CD90 Is Dispensable for White and Beige/Brown Adipocyte Differentiation
Abstract
1. Introduction
2. Results
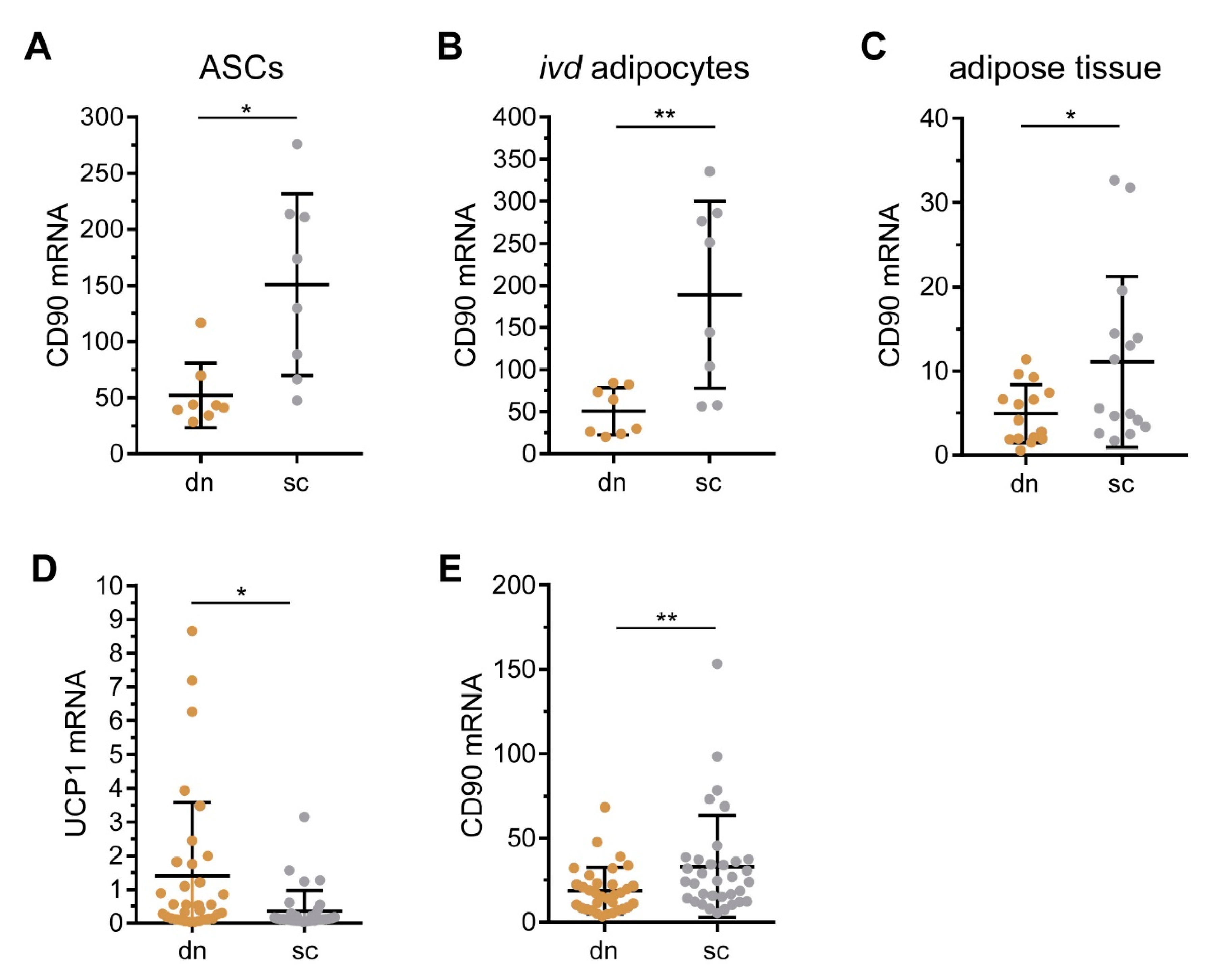
2.1. CD90/THY1 Expression in WAT and BAT
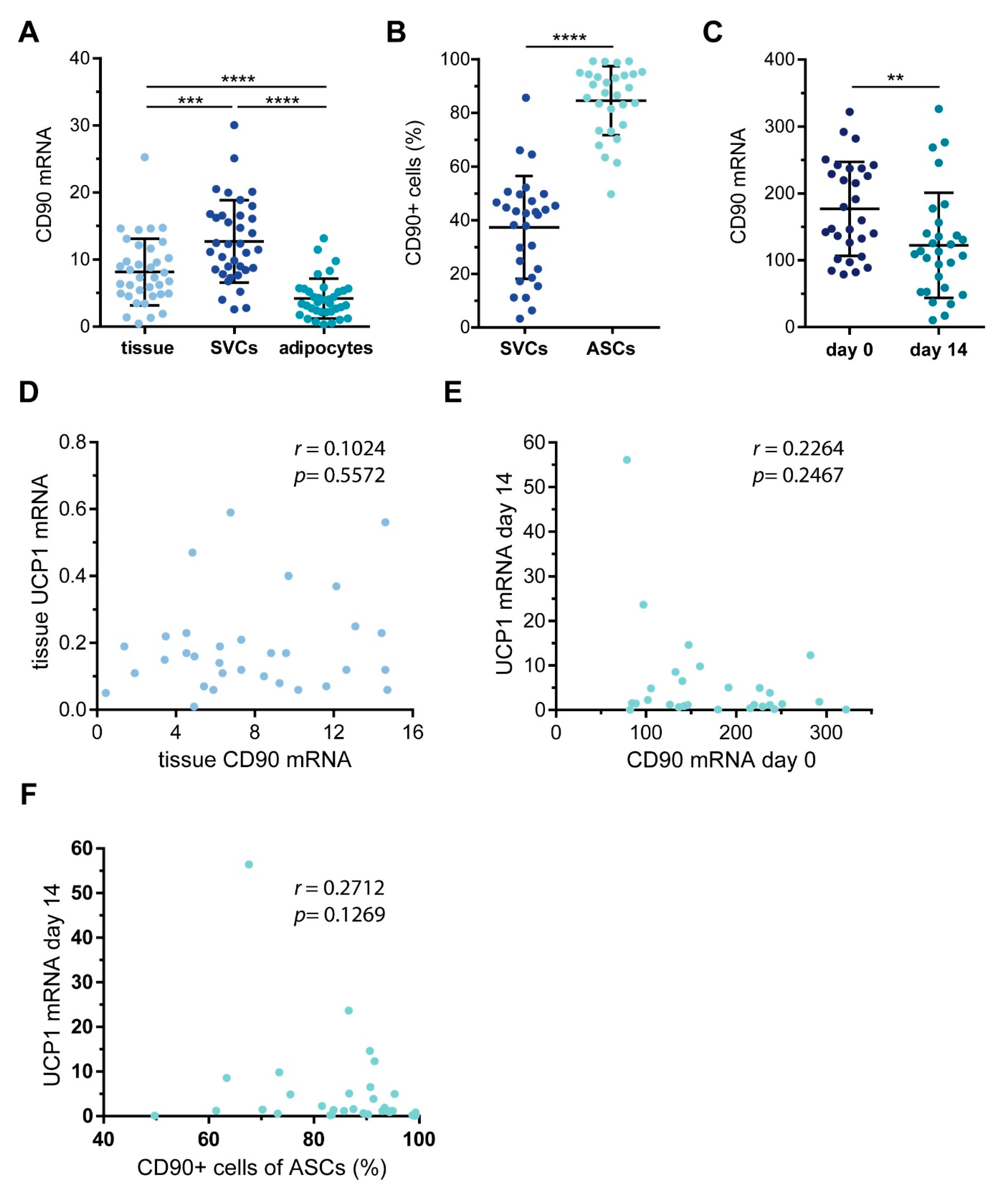
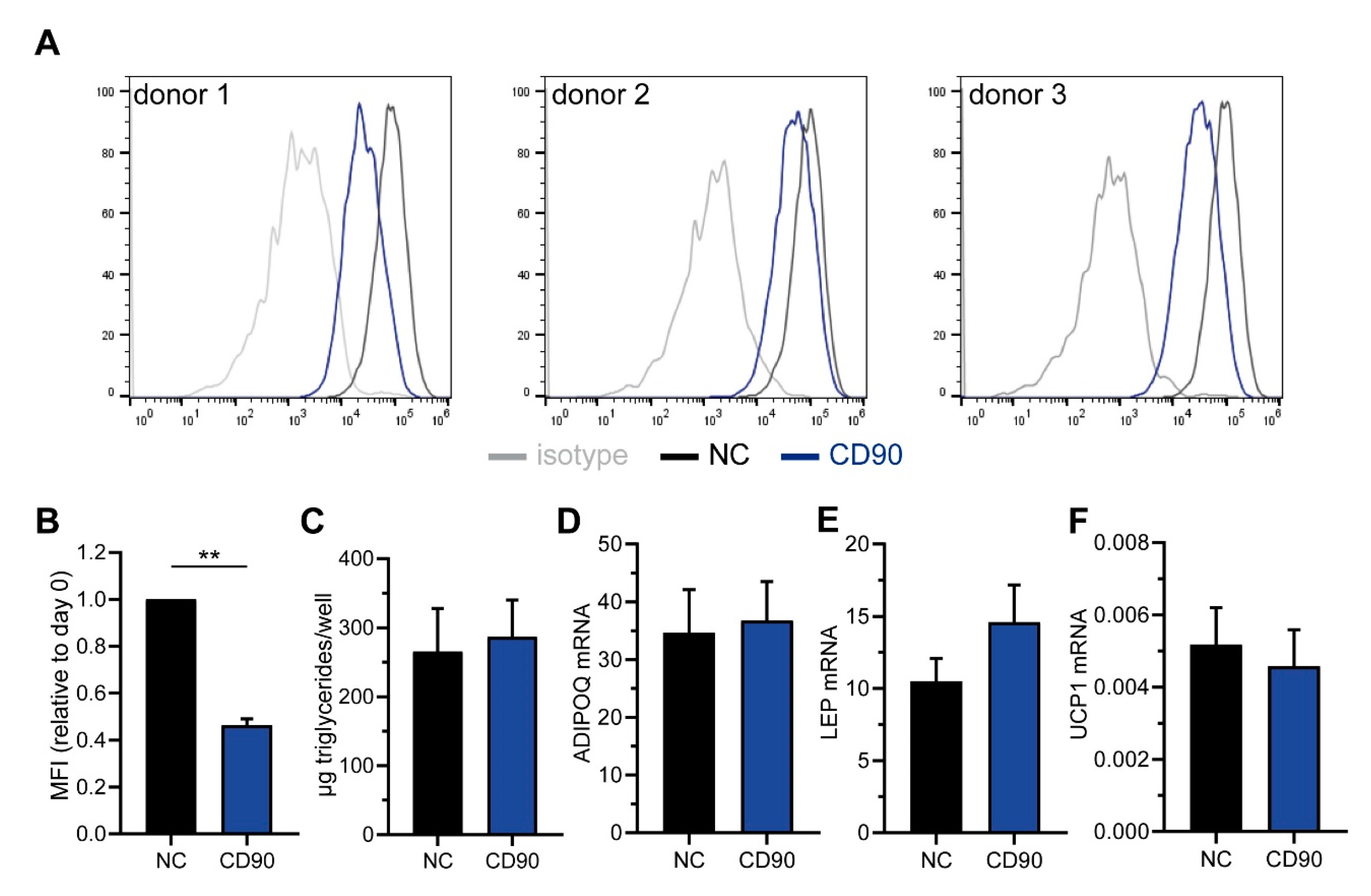
2.2. Characterization of CD90 Expression in WAT-Derived Stromal Cells
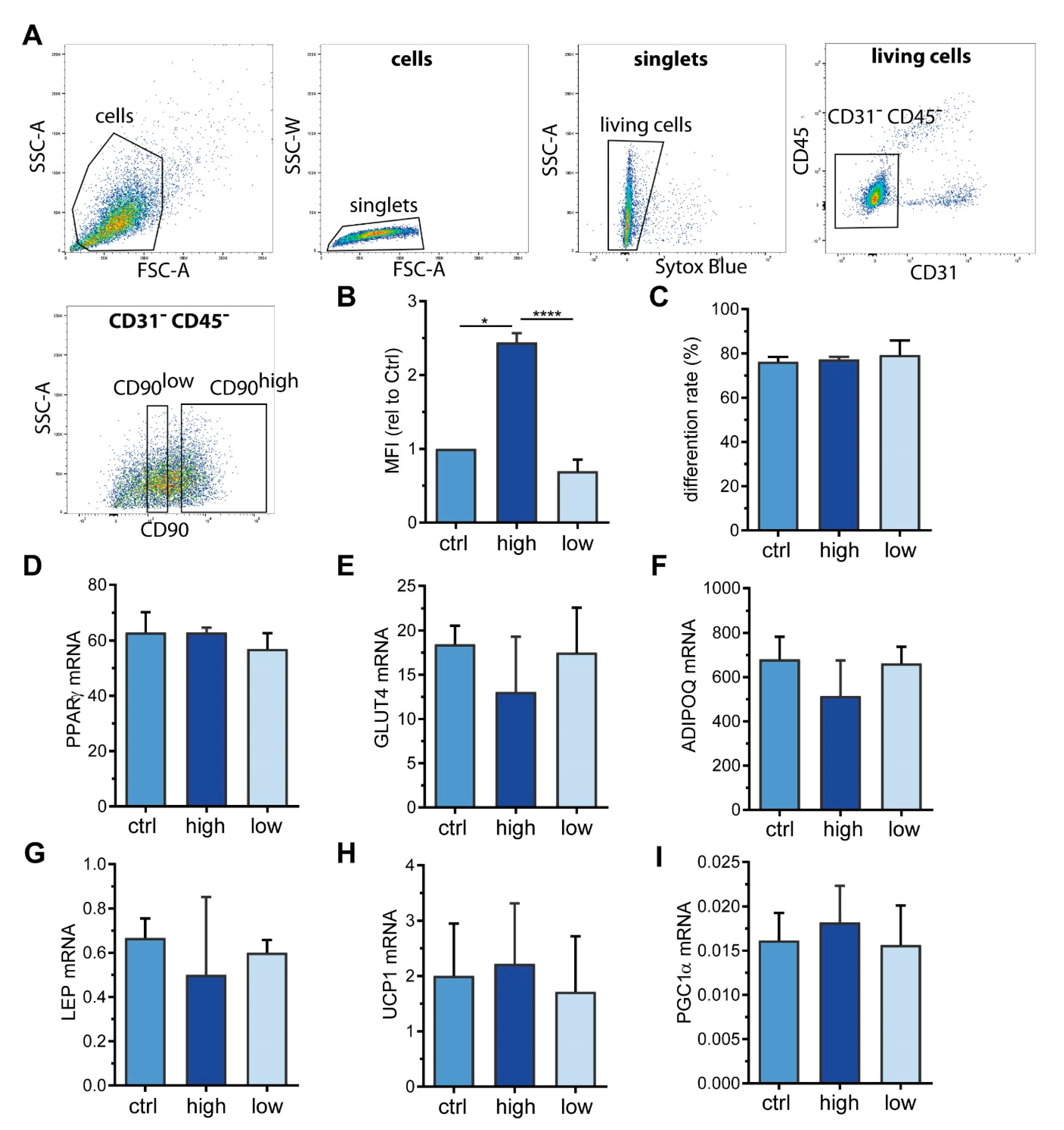
2.3. Sorting of ASC into CD90high and CD90low Populations
2.4. CRISPR/Cas9-Mediated Knockout of CD90 in Simpson-Golabi-Behmel Syndrome (SGBS) Cells
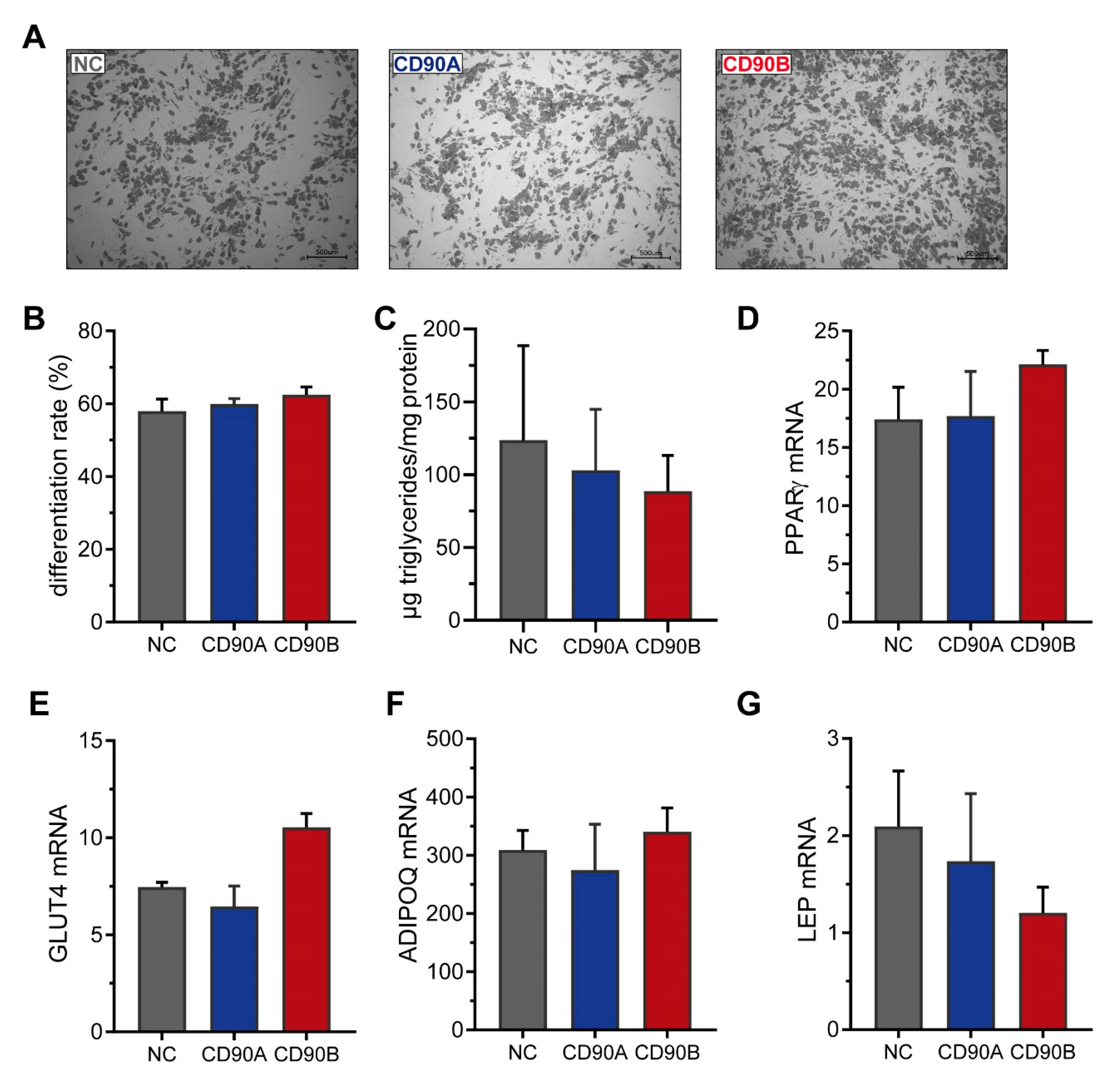
2.5. Knockdown of CD90 in Bone Marrow-Derived Mesenchymal Stromal Cells
3. Discussion
4. Materials and Methods
4.1. Patients and Primary Cell Isolation
4.2. Cell Culture Procedures
4.3. Flow Cytometry and Fluorescence-Activated Cell Sorting
4.4. Generation of CD90-Deficient SGBS Cells
4.5. siRNA-Mediated Silencing of CD90 in BMSCs
4.6. RNA Isolation, cDNA Synthesis, and Quantitative PCR
4.7. Protein Isolation and Western Blot
4.8. Determination of Triglyceride Content
4.9. Functional Extracellular Flux Analyses
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCs | Adipose tissue stromal cells |
| AT | Adipose tissue |
| BAT | Brown adipose tissue |
| BM | Bone marrow |
| BMI | Body mass index |
| BMSC | Bone marrow stromal cell |
| CD | Cluster of differentiation |
| Dn | Deep neck |
| ECAR | Extracellular acidification rate |
| FACS | Fluorescence-activated cell sorting |
