A Review of the Incidence Diagnosis and Treatment of Spontaneous Hemorrhage in Patients Treated with Direct Oral Anticoagulants
Abstract
1. Introduction
2. Indications for Modern Anticoagulants
3. Epidemiology
4. Bleeding
4.1. Bleeding Severity
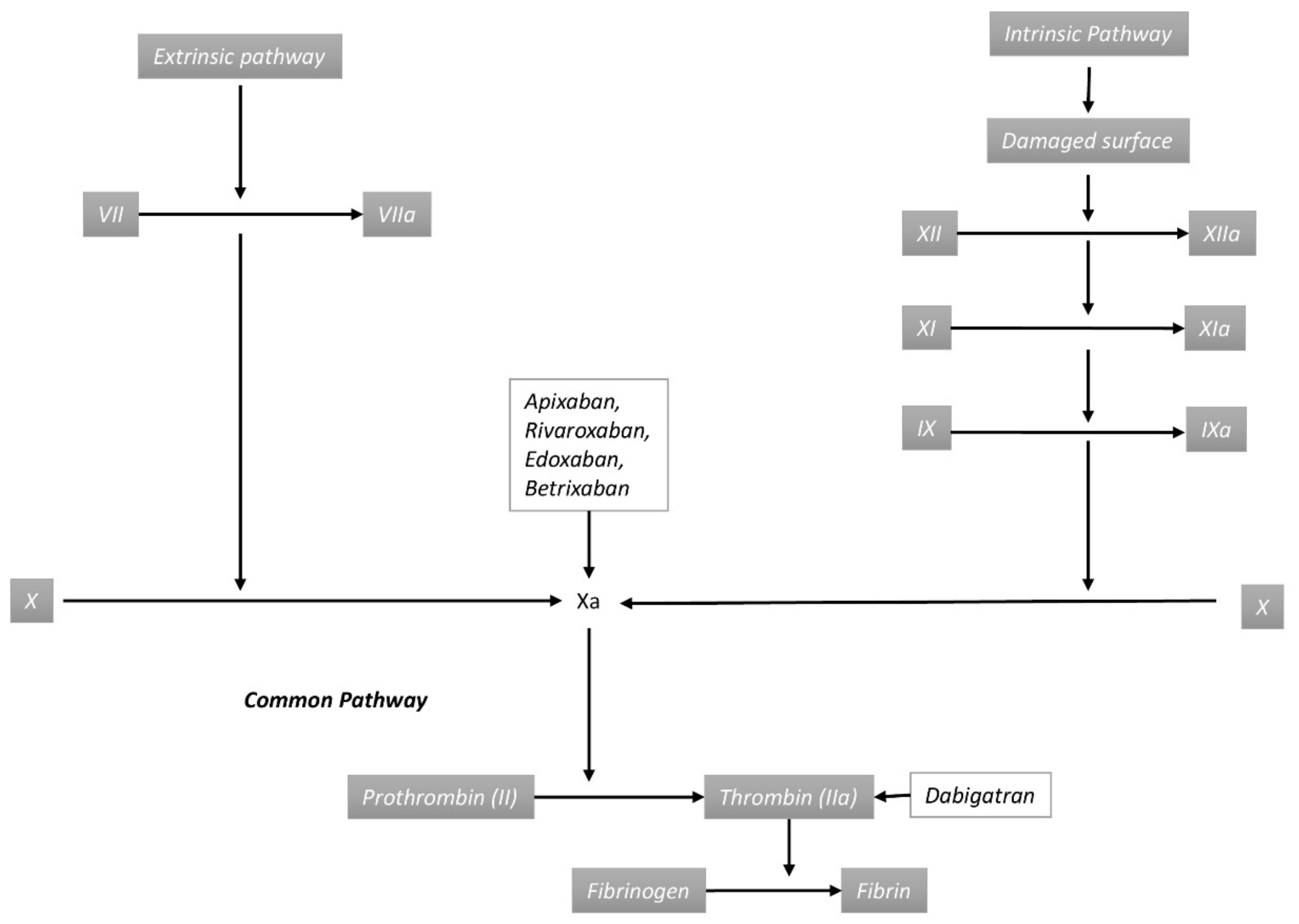
4.2. Pathogenesis of Direct Oral Anticoagulant-Associated Bleeding
4.3. Risk Factors
4.4. Risk Reduction Strategies
5. Management
5.1. Diagnosis
5.2. Treatment
5.3. Reversal Agents
5.3.1. Drug-Specific Reversal Agents
5.3.2. Non-Specific Reversal Agents
5.3.3. Future Antidotes
5.4. Restitution of Anticoagulation
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AF | Atrial Fibrillation |
| Anti-Xa | drug-specific assays |
| aPTT | Activated partial thromboplastin time |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRNMB | Clinically relevant non-major bleeding |
| CYP | Cytochromeombin time |
| DOACs | Direct Oral Anticoagulants |
| ECT | Ecarin clotting time |
| GI | Gastrointestinal |
| GI | Gastrointestinal |
| ICH | Intracranial hemorrhage |
| ICH | Intracranial Hemorrhage |
| LMWH | Low Molecular Weight Heparin |
| Nd | The total number of patients in the DOACs group |
| Nk | the total number of patients in the vitamin K antagonist group |
| NSAIDs | Nonsteroidal Anti-inflammatory Drugs |
| PT | Prothrombin time |
| UFH | Unfractionated Heparin |
| VKA | Vitamin K Antagonist |
| VTE | Venous Thromboembolism |
References
- Lim, G.B. Discovery and purification of heparin. Nat. Rev. Cardiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kustos, S.A.; Fasinu, P.S. Direct-Acting Oral Anticoagulants and Their Reversal Agents—An Update. Medicines 2019, 6, 103. [Google Scholar] [CrossRef]
- Weitz, J.I.; Bauersachs, R.; Becker, B.; Berkowitz, S.D.; Freitas, M.C.S.; Lassen, M.R.; Metzig, C.; Raskob, G.E. Effect of Osocimab in Preventing Venous Thromboembolism Among Patients Undergoing Knee Arthroplasty. JAMA 2020, 323, 130–139. [Google Scholar] [CrossRef]
- Wypasek, E.; Alhenc-Gelas, M.; Sydor, W.; Blecharczyk, A.; Zawilska, K.; Corral, J.; Iwaniec, T.; Celińska-Lowenhoff, M.; Potaczek, D.P.; Undas, J.; et al. Genetic characterization of antithrombin, protein C and protein S deficiencies in Polish patients. Pol. Arch. Intern. Med. 2017, 127, 512–523. [Google Scholar] [CrossRef][Green Version]
- Lancaster, T.R.; Singer, D.E.; Sheehan, M.A.; Oertel, L.B.; Maraventano, S.W.; Hughes, R.A.; Kistler, J.P. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch. Intern. Med. 1991, 151, 1944–1949. [Google Scholar] [CrossRef]
- Kaatz, S.; Mahan, C.E.; Nakhle, A.; Gunasekaran, K.; Ali, M.; Lavender, R.; Paje, D.G. Management of Elective Surgery and Emergent Bleeding with Direct Oral Anticoagulants. Curr. Cardiol. Rep. 2017, 19. [Google Scholar] [CrossRef]
- Undas, A.; Drabik, L.; Potpara, T. Bleeding in anticoagulated patients with atrial fibrillation. Pract. Consid. Kardiol. Pol. 2020, 78, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; A Akl, E.; Ornelas, J.; Blaivas, A.; Jiménez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140, 104–132. [Google Scholar]
