Lactobacillus reuteri DSM 17938 and Agave Inulin in Children with Cerebral Palsy and Chronic Constipation: A Double-Blind Randomized Placebo Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Intervention and Monitoring
2.3. Dietary Surveys
2.4. Anthropometry
2.5. Stool pH
2.6. Stool Consistency
2.7. DNA Extraction and Quantitative Real-Time PCR
2.8. Statistical Analysis
2.9. Ethics and Trial Registration
3. Results
3.1. Subject Characteristics
3.2. Rome IV Criteria
3.3. Stool Characteristics
3.4. Dietary Aspects
3.5. Gut Microbiota
3.6. Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A Report: The Definition and Classification of Cerebral Palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar] [PubMed]
- Sullivan, P.B.; Lambert, B.; Rose, M.; Ford-Adams, M.; Johnson, A.; Griffiths, P. Prevalence and Severity of Feeding and Nutritional Problems in Children with Neurological Impairment: Oxford Feeding Study. Dev. Med. Child Neurol. 2000, 42, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Giudice, E.D.; Staiano, A.; Capano, G.; Romano, A.; Florimonte, L.; Miele, E.; Ciarla, C.; Campanozzi, A.; Crisanti, A.F. Gastrointestinal Manifestations in Children with Cerebral Palsy. Brain Dev. 1999, 21, 307–311. [Google Scholar] [CrossRef]
- Yong, D.; Beattie, R.M. Normal bowel habit and prevalence of constipation in primary-school children. Ambul. Child Health 1998, 4, 277–282. [Google Scholar]
- Veugelers, R.; Benninga, M.A.; Calis, E.A.C.; Willemsen, S.P.; Evenhuis, H.; Tibboel, D.; Penning, C. Prevalence and Clinical Presentation of Constipation in Children with Severe Generalized Cerebral Palsy. Dev. Med. Child. Neurol. 2010, 52, e216–e221. [Google Scholar] [CrossRef]
- Sullivan, P.B. Gastrointestinal Disorders in Children with Neurodevelopmental Disabilities. Dev. Disabil. Res. Rev. 2008, 14, 128–136. [Google Scholar] [CrossRef]
- Park, E.S.; Park, C.I.; Cho, S.-R.; Na, S.-I.; Cho, Y.S. Colonic Transit Time and Constipation in Children with Spastic Cerebral Palsy. Arch. Phys. Med. Rehabil. 2004, 85, 453–456. [Google Scholar] [CrossRef]
- Coccorullo, P.; Strisciuglio, C.; Martinelli, M.; Miele, E.; Greco, L.; Staiano, A. Lactobacillus Reuteri (DSM 17938) in Infants with Functional Chronic Constipation: A Double-Blind, Randomized, Placebo-Controlled Study. J. Pediatr. 2010, 157, 598–602. [Google Scholar] [CrossRef]
- Choi, C.H.; Chang, S.K. Alteration of Gut Microbiota and Efficacy of Probiotics in Functional Constipation. J. Neurogastroenterol. Motil. 2015, 21, 4–7. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. 2001. Available online: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed on 25 February 2014).
