Value of Cardiac Biomarkers in the Early Diagnosis of Takotsubo Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Assessment
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Whole Cohort
3.2. Propensity Score Matching (Supplementary Files)
3.3. Outcomes
3.4. Value of the BNP and the Troponin I Kinetics in the Differential Diagnosis between TTS, STEMI, and NSTEMI
3.5. Value of the BNP/Troponin Ratio in the Differential Diagnosis between TTS, STEMI, and NSTEMI
3.6. Value of the BNP/Troponin Ratio in the Differential Diagnosis between TTS and STEMI
3.7. Value of the BNP/Troponin Ratio in the Differential Diagnosis between TTS and NSTEMI
3.8. Diagnostic Scores for TTS and STEMI Distinction
3.9. Diagnostic Scores for TTS and NSTEMI Distinction
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morel, O.; Sauer, F.; Imperiale, A.; Cimarelli, S.; Blondet, C.; Jesel, L. Importance of inflammation and neurohumoral activation in Takotsubo cardiomyopathy. J. Card. Fail. 2009, 15, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Lachmet-Thébaud, L.; Marchandot, B.; Matsushita, K.; Dagrenat, C.; Peillex, M.; Sato, C. Systemic Inflammatory Response Syndrome Is a Major Determinant of Cardiovascular Outcome in Takotsubo Syndrome. Circ. J. 2020, 84, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Athanasiadis, A.; Stöllberger, C.; Pistner, W.; Schwab, J.; Gottwald, U. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int. J. Cardiol. 2013, 166, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Girardey, M.; Jesel, L.; Campia, U.; Messas, N.; Hess, S.; Imperiale, A. Impact of Malignancies in the Early and Late Time Course of Takotsubo Cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2016, 80, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, N.D.; Tarantino, N.; Guastafierro, F.; De Gennaro, L.; Correale, M.; Stiermaier, T. Malignancies and outcome in Takotsubo syndrome: A meta-analysis study on cancer and stress cardiomyopathy. Heart Fail. Rev. 2019, 24, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W.; Maron, B.J. Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Messas, N.; Caspar, T.; Jesel, L.; Hess, S.; Girardey, M.; Radulescu, B. Takotsubo cardiomyopathy triggered by ischemic injury: When lateral myocardial infarction precipitate apical ballooning syndrome. Int. J. Cardiol. 