- Van Der Hulle, T.; Kooiman, J.; Exter, P.L.D.; Dekkers, O.M.; Klok, F.A.; Huisman, M.V. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: A systematic review and meta-analysis. J. Thromb. Haemost. 2014, 12, 320–328. [Google Scholar] [CrossRef]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.; Ashrani, A.; Botero, J.P.; Ferre, R.L.; Henkin, S.; Lenz, C.; Le-Rademacher, J.; Wysokinski, W.; Ii, R.D.M.; Ii, R.M. Apixaban and dalteparin in active malignancy associated venous thromboembolism. Thromb. Haemost. 2017, 117, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.; Van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.K.; Kwok, C.S. Dabigatran and rivaroxaban for prevention of venous thromboembolism—Systematic review and adjusted indirect comparison. J. Clin. Pharm. Ther. 2011, 36, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.S.; McDuffie, J.R.; Lachiewicz, P.F.; Ortel, T.L.; Williams, J.W. Comparative Effectiveness of New Oral Anticoagulants and Standard Thromboprophylaxis in Patients Having Total Hip or Knee Replacement. Ann. Intern. Med. 2013, 159, 275–284. [Google Scholar] [CrossRef]
- Gryn, O.J.; Nguyen, T.; Frankova, D. The Use of Rivaroxaban for Unprovoked Pulmonary Embolism in the Setting of Antithrombin Deficiency. Cureus 2020, 12, 8560. [Google Scholar] [CrossRef]
- Wypasek, E.; Potaczek, D.P.; Alhenc-Gelas, M.; Undas, A. PROS1 mutations associated with protein S deficiency in Polish patients with residual vein obstruction on rivaroxaban therapy. Thromb. Res. 2014, 134, 199–201. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Pai, M.; Linkins, L.-A. Direct oral anticoagulants for treatment of HIT: Update of Hamilton experience and literature review. Blood 2017, 130, 1104–1113. [Google Scholar] [CrossRef]
- Shatzel, J.J.; Crapster-Pregont, M.; Deloughery, T.G. Non-vitamin K antagonist oral anticoagulants for heparin-induced thrombocytopenia. A systematic review of 54 reported cases. Thromb. Haemost. 2016, 116, 397–400. [Google Scholar] [CrossRef]
- Chai-Adisaksopha, C.; Crowther, M.; Isayama, T.; Lim, W. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: A systematic review and meta-analysis. Blood 2014, 124, 2450–2458. [Google Scholar] [CrossRef]
- Wolfe, Z.; Nasir, F.; Subramanian, C.R.; Lash, B.; Khan, S.U. A systematic review and Bayesian network meta-analysis of risk of intracranial hemorrhage with direct oral anticoagulants. J. Thromb. Haemost. 2018, 16, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Huang, H.; Datta, Y.H. Direct oral anticoagulant use and the incidence of bleeding in the very elderly with atrial fibrillation. J. Thromb. Thrombolysis 2016, 42, 573–578. [Google Scholar] [CrossRef]
- Barra, M.E.; Fanikos, J.; Connors, J.M.; Sylvester, K.W.; Piazza, G.; Goldhaber, S.Z. Evaluation of Dose-Reduced Direct Oral Anticoagulant Therapy. Am. J. Med. 2016, 129, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.; Becattini, C.; Vanni, S.; Sbrojavacca, R.; Nitti, C.; Manina, G.; Masotti, L.; Pomero, F.; Cattinelli, S.; Cappelli, R.; et al. Clinically relevant non-major bleeding with oral anticoagulants: Non-major may not be trivial. Blood Transf. 2018, 16, 387–391. [Google Scholar]
- Treder, M.; Alnawaiseh, M.; Wirths, G.; Rosentreter, A.; Eter, N. Spontane intraokulare Blutungen unter oraler Antikoagulation. Der Ophthalmol. 2017, 115, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Senger, S.; Keiner, D.; Hendrix, P.; Oertel, J.; Information, P.E.K.F.C. New Target-Specific Oral Anticoagulants and Intracranial Bleeding: Management and Outcome in a Single-Center Case Series. World Neurosurg. 2016, 88, 132–139. [Google Scholar] [CrossRef]