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhu, Q.; Qu, X.; Qin, H. Microbial Treatment in Chronic Constipation. Sci. China Life Sci. 2018, 61, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Ianiro, G.; Tortora, A.; D’Angelo, G.; Di Rienzo, T.A.; Bibbò, S.; Migneco, A.; Gasbarrini, A. The Effect of Lactobacillus Reuteri Supplementation in Adults with Chronic Functional Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gastrointest. Liver Dis. JGLD 2014, 23, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Indrio, F.; Di Mauro, A.; Riezzo, G.; Civardi, E.; Intini, C.; Corvaglia, L.; Ballardini, E.; Bisceglia, M.; Cinquetti, M.; Brazzoduro, E.; et al. Prophylactic Use of a Probiotic in the Prevention of Colic, Regurgitation, and Functional Constipation: A Randomized Clinical Trial. JAMA Pediatr. 2014, 168, 228–233. [Google Scholar] [CrossRef]
- Closa-Monasterolo, R.; Ferré, N.; Castillejo-DeVillasante, G.; Luque, V.; Gispert-Llaurado, M.; Zaragoza-Jordana, M.; Theis, S.; Escribano, J. The Use of Inulin-Type Fructans Improves Stool Consistency in Constipated Children. A Randomised Clinical Trial: Pilot Study. Int. J. Food Sci. Nutr. 2017, 68, 587–594. [Google Scholar] [CrossRef]
- Collado Yurrita, L.; San Mauro Martín, I.; Ciudad-Cabañas, M.J.; Calle-Purón, M.E.; Hernández Cabria, M. Effectiveness of Inulin Intake on Indicators of Chronic Constipation; a Meta-Analysis of Controlled Randomized Clinical Trials. Nutr. Hosp. 2014, 30, 244–252. [Google Scholar] [CrossRef]
- Hod, K.; Ringel, Y. Probiotics in Functional Bowel Disorders. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 89–97. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Huys, G.; Daube, G. Probiotics: An Update. J. Pediatr. 2015, 91, 6–21. [Google Scholar] [CrossRef]
