Unconventional Yeasts Are Tolerant to Common Antifungals, and Aureobasidium pullulans Has Low Baseline Sensitivity to Captan, Cyprodinil, and Difenoconazole
Abstract
:1. Introduction
2. Results
2.1. Isolation and Identification of Naturally Occurring Yeasts Insensitive to Commonly Used Fungicides
2.1.1. Yeasts Are Tolerant to Commonly Used Antifungal Agents
2.1.2. Aureobasidium pullulans Is the Most Frequently Isolated Species in the Presence of Antifungals
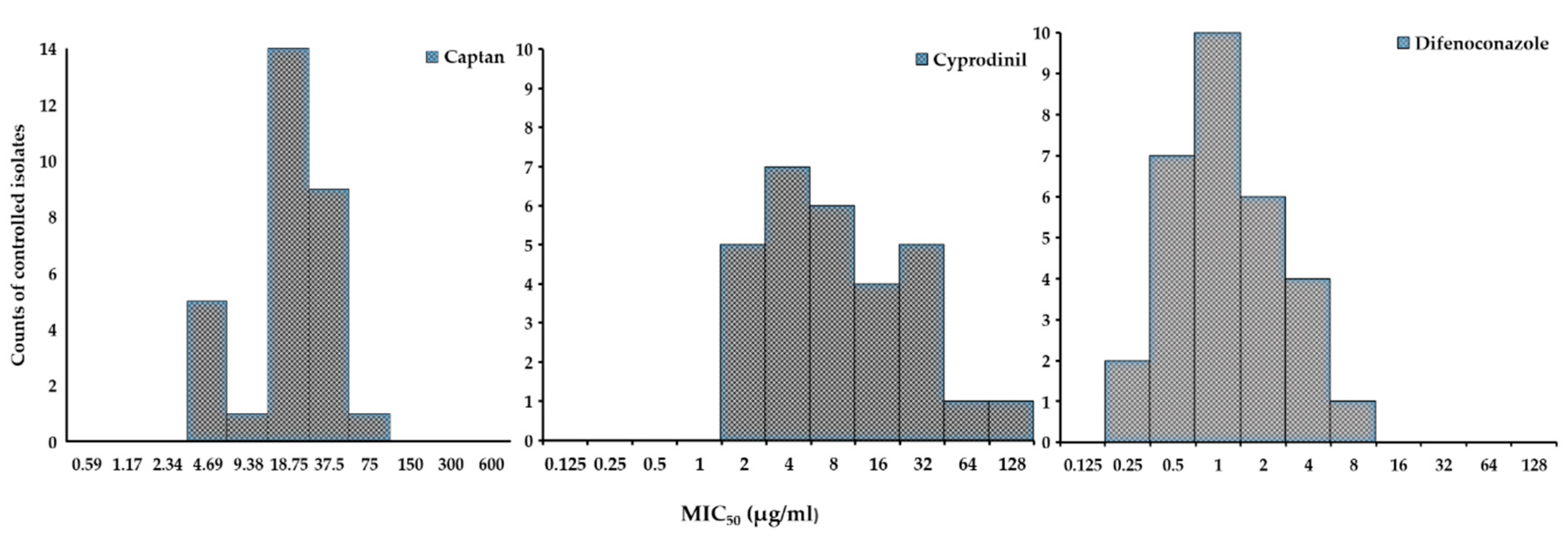
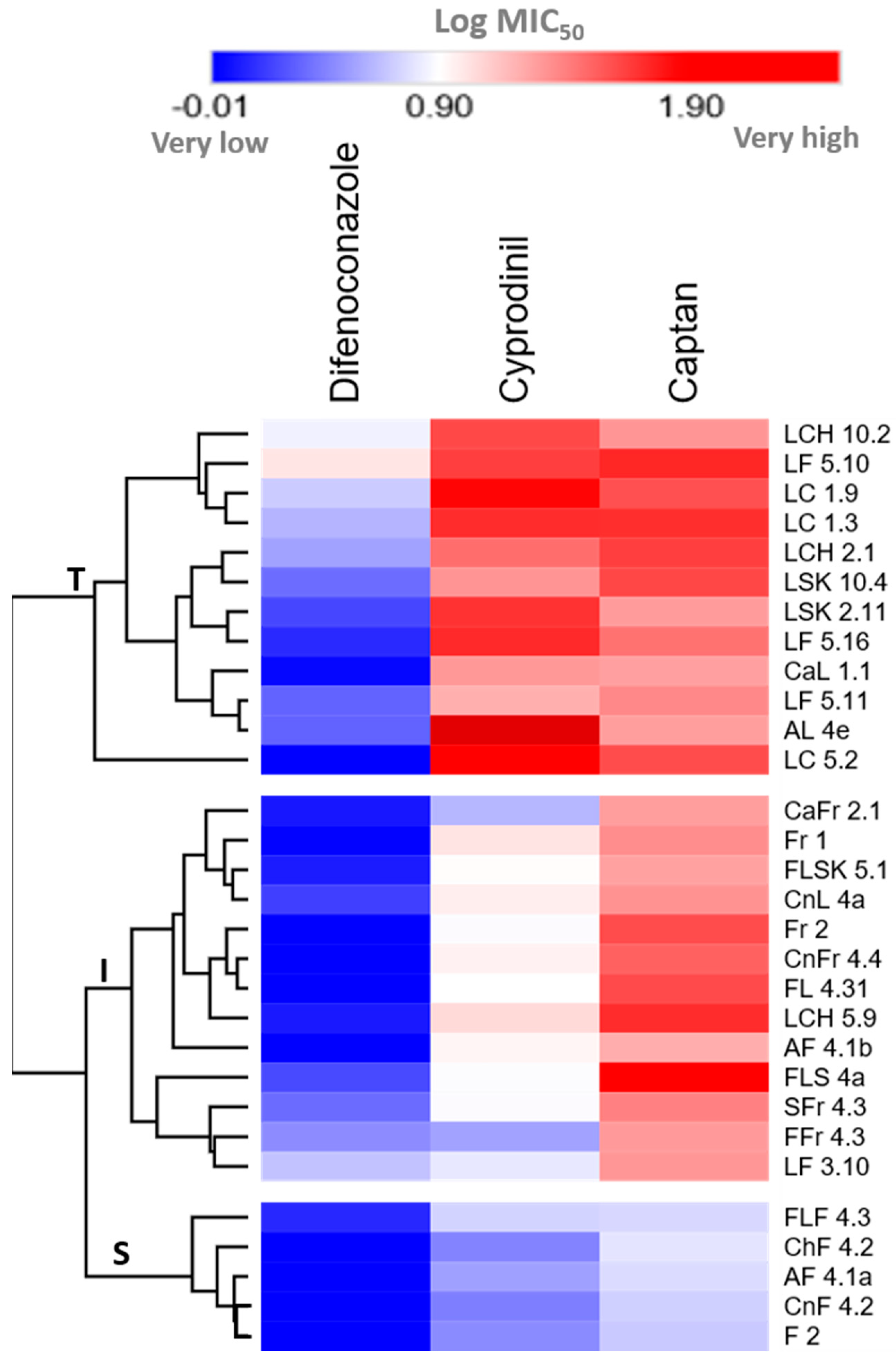
2.2. The Baseline Sensitivities and MIC50 of 30 A. pullulans Isolates
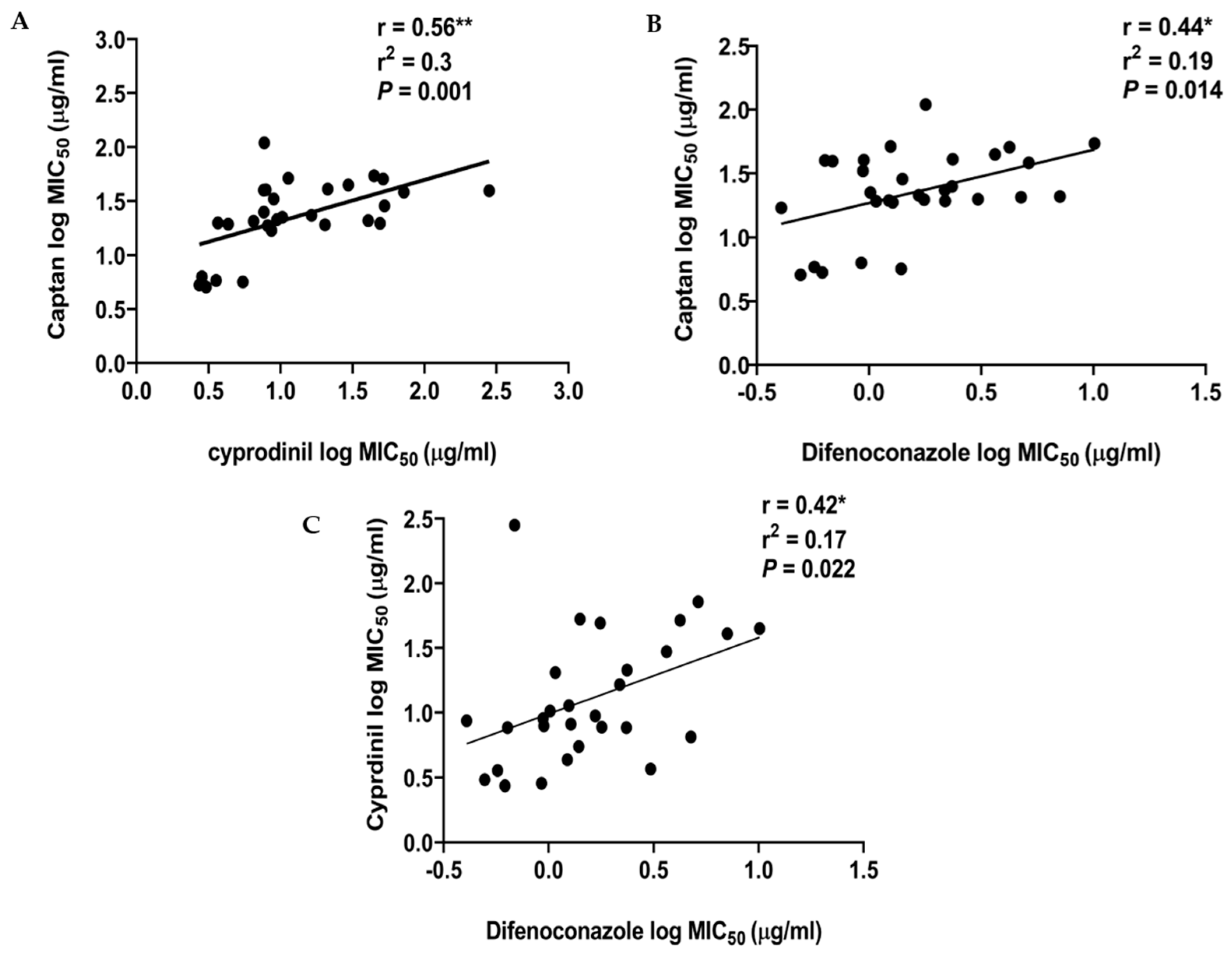
2.3. The MIC50 Values for the 30 A. pullulans Isolates for CPN, CYP, and DFN Show a Significant, Positive Correlation
3. Discussion
4. Materials and Methods
4.1. Fungal Isolate Collection and Storage
4.2. Fungal Identification
4.3. Determination of Baseline MIC50 Values
4.4. Statistical Analyses
4.5. Phylogenic Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boyce, K.; Morrissey, O.; Idnurm, A.; Macreadie, I. Insights into the global emergence of antifungal drug resistance. Microbiol. Aust. 2019. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 2015, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Ishii, H.; Holloman, D. Fungicide Resistance in Plant Pathogens; Ishii, H., Hollomon, D.W., Eds.; Springer: Tokyo, Japan, 2015; Volume 10, p. 481. [Google Scholar]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020. [Google Scholar] [CrossRef]