- Godin, R.; Marcoux, V.; Tagalakis, V. Abnormal uterine bleeding in women receiving direct oral anticoagulants for the treatment of venous thromboembolism. Vasc. Pharmacol. 2017, 93, 1–5. [Google Scholar] [CrossRef]
- Kurogi, R.; Nishimura, K.; Nakai, M.; Kada, A.; Kamitani, S.; Nakagawara, J.; Toyoda, K.; Ogasawara, K.; Ono, J.; Shiokawa, Y.; et al. Comparing intracerebral hemorrhages associated with direct oral anticoagulants or warfarin. Neurology 2018, 90, 1143–1149. [Google Scholar] [CrossRef]
- Caughey, G.E.; Ellett, L.K.; Barratt, J.D.; Shakib, S. Apixaban, concomitant medicines and spontaneous reports of haemorrhagic events. Ther. Adv. Drug Saf. 2017, 8, 157–164. [Google Scholar] [CrossRef]
- Zaarour, M.; Hassan, S.; Thumallapally, N.; Dai, Q. Rivaroxaban-Induced Nontraumatic Spinal Subdural Hematoma: An Uncommon Yet Life-Threatening Complication. Case Rep. Hematol. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Radcliff, K.; Ong, A.; Parvizi, J.; Post, Z.; Orozco, F. Rivaroxaban-induced Epidural Hematoma and Cauda Equina Syndrome after Total Knee Arthroplasty: A Case Report. Orthop. Surg. 2014, 6, 69–71. [Google Scholar] [CrossRef]
- Atia, R.; Bonnel, S.; Vallos, M.; Laroche, L.; Borderie, V.; Bouheraoua, N. Spontaneous choroidal hemorrhage associated with novel oral anticoagulants: A report of two cases and literature review. J. Fr. Ophtalmol. 2018, 41, 767–772. [Google Scholar] [CrossRef]
- Kham, N.M.; Song, M. Spontaneous, Life-Threatening Hemorrhagic Cardiac Tamponade Secondary to Rivaroxaban. Am. J. Ther. 2016, 23, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Burkland, D.; Mathuria, N. Isolated hemopericardium after initiation of rivaroxaban: Implications and potential mechanisms. Clin. Pr. 2019, 9, 1096. [Google Scholar] [CrossRef]
- Cinelli, M.; Uddin, A.; Duka, I.; Soomro, A.; Tamburrino, F.; Ghavami, F.; Lafferty, J. Spontaneous Hemorrhagic Pericardial and Pleural Effusion in a Patient Receiving Apixaban. Cardiol. Res. 2019, 10, 249–252. [Google Scholar] [CrossRef]
- Jun, J.H.; Hwang, J.C. Association of rivaroxaban anticoagulation and spontaneous vitreous hemorrhage. JAMA Ophthalmol. 2015, 133, 1184. [Google Scholar] [CrossRef] [PubMed]
- Basnet, S.; Mohanty, E.; Mir, I.; Dhital, R.; Koirala, A.; Tachamo, N. Atraumatic splenic rupture associated with apixaban. SAGE Open Medical Case Reports 2019, 7, 1–3. [Google Scholar] [CrossRef]
- Kwok, A.; Chern, T.Y.; Winn, R. Acute cholecystitis and gallbladder perforation leading to massive haemoperitoneum in a patient taking rivaroxaban. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Gunasekaran, K.; Winans, A.R.M.; Murthi, S.; Ahmad, M.R.; Kaatz, S. Rectus sheath hematoma associated with apixaban. Clin. Pract. 2017, 7, 957. [Google Scholar] [CrossRef] [PubMed]
- Elango, K.; Murthi, S.; Devasahayam, J.; Gunasekaran, K. Spontaneous rectus sheath haematoma due to cough on apixaban. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Aktas, H.; Inci, S.; Dogan, P.; Izgu, I. Spontaneous rectus sheath hematoma in a patient treated with apixaban. Intractable Rare Dis. Res. 2015, 5, 47–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, A.; Mastenbrook, J.; Bauler, L. Pain in the hip. Am. J. Emerg. Med. 2020, 38, 1046. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C. The subcommittee on control of anticoagulation of the scientific and standardization committee of the international society on thrombosis and haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [PubMed]