| FDG-PET/CT | fluorodeoxyglucose positron emission tomography/computed tomography |
| GLUT4 | Glucose transporter 4 |
| GPI | Glycosylphosphatidylinositol |
| MFI | Mean fluorescence intensity |
| MSC | Mesenchymal stromal cell |
| OCR | Oxygen consumption rare |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| Sc | Subcutaneous |
| SGBS | Simpson-Golabi-Behmel syndrome |
| UCP1 | Uncoupling protein-1 |
| SVCs | Stromal-vascular cells |
| WAT | White adipose tissue |
References
- Trayhurn, P. Brown Adipose Tissue-A Therapeutic Target in Obesity? Front. Physiol. 2018, 9, 1672. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Himms-Hagen, J.; Melnyk, A.; Zingaretti, M.C.; Ceresi, E.; Barbatelli, G.; Cinti, S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Physiol. 2000, 279, C670–C681. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic Peroxisome Proliferator-activated Receptor γ (PPARγ) Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-containing Adipocytes Molecularly Distinct from Classic Brown Adipocytes. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and beige fat: Physiological roles beyond heat generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef]
- Gilsanz, V.; Hu, H.H.; Kajimura, S. Relevance of brown adipose tissue in infancy and adolescence. Pediatr. Res. 2013, 73, 3–9. [Google Scholar] [CrossRef]
