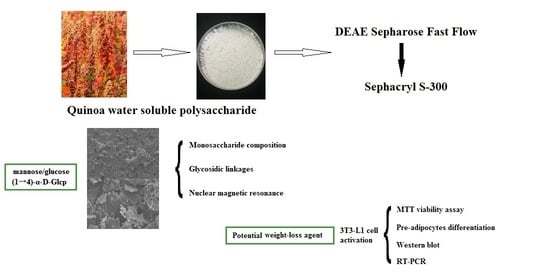
Structural Characterization of Quinoa Polysaccharide and Its Inhibitory Effects on 3T3-L1 Adipocyte Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample and Reagents
2.2. Extraction and Purification
2.3. Evaluation of the Structure Characteristics of the Water-Soluble Polysaccharide
2.3.1. Scanning Electron Microscopy
2.3.2. Molecular Weight Determination
2.3.3. Fourier-Transform Infrared Spectroscopy
2.3.4. Monosaccharide Composition Analysis
2.3.5. Analysis of Glycosidic Linkages
2.3.6. Nuclear Magnetic Resonance
2.4. Evaluation of the Inhibition of Adipocyte Differentiation by the Water-Soluble Polysaccharide
2.4.1. Cell Culture
2.4.2. Viability Assay
2.4.3. Cell Differentiation Assay
2.4.4. Oil-Red O Staining and Measurement of Optical Density
2.4.5. RNA Extraction and Real-Time Reverse Transcription Polymerase Chain Reaction
2.4.6. Western Blot Analysis
2.4.7. Statistical Analysis
3. Results
3.1. Extraction and Purification
3.2. Analysis of Surface Morphology
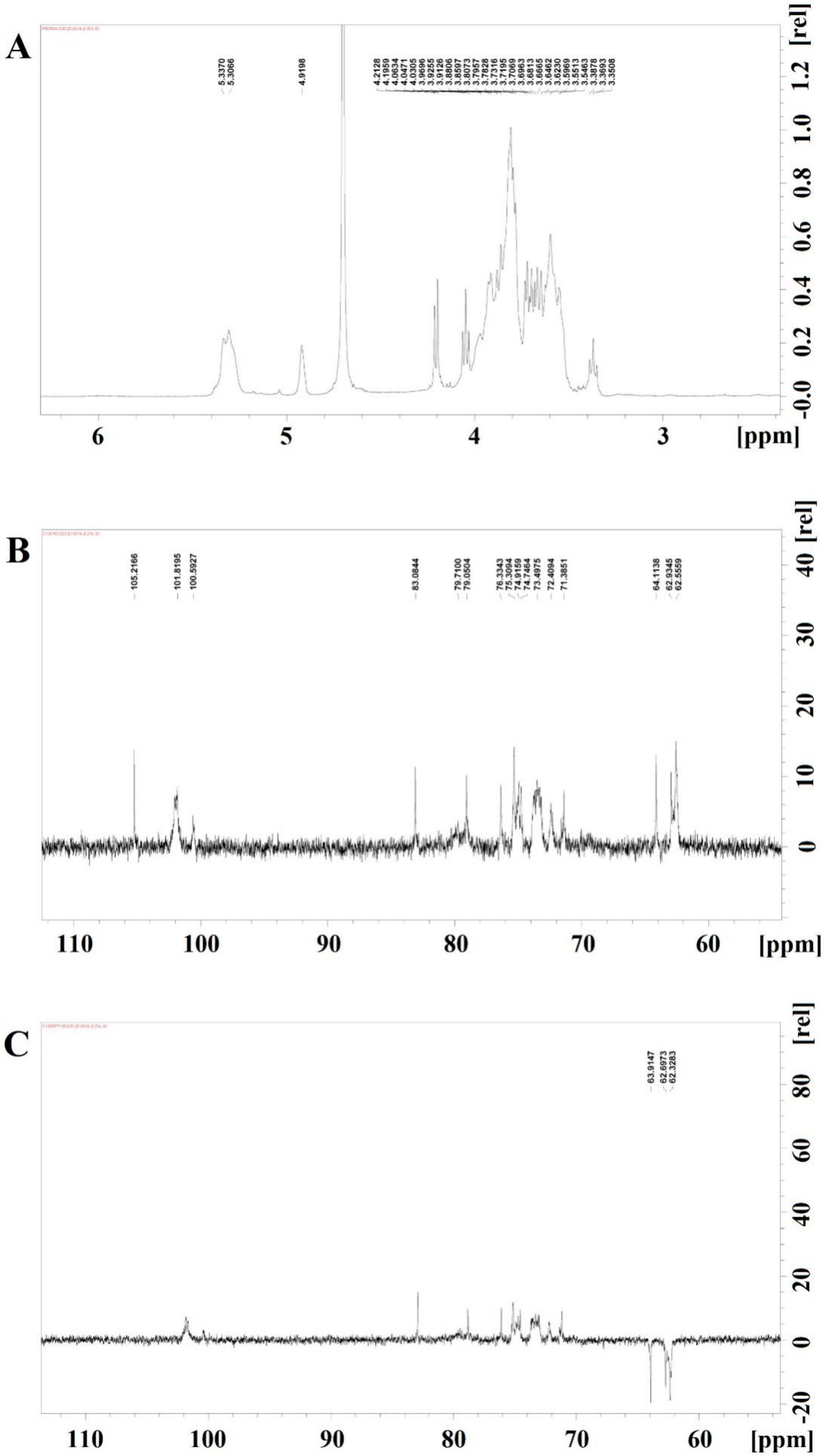
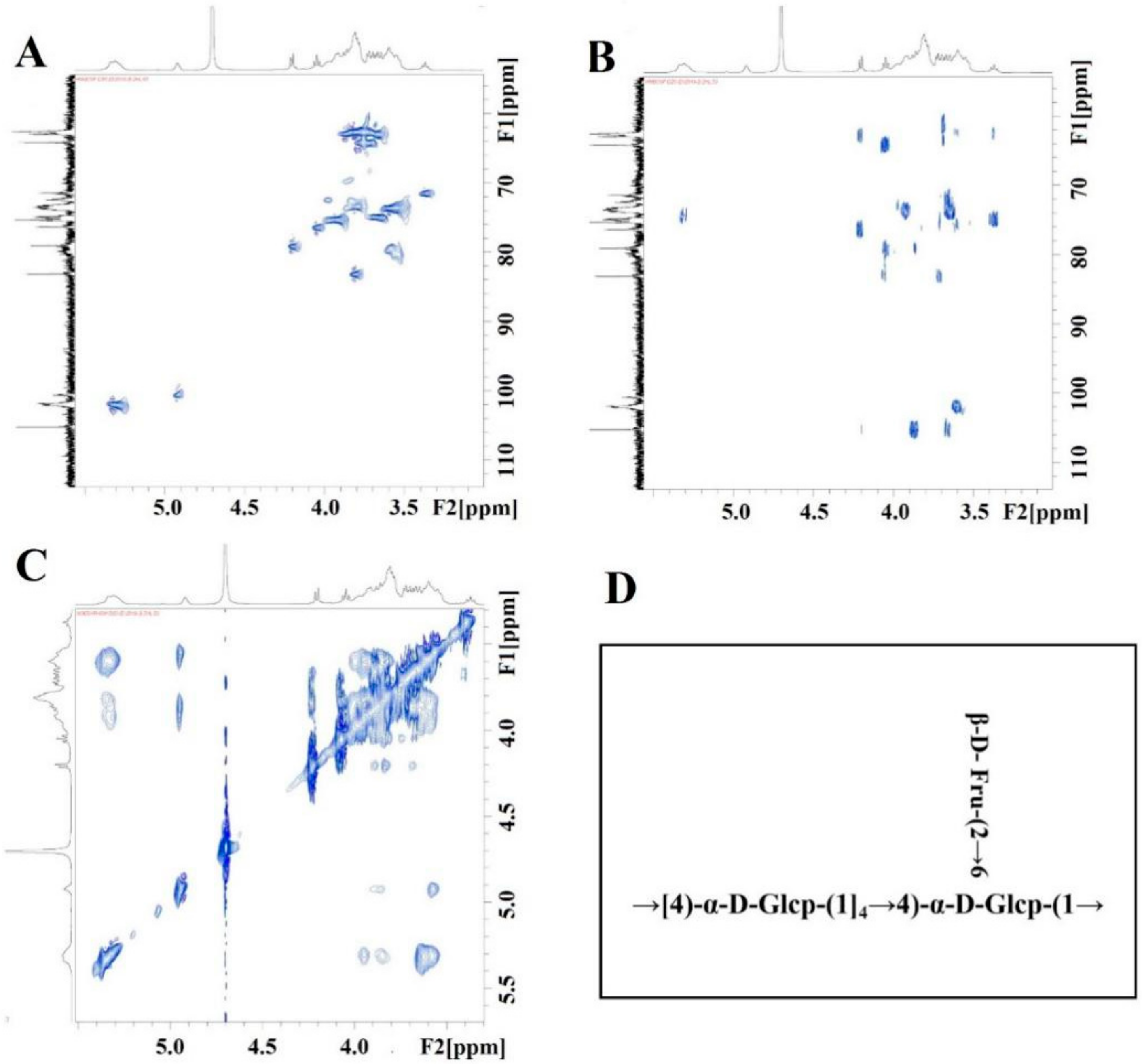
3.3. Analysis of Monosaccharide Composition and Glycosidic Linkages
3.4. Fourier-Transform Infrared Spectroscopy
3.5. Nuclear Magnetic Spectroscopy
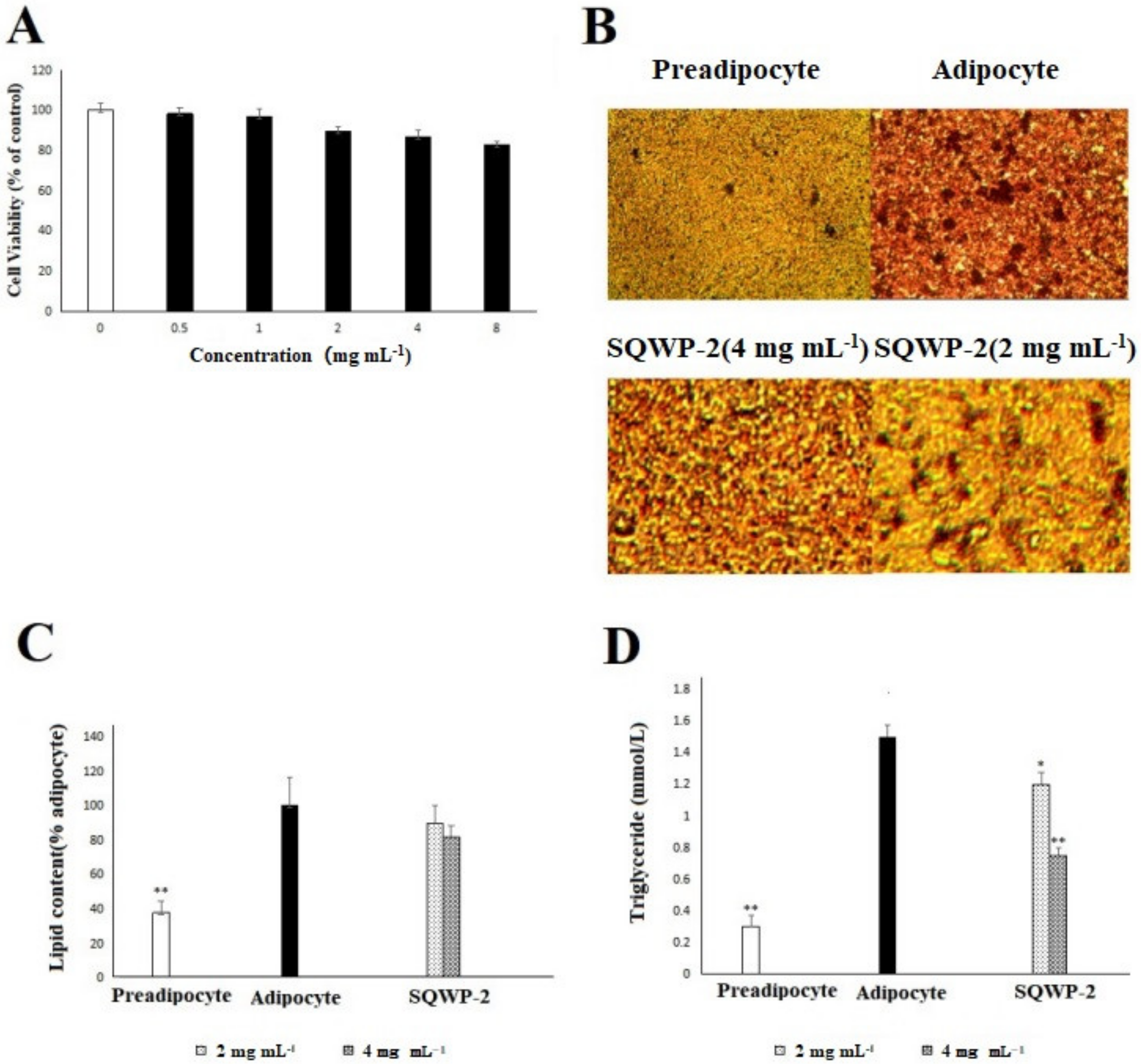
3.6. Cell Viability Analysis
3.7. Effect of the Water-Soluble Polysaccharide on Intracellular Lipid Accumulation in 3T3-L1 Cells
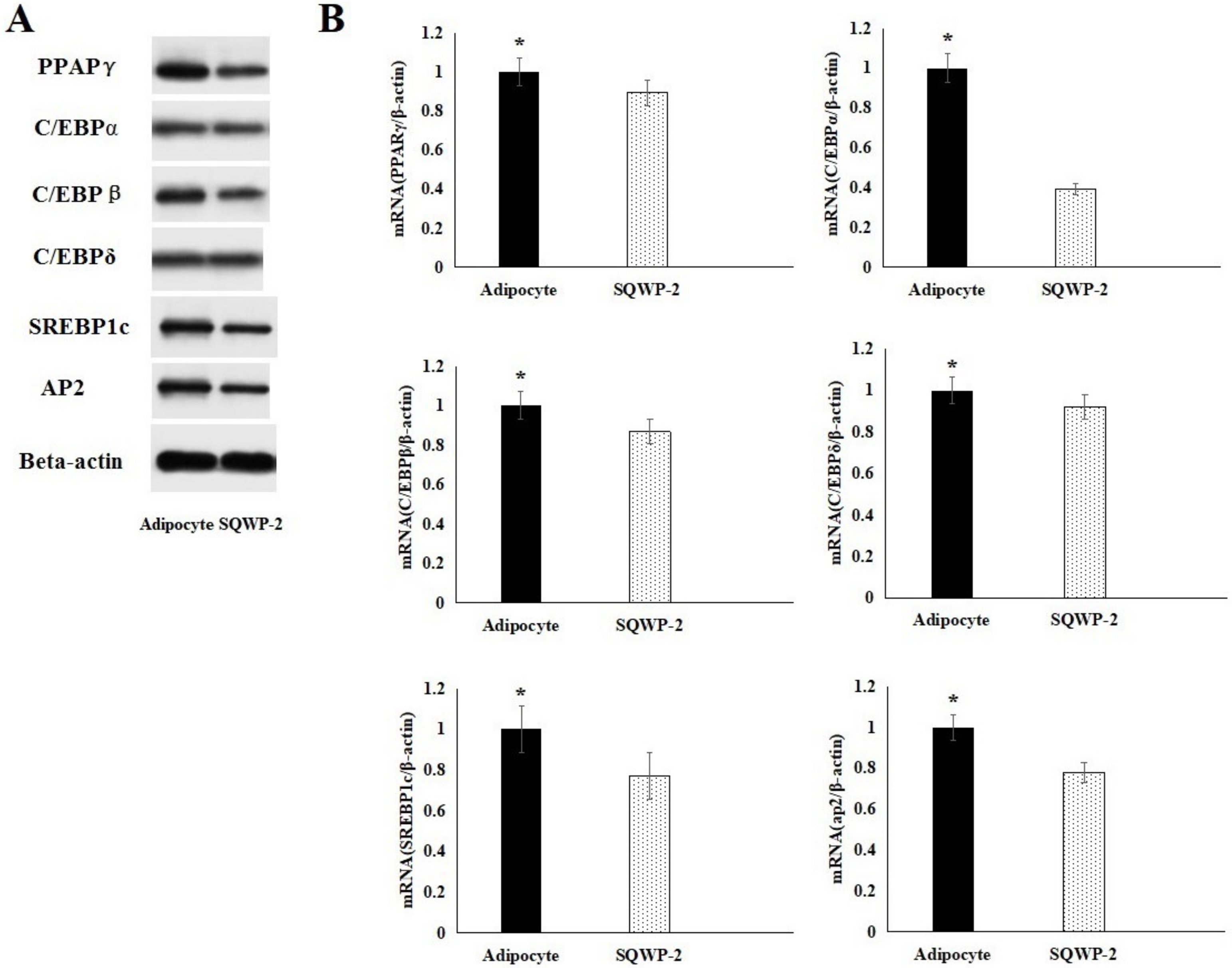
3.8. Effect of the Water-Soluble Polysaccharide on mRNA and Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| C/EBPα | CCAAT/enhancer-binding protein alpha |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| C/EBPδ | CCAAT/enhancer-binding protein delta |