- Korterink, J.J.; Ockeloen, L.; Benninga, M.A.; Tabbers, M.M.; Hilbink, M.; Deckers-Kocken, J.M. Probiotics for Childhood Functional Gastrointestinal Disorders: A Systematic Review and Meta-Analysis. Acta Paediatr. 2014, 103, 365–372. [Google Scholar] [CrossRef]
- Stevenson, R.D. Use of Segmental Measures to Estimate Stature in Children with Cerebral Palsy. Arch. Pediatr. Adolesc. Med. 1995, 149, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.; Day, S.; Shavelle, R.; Strauss, D. Low Weight, Morbidity, and Mortality in Children with Cerebral Palsy: New Clinical Growth Charts. Pediatrics 2011, 128, e299–e307. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; Bernal, M.-J.; Blasco, J.; Martínez, R.; Dalmau, J.; Ortuño, I.; Espín, B.; Vasallo, M.-I.; Gil, D.; Vidal, M.-L.; et al. Prebiotic Effect during the First Year of Life in Healthy Infants Fed Formula Containing GOS as the Only Prebiotic: A Multicentre, Randomised, Double-Blind and Placebo-Controlled Trial. Eur. J. Nutr. 2015, 54, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.; Candy, D.C.; Catto-Smith, A.G.; Clayden, G.; Loening-Baucke, V.; Di Lorenzo, C.; Nurko, S.; Staiano, A. The Paris Consensus on Childhood Constipation Terminology (PACCT) Group. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 273–275. [Google Scholar] [CrossRef]
- Song, Y.-L.; Kato, N.; Liu, C.-X.; Matsumiya, Y.; Kato, H.; Watanabe, K. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group-and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 2000, 187, 167–173. [Google Scholar] [CrossRef]
- Imanieh, M.H.; Golpayegan, M.R.; Sedighi, M.; Ahmadi, K.; Aghaie, A.; Dehghani, S.M.; Yousefi, G. Comparison of Three Therapeutic Interventions for Chronic Constipation in Paediatric Patients with Cerebral Palsy: A Randomised Clinical Trial. Prz. Gastroenterol. 2019, 14, 292–297. [Google Scholar] [CrossRef]