2016, 202, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W.; Lesser, J.R.; Menon, M.; Parpart, M.; Maron, M.S.; Maron, B.J. Spectrum and Significance of Electrocardiographic Patterns, Troponin Levels, and Thrombolysis in Myocardial Infarction Frame Count in Patients with Stress (Tako-tsubo) Cardiomyopathy and Comparison to Those in Patients with ST-Elevation Anterior Wall Myocardial Infarction. Am. J. Cardiol. 2008, 101, 1723–1728. [Google Scholar] [PubMed]
- Akashi, Y.J.; Musha, H.; Nakazawa, K.; Miyake, F. Plasma brain natriuretic peptide in takotsubo cardiomyopathy. QJM Mon. J. Assoc. Physicians 2004, 97, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Neil, C.J.; Sverdlov, A.L.; Mahadavan, G.; Chirkov, Y.Y.; Kucia, A.M. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am. J. Cardiol. 2011, 108, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Doyen, D.; Moceri, P.; Chiche, O.; Schouver, E.; Cerboni, P.; Chaussade, C. Cardiac biomarkers in Takotsubo cardiomyopathy. Int. J. Cardiol. 2014, 174, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.; Borlaug, B.A.; Lerman, A.; Rihal, C.S.; Prasad, A. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): Insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart Br. Card. Soc. 2009, 95, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, G.M.; Schoch, B.; Schmid, F.; Keller, P.; Sudano, I.; Lüscher, T.F. Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy. Int. J. Cardiol. 2012, 154, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.S.; Dhillon, A.S.; Taylor, H.C.; Sun, Z.; Desai, M.Y. Diagnostic utility of cardiac biomarkers in discriminating Takotsubo cardiomyopathy from acute myocardial infarction. J. Card. Fail. 2014, 20, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group, Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Cammann, V.L.; Jurisic, S.; Seifert, B.; Napp, L.C.; Diekmann, J. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: Results from the International Takotsubo Registry. Eur. J. Heart Fail. 2017, 19, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Kato, K.; Cammann, V.L.; Gili, S.; Jurisic, S.; Di Vece, D. Long-Term Prognosis of Patients With Takotsubo Syndrome. J. Am. Coll. Cardiol. 2018, 72, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Moeller, C.; Oehler, K.; Desch, S.; Graf, T.; Eitel, C. Long-term excess mortality in takotsubo cardiomyopathy: Predictors, causes and clinical consequences. Eur. J. Heart Fail. 2016, 18, 650. [Google Scholar] [CrossRef] [PubMed]




