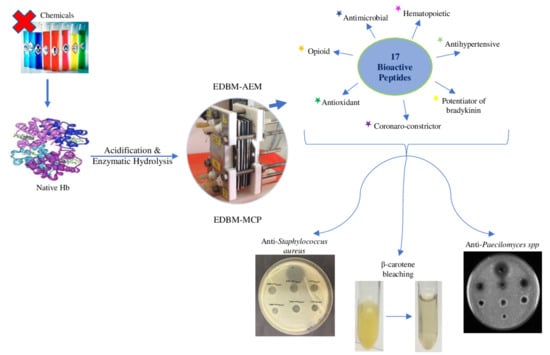
Bovine Hemoglobin Enzymatic Hydrolysis by a New Eco-Efficient Process-Part II: Production of Bioactive Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bovine Hemoglobin Hydrolysates
2.2.1. Stock Solution Preparation
2.2.2. Hydrolysis Process
2.3. Electrodialysis Cell
2.4. Analysis
2.4.1. RP-UPLC
Materials, Software and Elution Program Used
Identification of Peptide Population
Identification of Bioactive Peptides by Mass Spectrometry Analyses
2.4.2. Biological Activities
Antibacterial Activity
- Agar diffusion method
- Minimal inhibitory concentration (MIC) determination
Antifungal Activity
Antioxidant Bioactivities
- Antioxidant assay using the β-carotene bleaching method
- DPPH radical scavenging capacity
- Antioxidant properties products by ABTS assay
- Evaluation of total antioxidant capacity
2.4.3. Statistical Analyses
3. Results and Discussion
3.1. Identification and Characterization of the Peptide Population Derived from EDBM-MCP and EDBM-AEM
3.1.1. Schematic Representations
3.1.2. Venn Diagrams
3.2. Identification of Bioactive Peptides Resulting from Bovine Hemoglobin Hydrolysis by EDBM-MCP and EDBM-AEM
3.3. Biological Activities of the Hydrolysates Obtained from EDBM-MCP and EDBM-AEM
3.3.1. Antimicrobial Test
Antibacterial Activity
MIC Determination of Antibacterial Peptide Hydrolysates
3.3.2. Antifungal Test
3.3.3. Antioxidant Activities of Bovine Hemoglobin Hydrolysates
- β-carotene bleaching inhibition activity
- DPPH free radical scavenging capacity
- Antioxidant properties products by ABTS assay
- Evaluation of total antioxidant capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Hb | Hemoglobin |
| ED | Electrodialysis |
| AEM | Anion exchange membrane |
| MCP | Monovalent cation permselective membrane |
| BM | Bipolar membrane |
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Available online: https://openknowledge.worldbank.org/handle/10986/30317 (accessed on 4 April 2020).
- Anderson, R.C.; Yu, P.L. Pilot-scale extraction and antimicrobial activity of crude extract from ovine neutrophils. Process Biochem. 2008, 43, 882–886. [Google Scholar] [CrossRef]
- Yu, P.L.; Van der Linden, D.S.; Sugiarto, H.; Anderson, R.C. Antimicrobial peptides isolated from the blood of farm animals. Anim. Prod. Sci. 2010, 50, 660–669. [Google Scholar] [CrossRef]
- Del Hoyo, P.; Rendueles, M.; Díaz, M. Effect of processing on functional properties of animal blood plasma. Meat Sci. 2008, 78, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Bah, C.S.F.; Bekhit, A.D.A.; Carne, A.; McConnell, M.A. Slaughterhouse Blood: An Emerging Source of Bioactive Compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Adje, E.Y.; Balti, R.; Kouach, M.; Dhulster, P.; Guillochon, D.; Nedjar-Arroume, N. Obtaining antimicrobial peptides by controlled peptic hydrolysis of bovine hemoglobin. Int. J. Biol. Macromol. 2011, 49, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Dale, C.S.; Casten, K.; Geigner, M.A.; Gozzo, F.C.; Ferro, E.S.; Heimann, A.S.; Lakshmi, A.D. Hemoglobin-derived peptides as novel type of bioactive signaling molecules. AAPS J. 2010, 12, 658–668. [Google Scholar] [CrossRef] [Green Version]
- Nedjar-Arroume, N.; Dubois-Delval, V.; Adje, E.Y.; Traisnel, J.; Krier, F.; Mary, P.; Kouach, M.; Briand, G.; Guillochon, D. Bovine hemoglobin: An attractive source of antibacterial peptides. Peptides 2008, 29, 969–977. [Google Scholar] [CrossRef]
- Lignot, B.; Froidevaux, R.; Nedjar-Arroume, N.; Guillochon, D. Solvent effect on kinetics of appearance of neokyotorphin, VVh4 and a bradykinin-potentiating peptide in the course of peptic hydrolysis of bovine hemoglobin. Appl. Biochem. Biotechnol. 1999, 30, 201–207. [Google Scholar]