- Yu, T.; Zheng, Y.-P.; Tan, J.-C.; Xiong, W.-J.; Wang, Y.; Lin, L. Effects of Prebiotics and Synbiotics on Functional Constipation. Am. J. Med. Sci. 2017, 353, 282–292. [Google Scholar] [CrossRef]
- Wegner, A.; Banaszkiewicz, A.; Kierkus, J.; Landowski, P.; Korlatowicz-Bilar, A.; Wiecek, S.; Kwiecien, J.; Gawronska, A.; Dembinski, L.; Czaja-Bulsa, G.; et al. The Effectiveness of Lactobacillus Reuteri DSM 17938 as an Adjunct to Macrogol in the Treatment of Functional Constipation in Children. A Randomized, Double-Blind, Placebo-Controlled, Multicentre Trial. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 494–500. [Google Scholar] [CrossRef]
- Jadrešin, O.; Sila, S.; Trivić, I.; Mišak, Z.; Hojsak, I.; Kolaček, S. Lack of Benefit of Lactobacillus Reuteri DSM 17938 as an Addition to the Treatment of Functional Constipation. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 763–766. [Google Scholar] [CrossRef]
- Ojetti, V.; Petruzziello, C.; Migneco, A.; Gnarra, M.; Gasbarrini, A.; Franceschi, F. Effect of Lactobacillus Reuteri (DSM 17938) on Methane Production in Patients Affected by Functional Constipation: A Retrospective Study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1702–1708. [Google Scholar]
- Micka, A.; Siepelmeyer, A.; Holz, A.; Theis, S.; Schön, C. Effect of Consumption of Chicory Inulin on Bowel Function in Healthy Subjects with Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Food Sci. Nutr. 2017, 68, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.K.; Toporovski, M.S.; Tahan, S.; Neufeld, C.B.; de Morais, M.B. Dietary Fiber Mixture in Pediatric Patients with Controlled Chronic Constipation. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Chao, C.; Yang, L.-P.; Storm, H.; Oliva-Hemker, M.; Saavedra, J.M. Effects of Fructo-Oligosaccharide-Supplemented Infant Cereal: A Double-Blind, Randomized Trial. Br. J. Nutr. 2003, 90, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.C.M.; Costa-Ribeiro, H.; Almeida, P.S.; Pontes, M.V.; Leite, M.E.Q.; Filadelfo, L.R.; Khoury, J.C.; Bean, J.A.; Mitmesser, S.H.; Vanderhoof, J.A.; et al. Stool Pattern Changes in Toddlers Consuming a Follow-on Formula Supplemented with Polydextrose and Galactooligosaccharides. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Cherbut, C. Inulin and Oligofructose in the Dietary Fibre Concept. Br. J. Nutr. 2002, 87 (Suppl. 2), S159–S162. [Google Scholar] [CrossRef]
- García Contreras, A.A.; Vásquez Garibay, E.M.; Sánchez Ramírez, C.A.; Fafutis Morris, M.; Delgado Rizo, V. Factors Associated with the Stool Characteristics of Children with Cerebral Palsy and Chronic Constipation. Rev. Esp. Enfermedades Dig. 2020, 112, 41–46. [Google Scholar] [CrossRef]
- Caramico-Favero, D.C.O.; Guedes, Z.C.F.; Morais, M.B.D. Food intake, nutritional status and gastrointestinal symptoms in children with cerebral palsy. Arq. Gastroenterol. 2018, 55, 352–357. [Google Scholar] [CrossRef]
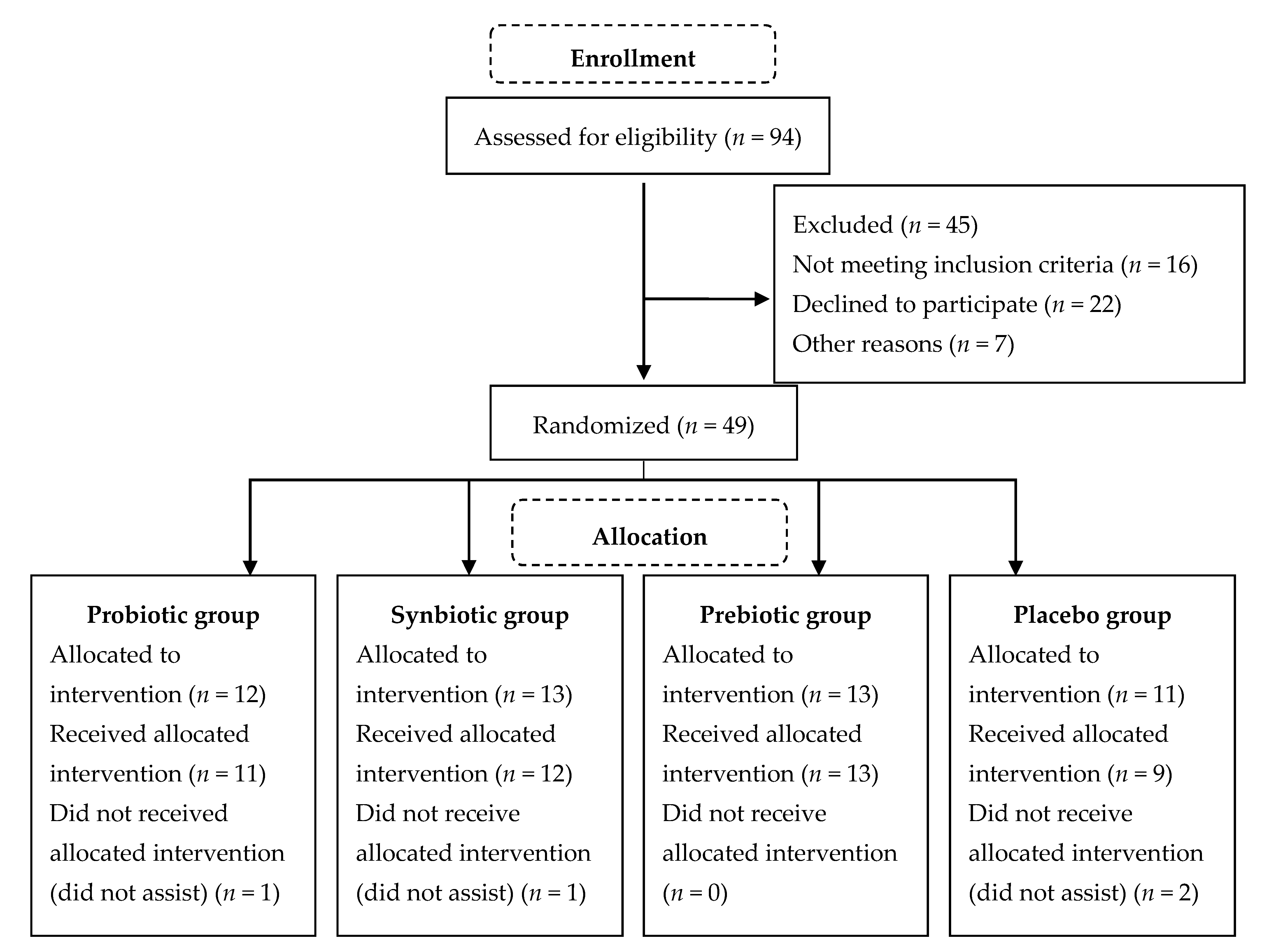
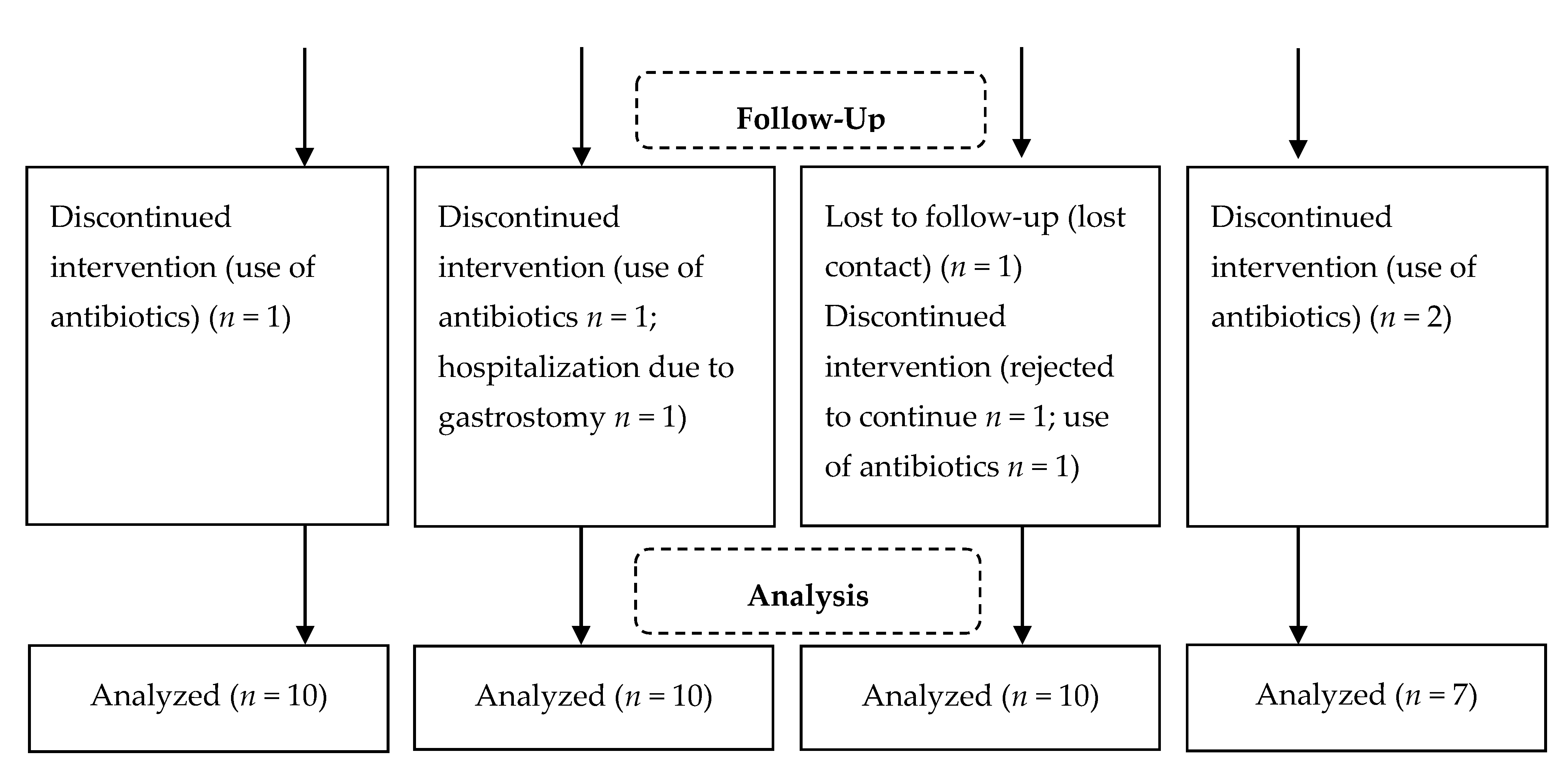
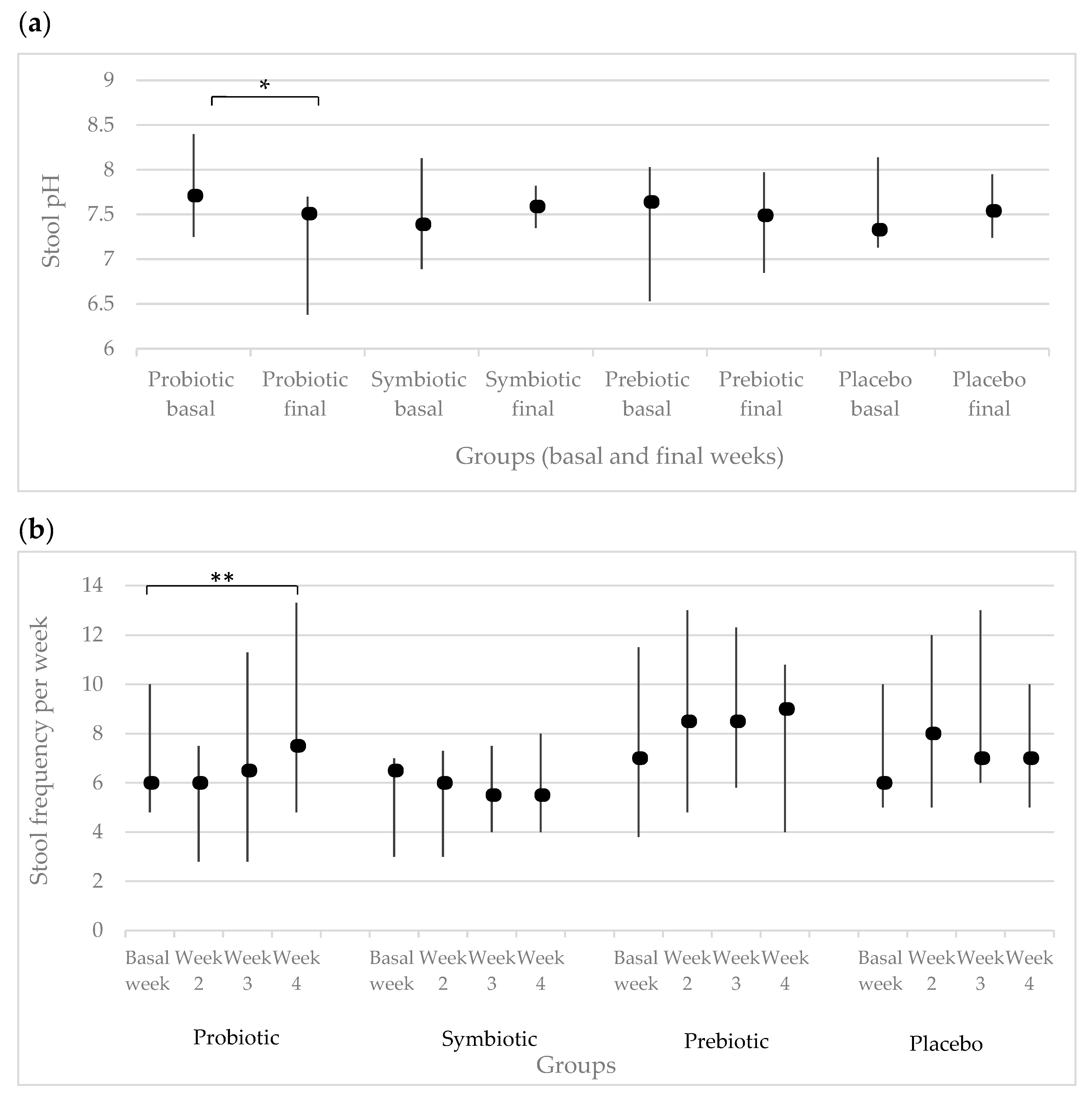
| Variable | Groups | p * | |||
|---|---|---|---|---|---|
| Probiotic (n = 10) | Synbiotic (n = 10) | Prebiotic (n = 10) | Placebo (n = 7) | ||
| Age (months) | 39 (28–51) | 42 (29–49) | 33 (22–53) | 37 (22–45) | 0.842 |
| Weight (kg) | 10.9 (10.0–13.4) | 9.2 (7.9–12.7) | 11.0 (8.9–14.2) | 9.9 (8.2–11.4) | 0.257 |
| Height (cm) | 88.1 (84.7–95.8) | 89.2 (81.8–93.4) | 89.6 (79.8–93.4) | 85.5 (80.7–92.5) | 0.844 |
| BMI (kg/m2) | 13.6 (12.6–14.4) | 12.7 (11.7–14.5) | 13.9 (12.4–15.6) | 13.2 (11.4–13.7) | 0.309 |
| BMI (Z) | −1.0 (−1.4–(−0.9)) | −1.4 (−1.9–(−0.6)) | −0.9 (−1.5–(−0.3)) | −1.2 (−1.9–(−0.9)) | 0.337 |
| Weight/age (Z) | −0.2 (−0.9–(−0.0)) | −1.2 (−1.7–(−0.0)) | −0.3 (−1.0–(−0.9)) | −0.9 (−1.5–(−0.2)) | 0.411 |