| AP2 | Adipocyte protein 2 |
| M-SREBP1C | Sterol regulatory element-binding protein-1c |
| Dept | Distortionless Enhancement Polarizationtransfer |
| HSQC | Heteronuclear Single Quantum Coherence |
| HMBC | Heteronuclear Multiple Bond Correlation |
| NOESY | Nuclear Overhauser Effect Spectroscopy |
| TG | Triglyceride |
References
- Ji, W.C.; Andriy, S.; Peter, C.; Roman, B.; Radek, P.; Yong, P. Structural analysis and anti-obesity effect of a pectic polysaccharideisolated from Korean mulberry fruit Oddi (Morus alba L.). Carbohydr. Polym. 2016, 146, 187–196. [Google Scholar] [CrossRef]
- James, W.P.T.; Jackson, L.R.; Mhurchu, C.N.; Kalamara, E.; Shayeghi, M.; Rigby, N.J. Overweight and obesity (high body mass index) comparative quantification of health risks: Global and regional burden of disease attributable to selected major risk factors. World Health Organ. 2014, 1, 497–596. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Guo, X. Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): A review. Int. J. Biol. Macromol. 2019, 135, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Chen, D.; Lanlan, H.; Yan, B.; Tiancun, X.; Guo, J.; Su, Z.Q. The effects of COST on the differentiation of 3T3-L1 preadipocytes and the mechanism of action. Saudi J. Biol. Sci. 2017, 2, 251–255. [Google Scholar] [CrossRef]
- Sven, E.J. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Antonio, M.M.F.; Monica, R.P.; Tomaz, D.S.B.; Helena, M.P.S.; Paes, C.; Jane, S.D.R.C. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 8, 1618–1630. [Google Scholar] [CrossRef]