| TTS Patients (n = 314) | STEMI Patients (n = 452) | NSTEMI Patients (n = 334) | p Value | p Value STEMI vs. TTS | p Value NSTEMI vs. TTS | p Value STEMI vs. NSTEMI | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age | 70.7 ± 13.4 | 62.1 ± 13.8 | 67.9 ± 13.9 | <0.001 | <0.001 | 0.024 | <0.001 |
| Female gender, n (%) | 257 (81.8) | 106 (23.4) | 81 (24.2) | <0.001 | <0.001 | <0.001 | 1 |
| CVD risk factors | |||||||
| Hypertension, n (%) | 180 (57.3) | 229 (50.7) | 234 (70.1) | <0.001 | 0.232 | 0.002 | <0.001 |
| Diabetes mellitus, n (%) | 65 (20.7) | 94 (20.8) | 96 (28.7) | 0.016 | 1 | 0.055 | 0.034 |
| Dyslipidemia, n (%) | 127 (40.5) | 185 (40.9) | 185 (55.4) | <0.001 | 1 | <0.001 | <0.001 |
| Current tobacco use, n (%) | 59 (18.8) | 208 (46) | 103 (30.8) | <0.001 | <0.001 | 0.001 | <0.001 |
| Past tobacco use, n (%) | 67 (21.3) | 119 (26.3) | 105 (31.4) | 0.014 | 0.370 | 0.013 | 0.387 |
| Background | |||||||
| History of malignancy, n (%) | 82 (26.1) | 31 (6.9) | 23 (6.9) | <0.001 | <0.001 | <0.001 | 1 |
| History of psychiatric disease, n (%) | 95 (30.4) | 10 (2.2) | 6 (1.8) | <0.001 | <0.001 | <0.001 | 1 |
| History of AF, n (%) | 50 (15.9) | 22 (4.9) | 39 (11.7) | <0.001 | <0.001 | 0.413 | 0.019 |
| History of stroke, n (%) | 32 (10.2) | 31 (6.9) | 43 (12.9) | 0.017 | 0.328 | 0.978 | 0.019 |
| History of PAD or ischemic heart disease, n (%) | 70 (22.4) | 77 (17.0) | 105 (31.4) | <0.001 | 0.228 | 0.031 | <0.001 |
| Chronic kidney disease, n (%) | <0.001 | 0.63 | <0.001 | 0.04 | |||
| moderate (DFG 30–60) | 70 (22.3) | 49 (10.8) | 53 (15.9) | ||||
| severe (15–30) | 11 (3.5) | 12 (2.6) | 9 (2.7) | ||||
| terminal (<15) | 9 (2.9) | 3 (0.7) | 2 (0.6) | ||||
| Hemodynamic parameters | |||||||
| Heart rate, bpm | 90.2 ± 23 | 77.5 ± 15.6 | 76 ± 21 | <0.001 | <0.001 | <0.001 | 1 |
| SBP, mmHg | 126.1 ± 28 | 128.6 ± 22 | 143.8 ± 28.1 | <0.001 | <0.001 | <0.001 | 1 |
| Electrocardiogram at admission | |||||||