- Lidell, M.E. Brown Adipose Tissue in Human Infants. In Brown Adipose Tissue; Springer: Cham, Switzerland, 2018; pp. 107–123. [Google Scholar]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Tews, D.; Schwar, V.; Scheithauer, M.; Weber, T.; Fromme, T.; Klingenspor, M.; Barth, T.F.; Möller, P.; Holzmann, K.; Debatin, K.M.; et al. Comparative gene array analysis of progenitor cells from human paired deep neck and subcutaneous adipose tissue. Mol. Cell. Endocrinol. 2014, 395, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Gagnon, J. Neuronal cell Thy-1 glycoprotein: Homology with immunoglobulin. Science 1982, 216, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Saalbach, A.; Anderegg, U. Thy-1: More than a marker for mesenchymal stromal cells. FASEB J. 2019, 33, 6689–6696. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Woeller, C.F.; O’Loughlin, C.W.; Pollock, S.J.; Thatcher, T.H.; Feldon, S.E.; Phipps, R.P. Thy1 (CD90) controls adipogenesis by regulating activity of the Src family kinase, Fyn. FASEB J. 2015, 29, 920–931. [Google Scholar] [CrossRef]
- Picke, A.-K.; Campbell, G.M.; Blüher, M.; Krügel, U.; Schmidt, F.N.; Tsourdi, E.; Winzer, M.; Rauner, M.; Vukicevic, V.; Busse, B.; et al. Thy-1 (CD90) promotes bone formation and protects against obesity. Sci. Transl. Med. 2018, 10, eaao6806. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Wabitsch, M.; Brenner, R.E.; Melzner, I.; Braun, M.; Möller, P.; Heinze, E.; Debatin, K.M.; Hauner, H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Posovszky, P.; Newell, F.S.; Wabitsch, M.; Tornqvist, H.E. Human SGBS cells—A unique tool for studies of human fat cell biology. Obes. Facts 2008, 1, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Halbgebauer, D.; Dahlhaus, M.; Wabitsch, M.; Fischer-Posovszky, P.; Tews, D. Browning capabilities of human primary adipose-derived stromal cells compared to SGBS cells. Sci. Rep. 2020, 10, 9632. [Google Scholar] [CrossRef] [PubMed]
- Reif AE, A.J. Immunological distinction of akr thymocytes. Nature 1964, 203, 886–887. [Google Scholar] [CrossRef] [PubMed]
- Reif, A.E.; Allen, J.M.V. The akr thymic antigen and its distribution in leukemias and nervous tissues. J. Exp. Med. 1964, 120, 413. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Lynes, M.D.; Dreyfuss, J.M.; Shamsi, F.; Schulz, T.J.; Zhang, H.; Huang, T.L.; Townsend, K.L.; Li, Y.; Takahashi, H.; et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat. Med. 2015, 21, 760–768. [Google Scholar] [CrossRef]
- Cypess, A.M.; White, A.P.; Vernochet, C.; Schulz, T.J.; Xue, R.; Sass, C.A.; Huang, T.L.; Roberts-Toler, C.; Weiner, L.S.; Sze, C.; et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 2013, 19, 635–639. [Google Scholar] [CrossRef]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef]
- Fujimori, K. Prostaglandins as PPAR γ modulators in adipogenesis. PPAR Res. 2012, 2012, 527607. [Google Scholar] [CrossRef]