- Buck, J. Combinations of fungicides with phylloplane yeasts for improved control of Botrytis cinerea on geranium seedlings. Phytopathology 2004, 94, 196–202. [Google Scholar] [CrossRef]
- Lima, G.; De Curtis, F.; Piedimonte, D.; Spina, A.M.; De Cicco, V. Integration of biocontrol yeast and thiabendazole protects stored apples from fungicide sensitive and resistant isolates of Botrytis cinerea. Postharvest Biol. Technol. 2006, 40, 301–307. [Google Scholar] [CrossRef]
- Schisler, D.A.; Boehm, M.J.; Paul, P.A.; Rooney, A.P.; Dunlap, C.A. Reduction of Fusarium head blight using prothioconazole and prothioconazole-tolerant variants of the Fusarium head blight antagonist Cryptococcus flavescens OH 182.9. Biol. Control 2015, 86, 36–45. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, T.; Sheng, K.; Zeng, L.; Ye, C.; Yu, T.; Zheng, X. Effect of pyrimethanil on Cryptococcus laurentii, Rhodosporidium paludigenum, and Rhodotorula glutinis biocontrol of Penicillium expansum infection in pear fruit. Int. J. Food Microbiol. 2013, 164, 155–160. [Google Scholar] [CrossRef]
- De Curtis, F.; De Cicco, V.; Lima, G. Efficacy of biocontrol yeasts combined with calcium silicate or sulphur for controlling durum wheat powdery mildew and increasing grain yield components. Field Crops Res. 2012, 134, 36–46. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Conway, W.S. Combining biological control with physical and chemical treatments to control fruit decay after harvest. Stewart Postharvest Rev. 2010, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- De Curtis, F.; Ianiri, G.; Raiola, A.; Ritieni, A.; Succi, M.; Tremonte, P.; Castoria, R. Integration of biological and chemical control of brown rot of stone fruits to reduce disease incidence on fruits and minimize fungicide residues in juice. J. Crop Prot. 2019, 119, 158–165. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.; Hamada, M.; Li, N.; Li, G.; Luo, C. Multiple fungicide resistance in Botrytis cinerea from greenhouse strawberries in Hubei Province, China. Plant Dis. 2017, 101, 601–606. [Google Scholar] [CrossRef] [Green Version]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 29–92. [Google Scholar]
- Brent, K.; Hollomon, D. Fungicide Resistance: The Assessment of Risk; Croplife International: Brussels, Belgium, 2007; Volume FRAC, Monograph 2. [Google Scholar]
- FRAC. FRAC Code List© 2020: Fungicides Sorted by Mode of Action (Including FRAC Code Numbering). Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2020-final.pdf?sfvrsn=8301499a_ (accessed on 16 March 2020).
