Development of HPLC Method for Quantification of Sinigrin from Raphanus sativus Roots and Evaluation of Its Anticancer Potential
Abstract
1. Introduction
2. Results
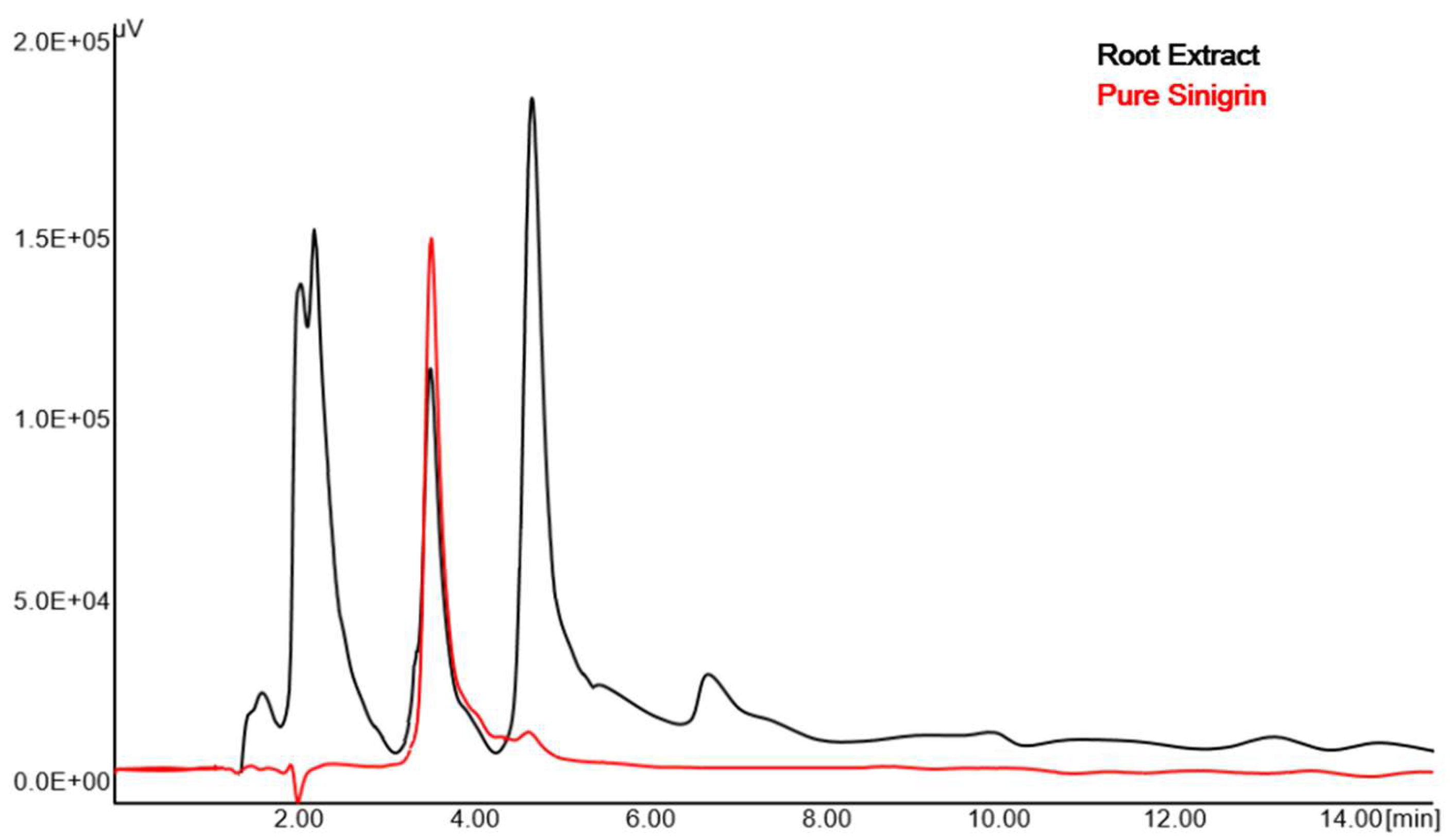
2.1. Quantification of Sinigrin Using RP-HPLC Method
2.2. Validation of RP-HPLC Method
2.2.1. System Suitability
2.2.2. Linearity and Range
2.2.3. Limit of Detection and Limit of Quantification
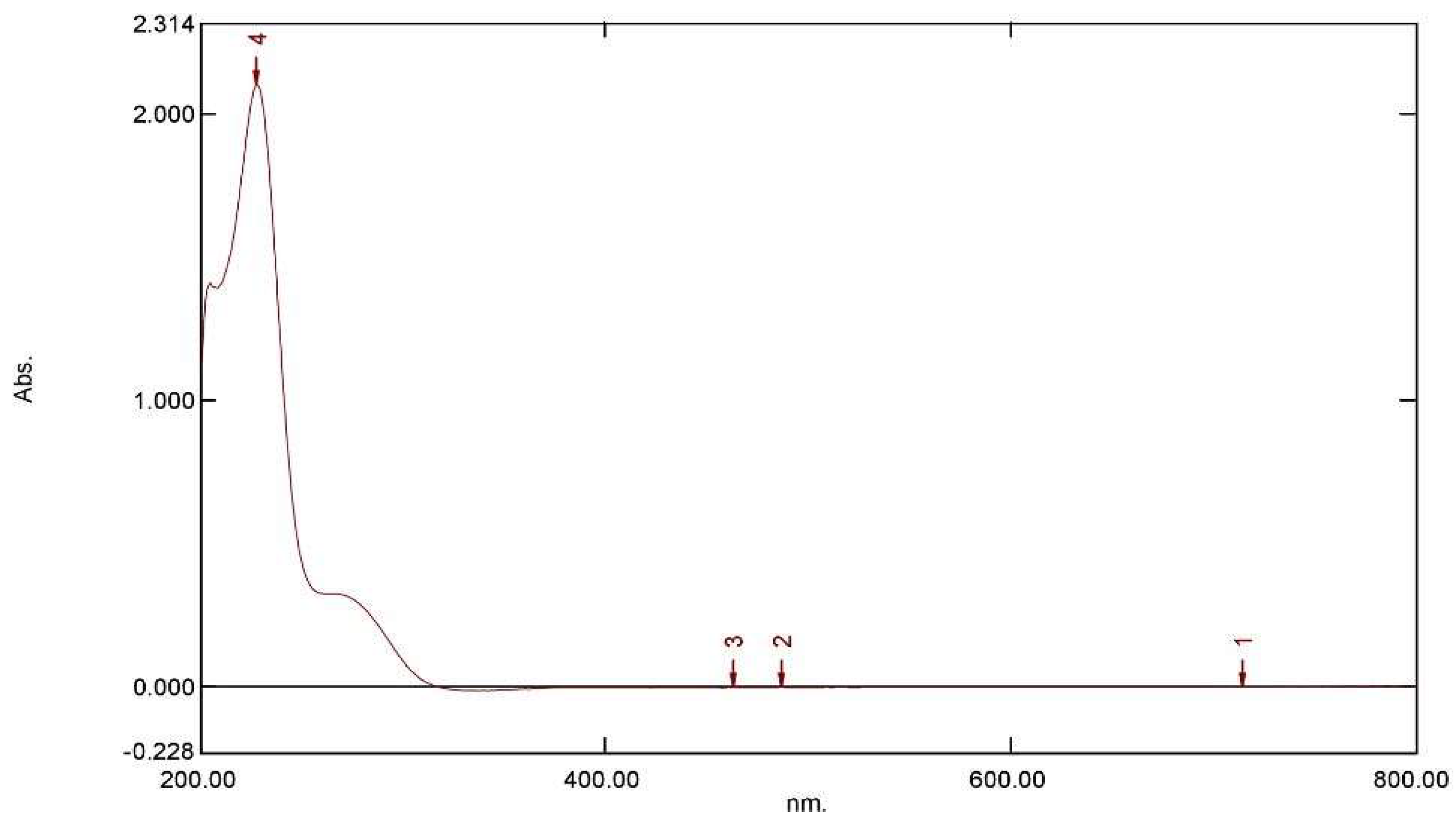
2.2.4. Specificity
2.2.5. Accuracy and Precision
2.2.6. Recovery Study
2.2.7. Stability
2.3. Anticancer Potential of Sinigrin on Different Cell Lines
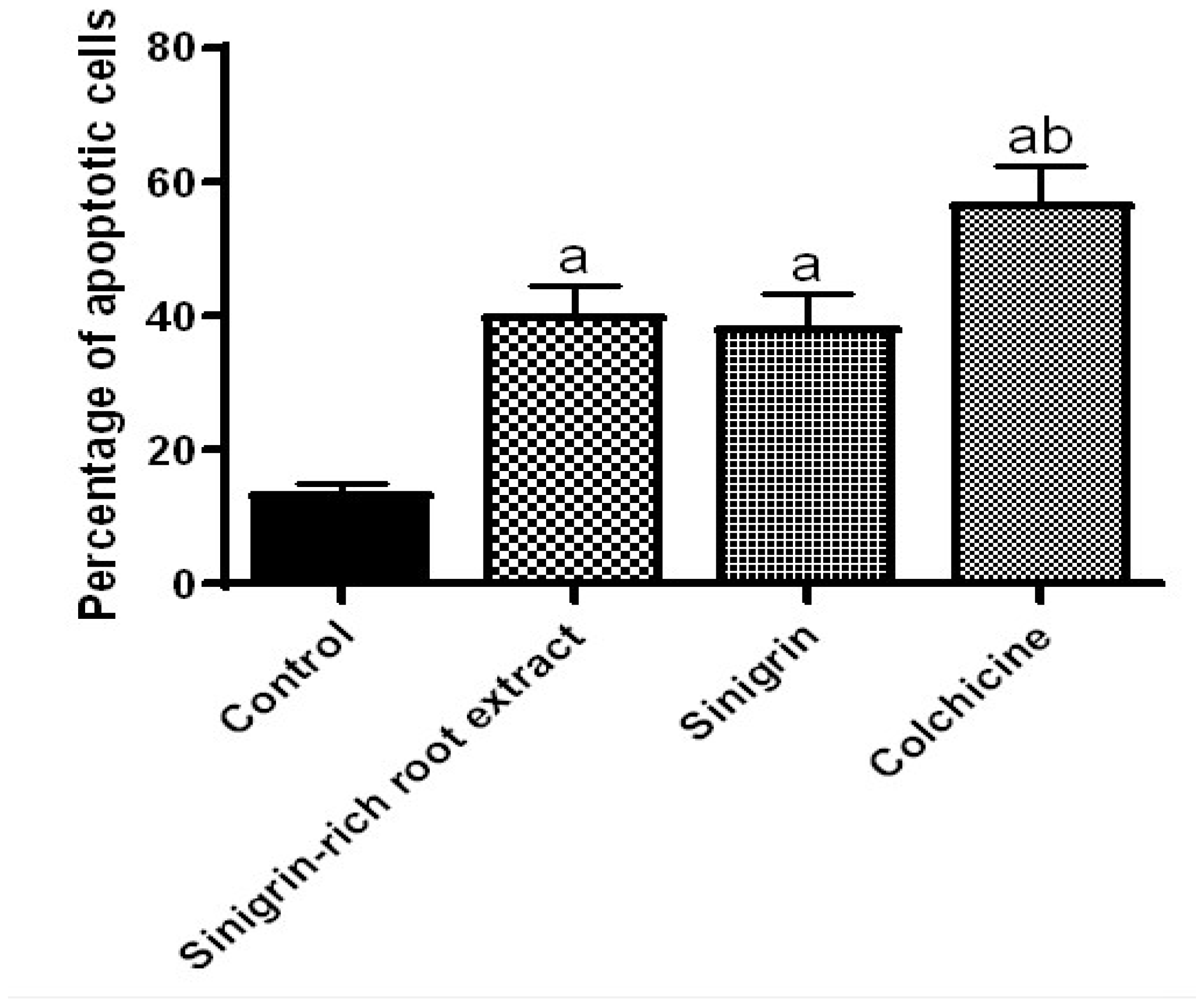
2.4. Effect of Roots Extract of Raphanus sativus and Sinigrin on Apoptotic Assay
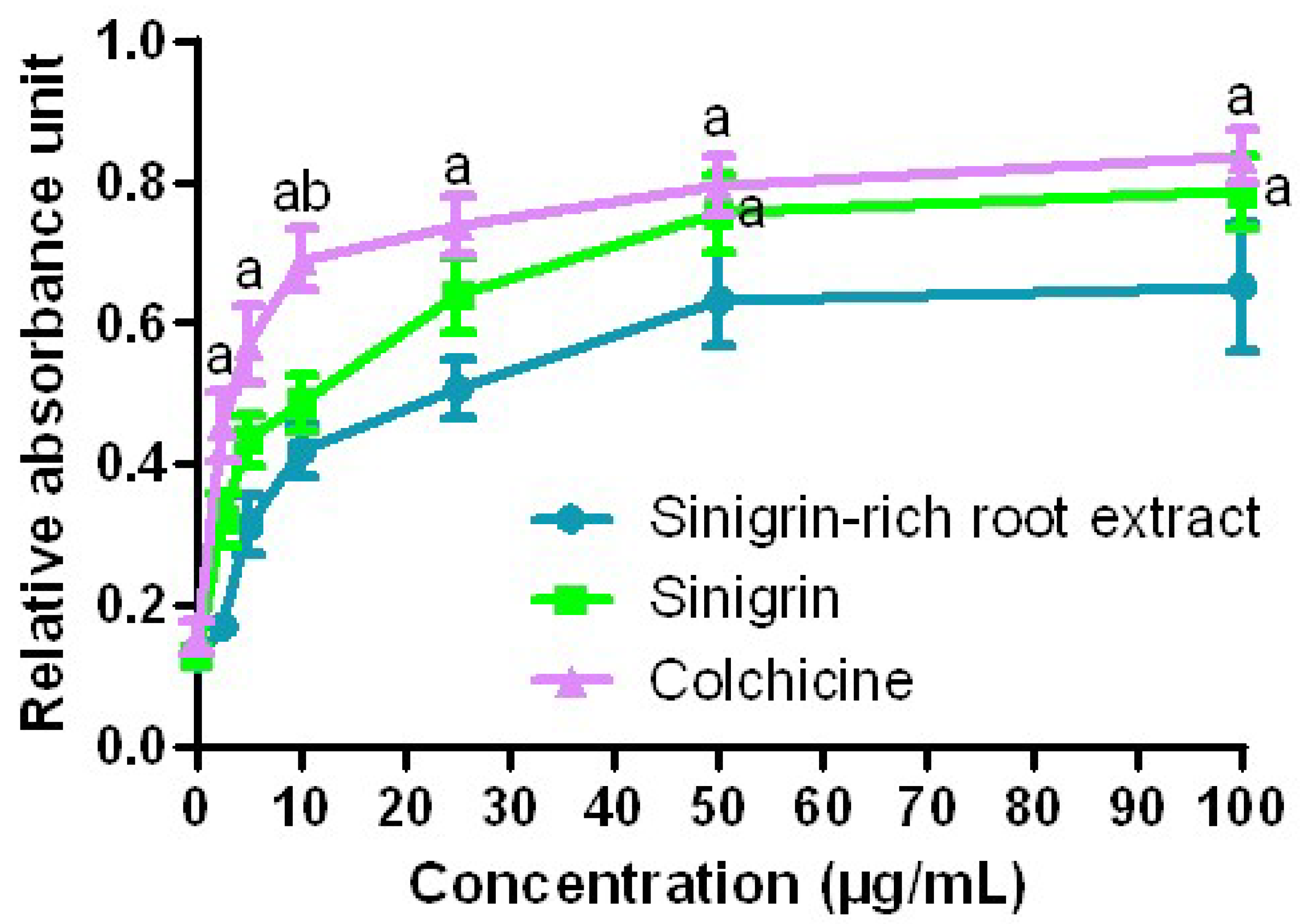
2.5. Effect of Roots Extract of Raphanus sativus and Sinigrin on Caspase-3 Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection of Plant Specimen
4.3. Preparation of Sample Extracts
4.4. Chromatographic Conditions
4.5. Preparation of Stock Solutions
4.6. Validation of Developed RP-HPLC Method
4.6.1. System Suitability
4.6.2. Linearity and Range
4.6.3. LOD and LOQ
4.6.4. Specificity
4.6.5. Accuracy and Precision
4.7. Recovery Study
4.8. Stability Study