- Ogungbenle, H.N. Nutritional evaluation and functional properties of quinoa flour. Int. J. Food Sci. Nutr. 2003, 54, 153–158. [Google Scholar] [CrossRef]
- Farinazzi-Machado, F.M.V.; Barbalho, S.M.; Oshiiwa, M.R.; Goulart, O. Pessan junior use of cereal bars with quinoa (Chenopodium quinoa W.) to reduce risk factors related to cardiova scular diseases. Cienc. Technol. Aliment. 2012, 32, 239–244. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.Y.; Gao, Y.; Shi, Z.X.; Hu, Y.B.; Ren, G.X. Suppressive effects of saponin-enriched extracts from quinoa on 3T3-L1 adipocyte differentiation. Food Funct. 2015, 6, 3282–3290. [Google Scholar] [CrossRef]
- Anne, S.F.; Véronique, M.; René, L.; Patrick, E.; Waly, D.; Stanislas, V.; Daniel, T.; Jean, F.H.; Dominique, H.; Annie, Q. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Adipocyte Biol. 2011, 20, 270–277. [Google Scholar] [CrossRef]
- Maha, A. Preparation of different formulae from quinoa and different sources dietary Fiber to treat obesity in rats. Nat. Sci. 2016, 14, 55–65. [Google Scholar] [CrossRef]
- Bin, K.; Xiao, K.; Xuesi, W.; Yubin, Y.; Yingjuan, H.; Jian, Q.; Chengheng, H.; Lin, S. Astragalus polysaccharides attenuates TNF-α-induced insulin resistance via suppression of miR-721 and activation of PPAR-γ and PI3K/AKT in 3T3-L1 adipocytes. Am. J. Transl. Res. 2017, 9, 2195–2206. [Google Scholar]
- Liu, J.L.; Yang, L.C.; Zhu, X.J.; Wang, W.J.; Zheng, G.D. Combinational effect of pine needle polysaccharide and kudzu flavonoids on cell differentiation and fat metabolism in 3T3-L1 cells. Food Sci. Technol. Res. 2018, 5, 903–910. