Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Extraction
2.2. β-Hexosaminidase Release
2.3. Antioxidant Activity
2.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
2.3.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.3.3. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.4. Nitric Oxide (NO) Assay
2.5. Total RNA Extraction and Quantitative RT-PCR
2.6. Western Blot Analysis
2.7. Flow Cytometric Analysis
2.8. Gas Chromatography/Mass Spectrometry (GC-MS) and Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.9. In Vivo Toxicity Evaluation
2.10. Molecular Docking
2.11. Statistical Analysis
3. Results
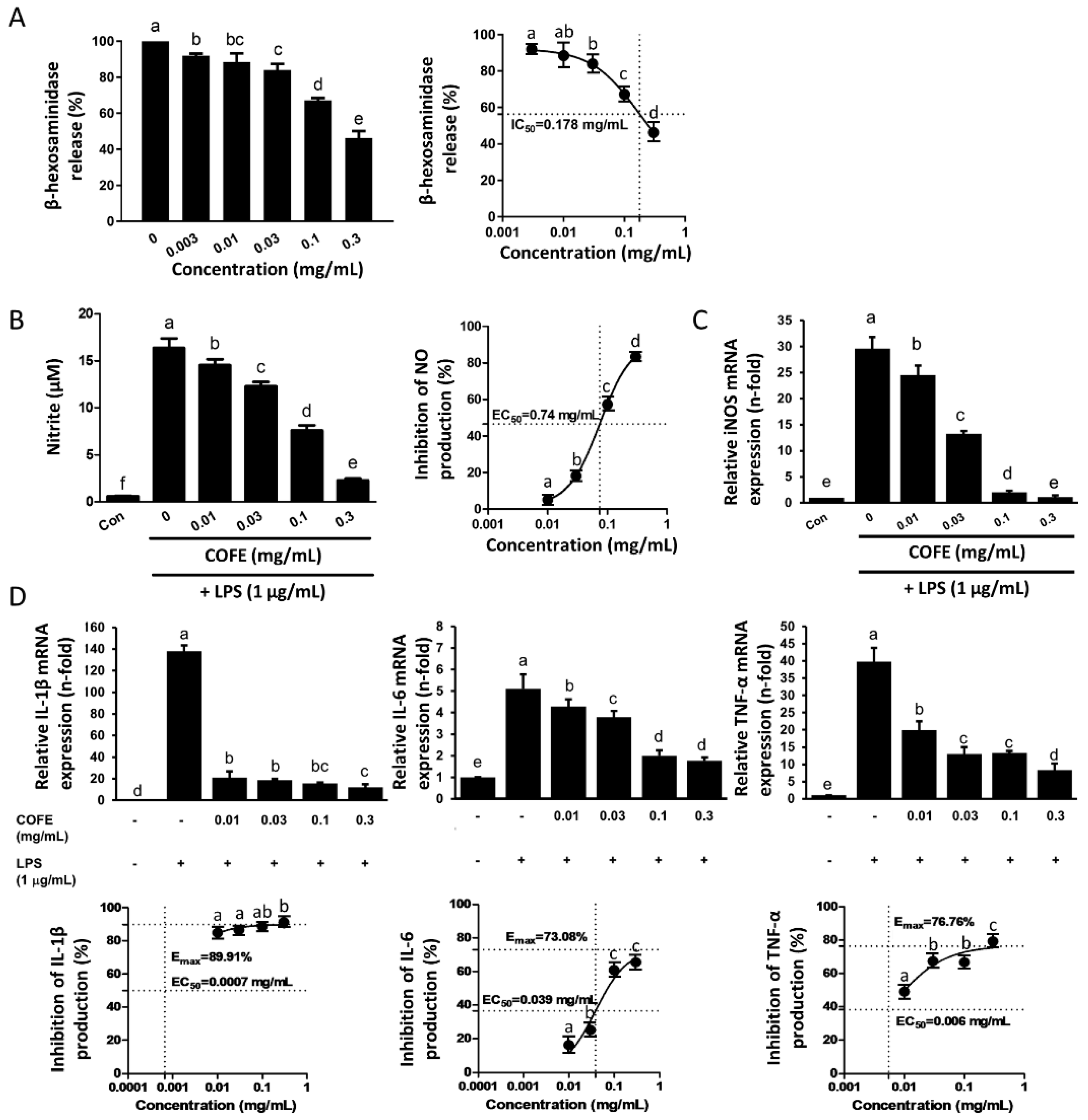
3.1. Effect of COFE on DNP/IgE-Induced Degranulation in RBL-2H3 Cells
3.2. Antioxidant Activity of COFE
3.3. Effect of COFE on LPS-Induced NO Production and Pro-Inflammatory Cytokines in RAW 264.7 Cells
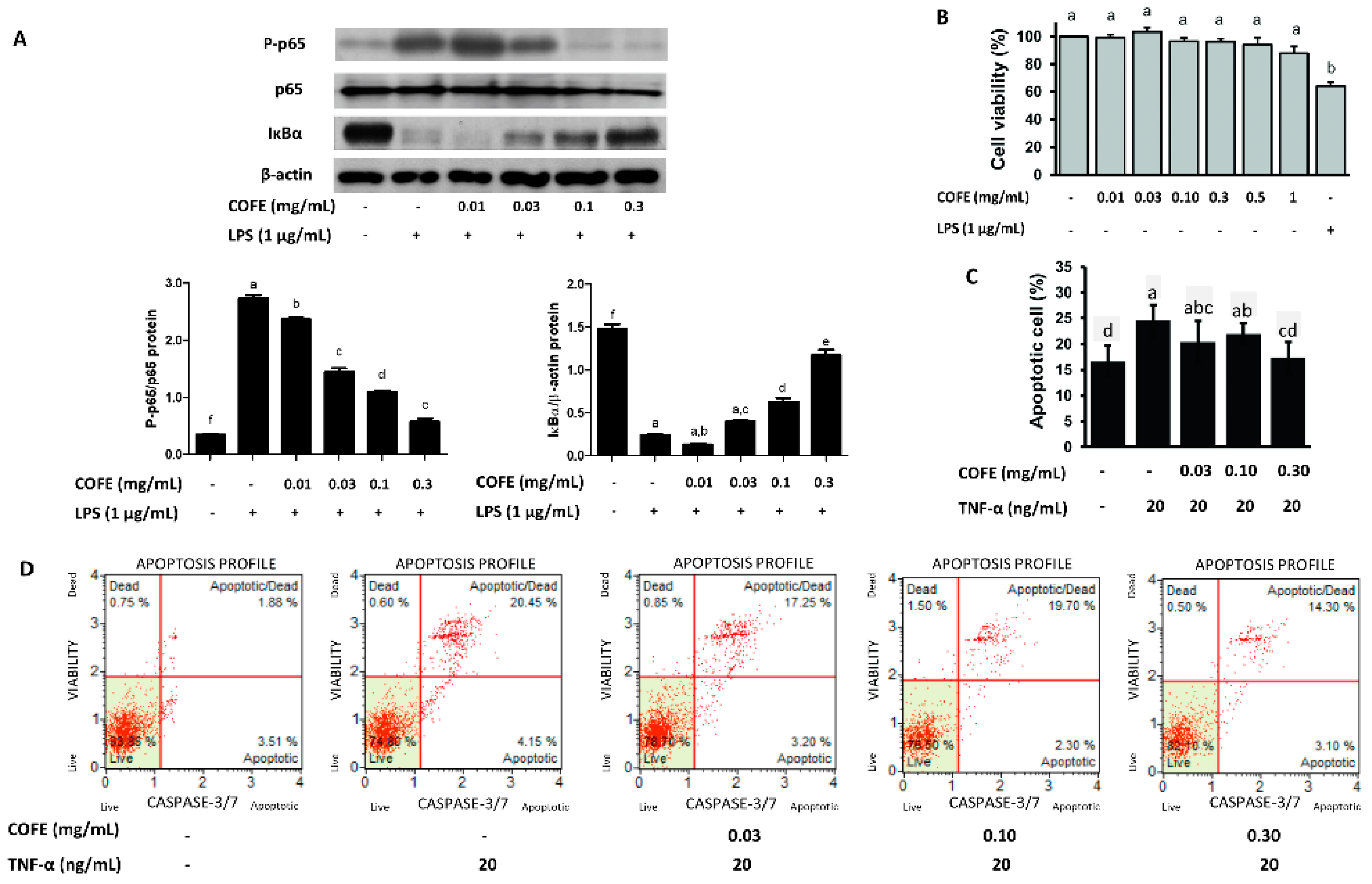
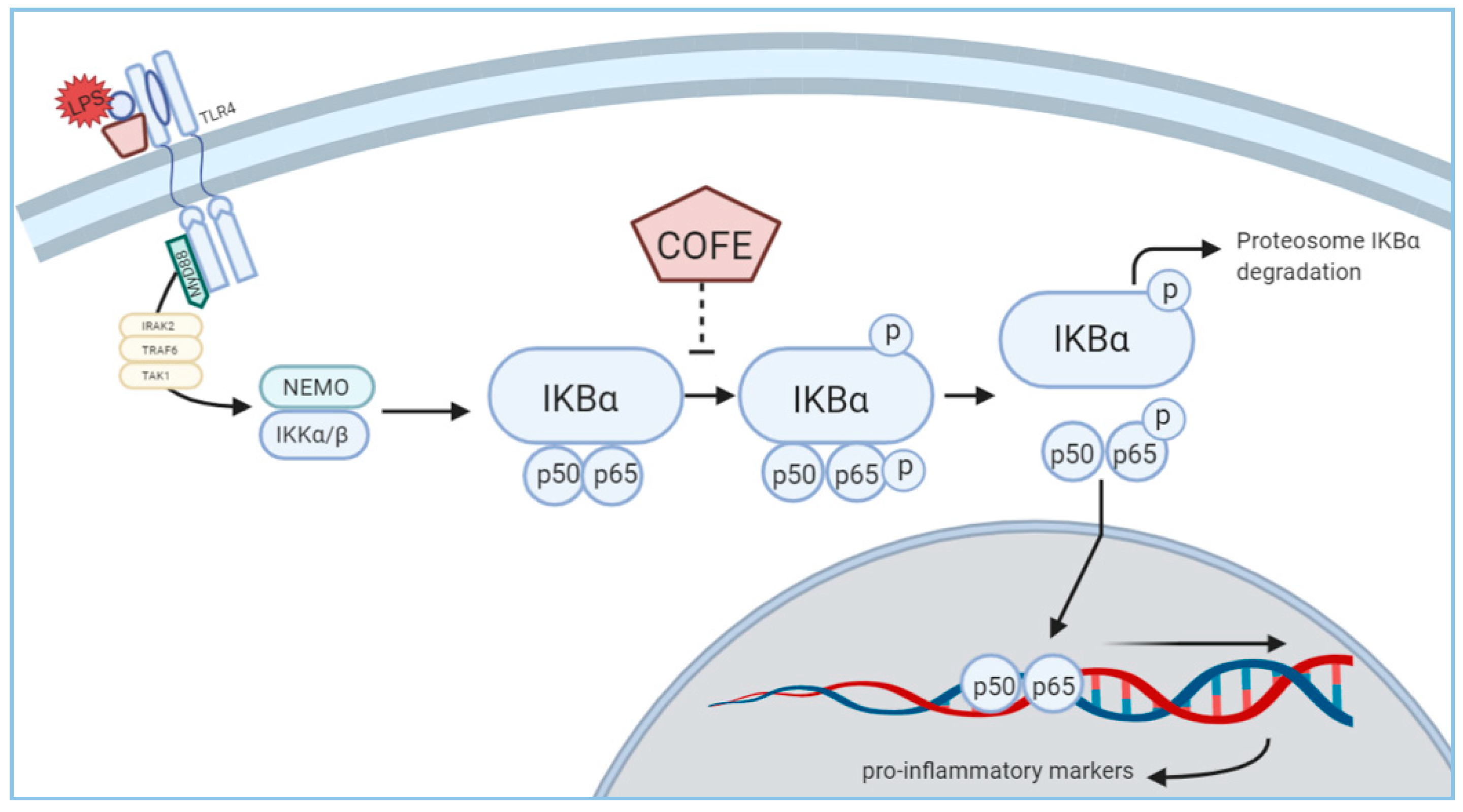
3.4. Effect of COFE on NF-κB Stimulation
3.5. Effect of COFE on TNF-α Induced Apoptosis in HaCaT Cells
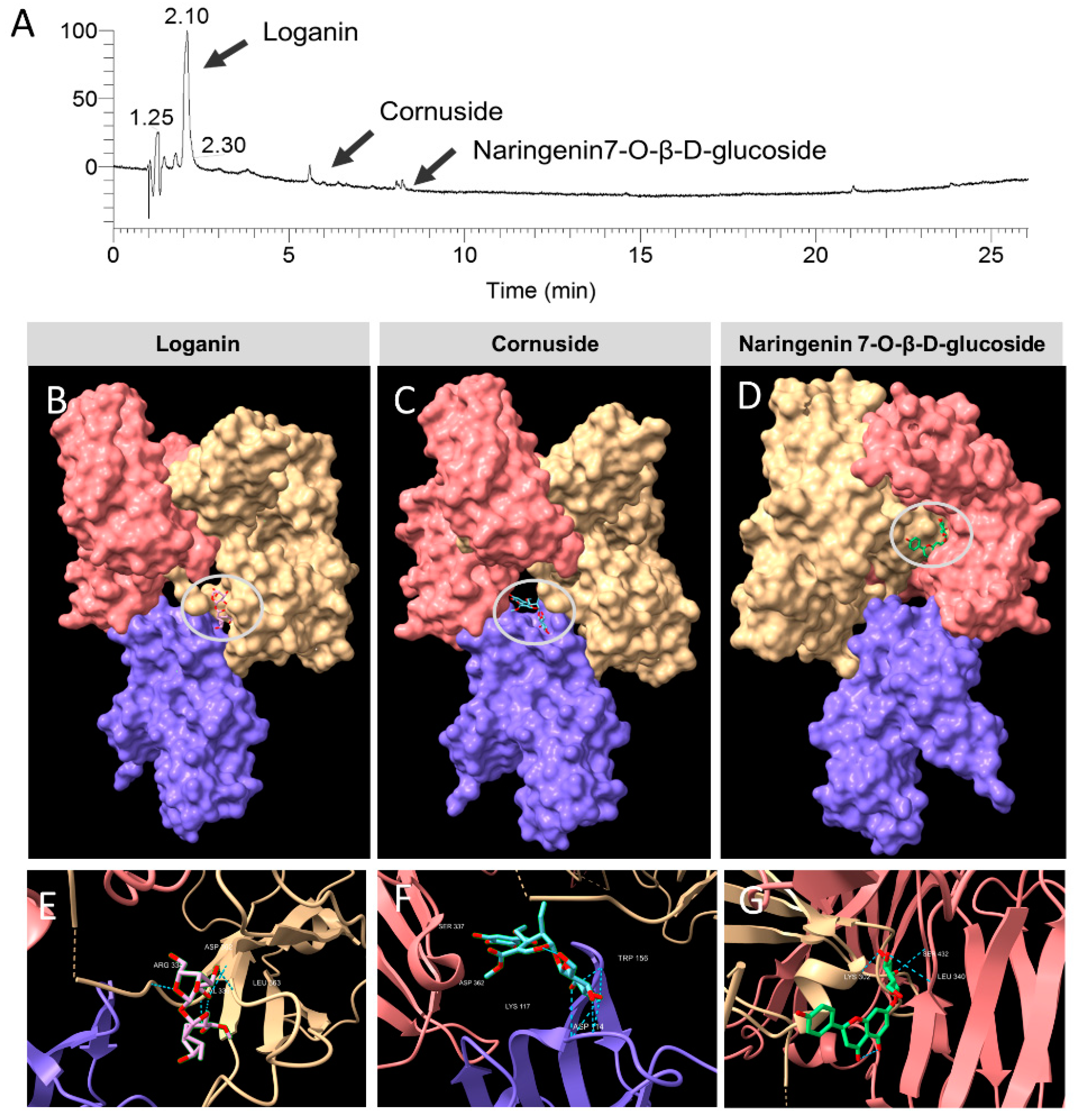
3.6. Identification of the Constituent Compounds of COFE
3.7. In Vivo Toxicity Evaluation of COFE
3.8. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011, 2, 110. [Google Scholar]
- Leung, D.Y.M.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar]
- Cooper, K.D. Atopic dermatitis: Recent trends in pathogenesis and therapy. J. Investig. Dermatol. 1994, 102, 128–137. [Google Scholar]