- Amiri, A.; Zuniga, A.; Peres, N. Potential impact of populations drift on Botrytis occurrence and resistance to multi-and single-site fungicides in Florida southern highbush blueberry fields. Plant Dis. 2018, 102, 2142–2148. [Google Scholar] [CrossRef] [Green Version]
- Barak, E.; Edgington, L. Botrytis cinerea resistant to captan: The effect of inoculum age and type on response to the fungicide. Can. J. Plant Pathol. 1984, 6, 211–214. [Google Scholar] [CrossRef]
- Prasongsuk, S.; Ployngam, S.; Wacharasindhu, S.; Lotrakul, P.; Punnapayak, H. Effects of sugar and amino acid supplementation on Aureobasidium pullulans NRRL 58536 antifungal activity against four Aspergillus species. Appl. Microbiol. Biotechnol. 2013, 97, 7821–7830. [Google Scholar] [CrossRef]
- Leathers, T.D.; Price, N.P.; Bischoff, K.M.; Manitchotpisit, P.; Skory, C.D. Production of novel types of antibacterial liamocins by diverse strains of Aureobasidium pullulans grown on different culture media. Biotechnol. Lett. 2015, 37, 2075–2081. [Google Scholar] [CrossRef]
- Prasongsuk, S.; Lotrakul, P.; Ali, I.; Bankeeree, W.; Punnapayak, H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2018, 63, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, T.; Chandrasekaran, A.; Kuttalam, S.; Senthilraja, G.; Samiyappan, R. Integrated control of fruit rot and powdery mildew of chilli using the biocontrol agent Pseudomonas fluorescens and a chemical fungicide. Biol. Control 2010, 52, 1–7. [Google Scholar] [CrossRef]
- Russell, P. Sensitivity Baselines in Fungicide Resistance Research and Management; Fungicide Resistance Action Committee Monograph 3; Crop Life International: Brussels, Belgium, 2002. [Google Scholar]
- Jurick, W.M.; Macarisin, O.; Gaskins, V.L.; Janisiewicz, W.J.; Peter, K.A.; Cox, K.D. Baseline sensitivity of Penicillium spp. to difenoconazole. Plant Dis. 2019, 103, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Keinath, A.P. Baseline sensitivity of Didymella bryoniae to cyprodinil and fludioxonil and field efficacy of these fungicides against isolates resistant to pyraclostrobin and boscalid. Plant Dis. 2015, 99, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Myresiotis, C.; Bardas, G.; Karaoglanidis, G. Baseline sensitivity of Botrytis cinerea to pyraclostrobin and boscalid and control of anilinopyrimidine-and benzimidazole-resistant strains by these fungicides. Plant Dis. 2008, 92, 1427–1431. [Google Scholar] [CrossRef] [Green Version]
- Villani, S.M.; Biggs, A.R.; Cooley, D.R.; Raes, J.J.; Cox, K.D. Prevalence of myclobutanil resistance and difenoconazole insensitivity in populations of Venturia inaequalis. Plant Dis. 2015, 99, 1526–1536. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T. The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Zalar, P.; Gostinčar, C.; De Hoog, G.; Uršič, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Leroux, P. Chemical control of Botrytis and its resistance to chemical fungicides. In Botrytis: Biology, Pathology and Control; Springer: Berlin, Germany, 2007; pp. 195–222. [Google Scholar] [CrossRef]
- Leroux, P.; Walker, A.-S. Activity of fungicides and modulators of membrane drug transporters in field strains of Botrytis cinerea displaying multidrug resistance. Eur. J. Plant Pathol. 2013, 135, 683–693. [Google Scholar] [CrossRef]
- Motulsky, H. Prism 5 statistics Guide, 2007; GraphPad Software: San Diego, CA, USA, 2007; Volume 31, pp. 39–42. [Google Scholar]
