Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke
Abstract
:1. Introduction
2. Pathophysiology of Ischemic Stroke and Therapeutic Targets
3. Potential Mechanisms of Stem Cell Therapy
3.1. Cell Differentiation
3.2. Bystander Effect of Stem Cells
4. Key Aspects of Clinical Trials
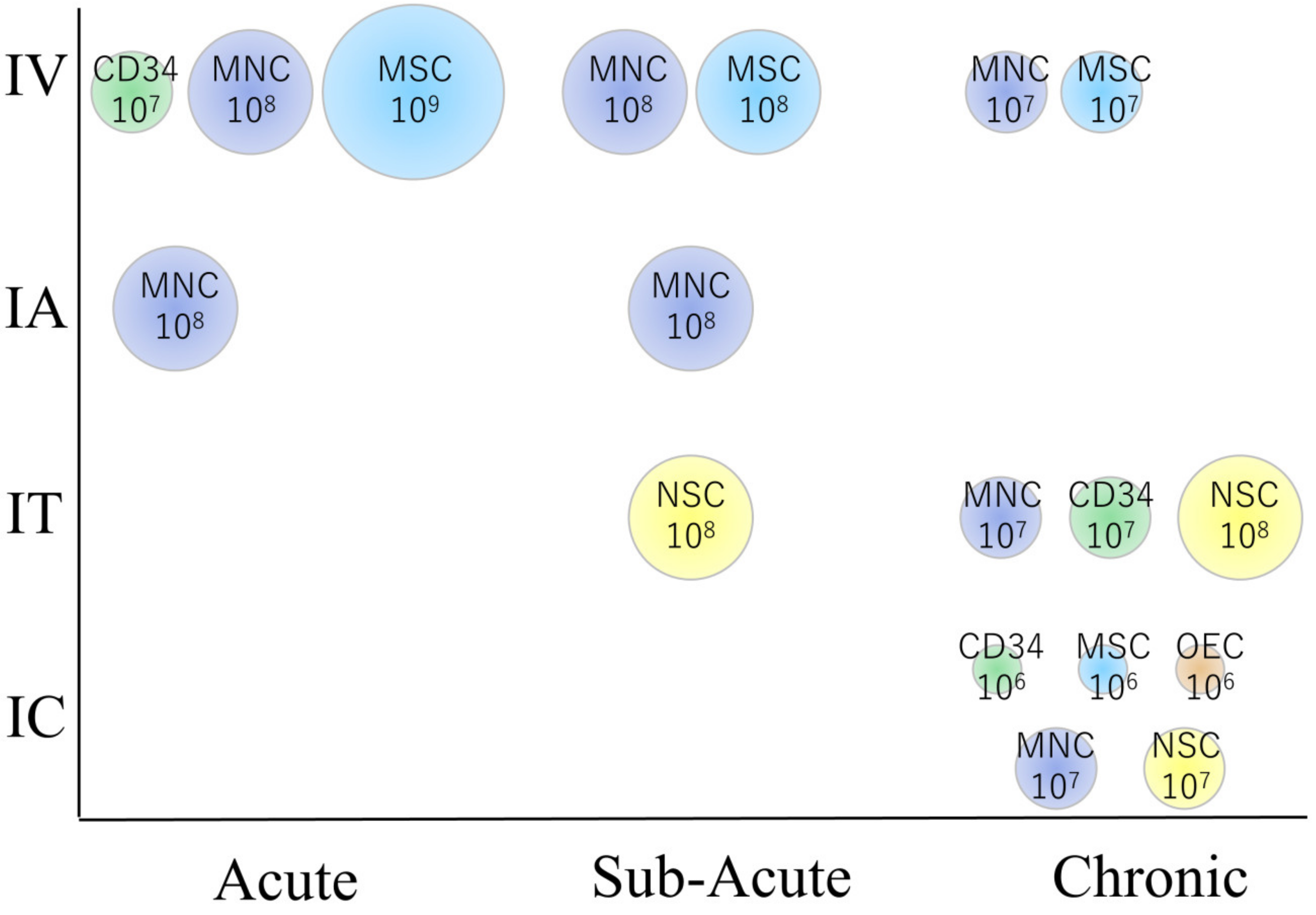
4.1. Overview of Clinical Trial Results
4.1.1. The Acute Phase of Stroke
4.1.2. The Sub-Acute Phase of Stroke
4.1.3. The Chronic Phase of Stroke
5. Unsolved Issues Associated with Optimal Treatment
5.1. Stem Cell Types
5.1.1. MNCs
5.1.2. Hematopoietic Stem Cells (CD34 Positive)
5.1.3. MSCs
5.1.4. NSCs
5.1.5. OECs
5.1.6. Other Cell Types
5.2. Cell Dose and Route
5.3. Patient Characteristics and Outcome Measure
6. Future Directions
6.1. Producing Good Cells (GMP Grade)
6.2. Producing Cells at Low Cost
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Barbosa da Fonseca, L.M.; Gutfilen, B.; Rosado de Castro, P.H.; Battistella, V.; Goldenberg, R.C.; Kasai-Brunswick, T.; Chagas, C.L.; Wajnberg, E.; Maiolino, A.; Salles Xavier, S.; et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp. Neurol. 2010, 221, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Battistella, V.; de Freitas, G.R.; da Fonseca, L.M.; Mercante, D.; Gutfilen, B.; Goldenberg, R.C.; Dias, J.V.; Kasai-Brunswick, T.H.; Wajnberg, E.; Rosado-de-Castro, P.H.; et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen. Med. 2011, 6, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.; Kumaran, S.S.; Bhatia, R.; Mohanty, S.; Srivastava, M.V.P. Safety and Feasibility of Autologous Mesenchymal Stem Cell Transplantation in Chronic Stroke in Indian patients. A four-year follow up. J. Stem Cells Regen. Med. 2017, 13, 14–19. [Google Scholar]
- Bhasin, A.; Srivastava, M.; Bhatia, R.; Mohanty, S.; Kumaran, S.; Bose, S. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J. Stem Cells Regen. Med. 2012, 8, 181–189. [Google Scholar]
- Bhasin, A.; Srivastava, M.V.; Kumaran, S.S.; Mohanty, S.; Bhatia, R.; Bose, S.; Gaikwad, S.; Garg, A.; Airan, B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc. Dis. Extra 2011, 1, 93–104. [Google Scholar] [CrossRef]
- Bhasin, A.; Srivastava, M.V.; Mohanty, S.; Bhatia, R.; Kumaran, S.S.; Bose, S. Stem cell therapy: A clinical trial of stroke. Clin. Neurol. Neurosurg. 2013, 115, 1003–1008. [Google Scholar] [CrossRef]
- Bhatia, V.; Gupta, V.; Khurana, D.; Sharma, R.R.; Khandelwal, N. Randomized Assessment of the Safety and Efficacy of Intra-Arterial Infusion of Autologous Stem Cells in Subacute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2018, 39, 899–904. [Google Scholar] [CrossRef] [Green Version]
- Correa, P.L.; Mesquita, C.T.; Felix, R.M.; Azevedo, J.C.; Barbirato, G.B.; Falcao, C.H.; Gonzalez, C.; Mendonca, M.L.; Manfrim, A.; de Freitas, G.; et al. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin. Nucl. Med. 2007, 32, 839–841. [Google Scholar] [CrossRef]
- Friedrich, M.A.; Martins, M.P.; Araujo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; Sartori El Ammar, J.; Machado, D.C.; Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012, 21, S13–S21. [Google Scholar] [CrossRef]
- Ghali, A.A.; Yousef, M.K.; Ragab, O.A.; ElZamarany, E.A. Intra-arterial Infusion of Autologous Bone Marrow Mononuclear Stem Cells in Subacute Ischemic Stroke Patients. Front. Neurol. 2016, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Moniche, F.; Gonzalez, A.; Gonzalez-Marcos, J.R.; Carmona, M.; Pinero, P.; Espigado, I.; Garcia-Solis, D.; Cayuela, A.; Montaner, J.; Boada, C.; et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: A pilot clinical trial. Stroke 2012, 43, 2242–2244. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mohanty, S.; Bhatia, R.; Srivastava, M.V.; Garg, A.; Srivastava, A.; Goyal, V.; Tripathi, M.; Kumar, A.; Bal, C.; et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: A pilot study. Indian J. Med. Res. 2012, 136, 221–228. [Google Scholar] [PubMed]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef] [Green Version]