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Yao, Y.; Gao, Y.; Hu, Y.B.; Shi, Z.X.; Ren, G.X. Suppressive effects of barley β-glucans with different molecular weight on 3T3-L1 adipocyte differentiation. J. Food Sci. 2016, 3, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating and anticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Wu, D.T.; Lv, G.P.; Zhao, J. Carbohydrates analysis in herbal glycomics. Trac Trends Anal. Chem. 2013, 52, 155–169. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.Y.; Ren, G.X. Immunoregulatory activities of polysaccharides from mung bean. Carbohydr. Polym. 2016, 139, 61–66. [Google Scholar] [CrossRef]
- Tatsuya, M.; Akio, M.; Norimasa, I.; Tokifumi, M.; Shin-Ichiro, N.; Yuan, C.L. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 1, 69–72. [Google Scholar] [CrossRef]
- Moshe, R.; Isaiah, J.K.; Yeshayahu, T. A scanning electron microscopy study of microencapsulation. J. Food Sci. 2010, 50, 139–144. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.; Zhu, S.; Yin, H.; Wang, M. Sugar compositional determination of polysaccharides from dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. Protoc. 2012, 7, 1590. [Google Scholar] [CrossRef] [PubMed]
- Meerloo, J.V.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The mtt assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, H.; Wang, C.; Bai, L.; Wang, Y.; Wang, W.; Wang, J. A comparison of methods for effective differentiation of the frozen-thawed 3T3-L1 cells. Anal. Biochem. 2019, 568, 57–64. [Google Scholar] [CrossRef]