- Lehmann, G.M.; Woeller, C.F.; Pollock, S.J.; O’Loughlin, C.W.; Gupta, S.; Feldon, S.E.; Phipps, R.P. Novel anti-adipogenic activity produced by human fibroblasts. Am. J. Physiol. Cell Physiol. 2010, 299, C672–C681. [Google Scholar] [CrossRef]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef]
- Puhl, A.C.; Milton, F.A.; Cvoro, A.; Sieglaff, D.H.; Campos, J.C.L.; Bernardes, A.; Filgueira, C.S.; Lindemann, J.L.; Deng, T.; Neves, F.A.R.; et al. Mechanisms of peroxisome proliferator activated receptor γ regulation by non-steroidal anti-inflammatory drugs. Nucl. Recept. Signal. 2015, 13, e004. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhou, Z.; Zhang, H.; Zhao, H.; Song, P.; Wang, D.; Yin, J.; Zhao, W.; Xie, Z.; Wang, F.; et al. CD90 serves as differential modulator of subcutaneous and visceral adipose-derived stem cells by regulating AKT activation that influences adipose tissue and metabolic homeostasis. Stem Cell Res. Ther. 2019, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Lee, J.H.; Dagur, P.K.; Yadav, H.; Shen, M.; Chen, W.; Kulkarni, A.B.; McCoy, J.P.; Finkel, T.; Cypess, A.M.; et al. TGF-β receptor 1 regulates progenitors that promote browning of white fat. Mol. Metab. 2018, 16, 160–171. [Google Scholar] [CrossRef]
- Yadav, H.; Rane, S.G. TGF-β/Smad3 signaling regulates brown adipocyte induction in white adipose tissue. Front. Endocrinol. (Lausanne) 2012, 3. [Google Scholar] [CrossRef]