- Rosado-de-Castro, P.H.; Schmidt Fda, R.; Battistella, V.; Lopes de Souza, S.A.; Gutfilen, B.; Goldenberg, R.C.; Kasai-Brunswick, T.H.; Vairo, L.; Silva, R.M.; Wajnberg, E.; et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen. Med. 2013, 8, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S., Jr.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69. [Google Scholar] [CrossRef]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F., Jr.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients With Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205. [Google Scholar] [CrossRef]
- Sharma, A.; Sane, H.; Gokulchandran, N.; Khopkar, D.; Paranjape, A.; Sundaram, J.; Gandhi, S.; Badhe, P. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res. Treat. 2014, 2014, 234095. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Monteagudo, C.; Hernandez-Ramirez, P.; Alvarez-Gonzalez, L.; Garcia-Maeso, I.; de la Cuetara-Bernal, K.; Castillo-Diaz, L.; Bringas-Vega, M.L.; Martinez-Aching, G.; Morales-Chacon, L.M.; Baez-Martin, M.M.; et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor. Neurol. Neurosci. 2009, 27, 151–161. [Google Scholar] [CrossRef]
- Taguchi, A.; Sakai, C.; Soma, T.; Kasahara, Y.; Stern, D.M.; Kajimoto, K.; Ihara, M.; Daimon, T.; Yamahara, K.; Doi, K.; et al. Intravenous Autologous Bone Marrow Mononuclear Cell Transplantation for Stroke: Phase1/2a Clinical Trial in a Homogeneous Group of Stroke Patients. Stem Cells Dev. 2015, 24, 2207–2218. [Google Scholar] [CrossRef] [Green Version]
- Bhasin, A.; Srivastava, M.V.P.; Mohanty, S.; Vivekanandhan, S.; Sharma, S.; Kumaran, S.; Bhatia, R. Paracrine Mechanisms of Intravenous Bone Marrow-Derived Mononuclear Stem Cells in Chronic Ischemic Stroke. Cerebrovasc. Dis. Extra 2016, 6, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Duma, C.; Kopyov, O.; Kopyov, A.; Berman, M.; Lander, E.; Elam, M.; Arata, M.; Weiland, D.; Cannell, R.; Caraway, C.; et al. Human intracerebroventricular (ICV) injection of autologous, non-engineered, adipose-derived stromal vascular fraction (ADSVF) for neurodegenerative disorders: Results of a 3-year phase 1 study of 113 injections in 31 patients. Mol. Biol. Rep. 2019, 46, 5257–5272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Guo, Y.; Tan, S.; Li, Z.; Xie, H.; Chen, P.; Wang, K.; He, Z.; He, P.; Ke, Y.; et al. Autologous Endothelial Progenitor Cells Transplantation for Acute Ischemic Stroke: A 4-Year Follow-Up Study. Stem Cells Transl. Med. 2019, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Honmou, O.; Houkin, K.; Matsunaga, T.; Niitsu, Y.; Ishiai, S.; Onodera, R.; Waxman, S.G.; Kocsis, J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011, 134, 1790–1807. [Google Scholar] [CrossRef] [Green Version]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef]
- Levy, M.L.; Crawford, J.R.; Dib, N.; Verkh, L.; Tankovich, N.; Cramer, S.C. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke 2019, 50, 2835–2841. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Kim, A.S.; Johnson, J.N.; Bates, D.; Poggio, G.; Case, C.; McGrogan, M.; et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): A phase 1/2a study. J. Neurosurg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bentley, P.; Hamady, M.; Marley, S.; Davis, J.; Shlebak, A.; Nicholls, J.; Williamson, D.A.; Jensen, S.L.; Gordon, M.; et al. Intra-Arterial Immunoselected CD34+ Stem Cells for Acute Ischemic Stroke. Stem Cells Transl. Med. 2014, 3, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.C.; Lin, S.Z.; Fan, J.R.; Lin, C.H.; Lee, W.; Lin, C.C.; Liu, Y.J.; Tsai, C.H.; Chen, J.C.; Cho, D.Y.; et al. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: A randomized phase II study. Cell Transplant. 2014, 23, 1599–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Zhu, W.; Zhu, J.; Wu, L.; Xu, G.; Liu, X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013, 22, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Laskowitz, D.T.; Bennett, E.R.; Durham, R.J.; Volpi, J.J.; Wiese, J.R.; Frankel, M.; Shpall, E.; Wilson, J.M.; Troy, J.; Kurtzberg, J. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase I Safety Study. Stem Cells Transl. Med. 2018, 7, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.Y.; Huang, F.J.; Zhao, M.; Xie, J.H.; Shi, J.; Wang, J.; Lin, X.Z.; Zuo, H.; Wang, Y.L.; Geng, T.C. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014, 23, S65–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ji, H.; Li, M.; Zhou, J.; Bai, W.; Zhong, Z.; Li, N.; Zhu, D.; Zhang, Z.; Liu, Y.; et al. Intrathecal Administration of Autologous CD34 Positive Cells in Patients with Past Cerebral Infarction: A Safety Study. ISRN Neurol. 2013, 2013, 128591. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xi, H.; Huang, H.; Zhang, F.; Liu, Y.; Chen, D.; Xiao, J. Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transplant. 2013, 22, S83–S91. [Google Scholar] [CrossRef]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Kondziolka, D.; Steinberg, G.K.; Wechsler, L.; Meltzer, C.C.; Elder, E.; Gebel, J.; Decesare, S.; Jovin, T.; Zafonte, R.; Lebowitz, J.; et al. Neurotransplantation for patients with subcortical motor stroke: A phase 2 randomized trial. J. Neurosurg. 2005, 103, 38–45. [Google Scholar] [CrossRef]
- Kondziolka, D.; Wechsler, L.; Goldstein, S.; Meltzer, C.; Thulborn, K.R.; Gebel, J.; Jannetta, P.; DeCesare, S.; Elder, E.M.; McGrogan, M.; et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000, 55, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, S.S.; Seledtsov, V.I.; Banul, N.V.; Poveshchenko, O.V.; Senyukov, V.V.; Astrakov, S.V.; Samarin, D.M.; Taraban, V.Y. Cell therapy of brain stroke. Bull. Exp. Biol. Med. 2005, 139, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 2020, 91, 396–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savitz, S.I.; Dinsmore, J.; Wu, J.; Henderson, G.V.; Stieg, P.; Caplan, L.R. Neurotransplantation of fetal porcine cells in patients with basal ganglia infarcts: A preliminary safety and feasibility study. Cerebrovasc. Dis. 2005, 20, 101–107. [Google Scholar] [CrossRef]
- Boltze, J.; Modo, M.M.; Mays, R.W.; Taguchi, A.; Jolkkonen, J.; Savitz, S.I.; Consortium, S. Stem Cells as an Emerging Paradigm in Stroke 4: Advancing and Accelerating Preclinical Research. Stroke 2019, 50, 3299–3306. [Google Scholar] [CrossRef]
- Savitz, S.I.; Chopp, M.; Deans, R.; Carmichael, T.; Phinney, D.; Wechsler, L.; Participants, S. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke 2011, 42, 825–829. [Google Scholar] [CrossRef] [Green Version]
- Savitz, S.I.; Cramer, S.C.; Wechsler, L.; Consortium, S. Stem cells as an emerging paradigm in stroke 3: Enhancing the development of clinical trials. Stroke 2014, 45, 634–639. [Google Scholar] [CrossRef] [Green Version]
- The STEPS Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009, 40, 510–515. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.Y.; Tarumi, T.; Liu, J.; Zhang, Y.; Turner, M.; Riley, J.; Tinajero, C.D.; Yuan, L.J.; Zhang, R. Distribution of cardiac output to the brain across the adult lifespan. J. Cereb. Blood Flow Metab. 2017, 37, 2848–2856. [Google Scholar] [CrossRef]
- Markus, H.S. Cerebral perfusion and stroke. J. Neurol. Neurosurg. Psychiatry 2004, 75, 353–361. [Google Scholar] [CrossRef]
- Powers, W.J.; Grubb, R.L., Jr.; Raichle, M.E. Physiological responses to focal cerebral ischemia in humans. Ann. Neurol. 1984, 16, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Yenari, M.A. Inflammatory responses in brain ischemia. Curr. Med. Chem. 2015, 22, 1258–1277. [Google Scholar] [CrossRef] [Green Version]
- Kawabori, M.; Yenari, M.A. The role of the microglia in acute CNS injury. Metab. Brain Dis. 2015, 30, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Lee, J.E.; Yenari, M.A. Stroke: Molecular mechanisms and potential targets for treatment. Curr. Mol. Med. 2003, 3, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Hokari, M.; Zheng, Z.; Kim, J.Y.; Calosing, C.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Triggering Receptor Expressed on Myeloid Cells-2 Correlates to Hypothermic Neuroprotection in Ischemic Stroke. Ther. Hypothermia Temp. Manag. 2013, 3, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabori, M.; Kacimi, R.; Kauppinen, T.; Calosing, C.; Kim, J.Y.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 2015, 35, 3384–3396. [Google Scholar] [CrossRef] [PubMed]