- Hwang, K.A.; Hwang, Y.J.; Song, J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complement. Altern. Med. 2016, 16, 196. [Google Scholar]
- Mechesso, A.F.; Lee, S.J.; Park, N.H.; Kim, J.Y.; Im, Z.E.; Suh, J.W.; Park, S.C. Preventive effects of a novel herbal mixture on atopic dermatitis-like skin lesions in BALB/c mice. BMC Complement. Altern. Med. 2019, 19, 25. [Google Scholar]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus officinalis—Analogies and differences of two medicinal plants traditionally used. Front. Pharmacol. 2018, 9, 894. [Google Scholar]
- Juckmeta, T.; Thongdeeying, P.; Itharat, A. Inhibitory effect on β -Hexosaminidase release from RBL-2H3 cells of extracts and some pure constituents of Benchalokawichian, a Thai herbal remedy, used for allergic disorders. Evid.-Based Complement. Altern. Med. ECAM 2014, 2014, 828760. [Google Scholar]
- Chang, Z.Q.; Hwang, M.H.; Rhee, M.H.; Kim, K.S.; Kim, J.C.; Lee, S.P.; Jo, W.S.; Park, S.C. The in vitro anti-platelet, antioxidant and cellular immunity activity of Phellinus gilvus fractional extracts. World J. Microbiol. Biotechnol. 2008, 24, 181–187. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar]