| Parameter | Groups | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Probiotic (n = 10) | Synbiotic (n = 10) | Prebiotic (n = 10) | Placebo (n = 7) | |||||||||||||
| Basal | Final | p * | % ** | Basal | Final | p * | % ** | Basal | Final | p * | % ** | Basal | Final | p * | % ** | |
| Bowel movements per week | ||||||||||||||||
| ≤2 times | 1 | 1 | 1.000 | 0 | 5 | – | 0.063 | 100 | 3 | – | 0.250 | 100 | 1 | – | 1.000 | 100 |
| >2 times | 9 | 9 | 5 | 10 | 7 | 10 | 6 | 7 | ||||||||
| History of excessive stool retention | ||||||||||||||||
| Yes | 7 | 1 | 0.031 | 85 | 8 | 1 | 0.016 | 88 | 7 | – | 0.016 | 100 | 4 | 2 | 0.500 | 50 |
| No | 3 | 9 | 2 | 9 | 3 | 10 | 3 | 5 | ||||||||
| History of painful defecation | ||||||||||||||||
| Yes | 8 | – | 0.008 | 100 | 9 | 1 | 0.008 | 89 | 7 | – | 0.002 | 100 | 7 | 3 | 0.125 | 57 |
| No | 2 | 10 | 1 | 9 | – | 10 | – | 4 | ||||||||
| Large stool diameter | ||||||||||||||||
| Yes | 10 | 5 | 0.063 | 50 | 10 | 4 | 0.031 | 60 | 10 | 3 | 0.016 | 70 | 7 | 5 | 0.500 | 29 |
| No | – | 5 | – | 6 | – | 7 | – | 2 | ||||||||
| Presence of a large fecal mass in the rectum | ||||||||||||||||
| Yes | 10 | 2 | 0.008 | 80 | 10 | 1 | 0.004 | 90 | 10 | 3 | 0.016 | 70 | 7 | 4 | 0.250 | 43 |
| No | – | 8 | – | 9 | – | 7 | – | 3 | ||||||||
| (a) Frequency of hard stool. | |||||
| Group | Basal Week n (%) | Week 2 n (%) | Week 3 n (%) | Week 4 n (%) | p * |
| Probiotic (n = 10) | 9 (90) | 7 (70) | 5 (50) | 6 (60) | 0.097 |
| Synbiotic (n = 10) | 7 (70) | 6 (60) | 6 (60) | 4 (40) | 0.453 |
| Prebiotic (n = 10) | 8 (80) | 5 (50) | 3 (30) | 3 (30) | 0.008 |
| Placebo (n = 7) | 3 (43) | 5 (71) | 3 (43) | 3 (42) | 0.646 |
| (b) Frequency of normal stool. | |||||
| Group | Basal Week n (%) | Week 2 n (%) | Week 3 n (%) | Week 4 n (%) | p * |
| Probiotic (n = 10) | 1 (10) | 3 (30) | 4 (40) | 4 (40) | 0.292 |
| Synbiotic (n = 10) | 1 (10) | 1 (10) | 3 (30) | 4 (40) | 0.266 |
| Prebiotic (n = 10) | 1 (10) | 3 (30) | 5 (50) | 7 (70) | 0.003 |