- Henninger, N.; Fisher, M. Extending the Time Window for Endovascular and Pharmacological Reperfusion. Transl. Stroke Res. 2016, 7, 284–293. [Google Scholar] [CrossRef]
- Kwak, K.A.; Kwon, H.B.; Lee, J.W.; Park, Y.S. Current Perspectives Regarding Stem Cell-based Therapy for Ischemic Stroke. Curr. Pharm. Des. 2018, 24, 3332–3340. [Google Scholar] [CrossRef]
- Barker, R.A.; Gotz, M.; Parmar, M. New approaches for brain repair-from rescue to reprogramming. Nature 2018, 557, 329–334. [Google Scholar] [CrossRef]
- Misra, V.; Ritchie, M.M.; Stone, L.L.; Low, W.C.; Janardhan, V. Stem cell therapy in ischemic stroke: Role of IV and intra-arterial therapy. Neurology 2012, 79, S207–S212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhang, Q.; Wang, W.; Lin, F.; Wang, S.; Zhao, J. Mesenchymal stem cell therapy for ischemic stroke: A look into treatment mechanism and therapeutic potential. J. Neurol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Shichinohe, H.; Houkin, K.; Iwasaki, Y. Autologous bone marrow stromal cell transplantation for central nervous system disorders—Recent progress and perspective for clinical application. J. Stem Cells Regen. Med. 2011, 7, 2–13. [Google Scholar] [PubMed]
- Cui, L.L.; Golubczyk, D.; Tolppanen, A.M.; Boltze, J.; Jolkkonen, J. Cell therapy for ischemic stroke: Are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann. Neurol. 2019, 86, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ceci, M.; Mariano, V.; Romano, N. Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment. Rev. Neurosci. 2018, 30, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qiu, R.; Li, L.; He, D.; Lv, H.; Wu, X.; Gu, N. The role of exogenous neural stem cells transplantation in cerebral ischemic stroke. J. Biomed. Nanotechnol. 2014, 10, 3219–3230. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.A.; Stokes, D.; Augelli, B.J.; DiGirolamo, C.; Prockop, D.J. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc. Natl. Acad. Sci. USA 1998, 95, 3908–3913. [Google Scholar] [CrossRef] [Green Version]
- Hokari, M.; Kuroda, S.; Chiba, Y.; Maruichi, K.; Iwasaki, Y. Synergistic effects of granulocyte-colony stimulating factor on bone marrow stromal cell transplantation for mice cerebral infarct. Cytokine 2009, 46, 260–266. [Google Scholar] [CrossRef]
- Hokari, M.; Kuroda, S.; Shichinohe, H.; Yano, S.; Hida, K.; Iwasaki, Y. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J. Neurosci. Res. 2008, 86, 1024–1035. [Google Scholar] [CrossRef]
- Kawabori, M.; Kuroda, S.; Ito, M.; Shichinohe, H.; Houkin, K.; Kuge, Y.; Tamaki, N. Timing and cell dose determine therapeutic effects of bone marrow stromal cell transplantation in rat model of cerebral infarct. Neuropathology 2013, 33, 140–148. [Google Scholar] [CrossRef]
- Kawabori, M.; Kuroda, S.; Sugiyama, T.; Ito, M.; Shichinohe, H.; Houkin, K.; Kuge, Y.; Tamaki, N. Intracerebral, but not intravenous, transplantation of bone marrow stromal cells enhances functional recovery in rat cerebral infarct: An optical imaging study. Neuropathology 2012, 32, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shichinohe, H.; Kuroda, S.; Yano, S.; Hida, K.; Iwasaki, Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007, 1183, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Kuroda, S.; Zhao, S.; Magota, K.; Shichinohe, H.; Houkin, K.; Kuge, Y.; Tamaki, N. Bone marrow stromal cell transplantation enhances recovery of local glucose metabolism after cerebral infarction in rats: A serial 18F-FDG PET study. J. Nucl. Med. 2013, 54, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shichinohe, H.; Ishihara, T.; Takahashi, K.; Tanaka, Y.; Miyamoto, M.; Yamauchi, T.; Saito, H.; Takemoto, H.; Houkin, K.; Kuroda, S. Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehabil. Neural Repair. 2015, 29, 80–89. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Katakowski, M.; Chen, X.; Wang, L.; Lu, D.; Lu, M.; Gautam, S.C.; Chopp, M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J. Neurosci. Res. 2003, 73, 778–786. [Google Scholar] [CrossRef]
