The Involvement of Energy Metabolism and Lipid Peroxidation in Lignin Accumulation of Postharvest Pumelos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of EC, NAD, and NADP Contents
2.3. Determination of Activities of SDH, CCO, and F0F1-ATPase
2.4. Measurement of H+-ATPase and Ca2+-ATPase Activities
2.5. Determination of Contents of H2O2, O2− and MDA, and –OH Scavenging Rate
2.6. Determination of Relative Amounts of Fatty Acids and U/S
2.7. Statistical Analysis
3. Results
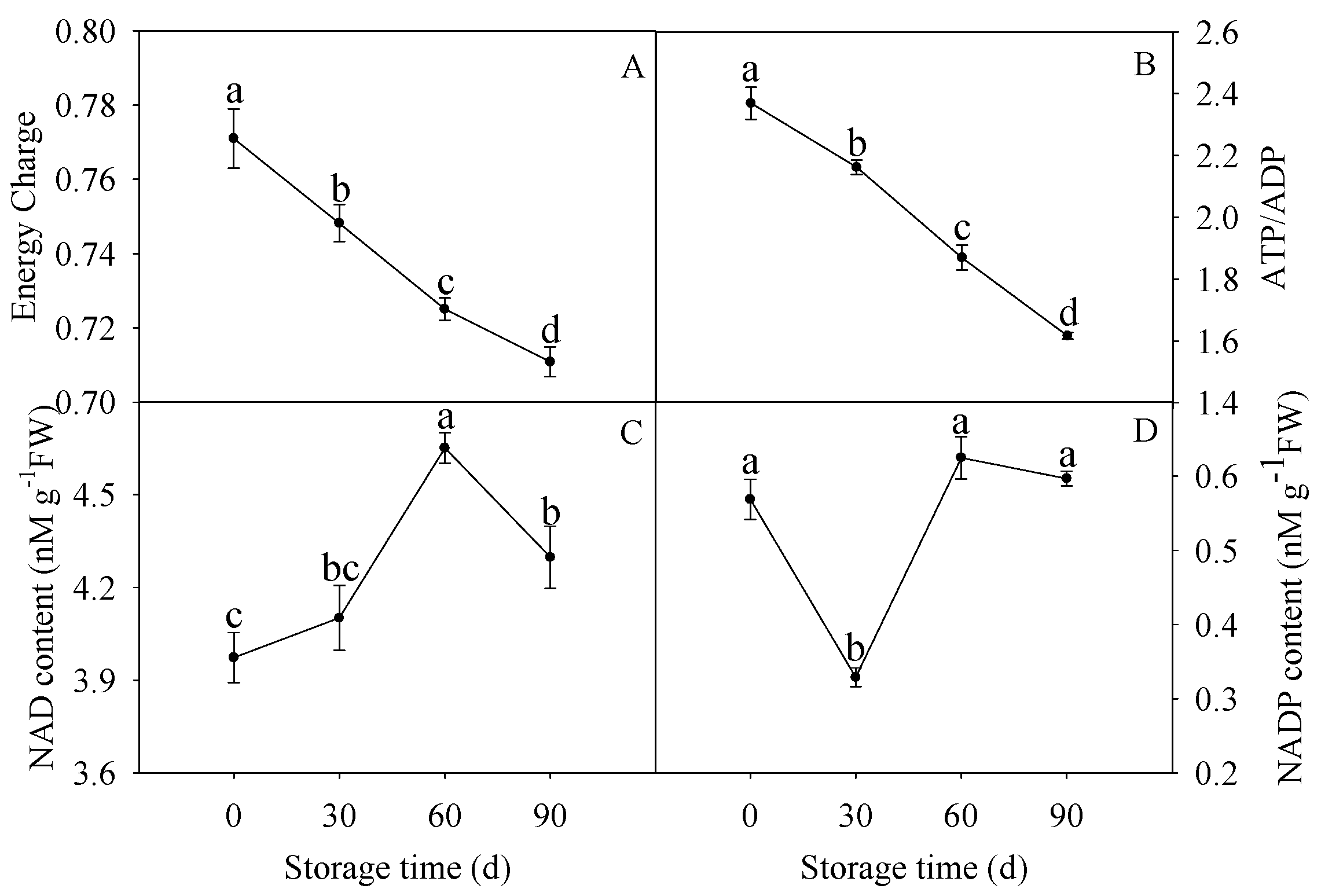
3.1. Changes in EC, ATP/ADP, NAD, and NADP Contents
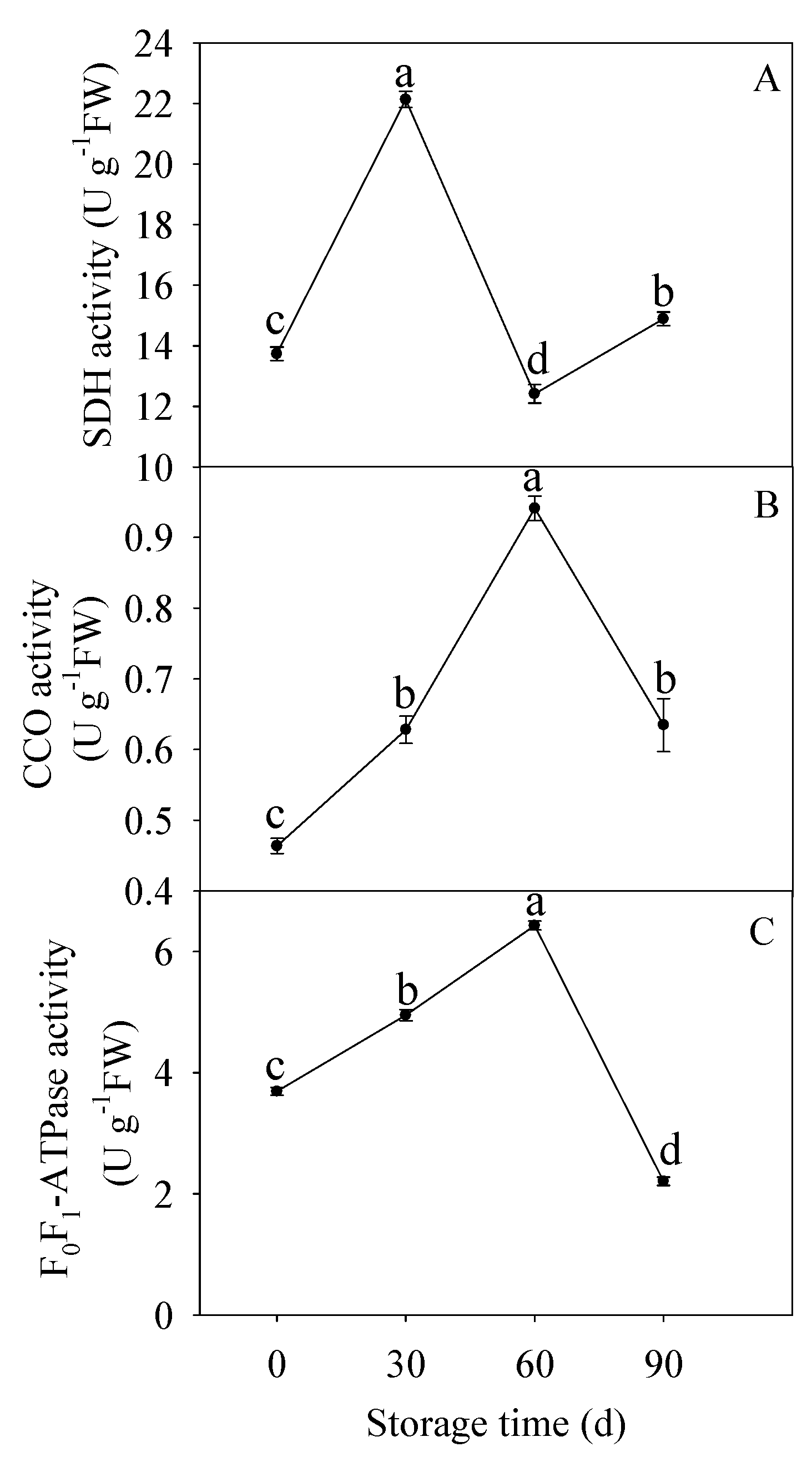
3.2. Changes in SDH, CCO, and F0F1-ATPase Activities
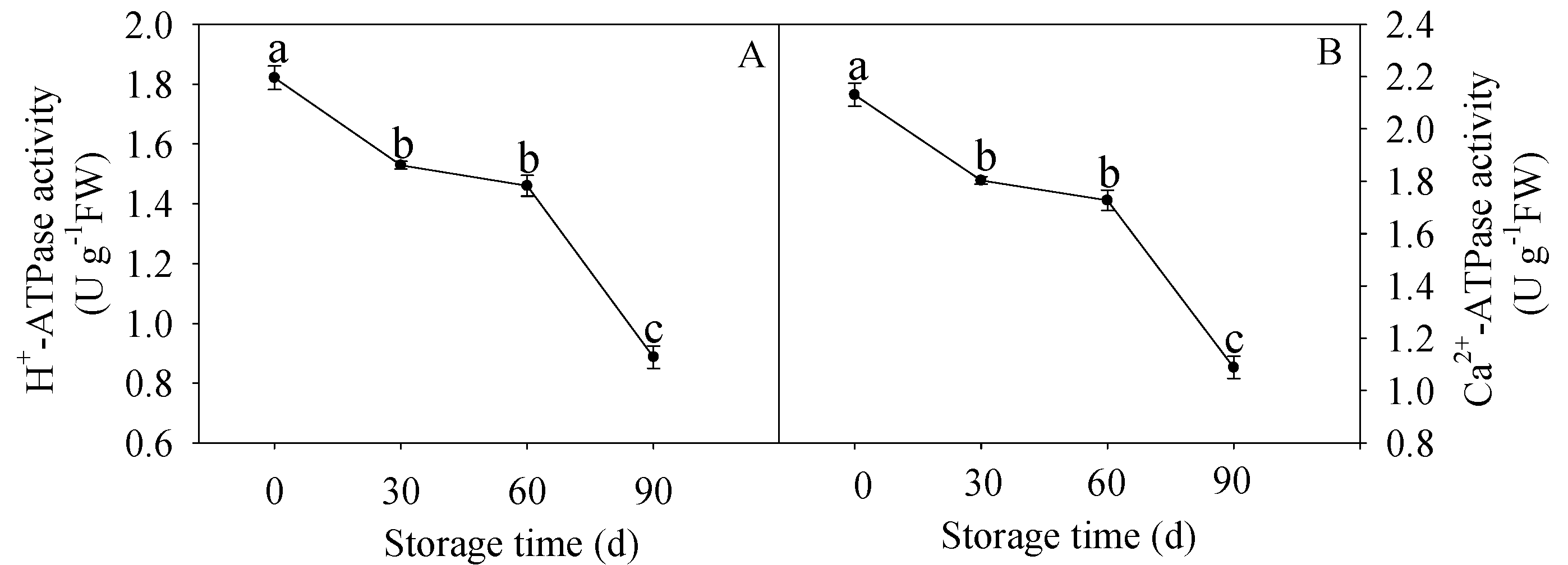
3.3. Changes in H+-ATPase and Ca2+-ATPase Activities
3.4. Changes in Contents of H2O2, O2−, and MDA, and -OH Scavenging Rate
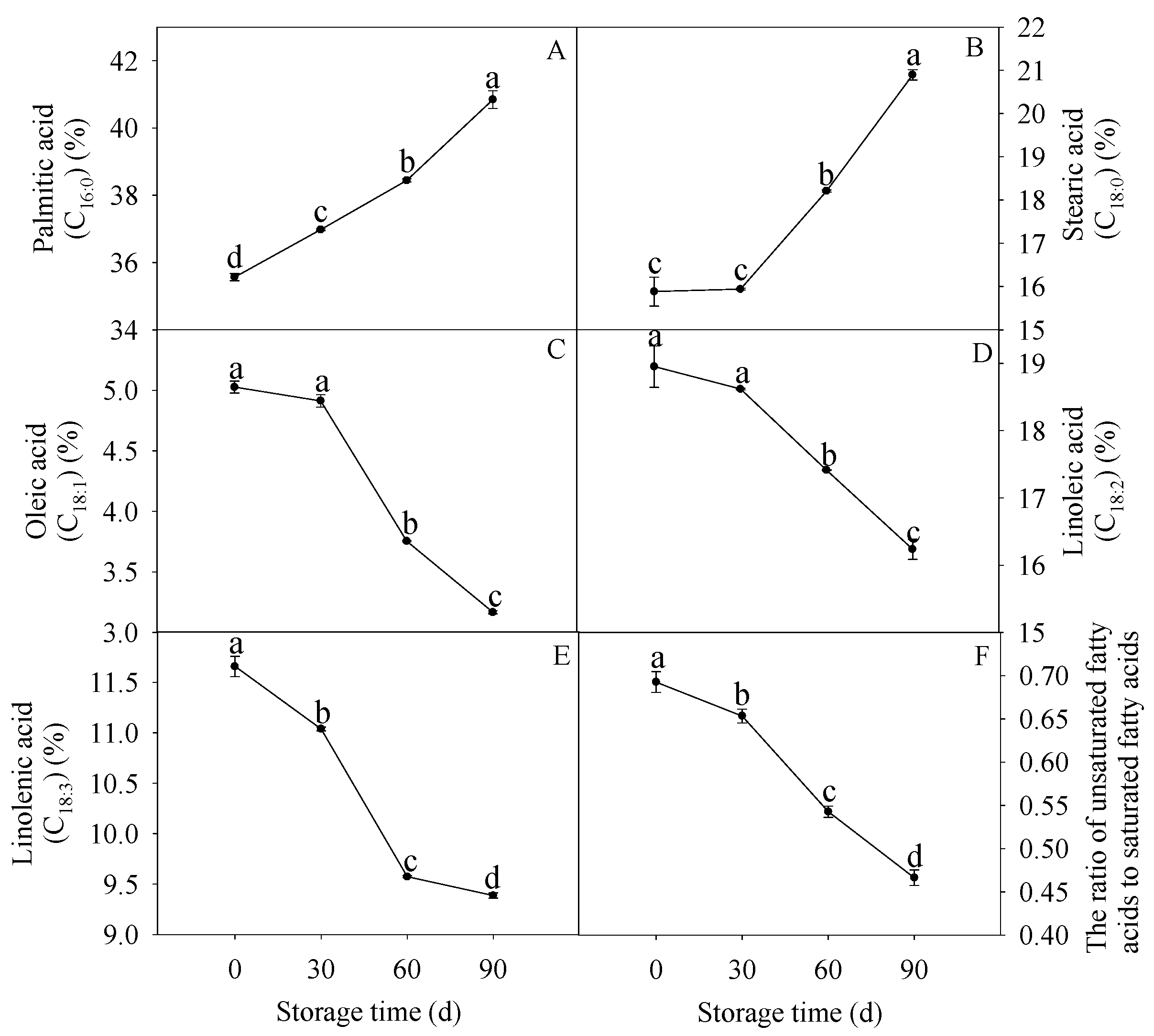
3.5. Changes in Membrane Fatty Acids
4. Discussions
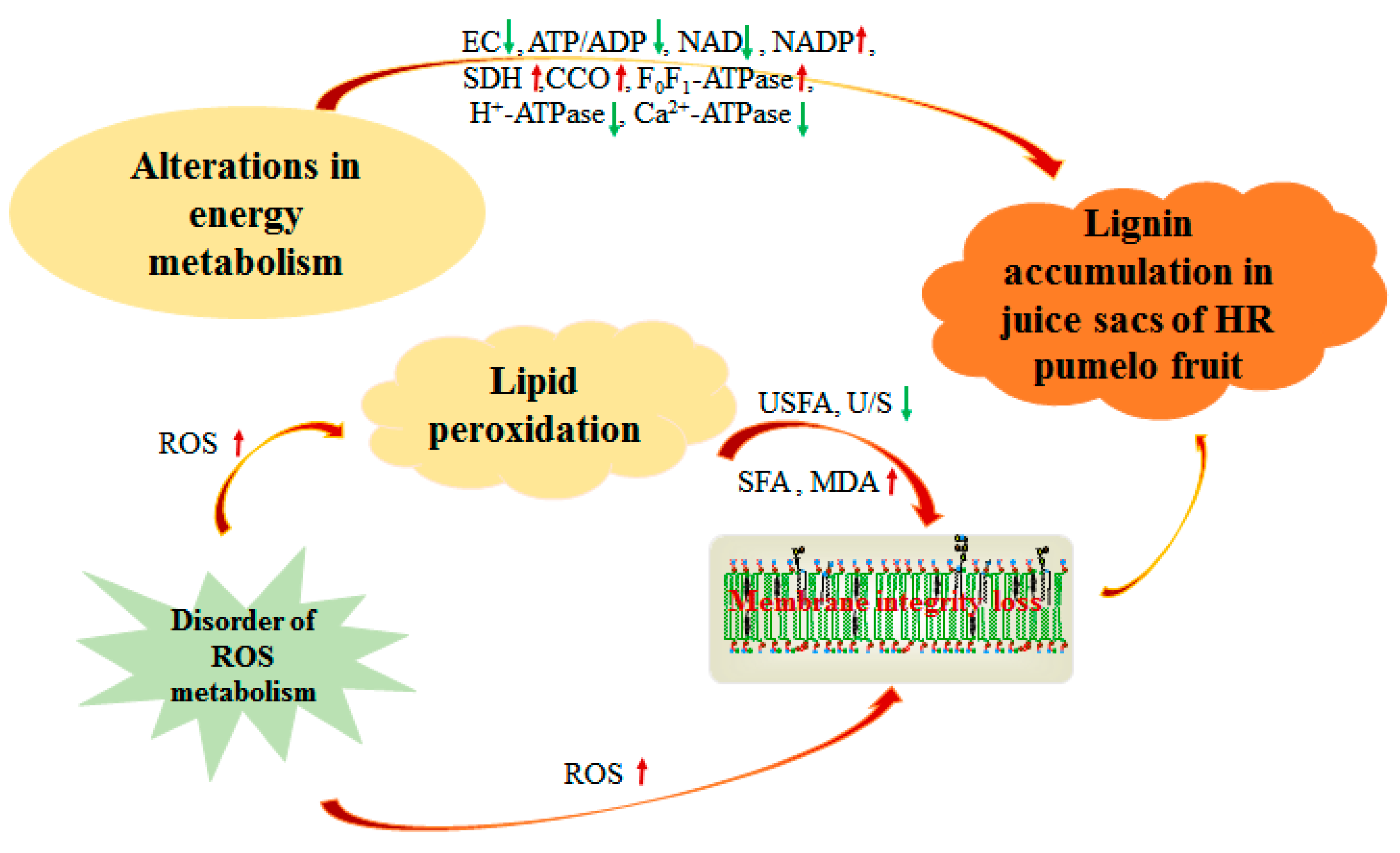
4.1. Lignin Accumulation Was Related to Energy Metabolism of Postharvest Pumelo Fruit
4.2. Lignin Accumulation Was Related to Lipid Peroxidation of Postharvest Pumelo Fruit
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burana-osot, J.; Soonthornchareonnon, N.; Chaidedgumjorn, A.; Hosoyama, S.; Toida, T. Determination of galacturonic acid from pumelo pectin in term of galactose by HPAEC with fluorescence detection. Carbohydr. Polym. 2010, 81, 461–465. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, Y.; Li, X.; Zhang, Z.; Hu, W. Research progress in postharvest physiology and storage technology of pumelo fruit. Food Sci. 2010, 31, 394–399. [Google Scholar]
- Lu, Z.; Zhang, Z.; Wu, H.; Zhou, Z.; Yu, J. Phenolic composition and antioxidant capacities of Chinese local pummelo cultivars’ peel. Hortic. Plant J. 2016, 2, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.L.; Pan, T.F.; Guo, Z.X.; Pan, D.M. Specific lignin accumulation in granulated juice sacs of Citrus maxima. J. Agric. Food Chem. 2014, 62, 12082–12089. [Google Scholar] [CrossRef]