4.9. Cell Lines and Culture
4.10. Screening of Test Compound by MTT Assay
4.11. Detection of Apoptosis by Flow Cytometry Assay
4.12. Measurement of Caspase-3 Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.-Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2019, 60, 2174–2211. [Google Scholar] [CrossRef]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Meng, X.; Li, Y.; Zhao, C.-N.; Liu, Q.; Li, H.-B. Effects of Vegetables on Cardiovascular Diseases and Related Mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Mandrich, L.; Caputo, E. Brassicaceae-Derived Anticancer Agents: Towards a Green Approach to Beat Cancer. Nutrients 2020, 12, 868. [Google Scholar] [CrossRef]
- Ramirez, D.; Abellán-Victorio, A.; Beretta, H.V.; Camargo, A.B.; Moreno, D.A. Functional Ingredients From Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-F.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A. Radish (Raphanus sativus) and Diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Kim, J.; Kim, D.-S.; Lee, E.-S.; Lee, H.-E. Deciphering the Nutraceutical Potential of Raphanus sativus—A Comprehensive Overview. Nutrients 2019, 11, 402. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, H.; Duan, Y.; Chen, G. Protective effects of radish (Raphanus sativus L.) leaves extract against hydrogen peroxide-induced oxidative damage in human fetal lung fibroblast (MRC-5) cells. Biomed. Pharmacother. 2018, 103, 406–414. [Google Scholar] [CrossRef]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef]
- Yi, G.; Lim, S.; Chae, W.B.; Park, J.E.; Park, H.R.; Lee, E.J.; Huh, J.H. Root Glucosinolate Profiles for Screening of Radish (Raphanus sativusL.) Genetic Resources. J. Agric. Food Chem. 2015, 64, 61–70. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef]
- Bellostas, N.; Kachlicki, P.; Sørensen, J.C.; Sørensen, H. Glucosinolate profiling of seeds and sprouts of B. oleracea varieties used for food. Sci. Hortic. 2007, 114, 234–242. [Google Scholar] [CrossRef]
- Mazumder, A.; Dwivedi, A.; Du Plessis, J. Sinigrin and Its Therapeutic Benefits. Molecules 2016, 21, 416. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Baenas, N.; Piegholdt, S.; Schloesser, A.; Moreno, D.A.; García-Viguera, C.; Rimbach, G.; Wagner, A.E. Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. Int. J. Mol. Sci. 2016, 17, 251. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Concentration of Sinigrin from Indian Mustard (Brassica juncea L.) Seeds Using Nanofiltration Membrane. In Lecture Notes in Electrical Engineering; Springer Science and Business Media LLC: Heidelberg, Germany, 2015; Volume 332, pp. 497–507. [Google Scholar]
- Faisal, W.; Farag, F.; Abdellatif, A.A.H.; Abbas, A. Taste Masking Approaches for Medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Lee, H.-W.; Lee, C.G.; Rhee, D.-K.; Um, S.H.; Pyo, S.; Pyo, S. Sinigrin inhibits production of inflammatory mediators by suppressing NF-κB/MAPK pathways or NLRP3 inflammasome activation in macrophages. Int. Immunopharmacol. 2017, 45, 163–173. [Google Scholar] [CrossRef]
- Grosser, K.; Van Dam, N.M. A Straightforward Method for Glucosinolate Extraction and Analysis with High-pressure Liquid Chromatography (HPLC). J. Vis. Exp. 2017, e55425. [Google Scholar] [CrossRef]
- Hu†, Y.; Liang†, H.; Yuan, Q.; Hong, Y. Determination of glucosinolates in 19 Chinese medicinal plants with spectrophotometry and high-pressure liquid chromatography. Nat. Prod. Res. 2010, 24, 1195–1205. [Google Scholar] [CrossRef]
- Herzallah, S.; Holley, R. Determination of sinigrin, sinalbin, allyl- and benzyl isothiocyanates by RP-HPLC in mustard powder extracts. LWT 2012, 47, 293–299. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Redeker, K.R.