- Ito, M.; Kuroda, S.; Sugiyama, T.; Maruichi, K.; Kawabori, M.; Nakayama, N.; Houkin, K.; Iwasaki, Y. Transplanted bone marrow stromal cells protect neurovascular units and ameliorate brain damage in stroke-prone spontaneously hypertensive rats. Neuropathology 2012, 32, 522–533. [Google Scholar] [CrossRef]
- Wang, Z.; He, D.; Zeng, Y.Y.; Zhu, L.; Yang, C.; Lu, Y.J.; Huang, J.Q.; Cheng, X.Y.; Huang, X.H.; Tan, X.J. The spleen may be an important target of stem cell therapy for stroke. J. Neuroinflamm. 2019, 16, 20. [Google Scholar] [CrossRef]
- Tan, C.; Zhao, S.; Higashikawa, K.; Wang, Z.; Kawabori, M.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Ukon, N.; Yasui, H.; et al. [(18)F]DPA-714 PET imaging shows immunomodulatory effect of intravenous administration of bone marrow stromal cells after transient focal ischemia. EJNMMI Res. 2018, 8, 35. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Doeppner, T.R.; Herz, J.; Gorgens, A.; Schlechter, J.; Ludwig, A.K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Ling, X.; Hu, G.; Zhu, Q.; Zhang, J.; Li, Q.; Zhao, B.; Wang, Y.; Deng, Z. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res. Ther. 2020, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Houkin, K.; Uchiyama, S.; Minematsu, K.; Taguchi, A.; Terasaka, S. Treatment evaluation of acute stroke for using in regenerative cell elements (TREASURE) trial: Rationale and design. Int. J. Stroke 2018, 13, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Stroncek, D.F.; Jin, P.; McKenna, D.H.; Takanashi, M.; Fontaine, M.J.; Pati, S.; Schafer, R.; Peterson, E.; Benedetti, E.; Reems, J.A. Human Mesenchymal Stromal Cell (MSC) Characteristics Vary Among Laboratories When Manufactured From the Same Source Material: A Report by the Cellular Therapy Team of the Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Front. Cell Dev. Biol. 2020, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Fernandez, M.; Rodriguez-Frutos, B.; Ramos-Cejudo, J.; Teresa Vallejo-Cremades, M.; Fuentes, B.; Cerdan, S.; Diez-Tejedor, E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res. Ther. 2013, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Shichinohe, H.; Kuroda, S.; Maruichi, K.; Osanai, T.; Sugiyama, T.; Chiba, Y.; Yamaguchi, A.; Iwasaki, Y. Bone marrow stromal cells and bone marrow-derived mononuclear cells: Which are suitable as cell source of transplantation for mice infarct brain? Neuropathology 2010, 30, 113–122. [Google Scholar] [CrossRef]
- Kolvenbach, R.; Kreissig, C.; Ludwig, E.; Cagiannos, C. Stem cell use in critical limb ischemia. J. Cardiovasc. Surg. 2007, 48, 39–44. [Google Scholar]
- Strauer, B.E.; Brehm, M.; Zeus, T.; Kostering, M.; Hernandez, A.; Sorg, R.V.; Kogler, G.; Wernet, P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002, 106, 1913–1918. [Google Scholar] [CrossRef] [Green Version]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Uemura, M.; Kasahara, Y.; Nagatsuka, K.; Taguchi, A. Cell-based therapy to promote angiogenesis in the brain following ischemic damage. Curr. Vasc. Pharmacol. 2012, 10, 285–288. [Google Scholar] [CrossRef]
- Inoue, T.; Croce, K.; Morooka, T.; Sakuma, M.; Node, K.; Simon, D.I. Vascular inflammation and repair: Implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc. Interv. 2011, 4, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular, T. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Bellagamba, B.C.; Grudzinski, P.B.; Ely, P.B.; Nader, P.J.H.; Nardi, N.B.; da Silva Meirelles, L. Induction of Expression of CD271 and CD34 in Mesenchymal Stromal Cells Cultured as Spheroids. Stem Cells Int. 2018, 2018, 7357213. [Google Scholar] [CrossRef] [PubMed]