- Lee, S.J.; Hossaine, M.D.A.; Park, S.C. A potential anti-inflammation activity and depigmentation effect of Lespedeza bicolor extract and its fractions. Saudi J. Biol. Sci. 2016, 23, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, J.; Zhu, D.; Huang, J.; Huang, Z.; Bai, J.; Qiu, X. Rapid separation and characterization of diterpenoid alkaloids in processed roots of Aconitum carmichaeli using ultra high performance liquid chromatography coupled with hybrid linear ion trap-Orbitrap tandem mass spectrometry. J. Sep. Sci. 2014, 37, 2864–2873. [Google Scholar] [PubMed]
- Hossain, M.A.; Lee, S.J.; Park, J.Y.; Reza, M.A.; Kim, T.H.; Lee, K.J.; Suh, J.W.; Park, S.C. Modulation of quorum sensing-controlled virulence factors by Nymphaea tetragona (water lily) extract. J. Ethnopharmacol. 2015, 174, 482–491. [Google Scholar] [CrossRef]
- OECD/OCDE. Acute Oral Toxicity-Acute Toxic Class. Method OECD Guideline for the Testing of Chemicals; OECD/OCDE: Paris, France, 2001; pp. 1–14. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [PubMed] [Green Version]
- Holdom, M.D.; Davies, A.M.; Nettleship, J.E.; Bagby, S.C.; Dhaliwal, B.; Girardi, E.; Hunt, J.; Gould, H.J.; Beavil, A.J.; McDonnell, J.M.; et al. Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcεRI. Nat. Struct. Mol. Biol. 2011, 18, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-H.; Hung, M.-H.; Chen, J.Y.-F.; Chang, H.-W.; Yu, M.-L.; Wan, L.; Tsai, F.J.; Wang, T.-P.; Fu, T.-F.; Chiu, C.-C. Anti-allergic activity of grapeseed extract (GSE) on RBL-2H3 mast cells. Food Chem. 2012, 132, 968–974. [Google Scholar] [CrossRef]