- Cordeiro, L.M.; Reinhardt, V.D.; Baggio, C.H.; Werner, M.F.; Burci, L.M.; Sassaki, G.L.; Iacomini, M. Arabinan and arabinan-rich pectic polysaccharides from quinoa (Chenopodium quinoa) seeds: Structure and gastroprotective activity. Food Chem. 2012, 130, 937–944. [Google Scholar] [CrossRef]
- Cao, Y.N.; Zou, L.; Li, W.; Song, Y.; Zhao, G.; Hu, Y.C. Dietary quinoa (Chenopodium quinoa Willd.) polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Int. J. Biol. Macromol. 2020, 163, 55–65. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.; Yang, Z.; Liu, A. Relationship between structural properties and antitumor activity of Astragalus polysaccharides extracted with different temperatures. Int. J. Biol. Macromol. 2019, 124, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Neiss, T.G. Solution NMR spectroscopy of food polysaccharides. Polym. Rev. 2012, 52, 81–114. [Google Scholar] [CrossRef]
- Hye, K.J.; Dongjoo, L.; Hong, P.K.; Seung-Hoon, B. Structure analysis and antioxidant activities of an amylopectin-type polysaccharide isolated from dried fruits of Terminalia chebula. Carbohydr. Polym. 2019, 211, 100–108. [Google Scholar] [CrossRef]
- Giuliana, D.N.; Kevin, M.; Boon, P.C. Quinoa intake reduces plasma and liver cholesterol, lessens obesityassociated inflammation, and helps to prevent hepatic steatosis inobese db/db mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef]
- Kim, K.; Lee, O.; Lee, B. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010, 86, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Mi-Ja, K.; Un-Jae, C.; Jin-Sil, L. Inhibitory effects of fucoidan in 3T3-L1 adipocyte differentiation. Mar. Biotechnol. 2009, 11, 557–562. [Google Scholar] [CrossRef]