- Romieu-Mourez, R.; Francois, M.; Boivin, M.N.; Stagg, J.; Galipeau, J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J. Immunol. 2007, 179, 1549–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagg, J.; Pommey, S.; Eliopoulos, N.; Galipeau, J. Interferon-gamma-stimulated marrow stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood 2006, 107, 2570–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Kopen, G.C.; Prockop, D.J.; Phinney, D.G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl. Acad. Sci. USA 1999, 96, 10711–10716. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Porada, C.D.; Zanjani, E.D.; Almeida-Porad, G. Adult mesenchymal stem cells: A pluripotent population with multiple applications. Curr. Stem Cell Res. Ther. 2006, 1, 365–369. [Google Scholar] [CrossRef]
- Tate, C.C.; Fonck, C.; McGrogan, M.; Case, C.C. Human mesenchymal stromal cells and their derivative, SB623 cells, rescue neural cells via trophic support following in vitro ischemia. Cell Transplant. 2010, 19, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Labusch, M.; Mancini, L.; Morizet, D.; Bally-Cuif, L. Conserved and Divergent Features of Adult Neurogenesis in Zebrafish. Front. Cell Dev. Biol. 2020, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Ottoboni, L.; von Wunster, B.; Martino, G. Therapeutic Plasticity of Neural Stem Cells. Front. Neurol. 2020, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleppner, S.R.; Robinson, K.A.; Trojanowski, J.Q.; Lee, V.M. Transplanted human neurons derived from a teratocarcinoma cell line (NTera-2) mature, integrate, and survive for over 1 year in the nude mouse brain. J. Comp. Neurol. 1995, 357, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.Q.; Mantione, J.R.; Lee, J.H.; Seid, D.P.; You, T.; Inge, L.J.; Lee, V.M. Neurons derived from a human teratocarcinoma cell line establish molecular and structural polarity following transplantation into the rodent brain. Exp. Neurol. 1993, 122, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Shichinohe, H.; Yamauchi, T.; Saito, H.; Houkin, K.; Kuroda, S. Bone marrow stromal cell transplantation enhances recovery of motor function after lacunar stroke in rats. Acta Neurobiol. Exp. 2013, 73, 354–363. [Google Scholar]
- Chiu, S.C.; Hung, H.S.; Lin, S.Z.; Chiang, E.; Liu, D.D. Therapeutic potential of olfactory ensheathing cells in neurodegenerative diseases. J. Mol. Med. 2009, 87, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Kang, Y.; Hu, Q.; Chen, C.; Yang, L.; Wang, K.; Chen, L.; Huang, H.; Zhou, C. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. 2010, 1317, 257–267. [Google Scholar] [CrossRef]
- Li, L.; Adnan, H.; Xu, B.; Wang, J.; Wang, C.; Li, F.; Tang, K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur. Spine J. 2015, 24, 919–930. [Google Scholar] [CrossRef]
- Lima, C.; Escada, P.; Pratas-Vital, J.; Branco, C.; Arcangeli, C.A.; Lazzeri, G.; Maia, C.A.; Capucho, C.; Hasse-Ferreira, A.; Peduzzi, J.D. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil. Neural Repair. 2010, 24, 10–22. [Google Scholar] [CrossRef]
- Lima, C.; Pratas-Vital, J.; Escada, P.; Hasse-Ferreira, A.; Capucho, C.; Peduzzi, J.D. Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. J. Spinal Cord Med. 2006, 29, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Mackay-Sim, A.; Feron, F.; Cochrane, J.; Bassingthwaighte, L.; Bayliss, C.; Davies, W.; Fronek, P.; Gray, C.; Kerr, G.; Licina, P.; et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain 2008, 131, 2376–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marei, H.E.; Hasan, A.; Rizzi, R.; Althani, A.; Afifi, N.; Cenciarelli, C.; Caceci, T.; Shuaib, A. Potential of Stem Cell-Based Therapy for Ischemic Stroke. Front. Neurol. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Rikhtegar, R.; Yousefi, M.; Dolati, S.; Kasmaei, H.D.; Charsouei, S.; Nouri, M.; Shakouri, S.K. Stem cell-based cell therapy for neuroprotection in stroke: A review. J. Cell. Biochem. 2019, 120, 8849–8862. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wei, Z.Z.; Jiang, M.Q.; Mohamad, O.; Yu, S.P. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog. Neurobiol. 2017, 157, 49–78. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Tanimori, A.; Kitta, S.; Shichinohe, H.; Houkin, K. Evaluation of Novel Stereotactic Cannula for Stem Cell Transplantation against Central Nervous System Disease. Stem Cells Int. 2020, 2020, 4085617. [Google Scholar] [CrossRef] [Green Version]