- Chung, K.J.; Chatzigeorgiou, A.; Economopoulou, M.; Garcia-Martin, R.; Alexaki, V.I.; Mitroulis, I.; Nati, M.; Gebler, J.; Ziemssen, T.; Goelz, S.E.; et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat. Immunol. 2017, 18, 654–664. [Google Scholar] [CrossRef]
- Tharp, K.M.; Jha, A.K.; Kraiczy, J.; Yesian, A.; Karateev, G.; Sinisi, R.; Dubikovskaya, E.A.; Healy, K.E.; Stahl, A. Matrix-assisted transplantation of functional beige adipose tissue. Diabetes 2015, 64, 3713–3724. [Google Scholar] [CrossRef]
- Payab, M.; Abedi, M.; Foroughi Heravani, N.; Hadavandkhani, M.; Arabi, M.; Tayanloo-Beik, A.; Sheikh Hosseini, M.; Gerami, H.; Khatami, F.; Larijani, B.; et al. Brown adipose tissue transplantation as a novel alternative to obesity treatment: A systematic review. Int. J. Obes. 2020. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P.; et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 2020, 12, aaz8664. [Google Scholar] [CrossRef]
- Shukla, L.; Yuan, Y.; Shayan, R.; Greening, D.W.; Karnezis, T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front. Pharmacol. 2020, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Skurk, T.; Wabitsch, M. Cultures of human adipose precursor cells. Methods Mol. Biol. 2001, 155, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Fekete, N.; Rojewski, M.T.; Fürst, D.; Kreja, L.; Ignatius, A.; Dausend, J.; Schrezenmeier, H. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS ONE 2012, 7, e43255. [Google Scholar] [CrossRef]