- Shomer, I.; Chalutz, E.; Vasiliver, R.; Lomaniec, E.; Berman, M. Sclerification of juice sacs in pummelo (Citrus-grandis) fruit. Can. J. Bot. Rev. Can. Bot. 1989, 67, 625–632. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Q.; Kang, P.; Liang, L.; Chen, J. Lignin accumulation in three pumelo cultivars in association with sucrose and energy depletion. Biomolecules 2019, 9, 701. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Fan, Z.; Ritenour, M.A.; Lin, Y. Hydrogen peroxide reduced ATPase activity and the levels of ATP, ADP, and energy charge and its association with pulp breakdown occurrence of longan fruit during storage. Food Chem. 2020, 311, 126008. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, J.; Chen, J.J.; Wang, X.L.; Zheng, Y.H. Effect of methyl jasmonate on energy metabolism in peach fruit during chilling stress. J. Sci. Food Agric. 2013, 93, 1827–1832. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, M.; Yun, Z.; Wang, J.; Feng, G.; Gao, Z.; Shi, X.; Jiang, Y. Effect of tea seed oil treatment on browning of litchi fruit in relation to energy status and metabolism. Postharvest Biol. Technol. 2017, 132, 97–104. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Du, R.; Luo, Z. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 2016, 208, 272–278. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.T.; Jiang, Y.M.; Zhang, S.; Lin, Y.F.; Wang, Z.H. Phomopsis longanae Chi-induced pericarp browning and disease development of harvested longan fruit in association with energy status. Postharvest Biol. Technol. 2014, 93, 24–28. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Lin, M.; Lin, Y.; Wang, H.; Hung, Y.-C. Salicylic acid reduces the incidence of Phomopsis longanae Chi infection in harvested longan fruit by affecting the energy status and respiratory metabolism. Postharvest Biol. Technol. 2020, 160, 111035. [Google Scholar] [CrossRef]
- Wang, J.; You, Y.; Chen, W.; Xu, Q.; Wang, J.; Liu, Y.; Song, L.; Wu, J. Optimal hypobaric treatment delays ripening of honey peach fruit via increasing endogenous energy status and enhancing antioxidant defence systems during storage. Postharvest Biol. Technol. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Song, L.-l.; Liu, H.; You, Y.-l.; Sun, J.; Yi, C.; Li, Y.-b.; Jiang, Y.-m.; Wu, J.-S. Quality deterioration of cut carnation flowers involves in antioxidant systems and energy status. Sci. Hortic. 2014, 170, 45–52. [Google Scholar] [CrossRef]