- Shichinohe, H.; Kawabori, M.; Iijima, H.; Teramoto, T.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Terasaka, S.; Arato, T.; Houkin, K. Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): A study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 179. [Google Scholar] [CrossRef]
| Reference Number | Country | Cell Type | Cell Source | Dose | Route | Transplant Timing | Treated Patient Number (Control) | Assessment Modality | Major Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Acute | |||||||||
| [16] | USA | Autologous | BMMNC | 4–6 × 108 | IV | 1–3 D | 10 | BI, mRS, NIHSS | showed good neurological recovery |
| [25] | USA | Allogeneic | BMSC | 1.2 × 109 | IV | 1–2 D | 65 (58) | mRS, NIHSS, BI | No difference for neurological recovery (primary endpoint), but earlier timing (24-36 h) may be beneficial |
| [35] | USA | Allogeneic | UCBC | 1.2 × 106 (CD34+) | IV | 3–9 D | 10 | mRS, NIHSS | Safe |
| [10] | Brazil | Autologous | BMMNC | 5–60 × 107 | IA | 3–10 D | 20 | mRS, NIHSS | 30% of the patients showed satisfactory clinical outcome |
| [12] | Spain | Autologous | BMMNC | 1.6 × 108 | IA | 5–9 D | 10(10) | mRS, BI, NIHSS | No difference in neurological function |
| [9] | Brazil | Autologous | BMMNC | 3 × 107 | IA | 9 D | 1 | SPECT | Brain/liver/spleen uptake at 8 h |
| [32] | UK | Autologous | CD34+ (BM) | 1–3 × 106 | IA | 1 W | 5 | mRS, NIHSS | Good recovery was observed |
| [36] | China | Allogeneic | UCBC & NPC | 3 × 107 (UC: IV), 1.5 × 107 (UC: IT), 1.8 × 107 (NPC: IT) | IV &IT | 1 W | 1 | NIHSS, BI, mRS | Showed some degree of neurological recovery |
| Sub-Acute | |||||||||
| [13] | India | Autologous | BMMNC | 2–19 × 108 | IV | 2–4 W | 11 | NIHSS, BI, mRS, PET | Favorable outcomes were mostly found in early treatment group |
| [5] | India | Autologous | BMMNC | 5 × 107 | IV | 3–4 M | 1(3) | FM, mBI | Safe |
| [15] | Brazil | Autologous | BMMNC | 2–5 × 108 | IV | 1–3 M | 5 | NIHSS | Cells in brain were scarce (1%), IV (21%) showed high cell distribution in lung compared with IV (7%) |
| [14] | India | Autologous | BMMNC | 2.8 × 10e7 | IV | 18 D | 59(59) | BI, mRS, NIHSS, PET | No significant recovery compared with control |
| [20] | Japan | Autologous | BMMNC | 2.5–3.4 × 108 | IV | 7–10 D | 12 | mRS, NIHSS, SPECT, PET | Better NIHSS (but not mRS, BI) recovery compared with historical control |
| [22] | Korea | Autologous | BMSC | 1 × 108 | IV | 1–2 M | 5 (25) | BI, mRS, NIHSS | Cell treatment group showed better neurological recovery than control |
| [28] | Korea | Autologous | BMSC | 1 × 108 | IV | 2 M | 16(36) | mRS, Survival | Better recovery, less mortality for 5 years |
| [26] | Japan | Autologous | BMSC | 0.8–1.5 × 108 | IV | 1–4 M | 12 | NIHSS | Recoveries were mainly seen 0–1 W from transplantation |
| [24] | China | Autologous | BMSC | 3 × 108 | IV | 1 M | 12 (6) | mRS, NIHSS, BI | No neurological difference compared with control |
| [27] | France | Autologous | BMSC | 1 or 3 × 108 | IV | 1–2 M | 16(15) | NIHSS, mRS, BI | No overall change, but motor functional evaluations indicated improvement |
| [36] | China | Allogeneic | UCBC & NPC | 1.2 × 108 (UC) | IV | 2 & 3 M | 2 | NIHSS, BI, mRS | Showed some degree of neurological recovery |
| [2] | Brazil | Autologous | BMMNC | 1–5 × 108 | IA | 2–3 M | 6 | SPECT | Cells were found in the brain after 2 h, but not after 24 h |
| [3] | Brazil | Autologous | BMMNC | 1–5 × 108 | IA | 2–3 M | 6 | NIHSS, SPECT | Safe, but cells could not be seen 24 h after injection in 4 out of 6 patients |
| [15] | Brazil | Autologous | BMMNC | 1-5 x 108 | IA | 1–3 M | 7 | NIHSS | Cells in brain were scarce (1%), IA (41%) showed high cell distribution in liver compared with IV (13%) |
| [11] | Egypt | Autologous | BMMNC | 1 × 106 | IA | 2–4 W | 21(18) | NIHSS, mRS, BI, | IA treatment did not improve neurological recovery compare with control |
| [8] | India | Autologous | BMMNC | 5 × 108 | IA | 1–2 W | 10 (10) | BI, NIHSS, mRS | Good recovery was observed in treatment group (P = 0.06) |
| [17] | USA | Autologous | BMMNC (ALD) | 3 × 106 | IA | 2–3 W | 29 (17) | mRS, NIHSS, BI | No statistical difference compared to control |