| Placebo (n = 7) | 3 (43) | 1 (14) | 2 (29) | 3 (43) | 0.564 |
| (c) Frequency of hard stool compared to placebo. | |||||
| Group | Basal Week | Final Week | Basal-Final Difference | p ** (Basal-Final vs. Placebo) | |
| Probiotic | 9 (90) | 6 (60) | 3 | 0.121 | |
| Synbiotic | 7 (70) | 4 (40) | 3 | 0.063 | |
| Prebiotic | 8 (80) | 3(30) | 5 | 0.031 | |
| Placebo | 3 (43) | 3 (43) | 0 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Contreras, A.A.; Vásquez Garibay, E.M.; Sánchez Ramírez, C.A.; Fafutis Morris, M.; Delgado Rizo, V. Lactobacillus reuteri DSM 17938 and Agave Inulin in Children with Cerebral Palsy and Chronic Constipation: A Double-Blind Randomized Placebo Controlled Clinical Trial. Nutrients 2020, 12, 2971. https://doi.org/10.3390/nu12102971
García Contreras AA, Vásquez Garibay EM, Sánchez Ramírez CA, Fafutis Morris M, Delgado Rizo V. Lactobacillus reuteri DSM 17938 and Agave Inulin in Children with Cerebral Palsy and Chronic Constipation: A Double-Blind Randomized Placebo Controlled Clinical Trial. Nutrients. 2020; 12(10):2971. https://doi.org/10.3390/nu12102971
Chicago/Turabian StyleGarcía Contreras, Andrea A., Edgar M. Vásquez Garibay, Carmen A. Sánchez Ramírez, Mary Fafutis Morris, and Vidal Delgado Rizo. 2020. "Lactobacillus reuteri DSM 17938 and Agave Inulin in Children with Cerebral Palsy and Chronic Constipation: A Double-Blind Randomized Placebo Controlled Clinical Trial" Nutrients 12, no. 10: 2971. https://doi.org/10.3390/nu12102971
APA StyleGarcía Contreras, A. A., Vásquez Garibay, E. M., Sánchez Ramírez, C. A., Fafutis Morris, M., & Delgado Rizo, V. (2020). Lactobacillus reuteri DSM 17938 and Agave Inulin in Children with Cerebral Palsy and Chronic Constipation: A Double-Blind Randomized Placebo Controlled Clinical Trial. Nutrients, 12(10), 2971. https://doi.org/10.3390/nu12102971





