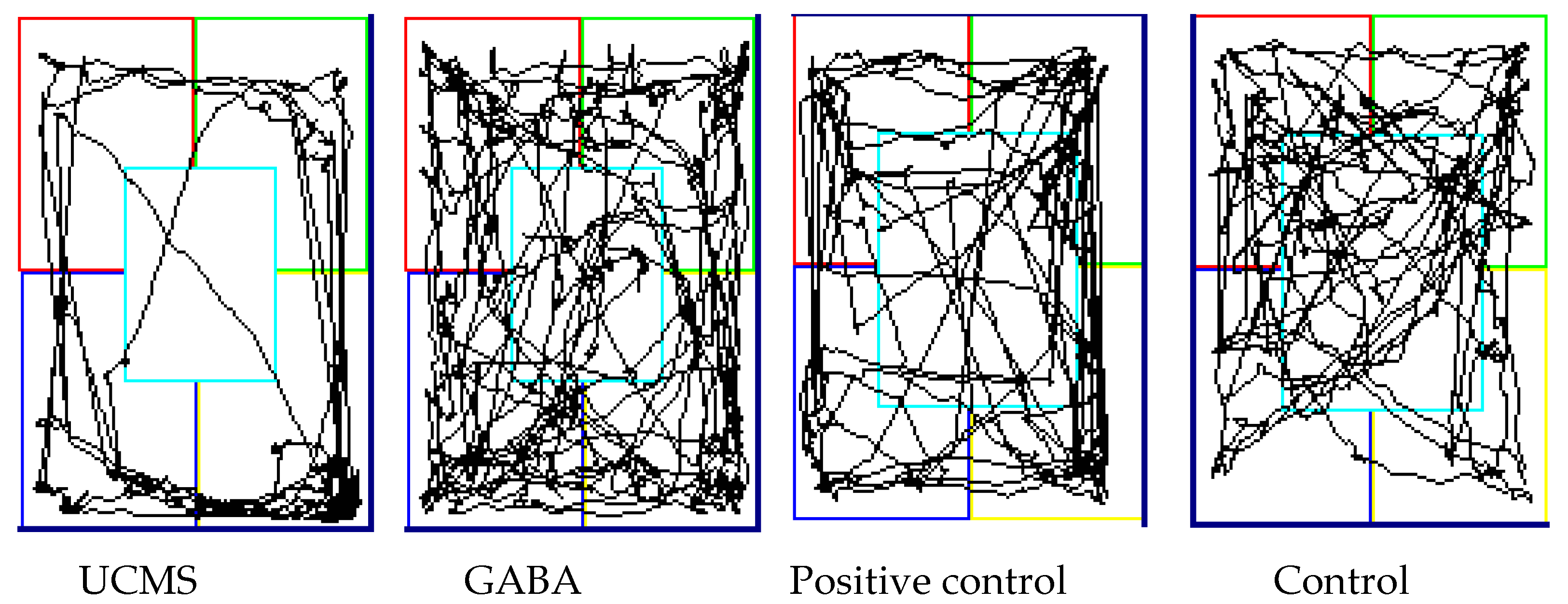
3.3.4. Open Field Test
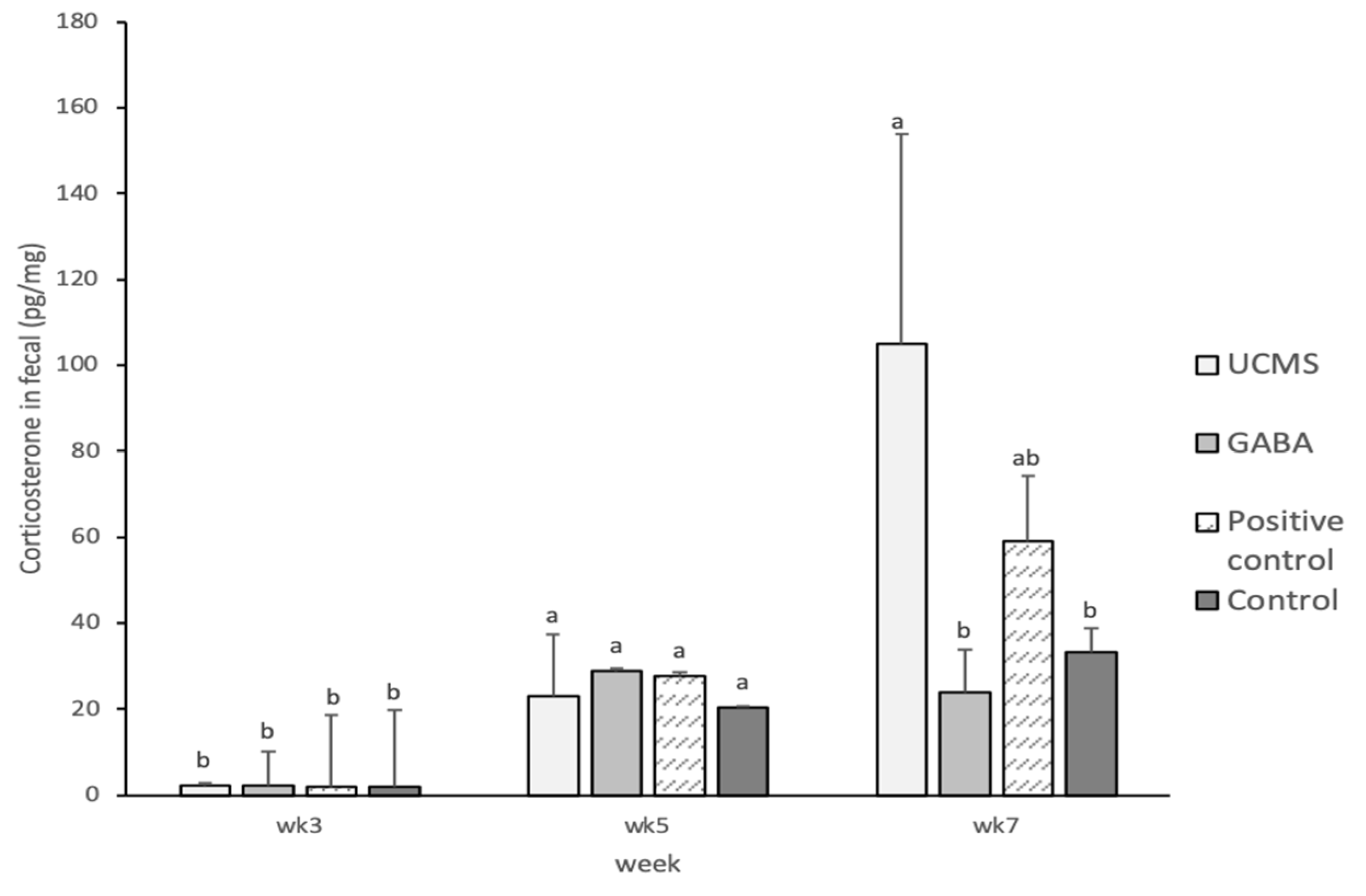
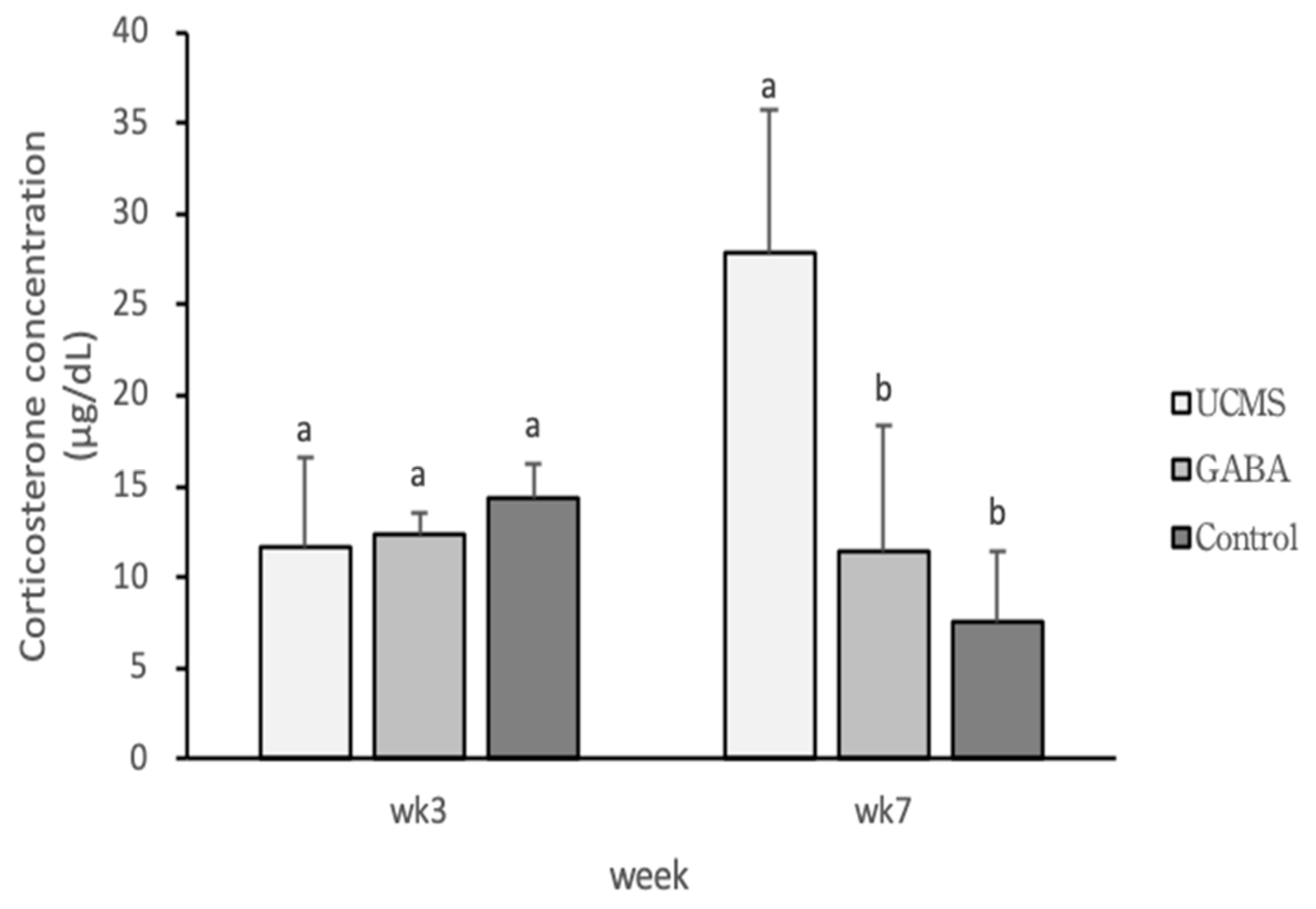
3.3.5. Biochemical Parameter Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, V.R. Soy protein in relation to human protein and amino acid nutrition. J. Am. Diet. Assoc. 1991, 91, 828–835. [Google Scholar] [PubMed]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Lian, D.J. The nutrition and utility of edamame. Tainan District Agric. Newsletter. 1998, 26, 4–7. [Google Scholar]
- Battistelli, N.; Tittarelli, F.; Perpetuini, G.; Suzzi, G.; Tofalo, R. Occurrence of Biogenic Amines in Fermented and Non-fermented Soybean Products. In Biogenic Amines in Food: Analysis, Occurrence and Toxicity; Royal Society of Chemistry: London, UK, 2019; Chapter 10; pp. 173–186. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.I.; Lim, Y.J.; Park, Y.J.; Kim, W.; Kim, D.O.; Kim, B.Y.; Eom, S.H.; Baik, M.Y. Antioxidant and phytoestrogenic activities of puffed black soybeans (Glycine max). LWT Food Sci. Technol. 2020, 118, 108780. [Google Scholar] [CrossRef]
- Kempa, S.; Krasensky, J.; Dal Santo, S.; Kopka, J.; Jonak, C. A central role of abscisic acid in stress-regulated carbohydrate metabolism. PLoS ONE 2008, 3, e3935. [Google Scholar] [CrossRef]
- Renault, H.; Roussel, V.; El Amrani, A.; Arzel, M.; Renault, D.; Bouchereau, A.; Deleu, C. The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant. Biol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Bettler, B.; Kaupmann, K.; Mosbacher, J.; Gassmann, M. Molecular structure and physiological functions of GABAB receptors. Physiol. Rev. 2004, 84, 835–867. [Google Scholar] [CrossRef]
- Cao, J.X.; Li, Y.P.; Xiong, X.Y.; Ye, J.; Liu, H. Applications of ϒ-aminobutyric acid in functional foods. J. Hebei Agric. Sci. 2008, 12, 52–54. [Google Scholar]
- Adeghate, E.; Ponery, A.S. GABA in the endocrine pancreas: Cellular localization and function in normal and diabetic rats. Tissue Cell 2002, 34, 1–6. [Google Scholar] [CrossRef]