- Van Den Bosch, F.; Paveley, N.; Shaw, M.; Hobbelen, P.; Oliver, R. The dose rate debate: Does the risk of fungicide resistance increase or decrease with dose? Plant Pathol. 2011, 60, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It be Managed? Citeseer: Forest Grove, OR, USA, 1995. [Google Scholar]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Dudášová, H.; Balaščáková, M. Yeasts in agricultural and managed soils. In Yeasts in Natural Ecosystems: Diversity; Springer: Berlin, Germany, 2017; pp. 117–144. [Google Scholar] [CrossRef]
- Yurkov, A.M. Yeasts of the soil–obscure but precious. Yeast 2018, 35, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Connell, L.; Redman, R.; Craig, S.; Rodriguez, R. Distribution and abundance of fungi in the soils of Taylor Valley, Antarctica. Soil Biol. Biochem. 2006, 38, 3083–3094. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Dudášová, H.; Stratilová, E.; Balaščáková, M. Diversity of yeasts in the soil adjacent to fruit trees of the Rosaceae family. Yeast 2019, 36, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Sláviková, E.; Vadkertiová, R. Effects of pesticides on yeasts isolated from agricultural soil. Z. Naturforsch. C. 2003, 58, 855–859. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Sláviková, E. Infl uence of Pesticides on Yeasts Colonizing Leaves. Z. Naturforsch. C 2011, 66, 588–594. [Google Scholar] [CrossRef]
- Teodoro, V.L.I.; Gullo, F.P.; Sardi, J.d.C.O.; Torres, E.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Environmental isolation, biochemical identification, and antifungal drug susceptibility of Cryptococcus species. Rev. Soc. Bras. Med. Trop. 2013, 46, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Najafzadeh, M.J.; Sutton, D.A.; Keisari, M.S.; Zarrinfar, H.; De Hoog, G.S.; Chowdhary, A.; Meis, J.F. In vitro activities of eight antifungal drugs against 104 environmental and clinical isolates of Aureobasidium pullulans. Antimicrob. Agents Chemother. 2014, 58, 5629–5631. [Google Scholar] [CrossRef] [Green Version]
- Sláviková, E.; Vadkertiová, R.; Vránová, D. Yeasts colonizing the leaf surfaces. J. Basic Microbiol. 2007, 47, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Vadkertiová, R.; Molnárová, J.; Vránová, D.; Sláviková, E. Yeasts and yeast-like organisms associated with fruits and blossoms of different fruit trees. Can. J. Microbiol. 2012, 58, 1344–1352. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, L.; Zhang, Z.; Huang, H.; Zhang, Z.; Jiang, L. Protective role of trehalose during radiation and heavy metal stress in Aureobasidium subglaciale F134. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zajc, J.; Zalar, P.; Gunde-Cimerman, N. Yeasts in hypersaline habitats. In Yeasts in Natural Ecosystems: Diversity; Springer: Berlin, Germany, 2017; pp. 293–329. [Google Scholar] [CrossRef]
- Gostinčar, C.; Ohm, R.A.; Kogej, T.; Sonjak, S.; Turk, M.; Zajc, J.; Zalar, P.; Grube, M.; Sun, H.; Han, J. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genom. 2014, 15, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brysch-Herzberg, M.; Seidel, M. Yeast diversity on grapes in two German wine growing regions. Int. J. Food Microbiol. 2015, 214, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Vadkertiová, R.; Sláviková, E. Metal tolerance of yeasts isolated from water, soil and plant environments. J. Basic Microbiol. 2006, 46, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Raghukumar, C.; Parvatkar, R.R.; Mascarenhas-Pereira, M. Heavy metal tolerance in the psychrotolerant Cryptococcus sp. isolated from deep-sea sediments of the Central Indian Basin. Yeast 2013, 30, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Beltran, G.; Torija, M.J. The role of the membrane lipid composition in the oxidative stress tolerance of different wine yeasts. Food Microbiol. 2019, 78, 143–154. [Google Scholar] [CrossRef]