| [34] | China | Allogeneic | UCBC & NPC | 2 × 107 | IA | 11–22 D | 3 | mRS | Showed neurological recovery in 2 out of 3 patients |
| [42] | Russia | Allogeneic | Fetus neuronal cell | 2 × 108 | IT | 4 M | 1 | Karnovskii score | Cell treatment showed 33% increase in Score |
| [36] | China | Allogeneic | UCBC & NPC | 3 × 107 (UC: IV), 1.5 × 107 (UC: IT), 1.8 × 107 (NPC: IT) | IV & IT | 2 W | 1 | NIHSS, BI, mRS | Showed some degree of neurological recovery |
| Chronic | |||||||||
| India | Autologous | BMMNC | 6–7 × 107 | IV | 5–14 M | 20(20) | FM, mBI, Ashworth | No difference compared with control | |
| [21] | India | Autologous | BMMNC | 5 × 107 | IV | 6–15 M | 11(9) | FM, mBI | Significant improvement in mBI, but not in FM |
| [4,5] | India | Autologous | BMSC | 5–6 × 107 | IV | 8–12 M | 6(6) | BI, FM, Ashworth | No significant difference compared with control up to 4 years |
| [29] | USA | Allogeneic | BMSC (hypoxia treated) | 1 × 108 | IV | 7 M-25 Y | 36 | NIHSS, BI | Significant recovery was observed compared with baseline |
| [7] | India | Autologous | BMSC/BMMNC | 5-6 × 107 | IV | 3 M-2 Y | 20(20) | FM, mBI | mBI showed significant improvement |
| [18] | India | Autologous | BMMNC | 6 × 107 | IT | 4 M-12 Y | 14 | FIM | Showed recovery, but this study included hemorrhagic stroke |
| [37] | China | Autologous | CD34+ (peripheral) | 1–3 × 107 | IT | 1–7 Y | 8 | NIHSS, BI | Patients showed recovery, but this may have been due to natural history |
| [42] | Russia | Allogeneic | Fetus neuronal cell | 2 × 108 | IT | 8 M-1.5 Y | 6 (6) | Karnovskii score | Cell treatment groups showed better recovery |
| [23] | USA | Autologous | ADSC (no culture) | N.D. | IT (ICV) | 1 Y | 1 | N.D. | Stable |
| [19] | Cuba | Autologous | BMMNC | 1–5 × 107 | IC | 3–5 Y | 3 | BI, NIHSS, SSS | Recovery compared with pre-operation was found |
| [33] | Taiwan | Autologous | CD34+ (peripheral) | 3–8 × 106 | IC | 6 M-5 Y | 15(15) | NIHSS, ESS, mRS | Statistically significant recovery |
| [30,31] | USA | Allogeneic | BMSC (Gene modified) | 2.5, 5, 10 × 106 | IC | 7–36 M | 18 | ESS, NIHSS, FM | Neurological recovery (ESS, NIHSS, F-M test) was observed up to 2 years |
| [41] | USA | Allogeneic | Fetus neuronal cell | 2 × 106 (n = 8) or 6 × 106 (n = 4) | IC | 7 M-5 Y | 12 | BI, ESS, NIHSS | 6 x 106 showed better recovery than 2 x 106 |
| [39] | UK | Allogeneic | Fetus neuronal cell | 2, 5, 10, 20 × 106 | IC | 1–4 Y | 11 | NIHSS, BI, Ashworth | Neurological recovery (median NIHSS of 2) was observed |
| [43] | UK | Allogeneic | Fetus neuronal cell | 2 × 107 | IC | 2M-1 Y | 23 | ARAT | Upper limb function recovered from baseline |
| [40] | USA | Allogeneic | Fetus neuronal cell | 5, 10 × 106 | IC | 1–6 Y | 18(4) | ESS, NIHSS, FM, ARAT | No difference for neurological recovery (primary endpoint), but showed partial recovery in some tests |
| [38] | China | Allogeneic | OEC | 1 × 106 | IC | 3 Y | 1 | BI | Recovery in speech and gait |
| [38] | China | Allogeneic | OEC & NPC | 1 × 106 & 2 × 106 | IC | 5 Y | 1 | BI | Recovery in motor function |
| [44] | USA | Xenogeneic | Fetal Porcine cell | 2 × 107 | IC | 1.5–10 Y | 5 | BI, RS, NIHSS | Slight recovery, but 2 patients exhibited adverse events (seizure and motor deficit) |
| [38] | China | Allogeneic | OEC & NPC | 1 × 106 & 2 × 106 | IC & IT (NPC) | 1–20 Y | 4 | BI | Recovery in gait |
| [36] | China | Allogeneic | UCBC & NPC | 3 × 107 (UC: IV), 1.5 × 107 (UC: IT), 1.8 × 107 (NPC: IT) | IV & IT | 10 M & 2 Y | 2 | NIHSS, BI, mRS | Showed some degree of neurological recovery |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. https://doi.org/10.3390/ijms21197380
Kawabori M, Shichinohe H, Kuroda S, Houkin K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. International Journal of Molecular Sciences. 2020; 21(19):7380. https://doi.org/10.3390/ijms21197380
Chicago/Turabian StyleKawabori, Masahito, Hideo Shichinohe, Satoshi Kuroda, and Kiyohiro Houkin. 2020. "Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke" International Journal of Molecular Sciences 21, no. 19: 7380. https://doi.org/10.3390/ijms21197380