- Roohinejad, S.; Omidizadeh, A.; Mirhosseini, H.; Rasti, B.; Saari, N.; Mustafa, S.; Mohd Yusof, R.; Shobirin Meor Hussin, A.; Hamid, A.; Abd Manap, M.Y. Effect of hypocholesterolemic properties of brown rice varieties containing different gamma aminobutyric acid (GABA) levels on Sprague-Dawley male rats. J. Food Agric. Environ. 2009, 7, 197–203. [Google Scholar]
- Shimada, M.; Hasegawa, T.; Nishimura, C.; Kan, H.; Kanno, T.; Nakamura, T.; Matsubayashi, T. Anti-hypertensive effect of γ-aminobutyric acid (GABA)-rich chlorella on high-normal blood pressure and borderline hypertension in placebo-controlled double blind study. Clin. Exp. Hypertens. 2009, 31, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhu, C.; Liu, C.H.; Xie, F.; Zhu, K.; Hu, S.Y. Gamma-aminobutyric acid binds to GABAB receptor to inhibit cholangiocarcinoma cells growth via the JAK/STAT3 pathway. Digest. Dis. Sci. 2013, 58, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Suwanmanon, K.; Hsieh, P.C. Effect of γ-aminobutyric acid and nattokinase-enriched fermented beans on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. J. Food Drug Anal. 2014, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Saito, S. Effect of intraventricular γ-aminobutyric acid (GABA) on discrimination learning in rats. Psychopharmacology 1978, 56, 127–132. [Google Scholar] [CrossRef]
- Okada, T.; Sugishita, T.; Murakami, T.; Murai, H.; Saikusa, T.; Horino, T.; Onoda, A.; Kajimoto, O.; Takahashi, R.; Takahashi, T. Effect of the defatted rice germ enriched with GABA for sleeplessness depression, autonomic disorder by oral administration. J. Jap. Soc. Food Sci. Technol. 2000, 47, 596–603. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-Aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Sánchez-López, Á.M.; Bahaji, A.; De Diego, N.; Baslam, M.; Li, J.; Muñoz, F.J.; Almagro, G.; García-Gómez, P.; Ameztoy, K.; Ricarte-Bermejo, A.; et al. Arabidopsis responds to alternata volatiles by triggering plastid phosphoglucose isomerase-independent mechanisms. Plant. Physiol. 2016, 172, 1989–2001. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, S.H.; Jang, G.Y.; Park, H.J.; Li, M.; Kim, S.; Lee, Y.R.; Noh, Y.H.; Lee, J.; Jeong, H.S. Effects of high hydrostatic pressure treatment on the enhancement of functional components of germinated rough rice (Oryza sativa L.). Food Chem. 2015, 166, 86–92. [Google Scholar] [CrossRef]