- Yoo, J.M.; Kim, N.Y.; Seo, J.M.; Kim, S.J.; Lee, S.Y.; Kim, S.K.; Kim, H.D.; Lee, S.W.; Kim, M.R. Inhibitory effects of mulberry fruit extract in combination with naringinase on the allergic response in IgE-activated RBL-2H3 cells. Int. J. Mol. Med. 2014, 33, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.M.; Yang, J.H.; Yang, H.J.; Cho, W.K.; Ma, J.Y. Inhibitory effect of fermented Arctium lappa fruit extract on the IgE-mediated allergic response in RBL-2H3 cells. Int. J. Mol. Med. 2016, 37, 501–508. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Lee, E.J.; Kim, H.R.; Hwang, K.A. In vitro antioxidant and anticancer effects of solvent fractions from Prunella vulgaris var. lilacina. BMC Complement. Altern. Med. 2013, 13, 310. [Google Scholar] [CrossRef] [Green Version]
- Valledor, A.F.; Comalada, M.; Santamaría-Babi, L.F.; Lloberas, J.; Celada, A. Macrophage proinflammatory activation and deactivation: A question of balance. Adv. Immunol. 2010, 108, 1–20. [Google Scholar]
- Shin, J.H.; Chung, M.J.; Seo, J.G. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4+Foxp3+ T cells. Food Nutr. Res. 2016, 60, 32550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef] [PubMed]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative stress and atopic dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Yidong, T.; Taihao, Q. Oxidative stress and human skin connective tissue aging. Cosmetics 2016, 3, 28. [Google Scholar]