- Wang, H.; Qian, Z.; Ma, S.; Zhou, Y.; Patrick, J.W.; Duan, X.; Jiang, Y.; Qu, H. Energy status of ripening and postharvest senescent fruit of litchi (Litchi chinensis Sonn.). BMC Plant Biol. 2013, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Liu, D.; Li, P. Methane delays the senescence and browning in daylily buds by re-established redox homeostasis. J. Sci. Food Agric. 2018, 98, 1977–1987. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Lin, M.; Ritenour, M.A.; Lin, Y. The role of ROS-induced change of respiratory metabolism in pulp breakdown development of longan fruit during storage. Food Chem. 2020, 305, 125439. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Ge, Y.; Wei, M.; Li, C.; Chen, Y.; Lv, J.; Li, J. Effect of acibenzolar-S-methyl on energy metabolism and blue mould of Nanguo pear fruit. Sci. Hortic. 2017, 225, 221–225. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.; Luo, Y.; Sun, X.; Wang, J.; Luo, T.; Zeng, Y.; Xu, J.; Deng, X.; Cheng, Y. Exogenous gamma-aminobutyric acid treatment affects citrate and amino acid accumulation to improve fruit quality and storage performance of postharvest citrus fruit. Food Chem. 2017, 216, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Cai, Y.; Yang, Z.; Joyce, D.C.; Zheng, Y. Effect of MeJA treatment on polyamine, energy status and anthracnose rot of loquat fruit. Food Chem. 2014, 145, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Ishikawa, T.; Ishikawa, T.; Sawa, Y.; Shibata, H. Intracellular energy depletion triggers programmed cell death during petal senescence in tulip. J. Exp. Bot. 2008, 59, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Qu, H.X.; Jiang, Y.M.; Shi, J.; Duan, X.W.; Joyce, D.C.; Li, Y.B. ATP-induced changes in energy status and membrane integrity of harvested litchi fruit and its relation to pathogen resistance. J. Phytopathol. 2008, 156, 365–371. [Google Scholar] [CrossRef]
- Li, P.; Yin, F.; Song, L.; Zheng, X. Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chem. 2016, 202, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, M.; Wu, G. Study of lignification’s delaying and its relationship with energy metabolism in loquat fruits after nitric oxide fumigation. Sci. Agric. Sin. 2014, 47, 2425–2434. [Google Scholar]