| Q wave, n (%) | 163 (52.8) | 96 (47.3) | 6 (1.9) | <0.001 | 0.723 | <0.001 | <0.001 |
| Echocardiography | |||||||
| LVEF at admission, % | 38.5 ± 12 | 50 ± 11.2 | 54.1 ± 11.2 | <0.001 | <0.001 | <0.001 | <0.001 |
| RV involvement, n (%) | 15 (5.1) | 48 (10.6) | 10 (3) | <0.001 | 0.029 | 0.659 | <0.001 |
| Biology at admission | |||||||
| Tn I, ng/L | 1 [2.7] | 1.3 [12] | 0.4 [1.5] | <0.001 | <0.001 | 1 | <0.001 |
| BNP, ng/mL | 427.5 [967] | 70 [183.2] | 99.5 [199] | <0.001 | <0.001 | <0.001 | 0.445 |
| CRP, mg/L | 13 [50.9] | 3 [9.7] | 3 [8.8] | <0.001 | <0.001 | <0.001 | 0.404 |
| Leukocytes, G/L | 12.1 ± 5.8 | 12 ± 4 | 9.8 ± 4.7 | <0.001 | 0.886 | <0.001 | <0.001 |
| Hemoglobin, g/dL | 12.8 ± 2.7 | 14.1 ± 1.8 | 13.9 ± 1.8 | <0.001 | <0.001 | <0.001 | 0.138 |
| Creatinine serum μmol/L | 68 [38.5] | 73.8 [25] | 76 [27.5] | 0.437 | - | - | - |
| GFR, mL/min/m2 | 73.9 ± 27.9 | 80.8 ± 17.8 | 77.6 ± 18.8 | <0.001 | <0.001 | 0.073 | 0.091 |
| Plasma sodium level, mmol/L | 137.4 ± 4.7 | 137.5 ± 3 | 138.2 ± 3.2 | 0.005 | 0.952 | 0.012 | 0.014 |
| TTS Patients (n= 314) | STEMI Patients (n= 452) | NSTEMI Patients (n= 334) | p Value | p Value STEMI vs. TTS | p Value NSTEMI vs. TTS | p Value STEMI vs. NSTEMI | |
|---|---|---|---|---|---|---|---|
| In-hospital events | |||||||
| Cardiogenic shock, n (%) | 44 (14) | 14 (3.1) | 1 (0.3) | <0.001 | <0.001 | <0.001 | 0.009 |
| SVT, n (%) | 78 (24.4) | 12 (2.6) | 10 (3) | <0.001 | <0.001 | <0.001 | 1 |
| Sustained VT, n (%) | 4 (1.3) | 1 (0.2) | 1 (0.3) | 0.115 | - | - | - |
| VF, n (%) | 3 (1.0) | 2 (0.4) | 2 (0.6) | 0.110 | - | - | - |
| Torsade de Pointe, n (%) | 2 (0.6) | 0 (0) | 0 (0) | 0.081 | - | - | - |
| 3-degree AV block, n (%) | 2 (0.6) | 8 (1.8) | 1 (0.3) | 0.092 | - | - | - |
| In hospital mortality, n (%) | 23 (7.3) | 9 (2) | 6 (1.8) | <0.001 | 0.001 | 0.003 | 1 |
| Hospital length of stay, n (days) | 8.0 [7.0] | 5.0 [5.0] | 4.0 [5.0] | 0.511 | <0.001 | <0.001 | 1 |
| Follow up events | |||||||
| Hospitalization for acute HF, n (%) | 33 (10.8) | 25 (5.8) | 17 (5.4) | 0.019 | 0.337 | 0.042 | 1 |
| 30-day mortality, n (%) | 20 (6.7) | 12 (2.7) | 8 (2.4) | 0.005 | 0.027 | 0.033 | 1 |
| 1-year mortality, n (%) | 43 (16.3) | 21 (4.7) | 22 (6.6) | <0.001 | <0.001 | <0.001 | 0.804 |