- Rojewski, M.T.; Lotfi, R.; Gjerde, C.; Mustafa, K.; Veronesi, E.; Ahmed, A.B.; Wiesneth, M.; Körper, S.; Sensebé, L.U.C.; Layrolle, P.; et al. Translation of a standardized manufacturing protocol for mesenchymal stromal cells: A systematic comparison of validation and manufacturing data. Cytotherapy 2019, 21, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.; Danzer, C.; Rechsteiner, M.; Lehmann, H.; Brandt, L.P.; Hejhal, T.; Catalano, A.; Busenhart, P.; Gonçalves, A.F.; Brandt, S.; et al. A versatile modular vector system for rapid combinatorial mammalian genetics. J. Clin. Investig. 2015, 125, 1603–1619. [Google Scholar] [CrossRef]
- Tews, D.; Pula, T.; Funcke, J.B.; Jastroch, M.; Keuper, M.; Debatin, K.M.; Wabitsch, M.; Fischer-Posovszky, P. Elevated UCP1 levels are sufficient to improve glucose uptake in human white adipocytes. Redox Biol. 2019, 26, 101286. [Google Scholar] [CrossRef] [PubMed]
- Raspotnig, G.; Fauler, G.; Jantscher, A.; Windischhofer, W.; Schachl, K.; Leis, H.J. Colorimetric Determination of Cell Numbers by Janus Green Staining. Anal. Biochem. 1999, 275, 74–83. [Google Scholar] [CrossRef]


| Marker | n | SVC Mean% ± SD | ASC Mean% ± SD | p Value * |
|---|---|---|---|---|
| CD31 | 29 | 11.8 ± 7.7 | 8.4 ± 5.7 | 0.126 |
| CD29 | 29 | 40.0 ± 18.8 | 77.5 ± 23.3 | <0.0001 |
| CD34 | 29 | 48.4 ± 15.6 | 92.6 ± 7.3 | <0.0001 |