- Zhao, Q.; Piot, J.M. Neokyotorphin formation and quantitative evolution following human hemoglobin hydrolysis with cathepsin D. Peptides 1997, 19, 759–766. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Piot, J.M. Investigation of inhibition angiotensin-converting enzyme (ACE) activity and opioid activity of two hemorphins, LVV-hemorphin-5 and VVhemorphin-5, isolated from a defined peptic hydrolysate of bovine hemoglobin. Neuropeptides 1997, 2, 147–153. [Google Scholar] [CrossRef]
- Vercaigne-Marko, D.; Kosciarz, E.; Nedjar-Arroume, N.; Guillochon, D. Improvement of Staphylococcus aureus-V8-protease hydrolysis of bovine hemoglobin by its adsorption on to a solid phase in the presence of SDS: Peptide mapping and obtaining of two haemopoietic peptides. Appl. Biochem. Biotechnol. 2000, 31, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Adje, E.Y.; Balti, R.; Kouach, M.; Guillochon, D.; Nedjar-Arroume, N. α67-106 of bovine hemoglobin: A new family of antimicrobial and angiotensin I-converting enzyme inhibitory peptides. Eur. Food Res. Technol. 2011, 232, 637–646. [Google Scholar] [CrossRef]
- Daoud, R.; Dubois, V.; Bors-Dodita, L.; Nedjar-Arroume, N.; Krier, F.; Chihib, N.-E.; Mary, P.; Kouach, M.; Briand, G.; Guillochon, D. New antibacterial peptide derived from bovine hemoglobin. Peptides 2005, 26, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Nedjar-Arroume, N.; Dubois-Delval, V.; Miloudi, K.; Daoud, R.; Krier, F.; Kouach, M.; Briand, G.; Guillochon, D. Isolation and characterization of four antibacterial peptides from bovine hemoglobin. Peptides 2006, 27, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Choisnard, L.; Froidevaux, R.; Nedjar-Arroume, N.; Lignot, B.; Vercaigne-Marko, D.; Krier, F.; Dhulster, P.; Guillochon, D. Kinetic study of the appearance of an anti-bacterial peptide in the course of bovine hemoglobin peptic hydrolysis. Appl. Biochem. Biotechnol. 2002, 36, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, R.; Firdaous, L.; Châtaigné, G.; Dhulster, P.; Nedjar, N. Production of an antimicrobial peptide derived from slaughterhouse by-product and its potential application on meat as preservative. Food Chem. 2016, 211, 306–313. [Google Scholar] [CrossRef]
- Bauer, A.K.; Dwyer-Nield, L.D.; Hankin, J.A.; Murphy, R.C.; Malkinson, A.M. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology 2011, 169, 1–15. [Google Scholar] [CrossRef]
- Imaida, K.; Fukishima, S.; Shirai, T.; Ohtami, M.; Nakamish, K.; Ito, N. Promoting activities of butylated hydroxyanisole and butylated hydroxytoluene on 2-stage urinary carcinogenesis and inhibition of gamma-glutamyl trans peptide positive for development in the liver of rats. Carcinogenesis 1983, 4, 895–899. [Google Scholar] [CrossRef]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT. Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar]
- Goot, A.J.V.D.; Pelgrom, P.J.M.; Berghout, J.A.M.; Geerts, M.E.J.; Jankowiak, L.; Hardt, N.A.; Boom, R.M. Concepts for further sustainable production of foods. J. Food Eng. 2016, 168, 42–51. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and byproducts: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylin, S.; Patouillard, L.; Margni, M.; Bazinet, L. Milk protein production by a more environmentally sustainable process: Bipolar membrane electrodialysis coupled with ultrafiltration. Green Chem. 2018, 20, 449–456. [Google Scholar] [CrossRef]
- Chaudron, C.; Faucher, M.; Bazinet, L.; Margni, M. The cost is not enough-An Alternative Eco-Efficiency Approach Applied to Cranberry De-Acidification. J. Clean. Prod. 2019, 232, 391–399. [Google Scholar] [CrossRef]
- Faucher, M.; Henaux, L.; Chaudron, C.; Mikhaylin, S.; Margni, M.; Bazinet, L. Electromembrane approach to substantially improve the ecoefficiency of deacidified cranberry juice production: Physicochemical properties, life cycle assessment and ecoefficiency score. J. Food Eng. 2020, 273, 109802. [Google Scholar] [CrossRef]