- Moongngarm, A.; Saetung, N. Comparison of chemical compositions and bioactive compounds of germinated rough rice and brown rice. Food Chem. 2010, 122, 782–788. [Google Scholar] [CrossRef]
- Tiansawang, K.; Luangpituksa, P.; Varanyanond, W.; Hansawasdi, C. GABA (γ-aminobutyric acid) production, antioxidant activity in some germinated dietary seeds and the effect of cooking on their GABA content. Food Sci. Technol. 2016, 36, 313–321. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; McLean, M.D. Metabolism and functions of gamma- aminobutyric acid. Trends Plant. Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Reggiani, R.; Cantu, C.A.; Brambilla, I.; Bertani, A. Accumulation and interconversion of amino acids in rice roots under anoxia. Plant Cell Physiol. 1988, 29, 981–987. [Google Scholar]
- Liao, W.C.; Wang, C.Y.; Shyu, Y.T.; Yu, R.C.; Ho, K.C. Influence of preprocessing methods and fermentation of adzuki beans on γ-aminobutyric acid (GABA) accumulation by lactic acid bacteria. J. Funct. Foods. 2013, 5, 1108–1115. [Google Scholar] [CrossRef]
- Seo, M.J.; Nam, Y.D.; Park, S.L.; Lee, S.Y.; Yi, S.H.; Lim, S.I. γ-aminobutyric acid production in skim milk co-fermented with Lactobacillus brevis 877G and Lactobacillus sakei 795. Food Sci. Biotechnol. 2013, 22, 751–755. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Battistelli, N.; Pepe, A.; Ianni, A.; Martino, G.; Suzzi, G. Accumulation γ-aminobutyric acid and biogenic amines in a traditional raw milk ewe’s cheese. Foods 2019, 8, 401. [Google Scholar] [CrossRef]
- Perpetuini, G.; Tittarelli, F.; Battistelli, N.; Suzzi, G.; Tofalo, R. γ-aminobutyric acid production by Kluyveromyces marxianus strains. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H.N. High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, Y.; Yu, K.W.; Imm, J.Y.; Suh, H.J. Enzyme-assisted extraction of cactus bioactive molecules under high hydrostatic pressure. J. Sci. Food Agric. 2014, 94, 850–856. [Google Scholar] [CrossRef]