- Chesebro, J.H.; Knatterud, G.; Roberts, R.; Borer, J.; Cohen, L.S.; Dalen, J.; Dodge, H.T.; Francis, C.K.; Hillis, D.; Ludbrook, P.; et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987, 76, 142–154. [Google Scholar] [CrossRef] [PubMed]
- An International Randomized Trial Comparing Four Thrombolytic Strategies for Acute Myocardial Infarction. N. Engl. J. Med. 1993, 329, 673–682. [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.W.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A Consensus Report From the Bleeding Academic Research Consortium. Circulaion 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef]
- White, N.J.; Ward, K.R.; Pati, S.; Strandenes, G.; Cap, A.P. Hemorrhagic blood failure. J. Trauma Acute Care Surg. 2017, 82, 41–49. [Google Scholar] [CrossRef]
- Yates, P.A.; Villemagne, V.L.; Ellis, K.A.; Desmond, P.; Masters, C.L.; Rowe, C.C. Cerebral Microbleeds: A Review of Clinical, Genetic, and Neuroimaging Associations. Front. Neurol. 2014, 4, 205. [Google Scholar] [CrossRef]
- Linkins, L.-A.; Choi, P.T.; Douketis, J.D. Clinical Impact of Bleeding in Patients Taking Oral Anticoagulant Therapy for Venous Thromboembolism. Ann. Intern. Med. 2003, 139, 893–900. [Google Scholar] [CrossRef]
- Garcia, D.; Lopes, R.D.; Hylek, E.M. New-onset atrial fibrillation and warfarin initiation: High risk periods and implications for new antithrombotic drugs. Thromb. Haemost. 2010, 104, 1099–1105. [Google Scholar]
- Benedetti, G.; Neccia, M.; Agati, L. Direct oral anticoagulants use in elderly patients with non valvular atrial fibrillation: State of evidence. Minerva Cardioangiol. 2017, 66, 301–313. [Google Scholar] [PubMed]
- Witt, D.M.; Nieuwlaat, R.; Clark, N.P.; Ansell, J.; Holbrook, A.; Skov, J.; Shehab, N.; Mock, J.; Myers, T.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism. Optimal management of anticoagulation therapy. Blood Adv. 2018, 2, 3257–3291. [Google Scholar] [CrossRef]
- Elhosseiny, S.; Al Moussawi, H.; Chalhoub, J.M.; Lafferty, J.; Deeb, L. Direct Oral Anticoagulants in Cirrhotic Patients: Current Evidence and Clinical Observations. Can. J. Gastroenterol. Hepatol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, J.; Patel, P.; Farrell, A.; Sivarajahkumar, S.; Cameron, K.; Ma, J.; Battistella, M. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol. Dial. Transplant. 2018, 34, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.T.; Neuen, B.L.; Cheng, L.P.; Jun, M.; Toyama, T.; Gallagher, M.P.; Jardine, M.J.; Sood, M.M.; Garg, A.X.; Palmer, S.C.; et al. Benefits and Harms of Oral Anticoagulant Therapy in Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 171, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Garcia, D.A.; Lyman, G.H.; Carrier, M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): A systematic review and meta-analysis. Thromb. Res. 2019, 173, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Janion-Sadowska, A.; Papuga-Szela, E.; Łukaszuk, R.; Chrapek, M.; Undas, A. Non–Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation and Thrombocytopenia. J. Cardiovasc. Pharmacol. 2018, 72, 153–160. [Google Scholar] [CrossRef]
- Mima, Y.; Sangatsuda, Y.; Yasaka, M.; Wakugawa, Y.; Nagata, S.; Okada, Y. Acute Thrombocytopenia after Initiating Anticoagulation with Rivaroxaban. Intern. Med. 2014, 53, 2523–2527. [Google Scholar] [CrossRef][Green Version]