- Abou-Diab, M.; Thibodeau, J.; Deracinois, B.; Flahaut, C.; Fliss, I.; Dhulster, P.; Nedjar, N.; Bazinet, L. Bovine hemoglobin enzymatic hydrolysis by a new ecoefficient process-Part I: Feasibility of electrodialysis with bipolar membrane and production of neokyotorphin (α137-141). Membranes 2020, 10, 257. [Google Scholar] [CrossRef]
- Crosby, W.H.; Mann, J.I.; Furth, F.W. Standardizing a method for clinical hemoglobinometry. U. S. Armed Forces Med. J. 1954, 5, 693–703. [Google Scholar]
- European Environment Agency. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 13 June 2020).
- Zhao, Q.Y.; Sannier, F.; Ricart, G.; Piot, J.M. A rapid detection and identification of hemorphins released from bovin hemoglobin enzymatic hydrolysate by use of HPLC coupled with Photodiode Array Detector. J. Liq. Chromatogr. 1995, 18, 93–103. [Google Scholar] [CrossRef]
- Takagi, H.; Shiomi, H.; Fukui, K.; Hayashi, K.; Kiso, Y.; Kitagawa, K. Isolation of a novel analgesic pentapeptide, neo-kyotorphin, from bovine brain. Life Sci. 1982, 31, 1733–1736. [Google Scholar] [CrossRef]
- Chang, R.C.C.; Huang, W.Y.; Redding, T.W.; Arimura, A.; Coy, D.H.; Schally, A.V. Isolation and structure of several peptides from porcine hypothalamic. Biochim. Biophys. Acta 1980, 625, 266–273. [Google Scholar] [CrossRef]
- Piot, J.M.; Zhao, Q.Y.; Guillochon, D.; Ricart, G.; Thomas, D. Isolation and characterization of a bradykinin potentiating peptide from a bovine peptic hemoglobin hydrolysate. FEBS Lett. 1992, 299, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Adje, E.Y. Hydrolyse Ménagée de L’hémoglobine Bovine par la Pepsine Porcine en Mélanges Hydroalcooliques et Obtention D’une Nouvelle Famille de Peptides Antimicrobiens; Lille University: Lille, France, 2010. [Google Scholar]
- Borch, E.; Arinder, P. Bacteriological safety issues in red meat and ready to-eat meat products, as well as control measures. Meat Sci. 2002, 62, 381–390. [Google Scholar] [CrossRef]
- Catiau, L.; Traisnel, J.; Delval-Dubois, V.; Chihib, N.-E.; Guillochon, D.; Nedjar- Arroume, N. Minimal antimicrobial peptide sequence from hemoglobin alpha chain: KYR. Peptides 2011, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, R.; Krier, F.; Nedjar-Arroume, N.; Vercaigne-Marko, D.; Kosciarz, E.; Ruckebusch, C.; Guillochon, D. Antibacterial activity of a pepsin derived bovine hemoglobin fragment. FEBS Lett. 2001, 491, 159–163. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009. [Google Scholar]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Sreening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bersuder, P.; Hole, M.; Smith, G. Antioxidants from a heated histidine glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J. Am. Oil Chem. Soc. 1998, 75, 181–187. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Pongsawatmanit, R.; Gordon, M.H. Characterisation of the phytochemicals and antioxidant properties of extracts from Teaw (Cratoxylum formosum Dyer). Food Chem. 2007, 100, 1620–1629. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay Free Radical. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Dubois, V.; Nedjar-Arroume, N.; Guillochon, D. Influence of pH on the appearance of active peptides in the course of peptic hydrolysis of bovine hemoglobin. Prep. Biochem. Biotechnol. 2005, 35, 85–102. [Google Scholar] [CrossRef]
- Barkhudaryan, N.; Oberthuer, W.; Lottspeich, F.; Galoyan, A. Structure of hypothalamic coronaro-constrictory peptide factors. Neurochem. Res. 1992, 17, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Zouari, O.; Przybylski, R.; Hannioui, M.; Sion, L.; Pascal Dhulster, P.; Nedjar-Arroume, N. High added-value co-product: The porcine cruor is an attractive source of active peptides. Rev. J. Nutr. 2020. [Google Scholar] [CrossRef]
- Jean, C. Activité Antimicrobienne de Peptides Provenant d’hydrolysats de Protéines de Babeurre, de Lactoferrine et de pois. Master’s Thesis, University of Montreal, Quebec, QC, Canada.