- Albalat, A.; Saera, V.A.; Capilla, E.; Gutiérrez, J.; Pérez, S.J.; Navarro, I. Insulin regulation of lipoprotein lipase (LPL) activity andexpression in gilthead sea bream (Sparus aurata). J. Biochem. Bolecular Biol. 2007, 2, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Carolina, O.P.; Élia, M.; Sónia, S.F.; Viviana, G.C.; Benedita, A.P.; Dmitry, V.E.; Angelina, S.P.; Alexandra, C.; Manuel, V.; Manuel, A.C.; et al. Structural analysisand potential immunostimulatory activity of Nannochloropsis oculata polysaccharides. Carbohydr. Polym. 2019, 222, 1–10. [Google Scholar] [CrossRef]
- Ji, X.; Yan, Y.; Hou, C.; Shi, M.; Liu, Y. Structural characterization of a galacturonicacid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int. J. Biol. Macromol. 2019, 147, 1–24. [Google Scholar] [CrossRef]
- Yang, M.; Tarun, B.; Hari, P.D.; Li, L.; Zisheng, L. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar] [CrossRef]


| Gene Name | Forward Primer | Reverse Primer | Accession No. |
|---|---|---|---|
| C/EBPα | TTACAACAGGCCAGGTTTCC | GGCTGGCGACATACAGTACA | NM_007678 |
| PPARγ | TTTTCAAGGGTGCCAGTTTC | AATCCTTGGCCCTCTGAGAT | NM_011146 |
| C/EBPβ | CCTTTAAATCCATGGAAGTGG | GGGCTGAAGTCGATGGC | NM_005194.2 |
| C/EBPδ | ACGACGAGAGCGCCATC | TCGCCGTCGCCCCAGTC | TRCN0000013697 |
| AP2 | GGCCAAGCCCAACATGATC | CACGCCCAGTTTGAAGGAAA | NM_024406 |
| M-SREBP1c | ACAGACAAACTGCCCATCCA | GCAAGAAGCGGATGTAGTCG | NC_010454.4 |
| β-actin | CCACAGCTGAGAGGGAAATC | AAGGAAGGCTGGAAAAGAGC | X03672 |
| Methylated Sugar | Mass Fragments (m/z) | Type of Linkage |
|---|---|---|
| 2,3,4,6-Me4-Glcp | 43,71,87,101,117,129,145,161,205 | Glc-(1→ |
| 1,3,4,6-Me4-Glc/Manp | 87,101,129,145,161 | Fru-(2→ |