- Alexander, J.H.; Lopes, R.D.; James, S.; Kilaru, R.; Zadionchenko, V.; Mohan, P.; Bhatt, D.L.; Goodman, S.; Verheugt, F.W.; Flather, M.; et al. Apixaban with Antiplatelet Therapy after Acute Coronary Syndrome. N. Engl. J. Med. 2011, 365, 699–70819. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Douros, A.; Renoux, C.; Yin, H.; Filion, K.B.; Suissa, S.; Azoulay, L. Concomitant Use of Direct Oral Anticoagulants with Antiplatelet Agents and the Risk of Major Bleeding in Patients with Nonvalvular Atrial Fibrillation. Am. J. Med. 2018, 132, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sindet-Pedersen, C.; Lamberts, M.; Staerk, L.; Bonde, A.N.; Berger, J.S.; Pallisgaard, J.L.; Hansen, M.L.M.; Madelaire, C.; Gislason, G.H.; Olesen, J.B. Combining Oral Anticoagulants With Platelet Inhibitors in Patients With Atrial Fibrillation and Coronary Disease. J. Am. Coll. Cardiol. 2018, 72, 1790–1800. [Google Scholar] [CrossRef]
- Roule, V.; Ardouin, P.; Briet, C.; Lemaitre, A.; Bignon, M.; Sabatier, R.; Champ-Rigot, L.; Milliez, P.; Blanchart, K.; Beygui, F. Vitamin K antagonist vs direct oral anticoagulants with antiplatelet therapy in dual or triple therapy after percutaneous coronary intervention or acute coronary syndrome in atrial fibrillation: Meta-analysis of randomized controlled trials. Clin. Cardiol. 2019, 42, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Ambler, G.; Shakeshaft, C.; Brown, M.M.; Charidimou, A.; Salman, R.A.-S.; Lip, G.Y.H.; Cohen, H.; Banerjee, G.; Houlden, H.; et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): A multicentre observational cohort study. Lancet Neurol. 2018, 17, 539–547. [Google Scholar] [CrossRef]
- Miller, C.S.; Dorreen, A.; Martel, M.; Huynh, T.; Barkun, A.N. Risk of Gastrointestinal Bleeding in Patients Taking Non–Vitamin K Antagonist Oral Anticoagulants: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1674–1683. [Google Scholar] [CrossRef]
- Kido, K.; Scalese, M.J. Management of Oral Anticoagulation Therapy After Gastrointestinal Bleeding: Whether to, When to, and How to Restart an Anticoagulation Therapy. Ann. Pharmacother. 2017, 51, 1000–1007. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; I Weitz, J.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Cohen, H.; Arachchillage, D.J.; Middeldorp, S.; Beyer-Westendorf, J.; Abdul-Kadir, R. Management of direct oral anticoagulants in women of childbearing potential: Guidance from the SSC of the ISTH: Reply. J. Thromb. Haemost. 2016, 15, 195–197. [Google Scholar] [CrossRef]
- Pengo, V.; Denas, G.; Zoppellaro, G.; Jose, S.P.; Hoxha, A.; Ruffatti, A.; Andreoli, L.; Tincani, A.; Cenci, C.; Prisco, D.; et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018, 132, 1365–1371. [Google Scholar] [CrossRef]
- Mueck, W.; Kubitza, D.; Becka, M. Co-administration of rivaroxaban with drugs that share its elimination pathways: Pharmacokinetic effects in healthy subjects. Br. J. Clin. Pharmacol. 2013, 76, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Borre, E.D.; Goode, A.; Raitz, G.; Shah, B.; Lowenstern, A.; Chatterjee, R.; Sharan, L.; Lapointe, N.A.; Yapa, R.; Davis, J.K.; et al. Predicting Thromboembolic and Bleeding Event Risk in Patients with Non-Valvular Atrial Fibrillation: A Systematic Review. Thromb. Haemost. 2018, 118, 2171–2187. [Google Scholar] [CrossRef] [PubMed]