- Oey, I. Effects of high pressure on enzymes. In High Pressure Processing of Food; Balasubramaniam, V.M., Barbosa-Cánovas, G.V., Lelieveld, H.L.M., Eds.; Springer: New York, NY, USA, 2016; pp. 391–431. [Google Scholar]
- Ueno, S.; Shigematsu, T.; Watanabe, T.; Nakajima, K.; Murakami, M.; Hayashi, M.; Fujii, T. Generation of free amino acids and γ-aminobutyric acid in water-soaked soybean by high-hydrostatic pressure processing. J. Agric. Food Chem. 2010, 58, 1208–1213. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, L.; Xu, C.; Mei, J.; Li, Y. Effects of germination and high hydrostatic pressure processing on mineral elements, amino acids and antioxidants in vitro bioaccessibility, as well as starch digestibility in brown rice (Oryza sativa L.). Food Chem. 2017, 214, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Rozan, P.; Lambein, F.; Frias, J.; Vidal-Valverde, C. Effects of different germination conditions on the contents of free protein and non-protein amino acids of commercial legumes. Food Chem. 2004, 86, 537–545. [Google Scholar] [CrossRef]
- Chen, K.Y. Using High Pressure Process and Other Enrichment Treatments to Increase γ-Aminobutyric Acid Contents in Legume Beans. Hort. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2015. [Google Scholar]
- Cohen, S.A.; Strydom, D.J. Amino acid analysis utilizing phenylisothiocyanate derivatives. Anal. Biochem. 1988, 174, 1–16. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 2005. [Google Scholar]
- Farooq, R.K.; Isingrini, E.; Tanti, A.; Le Guisquet, A.M.; Arlicot, N.; Minier, F.; Leman, S.; Chalon, S.; Belzung, C.; Camus, V. Is unpredictable chronic mild stress (UCMS) a reliable model to study depression-induced neuroinflammation. Behav. Brain Res. 2012, 231, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Willner, P.; Muscat, R. An animal model of anhedonia: Attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology 1991, 104, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Takao, K.; Nakanishi, K.; Yamasaki, N.; Tanda, K.; Miyakawa, T. Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 2010, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.F.; Doremus-Fitzwater, T.L.; Becker, H.C. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol 2011, 45, 355–364. [Google Scholar] [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Shiu, M.S.; Shyu, Y.T.; Wu, S.Z. Flooding stress and high-pressure treatment the GABA content of the vegetable soybean (Glycine max Merr.). Agriculture 2020, 10, 175. [Google Scholar] [CrossRef]