| 2,3,6-Me3-Glcp | 43,87,99,101,113,117,129,131,161,173,233 | →4)-Glcp-(1→ |
| 2,3-Me2-Glcp | 43,71,85,87,99,101,117,127,159,161,201 | →4,6)-Glcp-(1→ |
| Glycosyl Residues | H1 | H2 | H3 | H4 | H5 | H6a | H6b |
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | ||
| →4)-α-D-Glcp-(1→ | 5.31 | 3.55 | 3.94 | 3.57 | 3.78 | 3.76 | ns |
| 102.01 | 73.57 | 75.31 | 79.58 | 73.52 | 62.63 | ||
| →4,6)-α-D-Glcp-(1→ | 4.93 | 3.55 | 3.65 | 3.54 | 3.85 | 3.84 | ns |
| 100.7 | 73.53 | 74.02 | 78.2 | 74.6 | 69.71 | ||
| Fru-(2→ | 3.65/3.81 | 4.2 | 4.05 | 3.81 | 3.72/3.78 | ||
| 62.74 | 105.17 | 79.01 | 76.4 | 83.23 | 64.28 | ||
| α-D-Glcp-1→ | 5.26 | 3.36 | 3.66 | 3.95 | 3.98 | 3.6 | 3.82 |
| 101.33 | 71.4 | 75.1 | 70.61 | 72.41 | 62.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, C.; Shi, Z.; Yao, Y.; Ren, G. Structural Characterization of Quinoa Polysaccharide and Its Inhibitory Effects on 3T3-L1 Adipocyte Differentiation. Foods 2020, 9, 1511. https://doi.org/10.3390/foods9101511
Teng C, Shi Z, Yao Y, Ren G. Structural Characterization of Quinoa Polysaccharide and Its Inhibitory Effects on 3T3-L1 Adipocyte Differentiation. Foods. 2020; 9(10):1511. https://doi.org/10.3390/foods9101511
Chicago/Turabian StyleTeng, Cong, Zhenxing Shi, Yang Yao, and Guixing Ren. 2020. "Structural Characterization of Quinoa Polysaccharide and Its Inhibitory Effects on 3T3-L1 Adipocyte Differentiation" Foods 9, no. 10: 1511. https://doi.org/10.3390/foods9101511
APA StyleTeng, C., Shi, Z., Yao, Y., & Ren, G. (2020). Structural Characterization of Quinoa Polysaccharide and Its Inhibitory Effects on 3T3-L1 Adipocyte Differentiation. Foods, 9(10), 1511. https://doi.org/10.3390/foods9101511