- Hayes, B.M.; Anderson, M.A.; Traven, A.; van der Weerden, N.L.; Bleackley, M.R. Activation of stress signalling pathways enhances tolerance of fungi to chemical fungicides and antifungal proteins. Cell. Mol. Life Sci. 2014, 71, 2651–2666. [Google Scholar] [CrossRef]
- Hilber, U.W.; Schüepp, H. A reliable method for testing the sensitivity of Botryotinia fuckeliana to anilinopyrimidines in vitro. Pestic. Sci. 1996, 47, 241–247. [Google Scholar] [CrossRef]
- Johnson, K.A. Characterization and Fungicide Efficacy of North Carolina Colletotrichum Populations Causing Glomerella Leaf Spot and Fruit Rot on Apple. Master’s Thesis, Science North Carolina State University, Raleigh, NC, USA, 2018. [Google Scholar]
- Cowgill, W.; Oudamans, P.; Ward, D.; Rosenberger, D. Not Understanding phytotoxicity can damage your bottom line. Fruit Notes 2013, 78, 15–23. [Google Scholar]
- Arce, G.T.; Gordon, E.B.; Cohen, S.M.; Singh, P. Genetic toxicology of folpet and captan. Crit. Rev. Toxicol. 2010, 40, 546–574. [Google Scholar] [CrossRef] [Green Version]
- Gordon, E.B.; Tali, E.; Scott, M.; Michael, W. Measurement of the reaction between the fungicides captan or folpet and blood thiols. Toxicol. Methods 2001, 11, 209–223. [Google Scholar] [CrossRef]
- Kurz, M.H.; Batista, J.L.d.S.; de Oliveira, L.G.; Hoff, R.; Martins, M.L.; Gonçalves, F.F. Clean-up Procedure Development and Method Validation for Pesticide Residues Analysis in Carrots. Food Anal. Methods 2019, 12, 282–292. [Google Scholar] [CrossRef]
- Baroffio, C.A.; Siegfried, W.; Hilber, U.W. Long-term monitoring for resistance of Botryotinia fuckeliana to anilinopyrimidine, phenylpyrrole, and hydroxyanilide fungicides in Switzerland. Plant Dis. 2003, 87, 662–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseka, D.; Gudmestad, N. Spatial and temporal sensitivity of Alternaria species associated with potato foliar diseases to demethylation inhibiting and anilino-pyrimidine fungicides. Plant Dis. 2016, 100, 1848–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiaccadori, R. In Vitro, In Vivo and in Field Sensitivity of Venturia inaequalis to Anilinopyrimidine Fungicides with Different Types of Scab Management and Degree of Control. Open Access Libr. J. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Hou, Y.-P.; Mao, X.-W.; Qu, X.-P.; Wang, J.-X.; Chen, C.-J.; Zhou, M.-G. Molecular and biological characterization of Sclerotinia sclerotiorum resistant to the anilinopyrimidine fungicide cyprodinil. Pestic. Biochem. Physiol. 2018, 146, 80–89. [Google Scholar] [CrossRef]
- Mosbach, A.; Edel, D.; Farmer, A.D.; Widdison, S.; Barchietto, T.; Dietrich, R.A.; Corran, A.; Scalliet, G. Anilinopyrimidine resistance in Botrytis cinerea is linked to mitochondrial function. Front. Microbiol. 2017, 8, 2361. [Google Scholar] [CrossRef]
- Ali, E.M.; Amiri, A. Selection pressure pathways and mechanisms of resistance to the demethylation inhibitor-difenoconazole in Penicillium expansum. Front. Microbiol. 2018, 9, 2472. [Google Scholar] [CrossRef]
- Villani, S.M.; Hulvey, J.; Hily, J.-M.; Cox, K.D. Overexpression of the CYP51A1 gene and repeated elements are associated with differential sensitivity to DMI fungicides in Venturia inaequalis. Phytopathology 2016, 106, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeufer, E.E.; Ngugi, H.K. Orchard factors associated with resistance and cross resistance to sterol demethylation inhibitor fungicides in populations of Venturia inaequalis from Pennsylvania. Phytopathology 2012, 102, 272–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbelen, P.; Paveley, N.; Van den Bosch, F. Delaying selection for fungicide insensitivity by mixing fungicides at a low and high risk of resistance development: A modeling analysis. Phytopathology 2011, 101, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, F.; Paveley, N.; van den Berg, F.; Hobbelen, P.; Oliver, R. Mixtures as a fungicide resistance management tactic. Phytopathology 2014, 104, 1264–1273. [Google Scholar] [CrossRef]