- Sasagawa, A.; Naiki, Y.; Nagashima, S.; Yamakura, M.; Yamazaki, A.; Yamada, A. Process of producing brown rice with increased accumulation of GABA using high-pressure treatment and properties of GABA-increased brown rice. J. Appl. Glycosci. 2006, 53, 27–33. [Google Scholar] [CrossRef]
- Udovičić, M.; Baždarić, K.; Bilić-Zulle, L.; Petrovečki, M. What we need to know when calculating the coefficient of correlation? Biochem. Med. 2007, 17, 5–10. [Google Scholar]
- Serment-Moreno, V.; Jacobo-Velázquez, D.A.; Torres, J.A.; Welti-Chanes, J. Microstructural and physiological changes in plant cell induced by pressure: Their role on the availability and pressure-temperature stability of phytochemicals. Food Eng. Rev. 2017, 9, 314–334. [Google Scholar] [CrossRef]
- Yang, R.; Guo, Q.; Gu, Z. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 2013, 136, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.X.; Wu, T.J.; Li, H.Y.; Wang, W.; Liu, H.J. Dual effects of slightly acidic electrolyzed water (SAEW) treatment on the accumulation of γ-aminobutyric acid (GABA) and rutin in germinated buckwheat. Food Chem. 2016, 201, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bolarín, M.C.; Santa-Cruz, A.; Cayuela, E.; Pérez-Alfocea, F. Short-term solute changes in leaves and roots of cultivated and wild tomato seedlings under salinity. J. Plant Physiol. 1995, 147, 463–468. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Ueno, S.; Katayama, T.; Watanabe, T.; Nakajima, K.; Hayashi, M.; Shigematsu, T.; Fujii, T. Enzymatic production of γ-aminobutyric acid in soybeans using high hydrostatic pressure and precursor feeding. Biosci. Biotechnol. Biochem. 2013, 77, 706–713. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, R.; Chen, H.; Song, Y.; Gu, Z. Accumulation of γ- aminobutyric acid in germinated soybean (Glycine max L.) in relation to glutamate decarboxylase and diamine oxidase activity induced by additives under hypoxia. Eur. Food Res. Technol. 2012, 234, 679–687. [Google Scholar] [CrossRef]