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an efficient glucosinolate extraction method. Plant Methods 2017, 13, 17. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.; Bernal, J.L.; Bernal, J. Optimized extraction, separation and quantification of twelve intact glucosinolates in broccoli leaves. Food Chem. 2014, 152, 66–74. [Google Scholar] [CrossRef]
- Attimarad, M.; Nair, A.B.; Aldhubaib, B.E. Development of liquid chromatographic method for the simultaneous determination of metformin and miglitol in human plasma: Application to pharmacokinetic studies. J. Iran. Chem. Soc. 2015, 12, 1629–1636. [Google Scholar] [CrossRef]
- Satyavert; Gupta, S.; Nair, A.B.; Attimarad, M. Development and validation of bioanalytical method for the determination of hydrazinocurcumin in rat plasma and organs by HPLC-UV. J. Chromatogr. B 2020, 1156, 122310. [Google Scholar] [CrossRef]
- Mateos-Vivas, M.; Rodríguez-Gonzalo, E.; García-Gómez, D.; Carabias-Martínez, R. Hydrophilic interaction chromatography coupled to tandem mass spectrometry in the presence of hydrophilic ion-pairing reagents for the separation of nucleosides and nucleotide mono-, di- and triphosphates. J. Chromatogr. A 2015, 1414, 129–137. [Google Scholar] [CrossRef]
- Mvumi, C.; Ngadze, E.; Marais, D.; Dutoit, E.S.; Kugara, J. Determination and quantification of sinigrin glucosinolates in Alternaria solani susceptible tomato (Solanum lycopersicum) leaves treated with moringa (Moringa oleifera) leaf extract. Arch. Phytopathol. Plant Prot. 2018, 51, 432–444. [Google Scholar] [CrossRef]
- Tsao, R.; Yu, Q.; Potter, J.; Chiba, M. Direct and Simultaneous Analysis of Sinigrin and Allyl Isothiocyanate in Mustard Samples by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2002, 50, 4749–4753. [Google Scholar] [CrossRef]
- Attimarad, M.; Venugopala, K.N.; Sreeharsha, N.; Aldhubiab, B.E.; Nair, A.B. Validation of rapid RP-HPLC method for concurrent quantification of amlodipine and celecoxib in pure and formulation using an experimental design. Microchem. J. 2020, 152, 104365. [Google Scholar] [CrossRef]
- Betz, J.M.; Brown, P.N.; Roman, M.C. Accuracy, precision, and reliability of chemical measurements in natural products research. Fitoterapia 2011, 82, 44–52. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2(R1), ICH. 2005. Available online: http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed on 15 September 2020).