- Yokozawa, T.; Kang, K.S.; Park, C.H.; Noh, J.S.; Yamabe, N.; Shibahara, N.; Tanaka, T. Bioactive constituents of Corni Fructus: The therapeutic use of morroniside, loganin, and 7-O-galloyl-D-sedoheptulose as renoprotective agents in type 2 diabetes. Drug Discov. Ther. 2010, 4, 223–234. [Google Scholar] [PubMed]
- Li, M.; Wang, W.; Wang, P.; Yang, K.; Sun, H.; Wang, X. The pharmacological effects of morroniside and loganin isolated from Liuweidihuang Wan, on MC3T3-E1 cells. Molecules 2010, 15, 7403–7414. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.H.; Zhang, X.Y.; Zhang, L.Y.; Li, Q.; Ni, B.; Zheng, X.L.; Chen, A.J. Mast cell degranulation induced by chlorogenic acid. Acta Pharmacol. Sin. 2010, 31, 849–854. [Google Scholar] [CrossRef]
- Rigano, D.; Formisano, C.; Basile, A.; Lavitola, A.; Senatore, F.; Rosselli, S.; Bruno, M. Antibacterial activity of flavonoids and phenylpropanoids from Marrubium globosum ssp. libanoticum. Phytother. Res. 2007, 21, 395–397. [Google Scholar]
- Orhan, D.D. Novel flavanone glucoside with free radical scavenging properties from Galium fissurense. Pharm. Biol. 2003, 41, 475–478. [Google Scholar]
- Balbino, B.; Conde, E.; Marichal, T.; Starkl, P.; Reber, L.L. Approaches to target IgE antibodies in allergic diseases. Pharmacol. Ther. 2018, 191, 50–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [Green Version]
| RT (Retention Time) | Formula | % | ID | Activity |
|---|---|---|---|---|