- Kurlander, J.E.; Gu, X.; Scheiman, J.M.; Haymart, B.; Kline-Rogers, E.; Saini, S.D.; Kaatz, S.; Froehlich, J.B.; Richardson, C.R.; Barnes, G.D. Missed opportunities to prevent upper GI hemorrhage: The experience of the Michigan Anticoagulation Quality Improvement Initiative. Vasc. Med. 2019, 24, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Shah, N.D.; Sangaralingham, L.; Gersh, B.J.; Noseworthy, P.A. Non–Vitamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J. Am. Coll. Cardiol. 2017, 69, 2779–2790. [Google Scholar] [CrossRef]
- Ximenes, R.O.; Vieira Costa, J.P.; Pereira, E.S.K.T. Cullen’s Sign: Not Always Acute Pancreatitis. Gastroenterology 2018, 154, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, B.T.; Cuker, A.; Siegal, D.M.; Crowther, M.; Garcia, D.A. Laboratory Assessment of the Anticoagulant Activity of Direct Oral Anticoagulants. Chest 2016, 151, 127–138. [Google Scholar] [CrossRef]
- Neyens, R.; Bohm, N.; Cearley, M.; Andrews, C.; Chalela, J. Dabigatran-associated subdural hemorrhage: Using thromboelastography (TEG((R))) to guide decision-making. J. Thromb. Thrombolysis 2014, 37, 80–83. [Google Scholar] [CrossRef]
- Hemphill, J.C.; Greenberg, S.M.; Anderson, C.; Becker, K.J.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Cuker, A.; Burnett, A.; Triller, D.; Crowther, M.; Ansell, J.; Van Cott, E.M.; Wirth, D.; Kaatz, S. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am. J. Hematol. 2019, 94, 697–709. [Google Scholar] [CrossRef]
- Chin, P.K.; Doogue, M. Long-term prescribing of new oral anticoagulants. Aust. Prescr. 2016, 39, 200–204. [Google Scholar] [CrossRef]
- Charles, V.P., Jr.; Reilly, P.A.; Van Ryn, J.; Eikelboom, J.W.; Glund, S.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kam, C.-W.; et al. Idarucizumab for Dabigatran Reversal—Full Cohort Analysis. N. Engl. J. Med. 2017, 377, 431–441. [Google Scholar]
- Lu, G.; Conley, P.B.; Leeds, J.M.; Karbarz, M.J.; Levy, G.G.; Mathur, V.S.; Castillo, J.; Crowther, M.; Curnutte, J.T. A phase 2 PK/PD study of andexanet alfa for reversal of rivaroxaban and edoxaban anticoagulation in healthy volunteers. Blood Adv. 2020, 4, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Parasrampuria, D.A.; Marbury, T.; Matsushima, N.; Chen, S.; Wickremasingha, P.K.; He, L.; Dishy, V.; Brown, K.S. Pharmacokinetics, safety, and tolerability of edoxaban in end-stage renal disease subjects undergoing haemodialysis. Thromb. Haemost. 2015, 113, 719–727. [Google Scholar] [PubMed]
- Connolly, S.J.; Crowther, M.; Eikelboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Lawrence, J.H.; Yue, P.; Bronson, M.D.; Lu, G.; Conley, P.B.; et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med. 2019, 380, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Thalji, N.K.; Ivanciu, L.; Davidson, R.; A Gimotty, P.; Krishnaswamy, S.; Camire, R.M. A rapid pro-hemostatic approach to overcome direct oral anticoagulants. Nat. Med. 2016, 22, 924–932. [Google Scholar] [CrossRef]
- Ansell, J.; Bakhru, S.H.; Laulicht, B.E.; Steiner, S.S.; Dishy, V.; Costin, J.C.; Grosso, M.; Brown, K.; Noveck, R. Use of PER977 to Reverse the Anticoagulant Effect of Edoxaban. N. Engl. J. Med. 2014, 371, 2141–2142. [Google Scholar] [CrossRef]
- Lu, G.; Kotha, J.; Cardenas, J.M.; Herr, M.J.; Pandey, A.; Curnutte, J.; Conley, P.B.; Jennings, L.K. Abstract 18218: In Vitro Characterization of Andexanet Alfa (PRT064445), a Specific fXa Inhibitor Antidote versus Aripazine (PER977), a Non-specific Reversal Agent. Circulation 2014, 130, 18218. [Google Scholar]