- Aires, A.; Carvalho, R. Rapid Separation of Indole Glucosinolates in Roots of Chinese Cabbage (Brassica rapaSubsp. Pekinensis) by High-Performance Liquid Chromatography with Diode Array Detection. Int. J. Anal. Chem. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Yuan, D.; Shim, Y.Y.; Reaney, M.J.T.; Meda, V. Improved analysis of sinigrin in Ethiopian mustard. Int. J. Food Sci. Technol. 2016, 51, 690–699. [Google Scholar] [CrossRef]
- Fröhlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef]
- Anroop, B.; Bhatnagar, S.; Ghosh, B.; Parcha, V. Studies on Ocimum gratissimum seed mucilage: Evaluation of suspending properties. Indian J. Pharm. Sci. 2005, 67, 206. [Google Scholar]
- Balkrishna, A.; Das, S.K.; Pokhrel, S.; Joshi, A.; Laxmi; Verma, S.; Sharma, V.K. Colchicine: Isolation, LC–MS QTof Screening, and Anticancer Activity Study of Gloriosa superba Seeds. Molecules 2019, 24, 2772. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, W.; Jiang, X.; Liu, L.; Wei, K.; Du, H.; Wang, H.; Li, J. Anticancer effects and underlying mechanism of Colchicine on human gastric cancer cell lines in vitro and in vivo. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef]
- Jie, M.; Cheung, W.M.; Yu, V.; Zhou, Y.; Tong, P.H.; Ho, J.W.S. Anti-Proliferative Activities of Sinigrin on Carcinogen-Induced Hepatotoxicity in Rats. PLoS ONE 2014, 9, e110145. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Cell Biol. 2010, 2010, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Li, Y.; Wade, K.L.; Paonessa, J.D.; Fahey, J.W.; Zhang, Y. Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis 2010, 31, 2105–2110. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.W.; Minchinton, I.; Sang, J. The isolation and purification of indole glucosinolates from brassica species. J. Sci. Food Agric. 1983, 34, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Attimarad, M.; Alkadham, A.; Almosawi, M.H.; Venugopala, K.N. Development of Rapid and Validated RP-HPLC Method for Concurrent Quantification of Rosuvastatin and Aspirin form Solid Dosage Form. Indian J. Pharm. Educ. Res. 2018, 52, 151–158. [Google Scholar] [CrossRef]
- Shah, A.A.; Nayak, Y. Development, Optimisation and Validation of RP-HPLC Method for the Quantification of Resveratrol. Indian J. Pharm. Educ. Res. 2019, 53, s356–s363. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, R.; Nair, A.B.; Saini, G. Development and validation of RP-HPLC method for simultaneous estimation of nimesulide, phenylephrine hydrochloride, chlorpheniramine maleate and caffeine anhydrous in pharmaceutical dosage form. Acta Pol. Pharm. Drug Res. 2013, 69, 1017–1022. [Google Scholar]
- Attimarad, M.; Nagaraja, S.H.; Nair, A.B.; Aldhubaib, B.E.; Venugopala, K.N. Development of validated RP HPLC method with fluorescence detection for simultaneous quantification of sacubitril and valsartan from rat plasma. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 246–252. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; Al-Qubaisi, M.; Rasedee, A.; Hussein, M.Z. Evaluation of the Cytotoxic Effect of Ellagic Acid Nanocomposite in Lung Cancer A549 Cell Line and RAW 264.9 Cells. J. Bionanosci. 2017, 11, 578–583. [Google Scholar] [CrossRef]