- Luz, C.; Izzo, L.; Ritienib, A.; Mañesa, J.; Meca, G. Antifungal and antimycotoxigenic activity of hydrolyzed goat whey on Penicillium spp: An application as biopreservation agent in pita bread. LWT Food Sci. Technol. 2020, 118, 108717. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods. 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- Merouane, A.; Noui, A.; Medjahed, H.; Nedjari Benhadj Ali, K.; Saadi, A. Activité antioxydante des composés phénoliques d’huile d’olive extraite par méthode traditionnelle. Int. J. Biol. Chem. Sci. 2015, 8, 1865. [Google Scholar] [CrossRef] [Green Version]
- He Tang, C.; Peng, J.; Zhen, D.W.; Chen, Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009, 115, 672–678. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Kim, S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic processes: Market overview, membrane phenomena, recent developments and sustainable strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]






| Biological Activity | Position | Sequence | Molecular Weight (Da) | Control | EDBM-MCP | EDBM-AEM |
|---|---|---|---|---|---|---|
| Antimicrobial | α1-29 | VLSAADKGNVKAAWGKVGGHAAEYGAEAL | 2839 | + | + | + |
| α1-27 | VLSAADKGNVKAAWGKVGGHAAEYGAE | 2655 | + | + | + | |
| α1-23 | VLSAADKGNVKAAWGKVGGHAAE | 2235 | + | + | + | |
| α34-46 | LSFPTTKTYFPHF | 1585 | + | + | + | |
| α33-46 | FLSFPTTKTYFPHF | 1732 | − | + | − | |
| α37-46 | PTTKTYFPHF | 1238 | + | + | + | |
| α137-141 | TSKYR | 653 | + | + | + | |
| β1-13 | MLTAEEKAAVTAF | 1381 | + | + | + | |
| β126-145 | QADFQKVVAGVANALAHRYH | 2194 | + | + | + | |
| β140-145 | LAHRYH | 795 | + | + | + | |
| α99-105 | KLLSHSL | 796 | + | + | + | |
| α99-106 | KLLSHSLL | 910 | + | + | + | |
| Hematopoietic | α76-82 | LPGALSE | 685 | − | + | − |
| Opioid | α137-141 | TSKYR | 653 | + | + | + |
| β32-37 | VVYPWT | 763 | + | + | + | |
| β31-37 | LVVYPWT | 876 | + | + | + | |
| β32-40 | VVYPWTQRF | 1195 | + | + | + | |
| β31-40 | LVVYPWTQRF | 1308 | + | + | + | |
| Potentiator of bradykinin | α129-134 | LANVST | 603 | + | + | + |
| α110-125 | ASHLPSDFTPAVHASL | 1649 | + | + | + | |
| Coronaro-constrictor | β32-36 | VVYPW | 663 | − | + | − |
| Antihypertensive | α99-105 | KLLSHSL | 796 | + | + | + |
| Antioxidant | α137-141 | TSKYR | 653 | + | + | + |
| Bacteria Strains | Bovine Hemoglobin Hydrolysates | ||
|---|---|---|---|
| Control | EDBM-MCP | EDBM-AEM | |
| Staphylococcus aureus | +++ | +++ | +++ |
| Listeria monocytogenes | ++ | ++ | ++ |