| Drugs | Dabigatran | Apixaban | Betrixaban | Edoxaban | Rivaroxaban |
|---|---|---|---|---|---|
| Mechanism of action | Direct IIa (Thrombin) Inhibitor | Factor Xa Inhibitor | Factor Xa Inhibitor | Factor Xa Inhibitor | Factor Xa Inhibitor |
| Onset of action | Within 30 min | ~30 min | Within 30 min | Within 30 min | Within 30 min |
| Duration of action (h) | 24–36 | At least 24 | At least 24 | 24 | 24 |
| Baseline elimination half-life in hours | 12–17 | 9–14 | 19–27 | 10–14 | 5–9 (young)/11–13 (elderly) |
| Dosage | |||||
| Non-valvular AF | 150 mg twice daily | 5 mg twice daily ** | 60 mg once daily | 20 mg once daily with the evening meal | |
| VTE treatment | Parenteral anticoagulation for 5–10 days; then dabigatran 150 mg twice daily | 10 mg twice daily for one week, then 5 mg twice daily | Parenteral anticoagulation for 5–10 days; then edoxaban 60 mg once daily | 15 mg twice daily with food for three weeks; then 20 mg once daily with food | |
| VTE prophylaxis | 110 mg for the first day, then 220 mg once daily | 2.5 mg twice daily | 160 mg on the first day, followed by 80 mg once daily, with food | 10 mg once daily, with or without food | |
| Best laboratory measurement | dTT, ECT | Anti-Xa | Anti-Xa | Anti-Xa | Anti-Xa |
| Author (Year of Publication) | Study Inclusion | Fatal Bleeding | Major Bleeding | ICH | Major GI Bleeding | CRNMB |
|---|---|---|---|---|---|---|
| Van Der Hulle et al. (2014) | Five randomized controlled trials (2 evaluating rivaroxaban; 1, dabigatran; 1, apixaban; and 1, edoxaban) | 0.06% vs. 0.17% Nd = 12,197 Nk = 12,193 | 1.1% vs. 1.7% Nd = 12,197 Nk = 12,193 | 0.09% vs. 0.25% Nd = 12,197 Nk = 12,193 | 0.35% vs. 0.53% Nd = 12,197 Nk = 12,193 | 6.6% vs. 8.4% Nd = 12,197 Nk = 12,193 |
| Chai-Adisaksopha et al. (2014) | Twelve randomized controlled trials (4 evaluating dabigatran; 4, rivaroxaban; 2, apixaban; and 2, edoxaban) | 0.30% vs. 0.52% Nd = 57,850 Nk = 44,757 | 4% vs. 4.64% Nd = 57,850 Nk = 44,757 | 0.51% vs. 1.08% Nd = 57,850 Nk = 44,757 | 2.09% vs. 1.70% Nd = 53,753 Nk = 40,650 | 10.24% vs. 11.05% Nd = 45,774 Nk = 38,750 |
| BLEEDING COMPLICATIONS | |
|---|---|
| MAJOR BLEEDING | Intracranial bleeding (subarachnoid hemorrhage, epidural hemorrhage, subdural hemorrhage, and intraparenchymal hemorrhage) |
| Intraspinal hemorrhage | |
| Intraocular hemorrhage (retinal or vitreous hemorrhage) | |
| Hemorrhagic cardiac tamponade/hemopericardium | |
| Retroperitoneal hemorrhage | |
| Gastrointestinal hemorrhage | |
| Joint hematoma, traumatic or non-traumatic | |
| Hemoperitoneum, atraumatic splenic rupture | |
| CLINICALLY RELEVANT NON-MAJOR BLEEDING (CRNMB) | Genitourinary–Hematuria, vaginal bleeding, abnormal uterine bleeding |
| Respiratory tract–hemoptysis, gingival bleeding, epistaxis | |
| Intramuscular–Rectus sheath hematoma | |
| Skin/subcutaneous–Bruising | |
| BARC Definitions | ||
| Type 0 | No bleeding | |
| Type 1 | Bleeding that is not actionable and does not cause the patient to seek unscheduled intervention. | |
| Type 2 | Any overt, actionable sign of hemorrhage requiring non-surgical medical intervention by a healthcare professional. | |
| Type 3 | a | Overt bleeding plus hemoglobin drop of 3 to <5 g/dL (provided hemoglobin drop is related to bleed) |
| b | Overt bleeding plus hemoglobin drop ≥5 g/dL (provided hemoglobin drop is related to bleed) Cardiac tamponade Bleeding requiring surgical intervention for control or intravenous vasoactive agents | |