| 1-year CV mortality, n (%) | 15 (5.1) | 12 (2.7) | 9 (2.7) | 0.149 | - | - | - |
| Cut-Off Value | Se | Sp | NPV | PPV | AUC, (%) | 95% Confidence Interval, (%) | |
|---|---|---|---|---|---|---|---|
| TTS vs. STEMI | |||||||
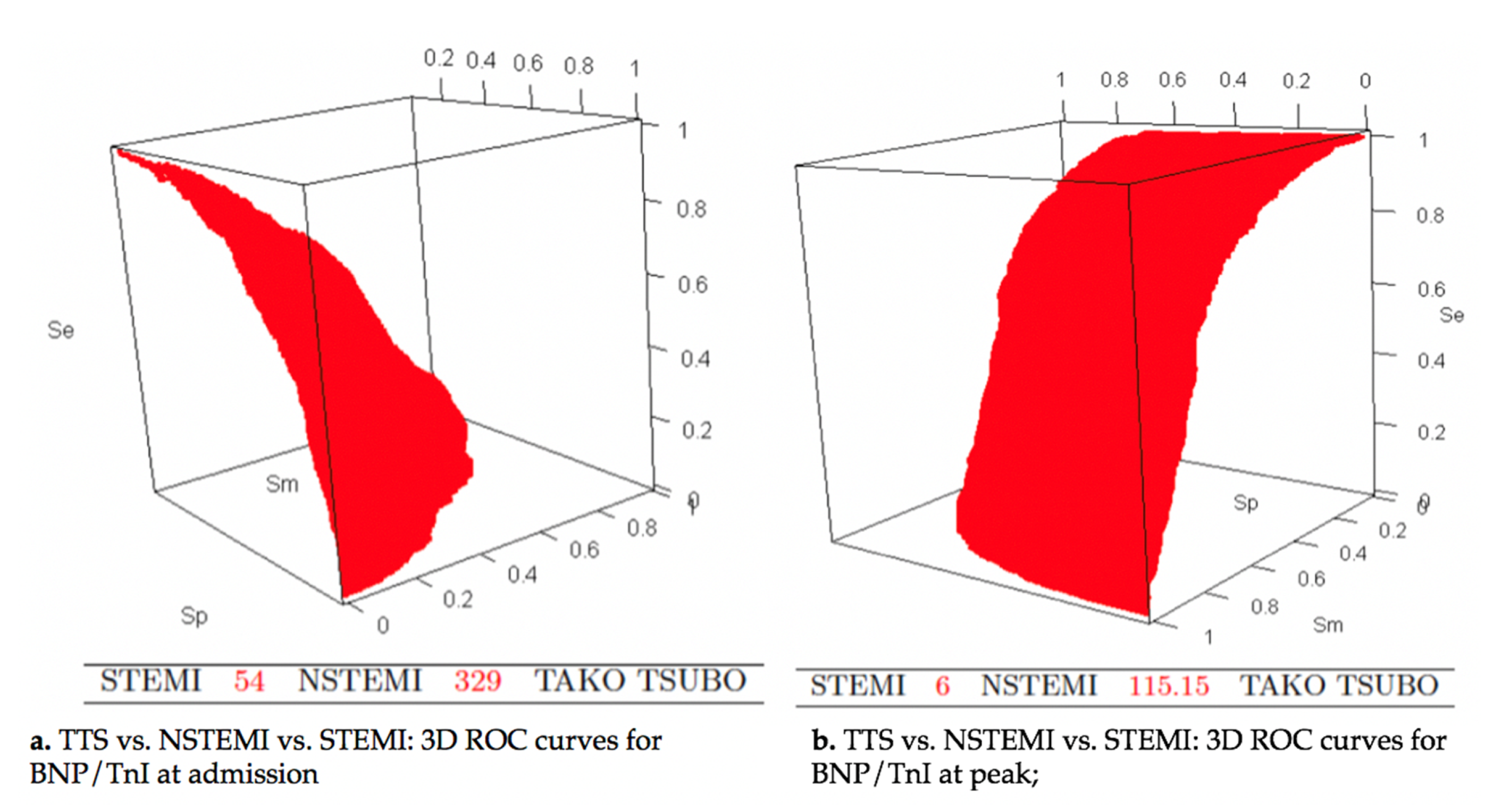
| BNP/Tn I at admission | 68.25 | 0.8216 | 0.6371 | 0.8395 | 0.6071 | 79.9 | (76.5–83.3) |
| Peak BNP/peak TnI | 39.25 | 0.8810 | 0.9362 | 0.9188 | 0.9056 | 96.0 | (94.6–97.3) |
| Diagnostic score | 0.3851 | 0.8788 | 0.8386 | 0.9158 | 0.7759 | 92.6 | (90.7–94.5) |
| Simplified diagnostic score | 43 | 0.9242 | 0.7735 | 0.9413 | 0.7219 | 93.3 | (91.5–95.1) |
| TTS vs. NSTEMI | |||||||
| BNP/TnI at admission | 196.25 | 0.658 | 0.5589 | 0.6434 | 0.5747 | 61.9 | (57.2–66.5) |
| Peak BNP/peak TnI | 76.5 | 0.7857 | 0.6578 | 0.7586 | 0.6916 | 75.8 | (71.9–79.6) |
| Diagnostic score | 0.4406 | 0.8951 | 0.8513 | 0.8936 | 0.8533 | 93.8 | (92.0–95.7) |
| Simplified diagnostic score | 42 | 0.8192 | 0.9138 | 0.8494 | 0.8950 | 92.4 | (90.2–94.5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagrenat, C.; Von Hunolstein, J.J.; Matsushita, K.; Thebaud, L.; Greciano, S.; Tuzin, N.; Meyer, N.; Trinh, A.; Jesel, L.; Ohlmann, P.; et al. Value of Cardiac Biomarkers in the Early Diagnosis of Takotsubo Syndrome. J. Clin. Med. 2020, 9, 2985. https://doi.org/10.3390/jcm9092985
Dagrenat C, Von Hunolstein JJ, Matsushita K, Thebaud L, Greciano S, Tuzin N, Meyer N, Trinh A, Jesel L, Ohlmann P, et al. Value of Cardiac Biomarkers in the Early Diagnosis of Takotsubo Syndrome. Journal of Clinical Medicine. 2020; 9(9):2985. https://doi.org/10.3390/jcm9092985
Chicago/Turabian StyleDagrenat, Charlotte, Jean Jacques Von Hunolstein, Kensuke Matsushita, Lucie Thebaud, Stéphane Greciano, Nicolas Tuzin, Nicolas Meyer, Annie Trinh, Laurence Jesel, Patrick Ohlmann, and et al. 2020. "Value of Cardiac Biomarkers in the Early Diagnosis of Takotsubo Syndrome" Journal of Clinical Medicine 9, no. 9: 2985. https://doi.org/10.3390/jcm9092985
APA StyleDagrenat, C., Von Hunolstein, J. J., Matsushita, K., Thebaud, L., Greciano, S., Tuzin, N., Meyer, N., Trinh, A., Jesel, L., Ohlmann, P., & Morel, O. (2020). Value of Cardiac Biomarkers in the Early Diagnosis of Takotsubo Syndrome. Journal of Clinical Medicine, 9(9), 2985. https://doi.org/10.3390/jcm9092985