| Micrococcus luteus | + | + | + |
| Kocuria rhizophila | +++ | +++ | +++ |
| Escherichia coli | + | + | + |
| Salmonelle Newport | + | + | + |
| Bacteria Strains | Bovine Hemoglobin Hydrolysates | ||
|---|---|---|---|
| MIC | |||
| Control | EDBM-MCP | EDBM-AEM | |
| mg/mL | mg/mL | mg/mL | |
| Staphylococcus aureus | 0.31 ± 0.0 a | 0.62 ± 0.0 b | 0.31 ± 0.0 a |
| Listeria monocytogenes | 1.25 ± 0.0 a | 2.5 ± 0.0 b | 2.5 ± 0.0 b |
| Micrococcus luteus | 5 ± 0.0 a | 10 ± 0.0 b | 10 ± 0.0 b |
| Kocuria rhizophila | 0.31 ± 0.0 a | 0.31 ± 0.0 a | 0.31 ± 0.0 a |
| Escherichia coli | 10 ± 0.0 a | 10 ± 0.0 a | 10 ± 0.0 a |
| Salmonelle Newport | 10 ± 0.0 a | 10 ± 0.0 a | 10 ± 0.0 a |
| DPPH | Control | EDBM-MCP | EDBM-AEM | NKT | Trolox |
|---|---|---|---|---|---|
| IC50 (mg/mL) | 2.55 ± 0.02 a | 2.86 ± 0.03 b | 2.72 ± 0.04 c | 0.61 ± 0.05 d | 0.38 ± 0.03 e |
| TEAC | 0.15 ± 0.03 a | 0.13 ± 0.02 b | 0.14 ± 0.01 c | 0.62 ± 0.09 d | 1 e |
| ABTS | Control | EDBM-MCP | EDBM-AEM | NKT | Trolox |
|---|---|---|---|---|---|
| IC50 (mg/mL) | 3.42 ± 0.01 a | 3.66 ± 0.03 b | 3.55 ± 0.04 c | 0.53 ± 0.05 d | 0.49 ± 0.09 e |
| TEAC | 0.14 ± 0.03 a | 0.13 ± 0.02 b | 0.14 ± 0.01 a | 0.92 ± 0.01 c | 1 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou-Diab, M.; Thibodeau, J.; Deracinois, B.; Flahaut, C.; Fliss, I.; Dhulster, P.; Bazinet, L.; Nedjar, N. Bovine Hemoglobin Enzymatic Hydrolysis by a New Eco-Efficient Process-Part II: Production of Bioactive Peptides. Membranes 2020, 10, 268. https://doi.org/10.3390/membranes10100268
Abou-Diab M, Thibodeau J, Deracinois B, Flahaut C, Fliss I, Dhulster P, Bazinet L, Nedjar N. Bovine Hemoglobin Enzymatic Hydrolysis by a New Eco-Efficient Process-Part II: Production of Bioactive Peptides. Membranes. 2020; 10(10):268. https://doi.org/10.3390/membranes10100268
Chicago/Turabian StyleAbou-Diab, Mira, Jacinthe Thibodeau, Barbara Deracinois, Christophe Flahaut, Ismail Fliss, Pascal Dhulster, Laurent Bazinet, and Naima Nedjar. 2020. "Bovine Hemoglobin Enzymatic Hydrolysis by a New Eco-Efficient Process-Part II: Production of Bioactive Peptides" Membranes 10, no. 10: 268. https://doi.org/10.3390/membranes10100268
APA StyleAbou-Diab, M., Thibodeau, J., Deracinois, B., Flahaut, C., Fliss, I., Dhulster, P., Bazinet, L., & Nedjar, N. (2020). Bovine Hemoglobin Enzymatic Hydrolysis by a New Eco-Efficient Process-Part II: Production of Bioactive Peptides. Membranes, 10(10), 268. https://doi.org/10.3390/membranes10100268