| 34.21 | C6H8O4 | 4.58 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | Antimicrobial, anti-inflammatory, antiproliferative |
| 38.23 | C6H6O3 | 39.9 | 2-furancarboxaldehyde | Antimicrobial, preservative |
| 43.38 | C4H6O5 | 9.71 | Malic acid | Antimicrobial |
| 50.41 | C7H6O | 1.41 | 2,4,6-cycloheptatrien-1-one | Antioxidant |
| Compound | Binding Energy (Kcal) | Van der Waals | Hydrogen Bond | Electrostatic |
|---|---|---|---|---|
| Loganin | −116.9 | −90.5332 | −26.3531 | 0 |
| Cornuside | −141.1 | −101.645 | −39.4547 | 0 |
| Naringenin 7-O-β-D-glucoside | −125.7 | −96.4237 | −29.3185 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quah, Y.; Lee, S.-J.; Lee, E.-B.; Birhanu, B.T.; Ali, M.S.; Abbas, M.A.; Boby, N.; Im, Z.-E.; Park, S.-C. Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities. Nutrients 2020, 12, 3317. https://doi.org/10.3390/nu12113317
Quah Y, Lee S-J, Lee E-B, Birhanu BT, Ali MS, Abbas MA, Boby N, Im Z-E, Park S-C. Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities. Nutrients. 2020; 12(11):3317. https://doi.org/10.3390/nu12113317
Chicago/Turabian StyleQuah, Yixian, Seung-Jin Lee, Eon-Bee Lee, Biruk Tesfaye Birhanu, Md. Sekendar Ali, Muhammad Aleem Abbas, Naila Boby, Zi-Eum Im, and Seung-Chun Park. 2020. "Cornus officinalis Ethanolic Extract with Potential Anti-Allergic, Anti-Inflammatory, and Antioxidant Activities" Nutrients 12, no. 11: 3317. https://doi.org/10.3390/nu12113317