- Song, L.; Chen, H.; Gao, H.; Fang, X.; Mu, H.; Yuan, Y.; Yang, Q.; Jiang, Y. Combined modified atmosphere packaging and low temperature storage delay lignification and improve the defense response of minimally processed water bamboo shoot. Chem. Cent. J. 2013, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Kitazawa, H.; Wang, X.; Sun, H. Regulation of respiratory pathway and electron transport chain in relation to senescence of postharvest white mushroom (Agaricus bisporus) under high O2/CO2 controlled atmospheres. J. Agric. Food Chem. 2017, 65, 3352–3360. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Ritenour, M.A.; Shi, J.; Zhang, S.; Chen, Y.; Wang, H. Hydrogen peroxide-induced pericarp browning of harvested longan fruit in association with energy metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef]
- Mnatsakanyan, N.; Krishnakumar, A.M.; Suzuki, T.; Weber, J. The role of the beta DELSEED-loop of ATP synthase. J. Biol. Chem. 2009, 284, 11336–11345. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Wei, B.; Zhou, Q.; Tan, D.; Ji, S. 1-Methylcyclopropene alleviates chilling injury by regulating energy metabolism and fatty acid content in ‘Nanguo’ pears. Postharvest Biol. Technol. 2015, 109, 130–136. [Google Scholar] [CrossRef]
- Affourtit, C.; Krab, K.; Leach, G.R.; Whitehouse, D.G.; Moore, A.L. New insights into the regulation of plant succinate dehydrogenase—On the role of the protonmotive force. J. Biol. Chem. 2001, 276, 32567–32574. [Google Scholar] [CrossRef] [Green Version]
- Brunori, M.; Antonini, G.; Malatesta, F.; Sarti, P.; Wilson, M.T. Cytochrome-c oxidase. Subunit structure and proton pumping. Eur. J. Biochem. 1987, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Soole, K.L.; Menz, R.I. ATP in plant mitochondria: Substrates, inhibitors, and uncouplers. Bioenergetics 2013, 2004, 128–132. [Google Scholar]
- Henriksson, J.; Reitman, J.S. Time course of changes in human skeletal-muscle succinate-dehydrogenase and cytochrome-oxidase activities and maximal oxygen-uptake with physical-activity and inactivity. Acta Physiol. Scand. 1977, 99, 91–97. [Google Scholar] [CrossRef]