- Elderfield, J.A.; Lopez-Ruiz, F.J.; van den Bosch, F.; Cunniffe, N.J. Using epidemiological principles to explain fungicide resistance management tactics: Why do mixtures outperform alternations? Phytopathology 2018, 108, 803–817. [Google Scholar] [CrossRef] [Green Version]
- Freimoser, F.M.; Hilber-Bodmer, M.; Brunisholz, R.; Drissner, D. Direct identification of Monilinia brown rot fungi on infected fruits by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. Chem. Biol. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Hilber-Bodmer, M.; Schmid, M.; Ahrens, C.H.; Freimoser, F.M. Competition assays and physiological experiments of soil and phyllosphere yeasts identify Candida subhashii as a novel antagonist of filamentous fungi. BMC Microbiol. 2017, 17, 4. [Google Scholar] [CrossRef] [Green Version]
- Koljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [Green Version]
- Motulsky, H. Prism 4 Statistics Guide—Statistical Analyses for Laboratory and Clinical Researchers; GraphPad Software Inc.: San Diego, CA, USA, 2003; pp. 122–126. [Google Scholar]
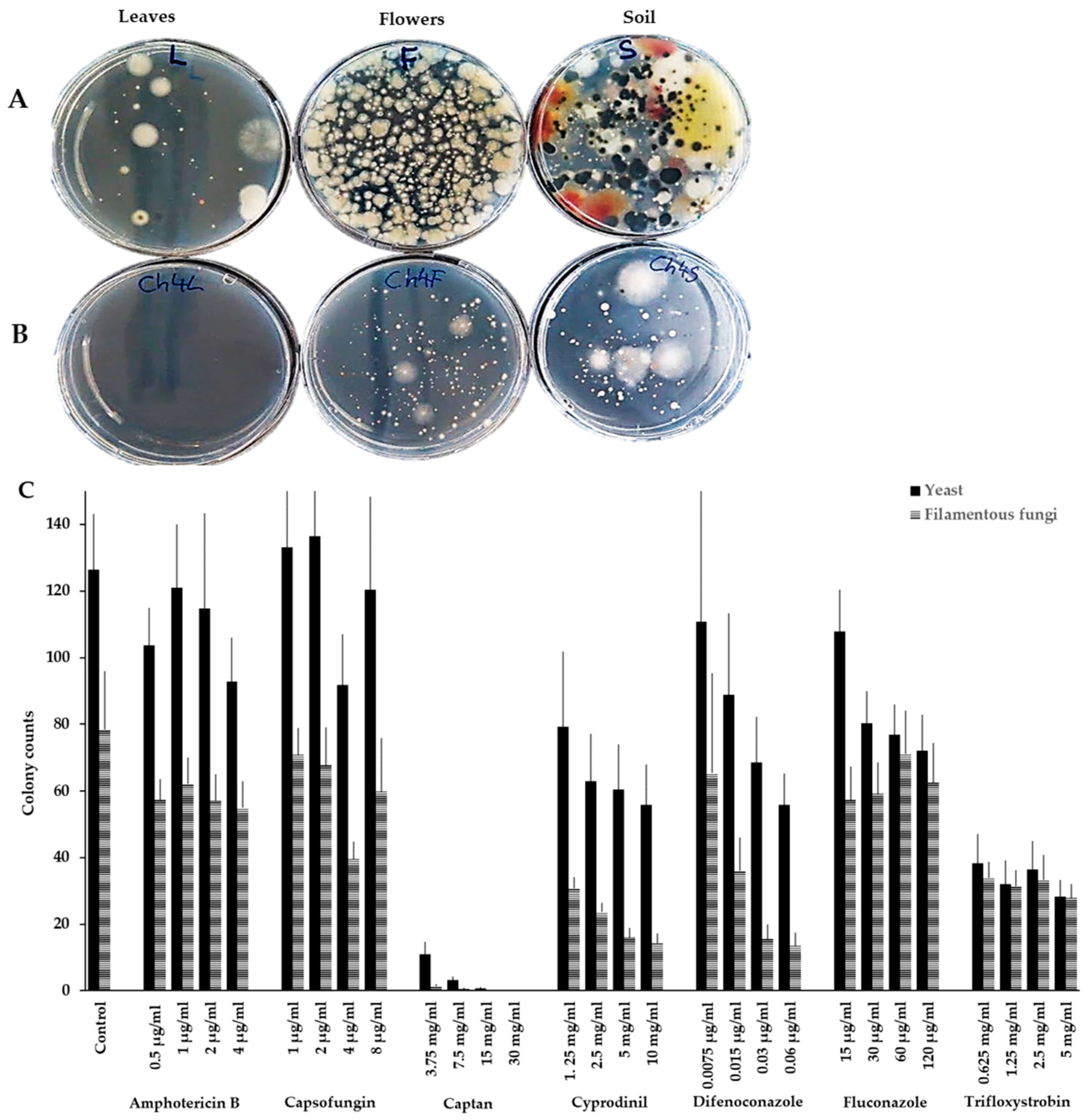


| Soil | Flower | Leaf | Fruit |
|---|---|---|---|
| Aureobasidium pullulans | Aureobasidium pullulans | Aureobasidium pullulans | Aureobasidium pullulans |
| Holtermaniella | Holtermaniella | Holtermaniella | Holtermaniella |
| Sporidiobulus metaroseus | Sporidiobulus metaroseus | Sporodiobolus metaroseus | Sporidiobolus metaroseus |
| Metschnikowia pulcherrima | Metschnikowia pulcherrima | Metschnikowia pulcherrima | |
| Cryptococcus laurentii | Cryptococcus laurentii | ||
| Cyberlindnera misumaiensis | Cyberlindneramisumaiensis | ||
| Hanseniaspora uvarum | Hanseniaspora uvarum | ||
| Cystofilobasidium macerans | Cystofilobasidium macerans | ||
| Bullera alba | Bullera alba | ||
| Sporidiobolaceae pararoseus | Sporodiobolaceae pararoseus | ||
| Schwanniomyces capriottii | Schwanniomyces capriottii | ||
| Rhodotorula | Rhodotorula | ||
| Apiotrichum porosum | |||
| Barnettozyma california | |||
| Candida californica | |||
| Coniochaeta | |||
| Cryptococcus | |||
| Cyberlindnera saturnus | |||
| Cystofilobasidiacea | |||
| Cystofilobasidium capitatum | |||
| Dipodascaceae | |||
| Dipodascus geotrichum | |||
| Hanseniospora | |||
| Kregervanrija fluxuum | |||
| Pichia mandshurica | |||
| Pichia sporocuriosa | |||
| Pichia terricola | |||
| Pichiaceae | |||
| Saccharomycopsis | |||
| Saccharomycopsis schoenii | |||
| Saccharomycopsis vini | |||
| Saitozyma podzolica | |||
| Schwanniomyces pseudopolymorphus | |||
| Sporidiobolaceae | |||
| Tremellomycetes | |||
| Trichosporon | |||
| Wickerhamomyces anomalus | |||
| Zygosaccharomyces microellipsoides | |||
| Erythrobasidium hasegawianum | |||
| Filobasidium | |||
| Filobasidium floriforme | |||
| Filobasidium magnum | |||
| Rhodotorula graminis | |||
| Curvibasidium | |||
| Isolate Name | MIC50 µg/mL | Isolating Fungicide | ||
|---|---|---|---|---|
| Captan | Cyprodinil | Difenoconazole | ||
| F2 | 5.1 | 2.8 | 0.5 | None |
| Fr1 | 22.5 | 9 | 1 | None |
| Fr2 | 40 | 7.4 | 0.6 | None |
| SFr4.3 | 25.1 | 7.9 | 2.4 | Slick (DFN) |
| LSK 2.11 | 19.7 | 49.3 | 1.8 | Slick (DFN) |