| CD105 | 29 | 20.4 ± 12.3 | 76.9 ± 15.4 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlhaus, M.; Roos, J.; Engel, D.; Tews, D.; Halbgebauer, D.; Funcke, J.-B.; Kiener, S.; Schuler, P.J.; Döscher, J.; Hoffmann, T.K.; et al. CD90 Is Dispensable for White and Beige/Brown Adipocyte Differentiation. Int. J. Mol. Sci. 2020, 21, 7907. https://doi.org/10.3390/ijms21217907
Dahlhaus M, Roos J, Engel D, Tews D, Halbgebauer D, Funcke J-B, Kiener S, Schuler PJ, Döscher J, Hoffmann TK, et al. CD90 Is Dispensable for White and Beige/Brown Adipocyte Differentiation. International Journal of Molecular Sciences. 2020; 21(21):7907. https://doi.org/10.3390/ijms21217907
Chicago/Turabian StyleDahlhaus, Meike, Julian Roos, Daniel Engel, Daniel Tews, Daniel Halbgebauer, Jan-Bernd Funcke, Sophie Kiener, Patrick J. Schuler, Johannes Döscher, Thomas K. Hoffmann, and et al. 2020. "CD90 Is Dispensable for White and Beige/Brown Adipocyte Differentiation" International Journal of Molecular Sciences 21, no. 21: 7907. https://doi.org/10.3390/ijms21217907
APA StyleDahlhaus, M., Roos, J., Engel, D., Tews, D., Halbgebauer, D., Funcke, J.-B., Kiener, S., Schuler, P. J., Döscher, J., Hoffmann, T. K., Zinngrebe, J., Rojewski, M., Schrezenmeier, H., Debatin, K.-M., Wabitsch, M., & Fischer-Posovszky, P. (2020). CD90 Is Dispensable for White and Beige/Brown Adipocyte Differentiation. International Journal of Molecular Sciences, 21(21), 7907. https://doi.org/10.3390/ijms21217907