- Li, L.; Lv, F.-Y.; Guo, Y.-Y.; Wang, Z.-Q. Respiratory pathway metabolism and energy metabolism associated with senescence in postharvest broccoli (Brassica oleracea L. var. italica) florets in response to O2/CO2 controlled atmospheres. Postharvest Biol. Technol. 2016, 111, 330–336. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Fan, Z.; Wang, H.; Lin, M.; Chen, Y.; Hung, Y.-C.; Lin, Y. Inhibitory effect of propyl gallate on pulp breakdown of longan fruit and its relationship with ROS metabolism. Postharvest Biol. Technol. 2020, 168, 111272. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, T.; Qin, G.; Li, B.; Tian, S. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit. Postharvest Biol. Technol. 2016, 111, 15–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; He, C.; Zhu, S. Postharvest exogenous application of abscisic acid reduces internal browning in pineapple. J. Agric. Food Chem. 2015, 63, 5313–5320. [Google Scholar] [CrossRef]
- Jiang, T.; Luo, Z.; Ying, T. Fumigation with essential oils improves sensory quality and enhanced antioxidant ability of shiitake mushroom (Lentinus edodes). Food Chem. 2015, 172, 692–698. [Google Scholar] [CrossRef]
- Jiang, X.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Lin, Y.; Shi, J.; Lin, Y. A novel chitosan formulation treatment induces disease resistance of harvested litchi fruit to Peronophythora litchi in association with ROS metabolism. Food Chem. 2018, 266, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, J.V.; Dixon, R.A.; Chen, F.; Mansfield, S.D.; Boerjan, W.; Ralph, J.; Crowley, M.F.; Beckham, G.T. Passive membrane transport of lignin-related compounds. Proc. Natl. Acad. Sci. USA 2019, 116, 23117–23123. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudet, A.M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Limwachiranon, J.; Li, L.; Zhang, L.; Xu, Y.; Fu, M.; Luo, Z. Hydrogen peroxide accelerated the lignification process of bamboo shoots by activating the phenylpropanoid pathway and programmed cell death in postharvest storage. Postharvest Biol. Technol. 2019, 153, 79–86. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, Y.X.; Lin, H.T.; Chen, Y.H.; Wang, H.; Shi, J. Application of propyl gallate alleviates pericarp browning in harvested longan fruit by modulating metabolisms of respiration and energy. Food Chem. 2018, 240, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zhang, Y.; Shan, T.M.; Huang, Y.P.; Xu, J.; Zheng, Y.H. Low-temperature conditioning alleviates chilling injury in loquat fruit and regulates glycine betaine content and energy status. J. Agric. Food Chem. 2015, 63, 3654–3659. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.J.; Liang, L.; Jiang, Y.M.; Chen, J.J. Fibroin delays chilling injury of postharvest banana fruit via enhanced antioxidant capability during cold storage. Metabolites 2019, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, Q.; Chen, J.; Jiang, Y. Revealing further insights on chilling injury of postharvest bananas by untargeted lipidomics. Foods 2020, 9, 894. [Google Scholar] [CrossRef]
- Gu, C.; Zhu, D.; Li, Q. Relationship between NAD kinase and NAD(H), NADP(H) and active oxygen during ripening and senescence of postharvested strawberry fruit. Sci. Agric. Sin. 2007, 40, 352–357. [Google Scholar]
- Stenuit, B.; Lamblin, G.; Cornelis, P.; Agathos, S.N. Aerobic denitration of 2,4,6-trinitrotoluene in the presence of phenazine compounds and reduced pyridine nucleotides. Environ. Sci. Technol. 2012, 46, 10605–10613. [Google Scholar] [CrossRef] [PubMed]
- Chumyam, A.; Shank, L.; Uthaibutra, J.; Saengnil, K. Effects of chlorine dioxide on mitochondrial energy levels and redox status of ‘Daw’ longan pericarp during storage. Postharvest Biol. Technol. 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Stein, L.R.; Imai, S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol. Metab. 2012, 23, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, W. NAD(+)/NADH and NADP(+)/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.F.; Cao, S.F.; Su, X.G.; Jiang, Y.M. Respiratory activity and mitochondrial membrane associated with fruit senescence in postharvest peaches in response to UV-C treatment. Food Chem. 2014, 161, 16–21. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Ge, Z.; Limwachiranon, J.; Ban, Z.; Yang, D.; Luo, Z. Effects of hydrogen sulfide on yellowing and energy metabolism in broccoli. Postharvest Biol. Technol. 2017, 129, 136–142. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Yuan, D.; Yun, Z.; Gao, Z.; Su, Z.; Zhang, Z. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Elmore, J.M.; Coaker, G. The role of the plasma membrane H+-ATPase in plant-microbe interactions. Mol. Plant 2011, 4, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Hu, H.; Wang, Y.; Zhou, H.; Zhang, Y.; Zhang, L.; Li, P. The role of melatonin in alleviating the postharvest browning of lotus seeds through energy metabolism and membrane lipid metabolism. Postharvest Biol. Technol. 2020, 167, 111243. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Khan, Z.U.; Mao, L.; Ying, T. Effect of nitric oxide on energy metabolism in postharvest banana fruit in response to chilling stress. Postharvest Biol. Technol. 2015, 108, 21–27. [Google Scholar] [CrossRef]
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, J.; Chen, Y.; Wei, J.; Wu, B. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 2019, 152, 9–18. [Google Scholar] [CrossRef]
- Jiao, W.; Shu, C.; Li, X.; Cao, J.; Fan, X.; Jiang, W. Preparation of a chitosan-chlorogenic acid conjugate and its application as edible coating in postharvest preservation of peach fruit. Postharvest Biol. Technol. 2019, 154, 129–136. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Chen, Z.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2020, 336, 127618. [Google Scholar] [CrossRef]
- Nie, Z.; Huang, Q.; Chen, C.; Wan, C.; Chen, J. Chitosan coating alleviates postharvest juice sac granulation by mitigating ROS accumulation in harvested pummelo (Citrus grandis L. Osbeck) during room temperature storage. Postharvest Biol. Technol. 2020, 169, 111309. [Google Scholar] [CrossRef]
- Chen, C.; Nie, Z.; Wan, C.; Chen, J. Preservation of Xinyu tangerines with an edible coating using Ficus hirta Vahl. fruits extract-incorporated chitosan. Biomolecules 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Jiang, W.B. Lignin deposition and effect of postharvest treatment on lignification of green asparagus (Asparagus officinalis L.). Plant Growth Regul. 2006, 48, 187–193. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Bernal, A.; Zayas, C.; Carmona, E.; Cordero, C.; Gonzalez, G.; Garcia, R.; Santana, I. Hydrogen peroxide induced phenylpropanoids pathway eliciting a defensive response in plants micropropagated in Temporary Immersion Bioreactors (TIBs). Plant Sci. 2012, 195, 71–79. [Google Scholar] [CrossRef]