- Shigematsu, T.; Murakami, M.; Nakajima, K.; Uno, Y.; Sakano, A.; Narahara, Y.; Hayashi, M.; Ueno, S.; Fujii, T. Bioconversion of glutamic acid to γ-aminobutyric acid (GABA) in brown rice grains induced by high pressure treatment. Jpn. J. Food Eng. 2010, 11, 189–199. [Google Scholar] [CrossRef]
- Pothion, S.; Bizot, J.C.; Trovero, F.; Belzung, C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav. Brain Res. 2004, 155, 135–146. [Google Scholar] [CrossRef]
- Mutlu, O.; Ulak, G.; Laugeray, A.; Belzung, C. Effects of neuronal and inducible NOS inhibitor 1-[2-(trifluoromethyl) phenyl] imidazole (TRIM) in unpredictable chronic mild stress procedure in mice. Pharmacol. Biochem. Behav. 2009, 92, 82–87. [Google Scholar] [CrossRef]
- Yalcin, I.; Belzung, C.; Surget, A. Mouse strain differences in the unpredictable chronic mild stress: A four-antidepressant survey. Behav. Brain Res. 2008, 193, 140–143. [Google Scholar] [CrossRef]
- Cannon, C.M.; Palmiter, R.D. Reward without Dopamine. J. Neurosci. 2003, 23, 10827–10831. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y.; Xue, J.S.; Li, H.Y.; Ma, Z.Q.; Fu, Q.; Qu, R.; Ma, S.P. Geraniol produces antidepressant-like effects in a chronic unpredictable mild stress mice model. Physiol. Behav. 2015, 152, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Taksande, B.G.; Faldu, D.S.; Dixit, M.P.; Sakaria, J.N.; Aglawe, M.M.; Umekar, M.J.; Kotagale, N.R. Agmatine attenuates chronic unpredictable mild stress induced behavioral alteration in mice. Eur. J. Pharmac. 2013, 720, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Dong, Z.; Huang, W.; Zhou, C.; Zhong, W.; Hu, P.; Wen, G.; Sun, X.; Hua, H.; Cao, H.; et al. Voluntary wheel running ameliorates depression-like behaviors and brain blood oxygen level-dependent signals in chronic unpredictable mild stress mice. Behav. Brain Res. 2017, 330, 17–24. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, J.; Zhang, Y.; Li, V.; Kong, J.; He, J.; Li, X.M. Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res. 2014, 1576, 81–90. [Google Scholar] [CrossRef]