- Kumar, R.T.K.; Liu, S.; Minna, J.D.; Prasad, S. Monitoring drug induced apoptosis and treatment sensitivity in non-small cell lung carcinoma using dielectrophoresis. Biochim. Biophys. Acta 2016, 1860, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; Zafeer, M.F.; Anis, E.; Ahmad, M.; Afzal, M. Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol. Rep. 2018, 5, 411–417. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Results |
|---|---|
| Retention time ± SD (min) | 3.59 ± 0.12 |
| Peak Area ± SD | 6,451,361 ± 20,673 |
| Theoretical plates ± SD | 4005.29 ± 42.56 |
| Symmetry ± SD | 1.39 ± 0.08 |
| Linearity range (μg/mL) | 50–800 |
| Slope ± SD | 15,005 ± 154 |
| Intercept ± SD | 467,329 ± 2475 |
| Correlation coefficient (R2) | 0.999 |
| Limit of detection (μg/mL) | 0.78 |
| Limit of quantification (μg/mL) | 2.45 |
| Analyte | Amount of Sinigrin Added (µg/mL) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| Amount Detected Mean ± SD a | %RSD | %RE | Amount Detected Mean ± SD b | %RSD | %RE | ||
| Sinigrin | 50 | 49.45 ± 0.87 | 1.76 | −1.11 | 49.45 ± 0.49 | 0.99 | −1.11 |
| 400 | 392.82 ± 5.98 | 1.53 | −1.82 | 394.54 ± 4.56 | 1.16 | −1.38 | |
| 800 | 790.55 ± 7.89 | 1.01 | −1.19 | 14 ± 5.02 | 0.64 | −1.37 | |
| Average | 1.43 | −1.37 | - | 0.94 | −1.29 | ||
| Analyte | Level (%) | Amount of Sinigrin (µg /mL) | Amount Detected (Mean ± SD) | % Recovery | % RE |
|---|---|---|---|---|---|
| Sinigrin | 50 | 200 | 189.45 ± 2.84 | 94.73 | −5.57 |
| 100 | 300 | 289.82 ± 3.42 | 96.61 | −3.51 | |
| 150 | 400 | 382.55 ± 4.79 | 95.64 | −4.56 | |
| Mean | 95.66 | −4.54 | |||
| Sample | IC50 Values (µg/mL) | ||
|---|---|---|---|
| DU-145 | HCT-15 | A-375 | |
| Colchicine | 11.92 | 12.17 | 10.17 |
| Sinigrin | 10.91 | 16.76 | 7.37 |
| Sinigrin-rich root extract | 15.88 | 21.42 | 24.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.B.; Gandhi, D.; Patel, S.S.; Morsy, M.A.; Gorain, B.; Attimarad, M.; Shah, J.N. Development of HPLC Method for Quantification of Sinigrin from Raphanus sativus Roots and Evaluation of Its Anticancer Potential. Molecules 2020, 25, 4947. https://doi.org/10.3390/molecules25214947
Nair AB, Gandhi D, Patel SS, Morsy MA, Gorain B, Attimarad M, Shah JN. Development of HPLC Method for Quantification of Sinigrin from Raphanus sativus Roots and Evaluation of Its Anticancer Potential. Molecules. 2020; 25(21):4947. https://doi.org/10.3390/molecules25214947
Chicago/Turabian StyleNair, Anroop B., Dipal Gandhi, Snehal S. Patel, Mohamed A. Morsy, Bapi Gorain, Mahesh Attimarad, and Jigar N. Shah. 2020. "Development of HPLC Method for Quantification of Sinigrin from Raphanus sativus Roots and Evaluation of Its Anticancer Potential" Molecules 25, no. 21: 4947. https://doi.org/10.3390/molecules25214947
APA StyleNair, A. B., Gandhi, D., Patel, S. S., Morsy, M. A., Gorain, B., Attimarad, M., & Shah, J. N. (2020). Development of HPLC Method for Quantification of Sinigrin from Raphanus sativus Roots and Evaluation of Its Anticancer Potential. Molecules, 25(21), 4947. https://doi.org/10.3390/molecules25214947