- Liu, J.; Liang, L.; Jiang, Y.; Chen, J. Changes in metabolisms of antioxidant and cell wall in three pummelo cultivars during postharvest storage. Biomolecules 2019, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Wei, B.; Gao, Z.; Zhou, Y.; Shi, F.; Zhou, X.; Zhou, Q.; Ji, S. Changes in membrane lipid composition and function accompanying chilling injury in bell peppers. Plant Cell Physiol. 2018, 59, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Maalekuu, K.; Elkind, Y.; Leikin-Frenkel, A.; Lurie, S.; Fallik, E. The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biol. Technol. 2006, 42, 248–255. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Wei, B.; Cheng, S.; Ji, S. Membrane lipid metabolism in relation to core browning during ambient storage of ‘Nanguo’ pears. Postharvest Biol. Technol. 2020, 169, 111288. [Google Scholar] [CrossRef]
- Holthuis, J.C.M.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Guo, L.; Wang, L.; Wang, H.; Ma, S.; Jiang, Y.; Qu, H. 1-Methylcyclopropene (1-MCP) slows ripening of kiwifruit and affects energy status, membrane fatty acid contents and cell membrane integrity. Postharvest Biol. Technol. 2019, 156, 110941. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, M.; Lin, H.; Lin, M.; Hung, Y.C.; Lin, Y.; Chen, Y.; Wang, H.; Ritenour, M.A. Phomopsis longanae-induced pericarp browning and disease development of longan fruit can be alleviated or aggravated by regulation of ATP-mediated membrane lipid metabolism. Food Chem. 2018, 269, 644–651. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Chen, J.; Liu, J. The Involvement of Energy Metabolism and Lipid Peroxidation in Lignin Accumulation of Postharvest Pumelos. Membranes 2020, 10, 269. https://doi.org/10.3390/membranes10100269
Yan H, Chen J, Liu J. The Involvement of Energy Metabolism and Lipid Peroxidation in Lignin Accumulation of Postharvest Pumelos. Membranes. 2020; 10(10):269. https://doi.org/10.3390/membranes10100269
Chicago/Turabian StyleYan, Huiling, Junjia Chen, and Juan Liu. 2020. "The Involvement of Energy Metabolism and Lipid Peroxidation in Lignin Accumulation of Postharvest Pumelos" Membranes 10, no. 10: 269. https://doi.org/10.3390/membranes10100269
APA StyleYan, H., Chen, J., & Liu, J. (2020). The Involvement of Energy Metabolism and Lipid Peroxidation in Lignin Accumulation of Postharvest Pumelos. Membranes, 10(10), 269. https://doi.org/10.3390/membranes10100269