- Gronli, J.; Murison, R.; Fiske, E.; Bjorvatn, B.; Sorensen, E.; Portas, C.M.; Ursin, R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol. Behav. 2005, 84, 571–577. [Google Scholar] [CrossRef]
- Makino, J.; Kato, K.; Maes, F.W. Temporal structure of open field behavior in inbred strains of mice. Jpn. Psychol. Res. 1991, 33, 145–152. [Google Scholar] [CrossRef][Green Version]
- Monteiro, S.; Roque, S.; de Sa-Calcada, D.; Sousa, N.; Correia-Neves, M.; Cerqueira, J.J. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatry. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, T.; Steinbusch, H. Factors of reproducibility of anhedonia induction in a chronic stress depression model in mice. In Mood and Anxiety Related Phenotypes in Mice; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 153–176. [Google Scholar]
- Johnson, S.A.; Fournier, N.M.; Kalynchuk, L.E. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav. Brain Res. 2006, 168, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Arango, V.; Underwood, M.D.; Mann, J.J. Serotonin brain circuits involved in major depression and suicide. Prog. Brain Res. 2002, 136, 443–453. [Google Scholar]
- Dranovsky, A.; Hen, R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol. Psychiatry 2006, 59, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.J. The medical management of depression. N. Engl. J. Med. 2005, 353, 1819–1834. [Google Scholar] [CrossRef]
- Young, A.H.; Goodwin, G.M.; Dick, H.; Fink, G. Effects of glucocorticoids on 5-HT 1A presynaptic function in the mouse. Psychopharmacology 1994, 114, 360–364. [Google Scholar] [CrossRef]
- Takao, K.; Nagatani, T.; Kitamura, Y.; Yamawaki, S. Effects of corticosterone on 5-HT1A and 5-HT2 receptor binding and on the receptor-mediated behavioral responses of rats. Eur. J. Pharmacol. 1997, 333, 123–128. [Google Scholar] [CrossRef]


| Storage Days | Control | 200 MPa | 400 MPa | 600 MPa | |
|---|---|---|---|---|---|
| GABA (mg/100 g) | 1 | 261.42 ± 3.66 a | 436.05 ± 20.57 c | 329.55 ± 23.20 a | 86.00 ± 6.58 b |
| 3 | 241.15 ± 3.93 a | 300.32 ± 19.79 a | 317.09 ± 28.24 a | 70.63 ± 2.96 a | |
| 5 | 245.1 ± 21.18 a | 383.66 ± 0.53 b | 288.46 ± 24.97 a | 107.14 ± 1.58 c | |