| c | Intracranial hemorrhage confirmed by autopsy or imaging or lumbar puncture Intraocular bleed compromising vision | |
| Type 4 | CABG-related or perioperative intracranial bleeding within 48 h | |
| Type 5 | a | Probable fatal bleeding |
| b | Definite fatal bleeding | |
| Patient-Related Risk Factors |
|---|
| Advanced age Low body mass Smoking Associated comorbidities like hypertension, chronic obstructive pulmonary disease, diabetes mellitus, renal failure, liver disease Previous gastrointestinal or intracranial bleeding Malignancies—tumor invasion Hematologic disorders Collagen vascular disorders Thrombocytopenia Concomitant use of other medications including steroids, nonsteroidal anti-inflammatory drugs, aspirin or clopidogrel |
| COMMON BLEEDING SCORES |
|---|
| HAS-BLED score |
| HEMORR2HAGES score |
| ATRIA score |
| ORBIT-AF score |
| ABC bleeding score |
| General Measures | Confirm DOAC intake history, the timing of the last dose, check for concomitant medicine, particularly antiplatelet drugs, assess for hemodynamic compromise, check the renal function, and oral activated charcoal (if the last dose within prior two hours) | |
| Minor Bleeding | Stop therapy, local hemostatic measures, supportive care, and monitoring | |
| Moderate Bleeding | All of the above and fluid resuscitation, blood transfusion, consider fresh frozen plasma transfusion, and consider hemodialysis for dabigatran | |
| Major Bleeding | All of the above; consider massive transfusion protocol with packed red blood cells, platelets, fresh frozen plasma, and other procedures/surgeries to achieve hemostasis | |
| Specific antidotes | Dabigatran–Idarazcizumab | |
| Xa inhibitors (Apixaban, Rivaroxaban, Edoxaban)–Andexant alfa | ||
| Non-specific reversal agents: 4 Prothrombin complex concentrates (PCC) [Factors II, VII, IX, and X], Tranexamic acid, epsilon- aminocaproic acid, Desmopressin | ||
| Future antidotes | FXa(116L) for both factor Xa as well as direct thrombin inhibitors | |
| PER977 (Arapazine/Chiraparantag) for factor Xa inhibitors | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasekaran, K.; Rajasurya, V.; Devasahayam, J.; Singh Rahi, M.; Chandran, A.; Elango, K.; Talari, G. A Review of the Incidence Diagnosis and Treatment of Spontaneous Hemorrhage in Patients Treated with Direct Oral Anticoagulants. J. Clin. Med. 2020, 9, 2984. https://doi.org/10.3390/jcm9092984
Gunasekaran K, Rajasurya V, Devasahayam J, Singh Rahi M, Chandran A, Elango K, Talari G. A Review of the Incidence Diagnosis and Treatment of Spontaneous Hemorrhage in Patients Treated with Direct Oral Anticoagulants. Journal of Clinical Medicine. 2020; 9(9):2984. https://doi.org/10.3390/jcm9092984
Chicago/Turabian StyleGunasekaran, Kulothungan, Venkat Rajasurya, Joe Devasahayam, Mandeep Singh Rahi, Arul Chandran, Kalaimani Elango, and Goutham Talari. 2020. "A Review of the Incidence Diagnosis and Treatment of Spontaneous Hemorrhage in Patients Treated with Direct Oral Anticoagulants" Journal of Clinical Medicine 9, no. 9: 2984. https://doi.org/10.3390/jcm9092984
APA StyleGunasekaran, K., Rajasurya, V., Devasahayam, J., Singh Rahi, M., Chandran, A., Elango, K., & Talari, G. (2020). A Review of the Incidence Diagnosis and Treatment of Spontaneous Hemorrhage in Patients Treated with Direct Oral Anticoagulants. Journal of Clinical Medicine, 9(9), 2984. https://doi.org/10.3390/jcm9092984