| FLSK 5.1 | 18.8 | 7.5 | 1.3 | Slick (DFN) |
| LSK 10.4 | 41 | 18.9 | 2.4 | Slick (DFN) |
| LCH 10.2 | 20.9 | 34.1 | 7.1 | Chorus (CYP) |
| LCH 5.9 | 51.5 | 11.9 | 1.3 | Chorus (CYP) |
| ChF4.2 | 6.3 | 2.2 | 0.9 | Chorus (CYP) |
| LCH 2.1 | 44.7 | 29.7 | 3.7 | Chorus (CYP) |
| CaL1.1 | 19.1 | 20.6 | 1.1 | Captan 80 WD (CPN) |
| CaFr2.1 | 19.4 | 3.8 | 1.2 | Captan 80 WD (CPN) |
| LC 5.2 | 39.5 | 186 | 0.7 | Captan 80 WD (CPN) |
| LC 1.9 | 38.4 | 59.6 | 5.2 | Captan 80 WD (CPN) |
| LC 1.3 | 50.8 | 50.5 | 4.2 | Captan 80 WD (CPN) |
| LF 3.10 | 20.6 | 3.4 | 4.8 | Flint (Trifloxystrobin) |
| LF 5.11 | 23.4 | 14.1 | 2.2 | Flint (Trifloxystrobin) |
| FFr4.3 | 19.9 | 3.2 | 3.1 | Flint (Trifloxystrobin) |
| LF 5.16 | 28.7 | 42.8 | 1.4 | Flint (Trifloxystrobin) |
| LF 5.10 | 54.4 | 41.9 | 10.1 | Flint (Trifloxystrobin) |
| AF4.1b | 17 | 8.9 | 0.4 | Amphotericin B |
| AL4e | 19.3 | 1.45 × 1039 | 2.2 | Amphotericin B |
| AF4.1a | 5.8 | 3.6 | 0.6 | Amphotericin B |
| CnF4.2 | 5.3 | 2 | 0.6 | Capsofungin |
| CnL4a | 21.4 | 6 | 1.7 | Capsofungin |
| CnFr4.4 | 33.1 | 9 | 0.9 | Capsofungin |
| FL4.31 | 40.2 | 8.3 | 1 | Fluconazole |
| FLF 4.3 | 5.7 | 4.7 | 1.4 | Fluconazole |
| FLS4a | 109.6 | 6.6 | 1.8 | Fluconazole |
| Mean | 28.9 | 22.6 * | 2.2 | |
| Median | 21.9 | 8.9 | 1.4 | |
| Range | 5.1–109.5 | 2.0–186 * | 0.4–10.1 | |
| No | Isolate Name | Sample | Season Isolated |
|---|---|---|---|
| 1 | F2 | Flower | Spring |
| 2 | Fr1 | Fruit | Summer |
| 3 | Fr2 | Fruit | Summer |
| 4 | AF4.1b | Flower | Summer |
| 5 | AL4e | Leaf | Summer |
| 6 | AF4.1a | Flower | Spring |
| 7 | LF 3.10 | Leaf | Autumn |
| 8 | LF 5.11 | Leaf | Autumn |
| 9 | FFr4.3 | Fruit | Summer |
| 10 | CaL1.1 | Leaf | Summer |
| 11 | CaFr2.1 | Fruit | Summer |
| 12 | LC 5.2 | Leaf | Autumn |
| 13 | CnF4.2 | Flower | Spring |
| 14 | CnL4a | Leaf | Summer |
| 15 | CnFr4.4 | Fruit | Summer |
| 16 | FL4.31 | Leaf | Summer |
| 17 | FLF 4.3 | Leaf | Spring |
| 18 | FLS4a | Leaf | Spring |
| 19 | LCH 10.2 | Leaf | Autumn |
| 20 | LCH 5.9 | Leaf | Autumn |
| 21 | ChF4.2 | Flower | Spring |
| 22 | SFr4.3 | Fruit | Summer |
| 23 | LSK 2.11 | Leaf | Autumn |
| 24 | FLSK 5.1 | Leaf | Winter |
| 25 | LC 1.9 | Leaf | Autumn |
| 26 | LC 1.3 | Leaf | Autumn |
| 27 | LCH 2.1 | Leaf | Autumn |
| 28 | LSK 10.4 | Leaf | Autumn |
| 29 | LF 5.16 | Leaf | Autumn |
| 30 | LF 5.10 | Leaf | Autumn |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magoye, E.; Hilber-Bodmer, M.; Pfister, M.; Freimoser, F.M. Unconventional Yeasts Are Tolerant to Common Antifungals, and Aureobasidium pullulans Has Low Baseline Sensitivity to Captan, Cyprodinil, and Difenoconazole. Antibiotics 2020, 9, 602. https://doi.org/10.3390/antibiotics9090602
Magoye E, Hilber-Bodmer M, Pfister M, Freimoser FM. Unconventional Yeasts Are Tolerant to Common Antifungals, and Aureobasidium pullulans Has Low Baseline Sensitivity to Captan, Cyprodinil, and Difenoconazole. Antibiotics. 2020; 9(9):602. https://doi.org/10.3390/antibiotics9090602
Chicago/Turabian StyleMagoye, Electine, Maja Hilber-Bodmer, Melanie Pfister, and Florian M. Freimoser. 2020. "Unconventional Yeasts Are Tolerant to Common Antifungals, and Aureobasidium pullulans Has Low Baseline Sensitivity to Captan, Cyprodinil, and Difenoconazole" Antibiotics 9, no. 9: 602. https://doi.org/10.3390/antibiotics9090602
APA StyleMagoye, E., Hilber-Bodmer, M., Pfister, M., & Freimoser, F. M. (2020). Unconventional Yeasts Are Tolerant to Common Antifungals, and Aureobasidium pullulans Has Low Baseline Sensitivity to Captan, Cyprodinil, and Difenoconazole. Antibiotics, 9(9), 602. https://doi.org/10.3390/antibiotics9090602