| Glutamate (mg/100 g) | 1 | 63.48 ± 3.38 a | 28.29 ± 1.92 a | 69.09 ± 11.18 a | 275.09 ± 6.61 a |
| 3 | 78.58 ± 1.21 b | 41.60 ± 0.95 b | 85.88 ± 9.15 a | 226.28 ± 15.59 a | |
| 5 | 51.51 ± 5.52 a | 41.05 ± 2.01 b | 51.45 ± 5.75 a | 431.7 ± 44.23 b |
| Treatment Group | Week4 | Week5 | Week6 | Week7 | |
|---|---|---|---|---|---|
| Distance in Zone (%) | UCMS | 20.32 ± 4.2 a | 21.81 ± 4.21 a | 14.41 ± 2.72 a | 15.54 ± 2.55 a |
| Control | 22.95 ± 3.18 a | 26.85 ± 1.88 a | 35.53 ± 5.01 b | 26.9 ± 6.77 b | |
| Positive control | 28.02 ± 1.81 b | 26.56 ± 5.67 a | 35.99 ± 7.37 b | 26.21 ± 4.55 b | |
| GABA | 32.8 ± 5.31 b | 40.75 ± 2.09 b | 31.39 ± 4.27 b | 27.52 ± 3.23 b | |
| Time in Zone (%) | UCMS | 18.9 ± 4.64 a | 19.81 ± 6.45 a | 10.93 ± 6.61 a | 13.62 ± 10.14 a |
| Control | 25.79 ± 2.4 b | 27.76 ± 4.06 ab | 42 ± 8.22 b | 37.15 ± 12.64 b | |
| Positive control | 33.88 ± 2.41 c | 36.99 ± 2.38 bc | 35.18 ± 6.69 b | 34.73 ± 6.95 b | |
| GABA | 29.44 ± 1.95 c | 41.94 ± 7.14 c | 44.71 ± 7.36 b | 32.46 ± 4.35 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-J.; Chang, C.-Y.; Lai, Y.-T.; Shyu, Y.-T. Increasing γ-Aminobutyric Acid Content in Vegetable Soybeans via High-Pressure Processing and Efficacy of Their Antidepressant-Like Activity in Mice. Foods 2020, 9, 1673. https://doi.org/10.3390/foods9111673
Wu S-J, Chang C-Y, Lai Y-T, Shyu Y-T. Increasing γ-Aminobutyric Acid Content in Vegetable Soybeans via High-Pressure Processing and Efficacy of Their Antidepressant-Like Activity in Mice. Foods. 2020; 9(11):1673. https://doi.org/10.3390/foods9111673
Chicago/Turabian StyleWu, Sz-Jie, Chia-Yu Chang, Yen-Ting Lai, and Yuan-Tay Shyu. 2020. "Increasing γ-Aminobutyric Acid Content in Vegetable Soybeans via High-Pressure Processing and Efficacy of Their Antidepressant-Like Activity in Mice" Foods 9, no. 11: 1673. https://doi.org/10.3390/foods9111673
APA StyleWu, S.-J., Chang, C.-Y., Lai, Y.-T., & Shyu, Y.-T. (2020). Increasing γ-Aminobutyric Acid Content in Vegetable Soybeans via High-Pressure Processing and Efficacy of Their Antidepressant-Like Activity in Mice. Foods, 9(11), 1673. https://doi.org/10.3390/foods9111673





